Abstract
Purpose
Fast-track surgery is a developing trend in medical care. It is a core challenge for clinical anesthesia to reasonably reduce the dosage of opioids and relieve postoperative pain. Serratus anterior plane block (SAPB) is a novel analgesic technique with such advantages as easy operation, good safety, and few side effects.
Patients and Methods
In total, 60 patients aged 18 to 65 years who were diagnosed with lung cancer and scheduled for thoracoscopic resection were randomly assigned to receive SABP or local infiltration anesthesia. We analyzed the time within 48 hrs after operation to visual analogue scale (VAS) pain score of 4 or higher and the number of patients requiring additional analgesics at 6 hrs and 12 hrs after operation.
Results
The estimated median time to VAS ≥4 was 4 hrs (1.32 to 6.68) in the control group and 11 hrs (6.71 to 15.29) in the SAPB group (log-rank test: P=0.008). The number of patients requiring additional analgesics at 6- and 12 hrs after operation was significantly lower in the SAPB group than that in the control group (P<0.05).
Conclusion
Compared with local infiltration, SAPB provided extended postoperative analgesia after thoracoscopic surgery with reduced consumption of additional analgesics in the early postoperative stage.
Keywords: fast-track surgery, video-assisted thoracic surgery, serratus anterior plane block, analgesia, clinical anesthesia, randomized clinical trial
Introduction
Video-assisted thoracoscopic surgery (VATS) has been maturing steadily and enables lobectomy, wedge resection, and mediastinal tumor resection. Wider application of modern VATS reduces operative trauma, allows earlier ambulation, and supports postoperative recovery of lung function,1–3 with potentially reduced medical costs. The pain after VATS is usually moderate and is mainly incisional pain at the intercostal muscles and soft tissues and pain at the chest tube site.4–11 Multi-modal analgesic management,12 which is increasingly the basis of pain control protocols, might include oral non-steroidal anti-inflammatory drugs (NSAIDs), intravenous opioids, and regional blocks.13,14 Ideal analgesia provides effective pain relief, increases patient comfort, and minimizes side effects. With advances in ultrasonic guidance, regional block techniques are becoming more common,3,15–19 and include thoracic epidural block (TEB), paravertebral block, and erector spinae block. TEB is still the gold standard for analgesia in thoracic surgery, but it requires normal coagulation function, and the failure rate in clinical practice is up to 30% due to difficult catheterization and catheter detachment.9,20 In addition, high epidural anesthesia easily induces hypotension requiring fluid replacement and possibly administration of vasoactive drugs, which can harm the microcirculation, to restore hemodynamic stability. Other possible adverse reactions include nausea, vomiting, and pruritus, perhaps related to the epidural administration of opioids.21 Paravertebral block can work well in relieving thoracic pain, but requires multi-site injection, potentially increasing the risk of overdose of local anesthetics, pneumothorax, and spinal cord or nerve injury.22,23 Finally, erector spinae block is mainly used for the treatment of posterior chest pain, and its effect on postoperative pain of the lateral and anterolateral chest wall remains to be further studied.24 Serratus anterior plane block (SAPB) is a novel chest wall block technique that was introduced by Blanco et al16 in 2013. SAPB is a regional block that was originally developed for analgesia after breast surgery. Its mechanism relies on the probable existence of two potential spaces on the surface of the serratus anterior muscles, a superficial serratus anterior plane between serratus anterior and latissimus dorsi and a deep serratus anterior plane between the serratus anterior and the intercostal muscles. Local anesthetics injected at these two planes block the intercostal nerves and thus alleviate chest wall pain. SABP targets the T2 to T9 intercostal nerve branches to establish analgesia at the lateral chest wall16. The technique is gradually being adopted for VATS, although there are as yet few randomized controlled trials to support its use in this context.25,26 Our primary aim in this randomized controlled study was to compare the time within 48 hrs postoperatively to visual analogue pain score (VAS) ≥4 between SABP and local infiltration anesthesia in patients undergoing VATS for lung cancer. The secondary aim was to compare the use of postoperative analgesics between the groups. We hypothesized that SAPB would provide longer lasting analgesia after VATS (vs. local infiltration).
