Abstract
Background
Tyrosine kinase inhibitors (tkis) have dramatically improved the survival of patients with ALK-rearranged (ALK+) non-small-cell lung cancer (nsclc). Clinical trial data can generally compare drugs in a pair-wise fashion. Real-world collection of health utility data, symptoms, and toxicities allows for the direct comparison between multiple tki therapies in the population with ALK+ nsclc.
Methods
In a prospective cohort study, outpatients with ALK+ recruited between 2014 and 2018, treated with a variety of tkis, were assessed every 3 months for clinico-demographic, patient-reported symptom and toxicity data and EQ-5D-derived health utility scores (hus).
Results
In 499 longitudinal encounters of 76 patients with ALK+ nsclc, each tki had stable longitudinal hus when disease was controlled, even after months to years: the mean overall hus for each tki ranged from 0.805 to 0.858, and longitudinally from 0.774 to 0.912, with higher values associated with second- or third-generation tkis of alectinib, brigatinib, and lorlatinib. Disease progression was associated with a mean hus decrease of 0.065 (95% confidence interval: 0.02 to 0.11). Health utility scores were inversely correlated to multiple symptoms or toxicities: rho values ranged from −0.094 to −0.557. Fewer symptoms and toxicities were associated with the second- and third-generation tkis compared with crizotinib. In multivariable analysis, only stable disease state and baseline Eastern Cooperative Oncology Group performance status were associated with improved hus.
Conclusions
There was no significant decrease in hus when patients with ALK+ disease were treated longitudinally with each tki, as long as patients were clinically stable. Alectinib, brigatinib, and lorlatinib had the best toxicity profiles and exhibited high mean hus longitudinally in the real-world setting.
Keywords: Health utility, real-world studies
BACKGROUND
The ALK chromosomal rearrangement, found in approximately 4% of all lung adenocarcinomas, leads to an alk fusion protein oncogenic driver1,2. It frequently occurs in younger nonsmokers with a greater likelihood of metastasis to the brain, pleura, and peritoneum3,4. Crizotinib was the first tyrosine kinase inhibitor (tki) targeting the ALK-rearrangement, and it demonstrated dramatically improved progression-free survival and higher objective response rates (that is, major shrinkage of the cancer) when compared with standard chemotherapy5,6. However, crizotinib eventually leads to drug resistance through several mechanisms, including secondary resistance ALK mutations7,8 and inadequate blood–brain barrier penetration by the drug9,10. Subsequent generations of alk inhibitors are more potent, cross the blood–brain barrier, and target different secondary resistance mutations. Ceritinib, alectinib, brigatinib, and lorlatinib have all demonstrated efficacy in second-line treatment, and several in first-line11–13. Clinical trials have compared individual tkis with chemotherapy5,6,11,14 or crizotinib15,16; however, direct comparisons of the activity and toxicity profiles of newer-generation alk inhibitors are lacking.
Health technology assessments (htas) weigh the costs, risks, and benefits of treatments to determine incremental benefit and often use quality-adjusted life–years (qalys), particularly in countries with publicly funded health care systems17. Health utility scores (hus), which summarize quality of life in a single value where 1.0 is perfect health and 0 is the worst health possible, are typically used to determine qaly18 and then used in economic analysis and modelling.
Studies have compared quality of life and utilities between tkis and chemotherapy through the use of clinical trial data19–22. Although meaningful, these results are not representative of the broader ALK-rearranged population, because patients enrolled in clinical trials are often healthier to meet inclusion criteria. Health utility scores derived from observational or real-world studies have typically generated aggregated values across broad groups of lung cancers and are not ALK-specific.23,24 The rarity of patients with ALK-rearranged lung cancer and its treatment with a variety of targeted therapies pose a challenge to the application of previous real-world utility values. Given that indirect measurements of hus are derived from healthy reference populations25, it becomes important not only to collect real-world health utility data prospectively, but also to demonstrate that hus are correlated with known factors that affect quality of life, such as symptoms and treatment toxicity. In this study, we
■ report hus longitudinally, by different tki treatments and disease states (defined as disease that is stable or progressing by imaging).
