Abstract
Objective
To evaluate serum tumor markers (STM) as predictive biomarkers in advanced non-small cell lung cancer (NSCLC) treated with chemo-immunotherapy.
Methods
Patients having received platinum-based chemo-(CHT) and PD-1/PD-L1-directed immune checkpoint inhibitor (ICI) combination therapy were retrospectively followed. Carcinoembryonic antigen (CEA), carbohydrate antigen 19–9 (CA19-9), cytokeratin-19 fragments (CYFRA 21–1) and neuron specific enolase (NSE) were routinely measured at NSCLC diagnosis. The marker with the highest relative elevation was defined “leading STM”, its change was assessed between CHT-ICI as well as mono-ICI maintenance initiation and the respective subsequent restaging. Corresponding computed tomography evaluations were analyzed using response evaluation criteria in solid tumors (RECIST). For CHT-ICI combination and subsequent mono-ICI-maintenance therapy, leading STM and RECIST response were evaluated regarding progression-free (PFS) and overall survival (OS) in Kaplan–Meier analyses.
Results
Among 80 CHT-ICI patients (41% women, mean age 63 years), median PFS was 5 months (M;4,9), median OS was 15M (10,/). PFS was significantly (p=0.042) longer, when the leading STM had decreased at first restaging under CHT-ICI combination therapy (9M (5,12; n=41) vs 5M (3,6; n=16)). In the 54 (67.5%) patients who received subsequent mono-ICI maintenance therapy, STM decrease was similarly associated with significantly (p<0.001) longer PFS (16M (7,/; n=16) vs 3.5M (2,6; n=22)). Patients with radiologically stable or progressive disease and concomitant leading STM decrease had similar PFS in the CHT-ICI combination phase (4M (3,7; n=16) vs 4.5M (2,6; n=14)), but longer PFS in the mono-ICI maintenance setting (13M (7,16; n=10) vs 3M (2,4; n=17)). Median OS was not reached in most subgroups.
Conclusion
Leading STM dynamics provide predictive biomarker information additional to radiological response evaluation patients receiving CHT-ICI combination therapy, especially in the mono-ICI maintenance setting.
Keywords: carcinoembryonic antigen, platinum doublet chemotherapy, pembrolizumab, CYFRA 21-1, RECIST, nivolumab
Introduction
The advent of programmed cell death protein 1/programmed death-ligand 1 (PD-1/PD-L1)-directed immune-checkpoint inhibitor (ICI) therapy for non-small cell lung cancer (NSCLC) has triggered a quest of finding biomarkers predicting response to such potentially effective treatment. Available baseline biomarkers like PD-L1 expression on tumor cells, tumor mutational burden or presence of a targetable genetic tumor alteration are being widely applied, but have only limited predictive properties on the individual patients’ level.1–4 For longitudinal follow-up in NSCLC therapy, radiological imaging by computed tomography (CT) is the only routine dynamic biomarker recommended.2 Radiological response to ICI therapy can however sometimes be misleading, as pseudo-progression, mixed responses or long-term stabilization can occur.2,5,6 Also, selected patients may benefit from ICI treatment beyond radiological progression, while others may incur rapid hyperprogression.2,5,7,8
Measurement of serum tumor markers (STM) is currently not recommended in diagnosis or management of advanced NSCLC.2 However, in chemotherapy (CHT) or tyrosine kinase inhibitor therapy, there is limited evidence that carcinoembryonic antigen (CEA), cytokeratin-19 fragments (CYFRA 21–1) or carbohydrate antigen 19–9 (CA19-9) can be used in treatment monitoring and longitudinal follow-up.9–11 Concerning ICI therapies, retrospective analyses suggest that baseline STM concentrations may have predictive relevance,12,13 and STM dynamics under ICI treatment could aid to estimate therapy outcomes.14–16
We have recently reported on the utility of STM dynamics in NSCLC patients undergoing ICI monotherapy, where we could demonstrate that the early change of the leading STM out of a panel of CEA, CYFRA 21–1, CA19-9 and neuron-specific enolase (NSE) predicted progression-free (PFS) and overall survival (OS). Of interest, a leading STM decrease upon concomitant stable or progressive disease in first radiological restaging could identify patients with considerably more advantageous outcomes.15
Within the last years, the application of ICI in advanced NSCLC without targetable genetic alterations has shifted away from monotherapy in second or later-line to first-line chemo-immunotherapy (CHT-ICI), especially in patients with a PD-L1 expression <50%.2,17–19 Given our previous results concerning STM as biomarkers for ICI monotherapy, we now aimed to evaluate, if early STM dynamics had similar predictive properties also in the CHT-ICI setting.