Methods
Patient Selection
This study was approved by the Ethics Committee of Shengjing Hospital of China Medical University (Shenyang, Liaoning, China) (protocol number: 2018PS087J) and conducted in accordance with the regulations of the Declaration of Helsinki for research involving human subjects. Written informed consent was obtained from all patients participating in the trial. The trial was registered with the Chinese Clinical Trial Registry prior to patient enrollment (ChiCTR1800016525; Principal investigator: Bo Long; Date of registration: June 6, 2018).
Patients who underwent VATS lobectomy under general anesthesia from June 20, 2018 to May 31, 2019 were eligible. The inclusion criteria were as follows: (1) elective thoracoscopic surgery under general anesthesia; (2) age 18 to 65 years with body mass index (BMI)=18 to 25 kg/m2 and American Society of Anesthesiologists (ASA) grade I–III. The exclusion criteria were: (1) long-time use of analgesic drugs; (2) past history of chest surgery; (3) allergy to local anesthetics; (4) systemic infection; (5) cognitive and language disorders precluding participation.
Randomization and Blinding
The patients were divided into SAPB and control groups using a random number table, and the group assignments were stored in a sealed envelope, known only to the physicians performing the nerve block procedures. The patients, surgeons, anesthetists responsible for the intraoperative anesthesia management (surgical anesthetists), and the physicians performing postoperative pain evaluations were all blinded to the group assignments, and group assignments were unsealed and blocks performed only with the surgeons and the surgical anesthetists away from the operating room.
Surgical Anesthesia Methods
Before operation, all patients received intramuscular midazolam (0.04 mg/kg) injection. Intravenous access was routinely established once the patients entered the operating room, and non-invasive blood pressure (BP), electrocardiogram (ECG), peripheral blood oxygen saturation by pulse oximetry (SpO2), bispectral index (BIS) (DRAGER, Germany), and pain threshold index (PTi) (Easymonitor, China) were monitored. Direct arterial blood pressure monitoring was performed by catheterization via radial artery puncture under local anesthesia. After preoxygenation, induction anesthesia was conducted by slow intravenous bolus of sufentanil, 0.3 µg/kg, propofol, 2 mg/kg, and rocuronium bromide, 0.6 mg/kg. Once the patients were asleep and showing muscle relaxation, a proper double-lumen endotracheal tube was inserted and mechanical ventilation to keep end-tidal carbon dioxide partial pressure (PetCO2) at 35 to 45 mmHg was initiated. During operation, general anesthesia was maintained with intravenous infusion of propofol and remifentanil by pump. The infusion rate of propofol and the concentration of remifentanil were adjusted to keep the BIS between 45 and 55 and the PTi between 40 and 60. The infusion of propofol and remifentanil was stopped before skin closing, and the patients were sent to the post anesthesia care unit (PACU) after waking, recovery from muscle relaxation, and removal of the endotracheal tube at the end of surgery.
Nerve Block Methods
After the induction of general anesthesia, the patients were kept in a lateral position. The surgeons identified and marked the incision site and then left the operating room together with the surgical anesthetists. Thereafter, local blocks were performed by anesthetists who were not participating in the intraoperative management. For the SAPB group, a 9 to 13.0 MHz high-frequency linear array ultrasonic probe (GE color Doppler ultrasonograph, USA) encased in sterile isolation film for ultra-thin endoscopes was placed longitudinally on the chest wall at the fifth rib in the midaxillary line for scanning. The probe was fixed after positioning to display latissimus dorsi, serratus anterior, and intercostal muscles, and a 22-gauge (80-mm) nerve block needle was inserted in the fifth intercostal space along the plane superior to the serratus anterior muscle. After withdrawing the plunger to check for blood and air, a 2-mL tracer was injected to confirm the position of the needle tip, followed by injection of 20 mL 0.5% ropivacaine. The same procedures were followed for the control group and the SAPB group, but rather than SAPB, the patients in the control group received local infiltration anesthesia, performed under direct vision by injection of a total of 20 mL 0.5% ropivacaine at the marked incision site. After these steps were completed, the surgeons and surgical anesthetists returned to the operating room and continued with routine sterile preparation, draping, and the planned surgical procedure, allowing 20 min after the block procedures for local anesthetic absorption and diffusion before starting the operation.