■ correlate hus with patient-reported symptoms and toxicities, especially given that the toxicities associated with the common alk-targeted tkis differ from toxicities associated with chemotherapy23,26,27.
■ determine other clinical factors associated with HUS in this unique patient population.
METHODS
In this prospective observational, single-institution, research-ethics approved (uhn reb no. 06-639CE) cohort study, eligible clinical outpatients had metastatic, histologically confirmed ALK+ nsclc and were capable of providing informed consent. Patients were required to be fluent in 1 of the more than 24 languages of the hus assessment tool EQ-5D-5L (EuroQol Research Foundation, Rotterdam, Netherlands).
Recruitment occurred at the Princess Margaret Cancer Centre (Toronto, Ontario) from November 2014 to July 2018 during outpatient clinic visits (encounters) as scheduled by the treating physician. Patients could enrol at any time during their disease course, before or during first-line or subsequent-line treatment.
After providing informed consent, patients completed the following surveys: a single baseline clinico-demographic survey, where baseline was defined as the date of study entry; the 5-question EQ-5D-5L survey that was used to generate hus based on Canadian reference values25; a visual analog scale on which patients rated their overall health that day on a scale from 0 to 10028; a modified version of the Patient-Reported Outcomes version of the Common Terminology Criteria for Adverse Events (pro-ctcae)29 to collect treatment toxicities; and the Edmonton Symptom Assessment System (esas)30 to assess cancer-related symptoms as previously validated. Excluding the baseline clinico-demographic questionnaire, all other surveys were administered every 3 months until death, last known follow-up, or patient withdrawal. Research coordinators approached patients for all surveys, but the surveys themselves were self-administered, sometimes with a family member or independent translator.
Clinical data were extracted from medical records to determine characteristics at diagnosis, such as diagnosis date, stage, and Eastern Cooperative Oncology Group (ecog) performance status (0–4, where 0 is fully active and able to carry out all activities without restriction); prior and current treatment history; and patient health status at every survey time point (or encounter), which was determined by review of radiologic imaging results, treatment information, and changes (such as dose modifications, dose discontinuation, and dose delays). Health states at each encounter were categorized as stable on a specific systemic (that is, drug) therapy, stable off systemic therapy (which were mostly assessments at the time of diagnosis), or having disease progression. To ensure validity, these health states were determined independently by multiple clinicians for a subset of patients, with discrepancies resolved by consensus.
Statistical Analysis
Descriptive summary statistics were used to report baseline patient characteristics of the cohort. Health utility scores were compared between treatments and between stable and progressing health states (within treatments) using t-tests. Longitudinal hus were stratified by treatment; trends in hus over time in each treatment group were visualized by fitting local regression models (locally estimated scatterplot smoothing).
Toxicities and symptoms were captured using pro-ctcae and esas tools as already described and compared between treatments using mean grades. Individual hus by specific symptoms or toxicities were presented in boxplots, and Spearman rank correlation coefficients, rho, were calculated. Cut-offs for correlational rho values were defined as mild: ± 0.2–0.39; moderate: ± 0.4–0.59; strong: ± 0.6 or greater.
In regression analysis, to account for multiple observations per patient, hus across time points were collapsed into a single mean hus per patient, per treatment, per disease state (stable disease or progression). Unadjusted linear regression analysis was performed to assess associations between clinico-demographic variables and hus. Predictors of hus were then identified by fitting a multivariable linear model; backwards model selection was applied to reduce the predictors in the final model to only those that significantly contributed to improved model fit. All tests were 2-sided; statistical significance was defined as p < 0.05. The statistical analysis was conducted in the R software application (version 3.5.2: The R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
The study included 76 patients. There were 499 encounters, a mean of 6.6 encounters per patient (range: 1–14). At the first encounter, 32 patients were receiving a tki, 6 were on chemotherapy, and 38 had no treatment or were newly diagnosed. Three patients withdrew consent to continue follow-up at 2, 4, and 7 months after study entry respectively; 4 patients periodically withdrew consent but then subsequently agreed to continue completing the surveys, of whom 2 repeatedly withdrew and agreed to continue multiple times. Overall, 17% of the planned baseline or longitudinal assessments were missing, mainly because of survey fatigue or being missed at random by the research coordinators during clinic visits. For patients with multiple encounters, the median time between encounters was 70.5 days (interquartile range: 77 days).