Patients and Methods
We retrospectively selected all consecutive patients having received at least one cycle of platinum-based doublet CHT and PD-1/PD-L1-directed ICI substances for advanced (stage IV or not otherwise treatable stage III)20 NSCLC at the lung cancer unit of Kepler University Hospital Linz, Austria until December 2019.
The patient registry as well as the present evaluation have been approved by the ethics committee of the federal state of Upper-Austria (EK Nr. 1139/2019). This study was conducted in accordance with the guidelines for the REporting of tumor MARKer studies (REMARK).21
Standard CHT-ICI combinations used were carboplatin/pemetrexed with pembrolizumab for non-squamous and carboplatin/paclitaxel with pembrolizumab for squamous-cell carcinomas.17,18 Few patients received carboplatin/paclitaxel and bevacizumab plus atezolizumab.19 According to our institutional standard proceedings, cisplatin was not used in these therapeutic regimens and CHT-ICI was routinely given for four cycles if tolerated. In maintenance therapy for patients having responded or stabilized on CHT-ICI, only the respective ICI but no CHT substance was applied. An earlier switch to maintenance mono-ICI could be performed if the treating physician deemed the continuation of combination therapy inappropriate due to CHT-associated toxicity. Treatment beyond radiological progression could be conducted in selected cases with significant clinical benefit.
Restaging was routinely conducted after every two cycles of CHT-ICI and after every three cycles of maintenance-ICI, examinations could be preponed due to symptoms suggesting disease progression or therapy-associated side-effects. Radiological response was routinely assessed by a CT scan of the chest and the upper abdomen using iodinated contrast medium unless contraindicated and additional imaging like cerebral magnetic resonance tomography if necessary, according to the clinician’s judgement. For this study, radiological response was reevaluated by two expert thoracic radiologists and graded by response evaluation criteria in solid tumors (RECIST, version 1.1) for first response under CHT-ICI and first response under mono-ICI maintenance therapy (CR-complete remission, PR-partial remission, SD-stable disease, PD-progressive disease).22
Routine blood sampling at primary lung cancer staging at our center includes analysis of CEA, CYFRA 21–1, CA19-9 and NSE. STM are not analyzed in hemolyzed blood samples according to the institutional standard laboratory proceedings. All STM initially elevated above the upper limit of normal are analyzed at every restaging together with imaging. For this study, STM analyses were conducted using a cobas e 801 immunoassay module (Roche Diagnostics, Rotkreuz, Switzerland) and the corresponding ElectroChemiLuminescence-ImmunoAssay (ECLIA) kits acquired from Roche. Upper limits of normal were 3.4ng/mL for CEA, 3.3ng/mL for CYFRA 21–1, 27U/mL for CA 19–9, and 16.3ng/mL for NSE. If more than one STM was available upon CHT-ICI initiation, the “leading” marker with the highest elevation in relation to the upper limit of normal was selected for follow-up. If none of the analyzed STM was elevated at CHT-ICI therapy initiation, the leading STM was determined as the one with the highest value in relation to the upper limit of normal. STM response was expressed as fraction of restaging versus baseline concentration.
Progression-free and overall survival were assessed separately for two individual therapeutic phases: From first CHT-ICI combination as well as from first mono-ICI-maintenance therapy application on to death or the date of the last verified contact. Disease progression was retrospectively defined by imaging and death, as well as by reviewing the relevant medical records. Therapy line was defined as treatment for non-curable (eg stage IV20 or not otherwise treatable stage III) disease, whereas previous therapies in potentially curable stages were not considered. We excluded patients in clinical trials and patients who had previously received ICI treatment.
For each therapy phase, Kaplan-Meier-analyses for PFS and OS were conducted for all patients, as well as according to the change in STM (decrease, increase) and to RECIST response criteria (CR/PR, SD, PD). Results were expressed as median in months (M) and 95% confidence interval (CI) unless otherwise specified. The resulting curves were compared using the Log rank test and a p-value<0.05 was regarded statistically significant. Uni- and multivariate models for predictive factors for PFS and OS in both therapeutic phases were calculated using Cox-regression analyses. Next to RECIST and STM response, variables analyzed were age (</≥70 years), sex, Eastern Cooperative Oncology Group (ECOG) performance status (0,1,≥2) and PD-L1 expression on tumor cells, determined using the 22C3 assay for Autostainer Link 48 by Dako (Agilent Technologies, Santa Clara, CA). A negative PD-L1 status was defined as membranous staining on <1% of viable tumor cells.