Analgesic Methods
Patients in both groups received intravenous sufentanil injection, 0.1 µg/kg, and intravenous flurbiprofen axetil infusion, 50 mg, plus ramosetron hydrochloride, 0.3 mg, at 30 min before completion of the operation. The postoperative intravenous analgesia pump was prepared as follows: butorphanol tartrate 0.1 mg/kg + flurbiprofen axetil 2.5 mg/kg in 0.9% NaCl injection for a total volume of 100 mL, flow rate 2 mL/h. If the postoperative VAS was ≥5, patients received an intravenous infusion of flurbiprofen axetil 50 mg, which was repeated after 6 hrs as needed. Oral oxycodone with acetaminophen was also given as needed. The VAS was based on a scale of 1 to 10, with 0 to 2=comfortable; 3 to 4=slight discomfort; 5 to 6=moderate discomfort; 7 to 8=severe discomfort; and 9 to 10=extreme discomfort.
Study Indicators
The primary indicator was the time within 48 hrs postoperatively to the first VAS score ≥4. The secondary indicators were remifentanil and propofol requirement during operation, the number of patients requiring additional analgesics at 6 hrs, 12 hrs, 24 hrs, and 48 hrs after operation, and the rate of adverse reactions.
Statistical Analysis
SPSS 17.0 software was used. The quantitative data, qualitative data, and event-time data were presented as means ± standard deviation (SD), percentages (%), and medians (interquartile range, IQR), respectively. Quantitative data of normal or abnormal distribution were analyzed with t-test or Mann–Whitney U-test. The qualitative data were compared using χ2 test or Fisher’s exact test. The event-time data were analyzed with Kaplan-Meier curve and log-rank test. P<0.05 suggested that a difference was statistically significant.
Sample Size
Sample size calculations were based on a pilot study (12 patients). The primary indicator was the time to VAS score 4 during the first 48 hrs postoperatively. The estimated median time after operation to the first VAS score of 4 was 4 h (SD=9.34) with local anesthetic infiltration in thoracoscopic surgery. We hypothesized that successful SAPB would last for 12 hrs (90% power and a 5% significance level), which would require 25 patients per group. To account for missing patient data, we included 30 patients in each group.
Results
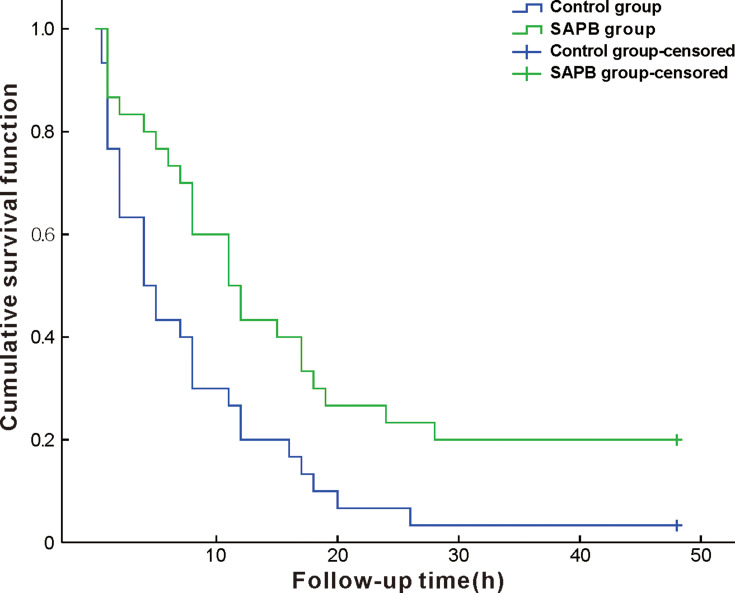
Total 76 patients were eligible for the study, of which 12 were excluded (6 did not sign informed consent, 2 with a history of chest surgery, 3 using analgesic drugs, and 1 with difficult language expression), and 64 were enrolled. After randomization, four additional cases were excluded because of intermediate switch to thoracotomy, and 60 patients (32 male and 28 female) were finally included in the statistical analysis (Figure 1). There were no statistically significant differences between groups in sex, age, BMI, or operation times (P>0.05)(Table 1). The estimated median time after operation to the first point VAS score 4 was 4 h (1.32 to 6.68) in the control group and 11 hrs (6.71 to 15.29) in the SAPB group (survival curve analysis [log rank test]: P=0.008), indicating longer lasting postoperative analgesia with ultrasound-guided SAPB vs. local infiltration anesthesia (Figure 2).