Table I shows the baseline clinico-demographic features of study participants at the time of diagnosis of stage iv disease. The majority were never-smokers, typically younger than the usual patient with lung cancer (median age was 60 years), equally divided between men (49%) and women (51%), with a mix of South Asian (9%), East Asian (47%), and white (38%) patients.
TABLE I.
Characteristics of 76 patients at the time of diagnosis (Dx) of metastatic (stage IV) disease
| Characteristic | Value |
|---|---|
| Age (years) | |
| Median | 60 |
| Range | 31–92 |
|
| |
| Sex [n (%) men] | 37 (49) |
|
| |
| Ethnicity [n (%)] | |
| East or Southeast Asian | 36 (47) |
| White | 29 (38) |
| Other | 11 (14) |
|
| |
| Smoking status [n (%) never-smoker] | 15 (20) |
|
| |
| Education [n (%) no postsecondary] | 22 (29) |
|
| |
| Employment [n (%) employed] | 32 (42) |
|
| |
| Marital status [n (%) married] | 53 (70) |
|
| |
| ecog PS | |
| 0 | 26 (34) |
| 1 | 38 (50) |
| 2 | 4 (5) |
| Not available | 8 (11) |
|
| |
| Histology [n (%) adenocarcinoma] | 71 (93) |
|
| |
| First-line drug [n (%) tki] | 44 (58) |
|
| |
| First encounter drug [n (%)] | |
| Tyrosine kinase inhibitor | 32 (42) |
| Chemotherapy | 6 (8) |
| At Dx or not on treatment | 38 (50) |
|
| |
| Sites of metastasis at first encounter [n (%)] | |
| 0 | 13 (17) |
| 1 | 28 (37) |
| ≥2 | 35 (46) |
| Pleura | 22 (29) |
| Lymph node | 25 (33) |
| Brain | 23 (30) |
| Liver | 9 (12) |
| Bone | 26 (34) |
| Adrenal gland | 2 (3) |
ecog PS = Eastern Cooperative Oncology Group performance status.
Longitudinal HUS, Treatment, and Health State
Mean hus for all encounters separated by disease state and treatment are reported in Table II. Mean hus were relatively high for all treatments when disease activity was stable, ranging from 0.805 to 0.858. Comparisons between disease states for individual tki treatments were not statistically significant. There was a significant difference in mean hus between patients who had stable compared with progressive disease, with a mean decrease of 0.065 (95% confidence interval: 0.02 to 0.11) as shown in Table III. Patients who were treatment-naïve had the lowest mean hus [0.733 (range: 0.62–0.84)]. Of patients who provided longitudinal hus values for both the pretreatment and stable-on-tki health states (n = 9), an improvement in the mean hus was documented: mean hus pre-tki were 0.694, and at first documentation of stability, mean hus were 0.816. Although there was a mean difference of 0.123 (95% confidence interval: −0.07 to −0.31), the lack of statistical significance, p = 0.176, was likely due to small numbers.
TABLE II.
Mean health utility scores (hus) between treatments, per encounter
| Current drug | Stable disease | Progressing disease | |||
|---|---|---|---|---|---|
|
|
|
||||
| Encounters (n) | Mean hus | p Valuea | Encounters (n) | Mean hus | |
| Crizotinib | 107 | 0.812 | Reference | 30 | 0.779 |
|
| |||||
| Ceritinib | 110 | 0.805 | 0.75 | 16 | 0.752 |
|
| |||||
| Alectinib | 37 | 0.852 | 0.08 | 10 | 0.838 |
|
| |||||
| Brigatinib | 21 | 0.834 | 0.20 | 10 | 0.707 |
|
| |||||
| Lorlatinib | 17 | 0.832 | 0.56 | 3 | 0.799 |
|
| |||||
| Single-agent CTxb | 37 | 0.827 | 0.60 | 16 | 0.729 |
By t-test, compared with crizotinib.
Because of small numbers, no data are presented for 7 platinum doublet chemotherapy encounters.
TABLE III.