Results
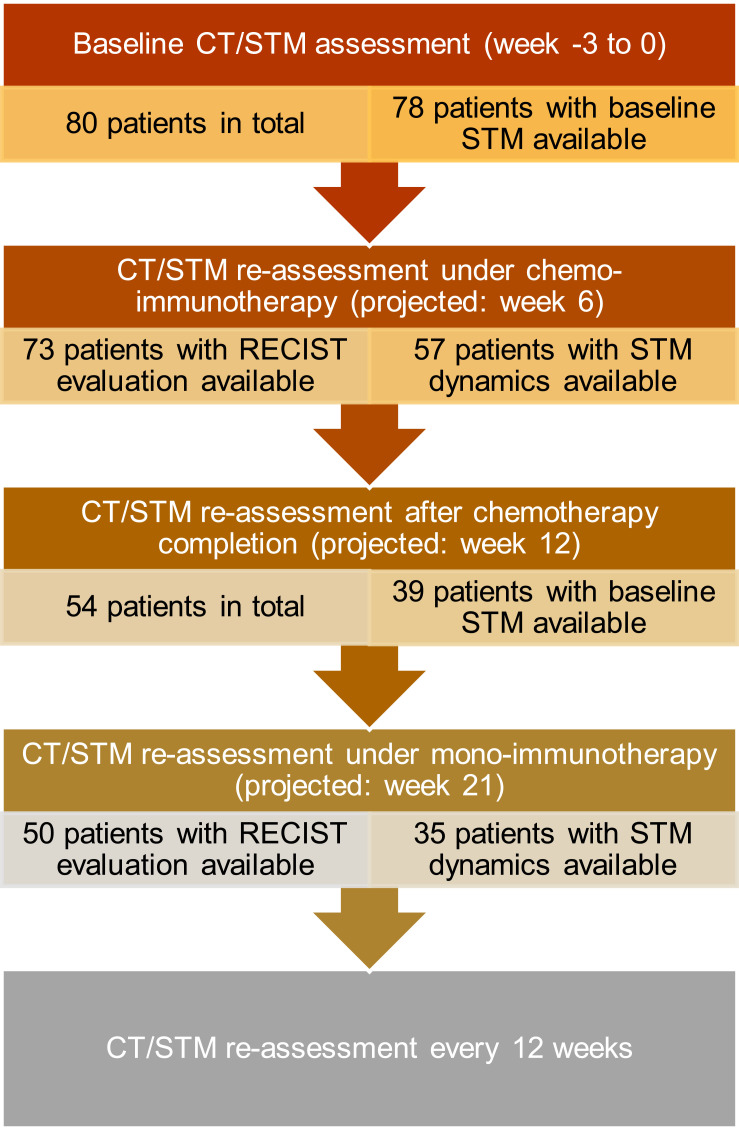
Eighty patients met the requirements to be included in the analysis. Baseline patient and tumor characteristics are shown in Table 1, the evaluation schedule including the respective patient numbers is shown in Figure 1.
Table 1.
Baseline Patient and Tumor Characteristics
| Mean age (SD) | 62.9 (1.07) | |
|---|---|---|
| Female sex (n, %) | 33 (41.3) | |
| Age categories (n, %) | <60 years | 28 (35.0) |
| 60–69 years | 34 (42.5) | |
| 70–79 years | 17 (21.3) | |
| 80+ years | 1 (1.3) | |
| ECOG (n, %) | 0 | 46 (58.2) |
| 1 | 22 (27.9) | |
| 2 | 11 (13.9) | |
| ICI substance (n, %) | Pembrolizumab | 77 (96.3) |
| Atezolizumab | 3 (3.8) | |
| Therapy line (n, %) | 1 | 76 (95) |
| 2 | 1 (1.3) | |
| ≥3 | 3 (3.8) | |
| Median chemo-immunotherapy cycles (IQR) | 4 (2) | |
| Median mono-immunotherapy cycles (IQR) | 3 (5,75) | |
| Smoking status (n, %) | Never/<5 py | 10 (12.5) |
| ≥5 py | 70 (87.5) | |
| Histological subtype (n, %) | Adenocarcinoma | 62 (77.5) |
| Squamous-cell carcinoma | 18 (22.5) | |
| Tumor stage (n, %) | III | 7 (8.8) |
| IV | 73 (91.3) | |
| PD-L1-status (n, %*) | positive | 41 (54.7) |
| PD-L1 expression (n, %) | n.a. | 5 (6.3) |
| <1% | 34 (42.5) | |
| 1–49% | 26 (32.5) | |
| ≥50% | 15 (18.6) | |
| Targetable mutation (n, %) | 3 (3.8) | |
Notes: Data are given as absolute number and percent within the respective group unless otherwise specified. *Percent of patients with PD-L1 status available.