Figure 1.
Flow diagram of the study design.
Abbreviation: SAPB, serratus anterior plane block
Table 1.
Comparison of General Clinical Data Between Two Groups
| Group | N | Male/Female (N) | Age (Years) (Mean±SD) | BMI (kg/m2) (Mean±SD) | Operation Time (min) (Mean±SD) |
|---|---|---|---|---|---|
| Control | 30 | 17/13 | 58.23±9.03 | 23.80±2.95 | 98.23±27.88 |
| SAPB | 30 | 15/15 | 56.20±7.20 | 24.47±2.87 | 107.73±27.16 |
| P | 0.612 | 0.339 | 0.378 | 0.186 |
Notes: Data were analyzed by t-test; continuous variables of normal distribution are expressed as mean ± standard deviation (SD).
Figure 2.
Kaplan–Meier curves comparing time to VAS =4 within 48 hrs after operation in two groups (patients were censored if VAS did not reach 4 or above within 48 hrs after surgery); survival curve analysis (log-rank test): P=0.008.
Abbreviations: SAPB, serratus anterior plane block; VAS, visual analogue scale.
The number of patients requiring additional analgesia at 6 hrs and 12 hrs after surgery was significantly higher (P<0.05) in the control group vs. the SAPB group (Table 2), but there was no significant difference between groups in analgesia requirement after 12 hrs, and there were no significant differences between groups in the intraoperative doses of remifentanil and propofol (P>0.05)(Table 3) or in the incidence of postoperative nausea and vomiting (P>0.05)(Table 4).
Table 2.
The Number of Patients Requiring Additional Analgesics at Different Time Points After Surgery (N)
| Group | 6hrs | 12hrs | 24hrs | 48hrs |
|---|---|---|---|---|
| Control | 11 | 15 | 3 | 1 |
| SAPB | 3 | 6 | 3 | 2 |
| P | 0.03 | 0.03 | 1 | 1 |
Note: The data were analyzed by Fisher’s exact test.
Table 3.
Intraoperative Remifentanil and Propofol Dose (mean±SD)
| Group | N | Intraoperative Remifentanil Dose Operation (μg) | Intraoperative Propofol Dose (mg) |
|---|---|---|---|
| Control | 30 | 242.76±98.42 | 762.24±102.61 |
| SAPB | 30 | 250.98±65.84 | 748.58±71.61 |
| P | 0.705 | 0.552 |
Notes: Data were analyzed by t-test, and continuous variables of normal distribution are expressed as means± standard deviation (SD).
Table 4.
Adverse Reactions
| Group | Nausea and Vomiting[n(%)] |
|---|---|
| Control | 3 (10%) |
| SAPB | 4 (13%) |
| P | 1.00 |
Notes: Data were analyzed by Fisher’s exact test, and the categorical variables are presented as n (%).
Discussion
Our study results suggest that SAPB with 20 mL 0.5% ropivacaine can reduce early postoperative pain after VATS lung resection with few side effects.