Univariable and multivariable regression analyses of clinico-demographic factors affecting health utility scores, by health state
| Variable | Health utility scores in univariable analysis | Change in health utility scores in multivariable analysis | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Mean | 95% ci | p Valuea | β | 95% ci | p Valuea | |
| Sex | ||||||
| Women | 0.759 | 0.72 to 0.79 | 0.053 | |||
| Men | 0.803 | 0.78 to 0.83 | ||||
|
| ||||||
| Age at Dx | ||||||
| <65 years | 0.787 | 0.76 to 0.81 | 0.557 | |||
| ≥65 years | 0.773 | 0.73 to 0.81 | ||||
|
| ||||||
| Health state | ||||||
| Stable | 0.810 | 0.79 to 0.83 | 0.012 | Reference | ||
| Progressing | 0.743 | 0.70 to 0.79 | −0.065 | −0.113 to −0.017 | 0.008 | |
| Treatment-naïve | 0.733 | 0.62 to 0.84 | −0.087 | −0.189 to 0.014 | 0.091 | |
|
| ||||||
| ecog PS at stage IV Dx | ||||||
| 0 | 0.826 | 0.80 to 0.86 | 0.003 | −0.075 | −0.121 to −0.028 | 0.002 |
| 1 | 0.744 | 0.71 to 0.78 | ||||
| 2 | 0.818 | 0.74 to 0.90 | ||||
|
| ||||||
| Line of treatment | ||||||
| 0 | 0.824 | 0.77 to 0.88 | 0.001 | |||
| 1 | 0.776 | 0.75 to 0.80 | ||||
| 2 | 0.813 | 0.79 to 0.84 | ||||
| 3 | 0.843 | 0.81 to 0.87 | ||||
| ≥4 | 0.762 | 0.72 to 0.80 | ||||
|
| ||||||
| Treatment at encounter | ||||||
| Crizotinib | 0.787 | 0.75 to 0.82 | 0.333 | |||
| Ceritinib | 0.748 | 0.67 to 0.82 | ||||
| Alectinib | 0.845 | 0.79 to 0.90 | ||||
| Other tki | 0.777 | 0.73 to 0.82 | ||||
| Chemotherapy | 0.763 | 0.68 to 0.85 | ||||
| None or other treatment | 0.775 | 0.73 to 0.82 | ||||
|
| ||||||
| Metastatic sites | ||||||
| 0 | 0.827 | 0.79 to 0.86 | 0.039 | |||
| 1 | 0.806 | 0.78 to 0.83 | ||||
| 2 | 0.751 | 0.70 to 0.80 | ||||
| 3 | 0.723 | 0.64 to 0.81 | ||||
| 4 | 0.789 | 0.73 to 0.84 | ||||
|
| ||||||
| Brain metastasis | ||||||
| No | 0.795 | 0.77 to 0.82 | 0.191 | |||
| Yes | 0.765 | 0.73 to 0.80 | ||||
|
| ||||||
| Bone metastasis | ||||||
| No | 0.803 | 0.78 to 0.83 | 0.025 | |||
| Yes | 0.751 | 0.71 to 0.79 | ||||
|
| ||||||
| Liver metastasis | ||||||
| No | 0.787 | 0.77 to 0.81 | 0.206 | |||
| Yes | 0.739 | 0.64 to 0.84 | ||||
|
| ||||||
| Pleural metastasis | ||||||
| No | 0.778 | 0.75 to 0.80 | 0.461 | |||
| Yes | 0.799 | 0.76 to 0.84 | ||||
|
| ||||||
| Lymph node metastasis | ||||||
| No | 0.795 | 0.77 to 0.82 | 0.102 | |||
| Yes | 0.755 | 0.71 to 0.80 | ||||
ci = confidence interval; Dx = diagnosis; ecog PS = Eastern Cooperative Oncology Group performance status; tki = tyrosine kinase inhibitor.
Significant values shown in boldface type.