Abbreviations: SD, standard deviation; ECOG, Eastern Cooperative Oncology Group; ICI, Immune checkpoint inhibitor; IQR, interquartile range; py, pack years; PD-L1, programmed death-ligand 1.
Figure 1.
Flowchart depicting the evaluation schedule and the respective patient numbers.
Abbreviations: CT, computed tomography; STM, serum tumor marker; RECIST, response evaluation criteria in solid tumors.
In the CHT-ICI phase, baseline STM were available in 78 (97.5%) patients, whereas CEA was leading STM in 45 (56.3%), CYFRA21-1 in 21 (26.3%), CA19-9 in 7 (8.8%) and NSE in 5 (6.3%) patients. The leading STM was elevated above the upper limit of normal in 74 (92.5%) patients. Among the four others, two had CYFRA 21–1 as leading STM with no STM follow-up available at re-staging and both had radiological PR. One patient had a decrease in CYFRA 21–1 with corresponding PR and one had a CA19-9 increase with radiological SD. Consecutive RECIST response evaluation was available in 73 (91.3%) patients, corresponding leading STM dynamics could be assessed in 57 (71.3%) patients.
A total of 54 (67.5%) patients received mono-ICI maintenance after CHT-ICI, 50 (62.5%) of them had a consecutive CT re-staging. Among those patients, baseline STM were available in 39 (48.8%), with CEA as the leading STM in 24 (30%), CYFRA 21–1 in 9 (11.3%), CA19-9 in 4 (5%) and NSE in 2 (2.5%) cases.
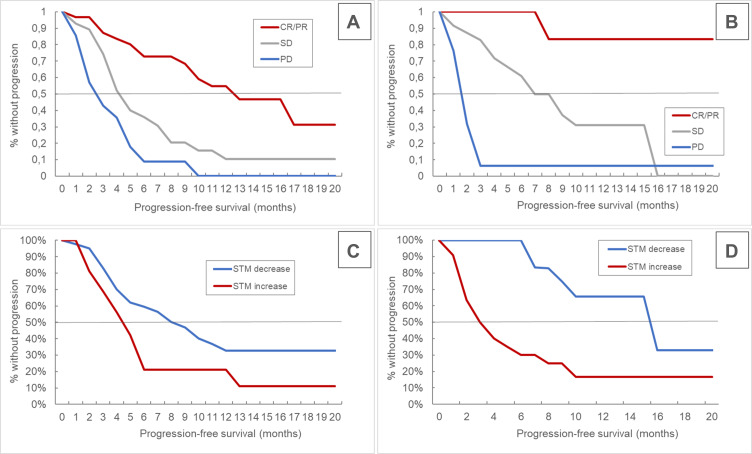
During the observational period, disease progression or death occurred in 56 (70%) patients, while 32 (40%) died. Overall median PFS from first CHT-ICI therapy on was 5M (4,9), median OS was 15M (10,/). In the mono-ICI phase, median PFS was 6M (3,10), while median OS was not reached (10,/). For the CHT-ICI as well as the mono-ICI phase, median PFS significantly differed between the RECIST and STM response categories. Median OS was not reached in most subgroups. (Table 2, Figure 2).
Table 2.
Progression-Free and Overall Survival According to Radiological Response and STM Response in the Respective Treatment Phases
| Chemo-Immunotherapy | n | Median PFS (M, 95% CI) | p | Median OS (M, 95% CI) | P | |
|---|---|---|---|---|---|---|
| CT response (RECIST) | PR | 31 | 13 (9,/) | <0.001 | n.r. (15,/) | 0.01 |
| SD | 28 | 5 (4,7) | n.r. (5,/) | |||
| PD | 14 | 3 (2,5) | 6 (3,10) | |||
| STM response | Decrease | 41 | 9 (5,12) | 0.042 | 15 (10,/) | 0.363 |
| Increase | 16 | 5 (3,6) | n.r. (10,/) | |||
| Mono-immunotherapy | n | Median PFS (95% CI) | p | Median OS (95% CI) | P | |
| CT response (RECIST) | CR/PR | 9 | n.r. (8,/) | <0.001 | n.r. (/,/) | 0.009 |
| SD | 24 | 7 (4,16) | n.r. (7,/) | |||
| PD | 17 | 2 (1,3) | 7 (2,/) | |||
| STM response | Decrease | 16 | 16 (7,/) | <0.001 | n.r. (/,/) | 0.055 |
| Increase | 22 | 3.5 (2,6) | n.r. (7,/) | |||
Notes: Data are given as time in months (95% confidence interval). Testing for significance between the subgroups was accomplished using a Log rank test, a p value of <0.05 was regarded statistically significant. Missing figures (/) are for values non assessable.