SAPB showed good results in randomized controlled trials for postoperative analgesia after breast surgery. There was no significant difference in VAS scores between the SAPB group and a placebo group during the first 24 hrs after operation, the doses of opioids and paracetamol in the SAPB group were significantly lower, and the time to the first use of analgesics was also evidently longer.27 These findings further supported the efficacy of SAPB for postoperative relief of chest wall pain and confirmed preliminarily studies proving the usefulness of SAPB in mitigating postoperative chest wall pain while reducing the postoperative dosage of opioids.10,25 Recent evaluations have also supported the use of SAPB in VATS. A comparison of SAPB and local infiltration anesthesia for postoperative analgesia after VATS found that patients in the SAPB group had better VAS scores during the first 8 h postoperatively, and the dose of opioids in SAPB group was far lower.28 A recent meta-analysis by De Cassai et al29 on the analgesic effect of SAPB in patients undergoing thoracoscopic surgery showed that general anesthesia combined with SAPB reduced perioperative pain compared with general anesthesia alone. The analgesic score in the SAPB group at 6-, 12-, and 24 hrs after surgery was lower than that of the control group, but the analgesic effect at 6 hrs was better than that at 24 hrs. In this study, we found that the time to VAS score 4 after the operation was significantly longer (median 11 hrs) in the SAPB group vs. the control group, and the number of patients requiring breakthrough analgesics during the first 12 hrs after operation was significantly lower, confirming that SAPB is superior to local infiltration at the incision site for postoperative pain management after VATS lung resection. The duration of analgesia in our SAPB group exceeded that reported by Chen,28 which might be explained by higher concentrations of local anesthetics used in our study.
There are two injection options for SAPB. Blanco et al16 held that local anesthetics injected at the superficial serratus anterior plane had a wider diffusion range. Piracha et al30 compared deep-plane vs. superficial plane injection for SAPB in four patients undergoing breast surgery and found that analgesia might be better after deep-plane injection, while Abdallah et al31 found similar analgesic effects at both planes after breast surgery. Effective analgesia has been documented with SAPB at either plane, and large-scale comparisons of the definite analgesic effects of SAPB in these two planes are yet to be published. In this study, we evaluated superficial SAPB in VATS patients and found that compared to local infiltration, SAPB provided longer lasting and more effective pain relief in the early postoperative period, possibly because the local anesthetics in SAPB diffuse slowly and are absorbed in the space between two layers of muscle, while the local anesthetics injected at the incision site are absorbed and metabolized rapidly in the subcutaneous tissues and muscles.
There is no consensus on the ideal injection volume of local anesthesia for SAPB. Kunigo et al32 compared SAPB with 20 mL 0.375% ropivacaine and 40 mL 0.375% ropivacaine for postoperative analgesia after breast cancer surgery and found that the higher volume produced a better analgesic effect, while the block effect of the lower dose was sufficient and might be safer. In this study, we did not explore the relation between the volume of local anesthetics and the block range, and we achieved a good analgesic effect using 20 mL of 0.5% ropivacaine.
One factor influencing the pain after VATS that is not addressed by previous trials of SAPB is the position of the chest tube and the visceral pain during breathing caused by stimulation of pleura by the tube. Park et al26 noted that their study of SABP may have been limited by variations in thoracoscopic operating techniques among participating surgeons. In our study, therefore, we examined cases from the same surgeon, the positioning of the thoracic drainage tubes was the same, and the κ-receptor agonist butorphanol was added in the analgesic pump to relieve the visceral pain. Additionally, compared with untreated control groups in Park et al26 and Kim et al33 this study compared SABP with local infiltration anesthesia, adding meaningful results.
This study has the following limitations. First, although nerve block or local anesthesia was performed in the absence of the operating surgeons and surgical anesthetists, the block methods could be judged according to the needle puncture trace. However, the surgical anesthetists adjusted the anesthesia based on sedation threshold index and PTi monitoring, which has little impact on the anesthesia depth and the administration of opioids. Self-reporting is considered as the gold standard for clinical determination of the presence and severity of pain but is unfeasible for patients under general anesthesia or with cognitive disorders or coma. Therefore, more and more objective evaluation methods are coming into use. General anesthesia significantly inhibits the activities of the cerebral cortex but keeps intact the activities of the subcortical autonomic nervous system. During general anesthesia, signs of the autonomic response to harmful stimuli may include changes in heart rate, blood pressure, pupil diameter and pulse wave amplitude, and diaphoresis, all of which are caused by suppression of or changes in the balance of sympathetic and parasympathetic nerve activity. Fluctuations in the PTi are thought to reflect these autonomic responses to noxious stimuli and might be more sensitive indicators of pain than the hemodynamic parameters. PTi was used to titer the dose of opioids in this study,34 although the reliability of PTi needs to be further validated. Second, in SAPB following general anesthesia, measurement of the anesthetic range is unfeasible, and the diffusion of local anesthetics on the surface of the serratus anterior muscles can only be observed by ultrasonography. Third, as postoperative pain management was guided by thoracic surgeons, the common formulae for analgesic pumps were conventionally used in this study, but the pain scores and medications were monitored and recorded. Finally, local infiltration anesthesia with ropivacaine was performed under direct vision at the marked incision site and the operation was started after local anesthetic absorption and diffusion. However, even if the surgeon could judge the grouping by signs of the local block at the incision site, it should have little influence on the final result, because the surgical anesthetist adjusted the anesthesia according to the monitoring, the dosage of the analgesia pump was configured postoperatively according to the patient’s weight, and the pain score was monitored by an anesthesiologist who was blinded to the treatment, and not the operating surgeon.