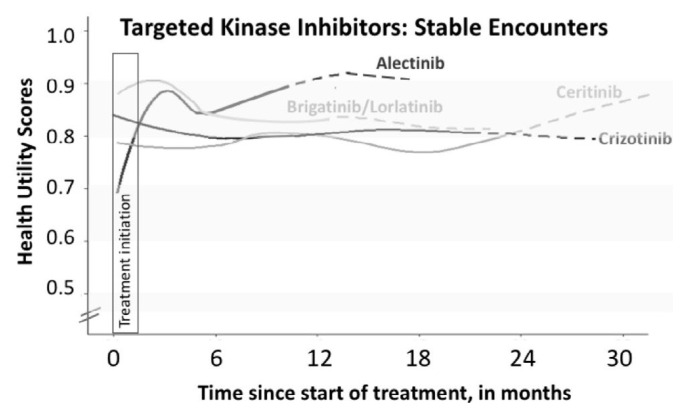
Figure 1 describes longitudinal hus while patients were stable on tki therapy. Most tkis had relatively similar mean hus and remained stable over a long treatment period. Although the mean HUS was numerically higher for patients treated with alectinib and brigatinib/lorlatinib than for those treated with crizotinib and ceritinib, there was considerable overlap of individual hus values.
FIGURE 1.
Mean health utility scores (HUS) over time, by tyrosine kinase inhibitor treatment, for patients clinically and radiologically stable on therapy. The HUS in each treatment group were modelled using locally estimated scatterplot smoothing. When fewer patients than 25% of the original number were present, lines are dotted to reflect potential survivor bias. The first 6 weeks of treatment are marked with a box (“Treatment initiation”) to represent the typical length of time required for patients to respond to therapy.
Correlation of Patient-Reported Toxicities and Symptoms and HUS
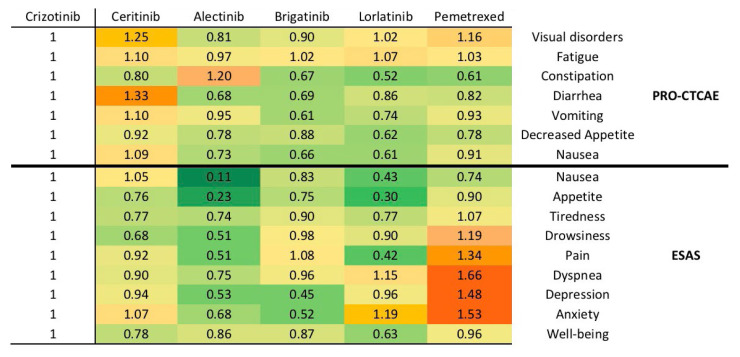
Mean toxicity and symptom scores of each treatment (as measured by pro-ctcae and esas) are shown in Figure 2 as mean ratios of symptom and toxicity raw scores relative to crizotinib; this figure also depicts the relative severity of symptoms and toxicities in a descriptive heat map, where green represented less severe or fewer symptoms or toxicities relative to crizotinib, and red represented greater or more severe symptoms or toxicities. Symptoms or toxicities were fewer with alectinib, brigatinib, and lorlatinib than with crizotinib. Relative to crizotinib, ceritinib was associated with some greater and some less severe symptoms or toxicities. As a comparator, single-agent chemotherapy (pemetrexed) demonstrated worse symptoms and toxicities relative to the tkis, including pain, dyspnea, depression, and anxiety. Within individual symptoms or toxicities, ceritinib had greater visual and gastrointestinal toxicity, while those receiving brigatinib and alectinib reported lower anxiety and depression scores.
FIGURE 2.
The mean ratio of symptoms and toxicities for second- and third-generation tyrosine kinase inhibitors compared with crizotinib. The mean ratios are generated using raw values from the Likert scales of individual items on the Patient-Reported Outcomes version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE: United States, National Cancer Institute) and the Edmonton Symptom Assessment System (ESAS). Mean ratios are presented as a “heat map,” in which green means symptoms that are less severe than those with crizotinib, and red means symptoms that are more severe, with yellow being neutral. All values for crizotinib are 1, because they serve as the reference for individual symptoms.