Abbreviations: PFS, progression-free survival; OS, overall survival; M, months; CI, confidence interval; CT, computed tomography; RECIST, response evaluation criteria in solid tumors; STM, serum tumor marker; CR, complete remission; PR, partial remission; SD, stable disease; PD, progressive disease.
Figure 2.
Kaplan-Meier curves for progression-free survival in the chemo-immunotherapy (A and C) and in the mono-immunotherapy maintenance phase (B and D) for RECIST- (A and B) and STM-response (C and D), respectively.
Abbreviations: RECIST, response evaluation criteria in solid tumors; CR, complete remission; PR, partial remission; SD, stable disease; PD, progressive disease; STM, serum tumor marker.
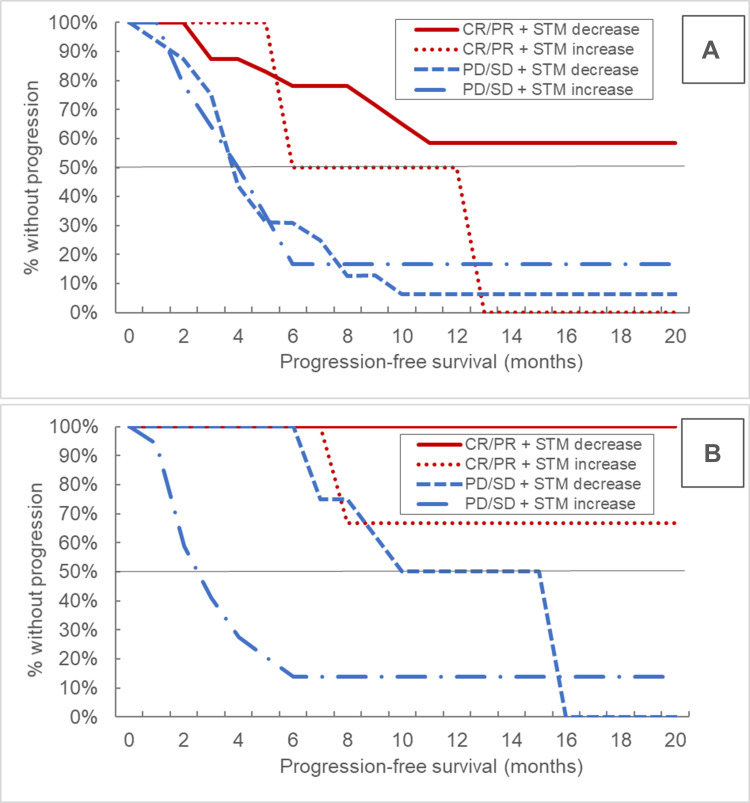
When separating individuals with radiological response (PR/CR) from those with stable or progressive disease (SD/PD), there was no relevant difference in PFS according to STM dynamics in CHT-ICI patients, however a significant difference was evident in the mono-ICI maintenance setting. Again, median OS was not reached in most subgroups. (Table 3, Figure 3, supplementary figure 1)
Table 3.
Progression-Free and Overall Survival According to Combined Radiological Response and STM Response in the Respective Treatment Phases
| Median PFS (M, 95% CI) | Median OS (M, 95% CI) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| STM Response | STM Response | ||||||||||
| Chemo-immunotherapy | N | Decrease | N | Increase | p | n | Decrease | n | Increase | p | |
| CT response (RECIST) | CR/PR | 24 | n.r. (9,/) | 2 | 9.5 (/,/) | <0.001 | 24 | n.r. (15,/) | 2 | n.r. (/,/) | 0.001 |
| SD/PD | 16 | 4 (3,7) | 14 | 4.5 (2,6) | 16 | 7.5 (4,/) | 14 | n.r. (10,/) | |||
| Mono-immunotherapy | N | Decrease | Increase | p | n | Decrease | n | Increase | p | ||
| CT response (RECIST) | CR/PR | 5 | n.r. (/,/) | 3 | n.r. (8,/) | <0.001 | 5 | n.r. (/,/) | 3 | n.r. (,/) | 0.22 |
| SD/PD | 10 | 13 (7,16) | 17 | 3 (2,4) | 10 | n.r. (7,/) | 17 | n.r. (5,/) | |||
Notes: Data are given as time in months (95% confidence interval). Testing for significance between the subgroups was accomplished using a Log rank test, a p value of <0.05 was regarded statistically significant. Missing figures (/) are for values non assessable.