Conclusion
In summary, ultrasound-guided SAPB is a safe and convenient regional nerve block technique for VATS that effectively relieves early wound pain, provides longer-lasting analgesia than local infiltration at the incision, and limits the need for early postoperative breakthrough analgesia.
Acknowledgments
We would like to acknowledge and express our deepest gratitude to the participants of this study.
Funding Statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. There is no funding to report.
Abbreviations
ASA, American Society of Anesthesiologists; BIS, bispectral index; BMI, body mass index; BP, blood pressure; ECG, electrocardiogram; IQR, interquartile range; NSAIDs, non-steroidal anti-inflammatory drugs; PACU, post-anesthesia care unit; PetCO2, end-tidal carbon dioxide partial pressure; PTi, pain threshold index; SAPB, serratus anterior plane block; SD, standard deviation; SpO2, oxygen saturation; TEB, thoracic epidural block; VAS, visual analogue scale; VATS, video-assisted thoracoscopic surgery.
Data Sharing Statement
We intend to share individual deidentified patient data which is included in the manuscript. No further specific data or study documents will be shared. The data shared will be accessible on the website (http://www.chictr.org.cn/addproject2.aspx) within 6 months after the study is received.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from every patient included in the study.
Author Contributions
All authors made a significant contribution to the work reported, whether in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all of these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest for this work.
References
- 1.McKenna RJ Jr., Houck W, Fuller CB. Video-assisted thoracic surgery lobectomy: experience with 1100 cases. Ann Thorac Surg. 2006;81(2):421–5; discussion 425–6. doi: 10.1016/j.athoracsur.2005.07.078 [DOI] [PubMed] [Google Scholar]
- 2.Nagahiro I, Andou A, Aoe M, et al. Pulmonary function, postoperative pain, and serum cytokine level after lobectomy: a comparison of VATS and conventional procedure. Ann Thorac Surg. 2001;72(2):362–365. doi: 10.1016/S0003-4975(01)02804-1 [DOI] [PubMed] [Google Scholar]
- 3.Sugiura H, Morikawa T, Kaji M, et al. Long-term benefits for the quality of life after video-assisted thoracoscopic lobectomy in patients with lung cancer. Surg Laparosc Endosc Percutan Tech. 1999;9(6):403–408. doi: 10.1097/00129689-199912000-00007 [DOI] [PubMed] [Google Scholar]
- 4.Corcoran JP, Psallidas I, Wrightson JM, Hallifax RJ, Rahman NM. Pleural procedural complications: prevention and management. J Thorac Dis. 2015;7(6):1058–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gerner P. Postthoracotomy pain management problems. Anesthesiol Clin. 2008;26(2):355–67, vii. doi: 10.1016/j.anclin.2008.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blanco R, Fajardo M, Parras Maldonado T. Ultrasound description of Pecs II (modified Pecs I): a novel approach to breast surgery. Rev Esp Anestesiol Reanim. 2012;59(9):470–475. doi: 10.1016/j.redar.2012.07.003 [DOI] [PubMed] [Google Scholar]
- 7.Faccenda KA, Finucane BT. Complications of regional anaesthesia incidence and prevention. Drug Saf. 2001;24(6):413–442. doi: 10.2165/00002018-200124060-00002 [DOI] [PubMed] [Google Scholar]