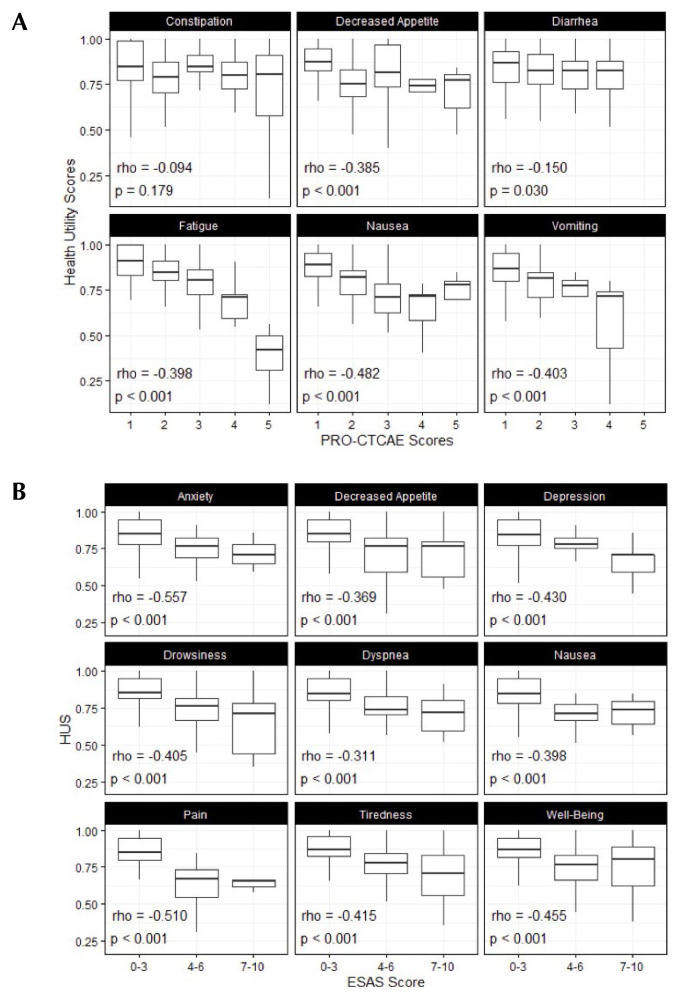
Figure 3 demonstrates the correlation between severity of toxicities or symptoms and hus among patients with stable disease. Higher severities of 5 out of 6 pro-ctcae and all 9 esas items were each mildly to moderately associated with lower hus (Spearman rho: −0.15 to −0.557); these were all statistically significant (p < 0.05). There was no significant correlation between hus and constipation (rho: −0.094; p = 0.18). Anxiety and pain generated the correlations with the largest magnitude, each with rho values below −0.50.
FIGURE 3.
Association between the severity of health utility scores (HUS) and either (A) the toxicities on the Patient-Reported Outcomes version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE: United States, National Cancer Institute) or (B) symptom scores on the Edmonton Symptom Assessment System (ESAS) for patients stable on treatment.
Clinical Factors Affecting HUS
The association between clinico-demographic factors and hus were assessed using linear regression (Table III). Univariable analysis showed that the number of overall sites of metastases was inversely associated with hus (p = 0.039). Bone metastases were the only specific metastatic site that demonstrated a significant association with hus (p = 0.025). Men also trended toward significantly higher hus in univariable analysis (p = 0.053). Multivariable analysis with backwards selection identified only progressive disease and ecog for retention in the final model. Compared with patients with stable disease, patients with progressive disease had significantly lower hus (p = 0.008), whereas an inverse relationship was seen between ecog, status and hus (p = 0.002). Disease progression was associated with a mean hus drop of 0.065, while increasing ecog score was associated with a mean hus drop of 0.075.
DISCUSSION
The population with ALK+ lung cancer is unique in its clinico-demographic characteristics. Our patient cohort was representative of this population6,11–13, including having a younger median age at diagnosis31, a high proportion of Asian patients, and a high proportion of patients presenting with brain metastases32,33. This prospective evaluation assessed the longitudinal hus of patients taking any of the available alk-targeted tkis. Despite variable baseline hus values, once alk-targeted therapy was started, regardless of the line of therapy, mean hus values ranged from 0.770 to 0.920 longitudinally for the various alk-targeted drugs. The more recently developed alk inhibitors of alectinib, brigatinib, and lorlatinib had the highest sustained mean hus, with values exceeding 0.830, while crizotinib and ceritinib had lower mean hus values, although these remained above 0.760.