Abbreviations: PFS, progression-free survival; OS, overall survival; M, months; CI, confidence interval; N, number; STM, serum tumor marker; CT, computed tomography; RECIST, response evaluation criteria in solid tumors; CR, complete remission; PR, partial remission; SD, stable disease; PD, progressive disease.
Figure 3.
Kaplan-Meier curves for progression-free survival according to RECIST response categories and STM dynamics in the chemo-immunotherapy (A) as well as the mono-immunotherapy maintenance phase (B).
Abbreviations: RECIST, response evaluation criteria in solid tumors; CR, complete remission; PR, partial remission; SD, stable disease; PD, progressive disease; STM, serum tumor marker.
Univariate and stepwise multivariate Cox-regression analyses for variables influencing PFS and OS including RECIST and STM response are depicted in supplementary table 1.
Discussion
Our analyses implicate that in chemoimmunotherapy for advanced NSCLC, leading STM dynamics parallel radiological response. The results for the mono-ICI maintenance phase resemble our previously reported findings for ICI monotherapy and underline, that especially upon radiologically stable or progressive disease, a concomitant STM decrease may identify patients with further therapeutic benefit of ICI therapy.
In line with our published findings concerning the utility of STM in mono-immunotherapy, we suggest that our model of a leading STM out of a panel of CEA, CYFRA 21–1, CA 19–9 and NSE may be a valuable additional tool in monitoring CHT-ICI patients. Importantly, the application of such blood-based biomarker does in no way challenge the gold standard of CT imaging in baseline evaluations as well as for restaging. Rather, STM could complement radiological assessment and may be especially helpful in situations when the clinician needs additional biomarker information upon ambiguous radiological response.
As an example, in a patient with insignificant progression of some lesions but with a clinical benefit of ICI therapy, a decrease in leading STM levels would clearly encourage continuation of therapy. Conversely, simultaneous radiological tumor progression and STM increase would clearly speak against the possibility of pseudoprogression but should encourage a switch in the therapeutic regimen, as such situation is associated with inferior PFS and OS.
Although PFS differed between STM response categories for both CHT-ICI as well as the ICI monotherapy maintenance, out data suggest, that the predictive value of STM is considerably larger in the mono-ICI setting than in the combination phase. It is well known, that STM can be influenced by factors apart from malignancies, like infection, renal function impairment or trauma.23–26 Thus, it seems likely, that CHT and associated complications like neutropenia, infections or tumor response to CHT itself may affect STM response and could explain the smaller effect on PFS in the CHT-ICI setting. Also, a majority of patients responded to CHT-ICI radiologically as well as concerning STM which leads to a clear imbalance in subgroups sample sizes that confines the validity of the reported results.
Next to the these mentioned limitations of sample size in subgroup analyses and the strengths concerning reproducibility of our previous results, several other issues need to be discussed that may limit the significance of our reported findings: Data were retrieved from a single-center, retrospective patient registry and the patient collective represents real-life situation: Not all patients received the full four cycles of CHT-ICI for reasons of toxicity (although median number of CHT/ICI cycles was four), which may have influenced PFS and OS analyses for both the CHT-ICI and the ICI maintenance therapy. On the other hand, however, as all patients received carboplatin and no patient received additional chemotherapy maintenance, bias due to these factors can be excluded. Overall survival could not be assessed in most subgroup analyses due to the small number of patients having died especially in well-responding subgroups, OS results therefore must only be interpreted cautiously. Also, the different CHT therapy regimens varied due to histological subtypes and have not been compared head-to-head in clinical trials, which may have caused bias to our results. Furthermore it is clear, that the patient cohort receiving ICI-maintenance therapy is not comparable to our previously reported patient collective receiving sole mono-immunotherapy in various therapy lines, as the present mono-ICI maintenance cohort consists of patients already selected by the directly previous CHT-ICI combination therapy and associated response or toxicity. It is therefore important to clarify, that it was not our aim to evaluate PFS and OS for either therapy phase, but rather to evaluate the utility of STM analyses in the therapeutic setting of chemo-immunotherapy in general.
Conclusions
At present, we believe that like in mono-ICI therapy, STM can also be a valuable dynamic biomarker in addition to CT imaging in patients receiving CHT-ICI combination therapy for advanced NSCLC. The clinical impact of STM dynamics may be considerably larger in the mono-ICI maintenance therapy setting than in initial CHT-ICI combination therapy. Future research will likely provide new, possibly composite, biomarkers based on molecular tumor profiling as well as on the patients’ immunological status. We suggest that STM should be included in such considerations, whereupon larger-scale and prospective, biomarker-driven clinical trials would be the next step warranted.