- 8.Jeng CL, Torrillo TM, Rosenblatt MA. Complications of peripheral nerve blocks. Br J Anaesth. 2010;105(Suppl 1):i97–i107. doi: 10.1093/bja/aeq273 [DOI] [PubMed] [Google Scholar]
- 9.Khalil AE, Abdallah NM, Bashandy GM, Kaddah TA. Ultrasound-guided serratus anterior plane block versus thoracic epidural analgesia for thoracotomy pain. J Cardiothorac Vasc Anesth. 2017;31(1):152–158. doi: 10.1053/j.jvca.2016.08.023 [DOI] [PubMed] [Google Scholar]
- 10.Okmen K, Okmen BM. The efficacy of serratus anterior plane block in analgesia for thoracotomy: a retrospective study. J Anesth. 2017;31(4):579–585. [DOI] [PubMed] [Google Scholar]
- 11.Okmen K, Okmen BM, Uysal S. Serratus Anterior Plane (SAP) block used for thoracotomy analgesia: a case report. Korean J Pain. 2016;29(3):189–192. doi: 10.3344/kjp.2016.29.3.189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Umari M, Carpanese V, Moro V, et al. Postoperative analgesia after pulmonary resection with a focus on video-assisted thoracoscopic surgery. Eur J Cardiothorac Surg. 2018;53(5):932–938. doi: 10.1093/ejcts/ezx413 [DOI] [PubMed] [Google Scholar]
- 13.Papagiannopoulou P, Argiriadou H, Georgiou M, et al. Preincisional local infiltration of levobupivacaine vs ropivacaine for pain control after laparoscopic cholecystectomy. Surg Endosc. 2003;17(12):1961–1964. doi: 10.1007/s00464-002-9256-1 [DOI] [PubMed] [Google Scholar]
- 14.Sihoe AD, Manlulu AV, Lee TW, Thung KH, Yim AP. Pre-emptive local anesthesia for needlescopic video-assisted thoracic surgery: a randomized controlled trial. Eur J Cardiothorac Surg. 2007;31(1):103–108. doi: 10.1016/j.ejcts.2006.09.035 [DOI] [PubMed] [Google Scholar]
- 15.Blanco R. The “pecs block” a novel technique for providing analgesia after breast surgery. Anaesthesia. 2011;66(9):847–848. doi: 10.1111/j.1365-2044.2011.06838.x [DOI] [PubMed] [Google Scholar]
- 16.Blanco R, Parras T, McDonnell JG, Prats-Galino A. Serratus plane block: a novel ultrasound-guided thoracic wall nerve block. Anaesthesia. 2013;68(11):1107–1113. doi: 10.1111/anae.12344 [DOI] [PubMed] [Google Scholar]
- 17.Hebbard P, Fujiwara Y, Shibata Y, Royse C. Ultrasound-guided transversus abdominis plane (TAP) block. Anaesth Intensive Care. 2007;35(4):616–617. [PubMed] [Google Scholar]
- 18.Luketich JD, Land SR, Sullivan EA, et al. Thoracic epidural versus intercostal nerve catheter plus patient-controlled analgesia: a randomized study. Ann Thorac Surg. 2005;79(6):1845–9; discussion 1849–50. doi: 10.1016/j.athoracsur.2004.10.055 [DOI] [PubMed] [Google Scholar]
- 19.Senturk M, Ozcan PE, Talu GK, et al. The effects of three different analgesia techniques on long-term postthoracotomy pain. Anesth Analg. 2002;94(1):11–5, table of contents. doi: 10.1213/00000539-200201000-00003 [DOI] [PubMed] [Google Scholar]
- 20.Rosero EB, Joshi GP. Nationwide incidence of serious complications of epidural analgesia in the United States. Acta Anaesthesiol Scand. 2016;60(6):810–820. doi: 10.1111/aas.12702 [DOI] [PubMed] [Google Scholar]
- 21.Hermanides J, Hollmann MW, Stevens MF, Lirk P. Failed epidural: causes and management. Br J Anaesth. 2012;109(2):144–154. doi: 10.1093/bja/aes214 [DOI] [PubMed] [Google Scholar]
- 22.Lonnqvist PA, MacKenzie J, Soni AK, Conacher ID. Paravertebral blockade. Failure rate and complications. Anaesthesia. 1995;50(9):813–815. doi: 10.1111/j.1365-2044.1995.tb06148.x [DOI] [PubMed] [Google Scholar]