Acknowledgments
This publication was supported by Johanne Kepler Open Access Publishing Fund.
Funding Statement
No external funding was used in conducting the study or in writing of this manuscript.
Data Sharing Statement
According to the terms imposed by the ethics committees, the full datasets analyzed during the current study cannot be made publicly available, as they contain possibly identifiable patient data. Upon reasonable request to the corresponding author and if approved as an amendment by the responsible local ethics committee, selected anonymized data can however be shared.
Ethics Approval and Informed Consent
This study was conducted in accordance with the Declaration of Helsinki and approved by the ethics committee of the federal state of Upper-Austria (EK Nr. 1139/2019).
The study was conducted in an entirely retrospective fashion, without an experimental approach or additional patient contact. Only patient data assessed in clinical routine were analyzed. Patient data were collected in an anonymized fashion and securely electronically stored in a way, that only the authors had access to the data. No identifiable patient data has been or will ever be published by the authors.
Thus, according to the ethic committee approval, no patient consent was necessary for participation in this study.
Consent for Publication
Due to the ethics committee approval and the study characteristics, no patient consent for publication was needed.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
DL has served as consultant/advisor to Roche and received travel/accommodation funding from Roche and Merck Sharp & Dohme, outside the submitted work. AH has received travel/accommodation funding from Roche, outside the submitted work. RW has received speakers’ honoraria and travel/accommodation funding from and served as consultant/advisor to Roche, Merck Sharp & Dohme and Bristol-Myers Squibb, and reports personal fees from Roche, Merck Sharp & Dhome, and Bristol Myers Squibb, outside the submitted work. EB has received speakers’ honoraria from and served as consultant/advisor to Roche, Merck Sharp & Dohme, and Bristol-Myers Squibb; he has received travel/accommodation funding from Roche and Merck Sharp & Dohme. BL has received speakers’ honoraria from and has served as consultant/advisor to Roche, Merck Sharp & Dohme and Bristol-Myers Squibb. The aforementioned authors report no other potential conflicts of interest for this work. WH, KA, MS, BH, CA and BK declare that they have no conflicts of interest that might be relevant to the contents of this manuscript.
References
- 1.Cyriac G, Gandhi L. Emerging biomarkers for immune checkpoint inhibition in lung cancer. Semin Cancer Biol. 2018;52:269–277. doi: 10.1016/j.semcancer.2018.05.006 [DOI] [PubMed] [Google Scholar]
- 2.Planchard D, Popat S, Kerr K, et al. Metastatic non-small cell lung cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29:iv192–iv237. doi: 10.1093/annonc/mdy275 [DOI] [PubMed] [Google Scholar]
- 3.Prelaj A, Tay R, Ferrara R, et al. Predictive biomarkers of response for immune checkpoint inhibitors in non–small-cell lung cancer. Eur J Cancer. 2019;106:144–159. doi: 10.1016/j.ejca.2018.11.002 [DOI] [PubMed] [Google Scholar]
- 4.Lee CK, Man J, Lord S, et al. Clinical and molecular characteristics associated with survival among patients treated with checkpoint inhibitors for advanced non–small cell lung carcinoma. JAMA Oncol. 2018;4:210. doi: 10.1001/jamaoncol.2017.4427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borcoman E, Kanjanapan Y, Champiat S, et al. Novel patterns of response under immunotherapy. Ann Oncol. 2019;30:385–396. doi: 10.1093/annonc/mdz003 [DOI] [PubMed] [Google Scholar]
- 6.Chiou VL, Burotto M. Pseudoprogression and immune-related response in solid tumors. J Clin Oncol. 2015;33:3541–3543. doi: 10.1200/JCO.2015.61.6870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferrara R, Mezquita L, Texier M, et al. Hyperprogressive disease in patients with advanced non–small cell lung cancer treated with PD-1/PD-l1 inhibitors or with single-agent chemotherapy. JAMA Oncol. 2018;4:1543. doi: 10.1001/jamaoncol.2018.3676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gandara DR, von Pawel J, Mazieres J, et al. Atezolizumab treatment beyond progression in advanced nsclc: results from the randomized, phase iii oak study. J Thorac Oncol. 2018;13:1906–1918. doi: 10.1016/j.jtho.2018.08.2027 [DOI] [PubMed] [Google Scholar]