- 23.Michelet P, Guervilly C, Helaine A, et al. Adding ketamine to morphine for patient-controlled analgesia after thoracic surgery: influence on morphine consumption, respiratory function, and nocturnal desaturation. Br J Anaesth. 2007;99(3):396–403. doi: 10.1093/bja/aem168 [DOI] [PubMed] [Google Scholar]
- 24.Luftig J, Mantuani D, Herring AA, et al. Successful emergency pain control for posterior rib fractures with ultrasound-guided erector spinae plane block. Am J Emerg Med. 2018;36(8):1391–1396. doi: 10.1016/j.ajem.2017.12.060 [DOI] [PubMed] [Google Scholar]
- 25.Okmen K, Metin Okmen B. Evaluation of the effect of serratus anterior plane block for pain treatment after video-assisted thoracoscopic surgery. Anaesth Crit Care Pain Med. 2018;37(4):349–353. doi: 10.1016/j.accpm.2017.09.005 [DOI] [PubMed] [Google Scholar]
- 26.Park MH, Kim JA, Ahn HJ, et al. A randomised trial of serratus anterior plane block for analgesia after thoracoscopic surgery. Anaesthesia. 2018;73(10):1260–1264. doi: 10.1111/anae.14424 [DOI] [PubMed] [Google Scholar]
- 27.Rahimzadeh P, Imani F, Faiz SHR, Boroujeni BV. Impact of the ultrasound-guided serratus anterior plane block on post-mastectomy pain: a Randomised Clinical Study. Turk J Anaesthesiol Reanim. 2018;46(5):388–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen G, Li Y, Zhang Y, Fang X. Effects of serratus anterior plane block for postoperative analgesia after thoracoscopic surgery compared with local anesthetic infiltration: a randomized clinical trial. J Pain Res. 2019;12:2411–2417. doi: 10.2147/JPR.S207116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.De Cassai A, Boscolo A, Zarantonello F, et al. Serratus anterior plane block for video-assisted thoracoscopic surgery: a meta-analysis of randomised controlled trials. Eur J Anaesthesiol. 2020. doi: 10.1097/EJA.0000000000001290 [DOI] [PubMed] [Google Scholar]
- 30.Piracha MM, Thorp SL, Puttanniah V, Gulati A. “A Tale of Two Planes”:Deep Versus Superficial Serratus Plane Block for Postmastectomy Pain Syndrome. Reg Anesth Pain Med. 2017;42(2):259–262. [DOI] [PubMed] [Google Scholar]
- 31.Abdallah FW, Cil T, Maclean D, et al. Too deep or not too deep?: a propensity-matched comparison of the analgesic effects of a superficial versus deep serratus fascial plane block for ambulatory breast cancer surgery. Reg Anesth Pain Med. 2018;43(5):480–487. [DOI] [PubMed] [Google Scholar]
- 32.Kunigo T, Murouchi T, Yamamoto S, Yamakage M. Injection volume and anesthetic effect in serratus plane block. Reg Anesth Pain Med. 2017;42(6):737–740. doi: 10.1097/AAP.0000000000000649 [DOI] [PubMed] [Google Scholar]
- 33.Kim D-H, Oh YJ, Lee JG, et al. Efficacy of ultrasound-guided serratus plane block on postoperative quality of recovery and analgesia after video-assisted thoracic surgery: a randomized, triple-blind, Placebo-Controlled Study. Anesth Analg. 2018;126(4):1353–1361. doi: 10.1213/ANE.0000000000002779 [DOI] [PubMed] [Google Scholar]
- 34.Wu L, Wang S, Wang Y, et al. Prediction of hemodynamic reactivity by electroencephalographically derived pain threshold index in children undergoing general anesthesia: a Prospective Observational Study. J Pain Res. 2019;12:3245–3255. [DOI] [PMC free article] [PubMed] [Google Scholar]