- 9.Noonan SA, Patil T, Gao D, et al. Baseline and on-treatment characteristics of serum tumor markers in stage iv oncogene-addicted adenocarcinoma of the lung. J Thorac Oncol. 2018;13:134–138. doi: 10.1016/j.jtho.2017.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holdenrieder S, Wehnl B, Hettwer K, et al. Carcinoembryonic antigen and cytokeratin-19 fragments for assessment of therapy response in non-small cell lung cancer: a systematic review and meta-analysis. Br J Cancer. 2017;116:1037–1045. doi: 10.1038/bjc.2017.45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Jong C, Deneer VHM, Kelder JC, et al. Association between serum biomarkers CEA and LDH and response in advanced non‐small cell lung cancer patients treated with platinum‐based chemotherapy. Thorac Cancer. 2020;1759–7714:13449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kataoka Y, Hirano K, Narabayashi T, et al. Carcinoembryonic antigen as a predictive biomarker of response to nivolumab in non-small cell lung cancer. Anticancer Res. 2018;38:559–563. [DOI] [PubMed] [Google Scholar]
- 13.Shirasu H, Ono A, Omae K, et al. CYFRA 21-1 predicts the efficacy of nivolumab in patients with advanced lung adenocarcinoma. Tumor Biol. 2018;40:101042831876042. doi: 10.1177/1010428318760420 [DOI] [PubMed] [Google Scholar]
- 14.Dal Bello MG, Filiberti RA, Alama A, et al. The role of CEA, CYFRA21-1 and NSE in monitoring tumor response to Nivolumab in advanced non-small cell lung cancer (NSCLC) patients. J Transl Med. 2019;17:74. doi: 10.1186/s12967-019-1828-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lang D, Horner A, Brehm E, et al. Early serum tumor marker dynamics predict progression-free and overall survival in single PD-1/PD-L1 inhibitor treated advanced NSCLC—A retrospective cohort study. Lung Cancer. 2019;134:59–65. doi: 10.1016/j.lungcan.2019.05.033 [DOI] [PubMed] [Google Scholar]
- 16.Zhang Z, Yuan F, Chen R, et al. Dynamics of serum tumor markers can serve as a prognostic biomarker for Chinese advanced non-small cell lung cancer patients treated with immune checkpoint inhibitors. Front Immunol. 2020. doi: 10.3389/fimmu.2020.01173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gandhi L, Rodríguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus chemotherapy in metastatic non–small-cell lung cancer. N Engl J Med. 2018;378:2078–2092. doi: 10.1056/NEJMoa1801005 [DOI] [PubMed] [Google Scholar]
- 18.Paz-Ares L, Luft A, Vicente D, et al. Pembrolizumab plus chemotherapy for squamous non–small-cell lung cancer. N Engl J Med. 2018;379:2040–2051. doi: 10.1056/NEJMoa1810865 [DOI] [PubMed] [Google Scholar]
- 19.Socinski MA, Jotte RM, Cappuzzo F, et al. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med. 2018;378:2288–2301. doi: 10.1056/NEJMoa1716948 [DOI] [PubMed] [Google Scholar]
- 20.Chansky K, Detterbeck FC, Nicholson AG, et al. The IASLC lung cancer staging project: external validation of the revision of the TNM stage groupings in the eighth edition of the TNM classification of lung cancer. J Thorac Oncol. 2017;12:1109–1121. doi: 10.1016/j.jtho.2017.04.011 [DOI] [PubMed] [Google Scholar]
- 21.McShane LM, Altman DG, Sauerbrei W, et al. Reporting recommendations for tumor marker prognostic studies. J Clin Oncol. 2005;23:9067–9072. doi: 10.1200/JCO.2004.01.0454 [DOI] [PubMed] [Google Scholar]
- 22.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026 [DOI] [PubMed] [Google Scholar]
- 23.Chen J, Tao F, Zhang B, et al. Elevated squamous cell carcinoma antigen, cytokeratin 19 fragment, and carcinoembryonic antigen levels in diabetic nephropathy. Int J Endocrinol. 2017;2017:1–6. doi: 10.1155/2017/5304391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anderson BJ, Reilly JP, Shashaty MGS, et al. Admission plasma levels of the neuronal injury marker neuron-specific enolase are associated with mortality and delirium in sepsis. J Crit Care. 2016;36:18–23. doi: 10.1016/j.jcrc.2016.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kwon Y-J, Lee H-S, Shim J-Y, et al. Serum carcinoembryonic antigen is positively associated with leukocyte count in Korean adults. J Clin Lab Anal. 2018;32:e22291. doi: 10.1002/jcla.22291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weber TH, Kerttula Y. Carcinoembryonic antigen (CEA) in blood in cases of pneumonia. Scand J Infect Dis. 1986;18:547–550. doi: 10.3109/00365548609021660 [DOI] [PubMed] [Google Scholar]