A visual summary of hus values using a heat map revealed informative associations across therapies. Our results were also consistent with previously reported clinical trial data: patients receiving alectinib have been shown to have lower rates of adverse events when compared with crizotinib in the alex trial15, and likewise with brigatinib and gastrointestinal symptoms of all grades or severity in the alta-1L trial16. Chemotherapy is generally reserved for ALK patients who have exhausted all tki treatments; this may explain the worse symptoms and toxicities seen with pemetrexed chemotherapy, when compared with tkis.
Disease progression was associated with statistically significant lower hus in all treatments, when compared with stable disease. This difference was also clinically significant. This demonstrated that the excellent state of health enjoyed by stable ALK+ patients on targeted therapy was not due to inherent disease characteristics, but rather due to disease control with alk-targeted drugs. The observed difference in hus between disease states also supports a quality-of-life–based clinical benefit of receiving alk-targeted therapy that was in addition to improved progression-free survival.
There were mild to moderately strong correlations between hus and symptoms and toxicities that were assessed by esas and pro-ctcae tools respectively. These associations also affirmed the validity of the EQ-5D-5L–derived hus values in our patient population. However, equally important is that these correlations support the sensitivity of EQ-5D-5L–derived hus measurements to differences in clinico-demographic factors, symptoms, and toxicities expected to be associated with health utility. Across all symptoms and toxicities except for constipation, a greater severity score was associated with lower hus scores, with most associations both statistically significant and with moderate correlational rho values between −0.4 and −0.5 that would be considered clinically relevant.
In our multivariable analysis of factors associated with hus, we identified that having stable disease state and better baseline ecog performance scores were significantly and independently associated with higher hus. As both of these factors are clinically relevant and associated with hus, this further validates EQ-5D-5L as an appropriate instrument to capture hus in the population with ALK+ lung cancer.
Cost-effectiveness evaluations have generally used data from clinical trials20,34,35. The mean hus value of 0.820 for crizotinib treatment from the profile 1007 trial was derived from EQ-5D19, which was similar to our prospective observational EQ-5D-5L–derived mean hus of 0.812. In contrast, the alta trial attempted to convert the European Organisation for Research and Treatment of Cancer’s qlq-30 scores into hus and found derived hus values ranging from 0.710 at baseline to 0.780 at the 5th cycle (5 months after initiation of brigatinib)36. These alta conversions might have been overly conservative, given that hus values in this present study of patients on brigatinib measured during stable disease had mean values of 0.834. Further, we generated values within a single cohort for each of the 5 commercially available alk-targeted agents, something not possible in the clinical trial setting that usually compares two tkis at one time.
Nonetheless, there are several limitations in this study. The low prevalence of patients with ALK+ lung cancer resulted in a modest sample size for our single-institution study, despite the fact that our centre is a comprehensive cancer centre focused on molecularly targeted lung cancers, such as ALK+ disease. As some of the agents are newly commercially available, our longitudinal assessments provide stable data between 10 and 22 months after treatment initiation, depending on the targeted agent. In the longitudinal analysis, patients contributed different amounts of data at different time points in their treatment; this may contribute to bias in the locally estimated scatterplot smoothing curve (Figure 1). There may have been slight referral biases towards fitter patients, given that our institution is a tertiary referral centre for patients with lung cancer. Finally, our study was conducted at a single site and, as such, our findings may not be directly applicable to other cancer centres with different resources and patient populations, which might influence general quality of life experienced by patients.
CONCLUSIONS
The routine capture of symptom, toxicity, and hus data prospectively in all patients with ALK+ is imperative for economic analyses where many therapeutic options are available. Health utility values were similar to clinical trial–based EQ-5D-5L–derived hus. These hus values correlated with most individual symptoms and toxicities associated with alk-targeted agents. Symptoms, toxicities, and hus values were all consistent: ceritinib and crizotinib had greater gastrointestinal symptoms and toxicities leading to slightly lower hus values, while alectinib, brigatinib, and lorlatinib had fewer gastrointestinal symptoms and toxicities. This partly contributed to higher longitudinal hus values. In the absence of population-based studies, our single-institution study serves as a surrogate for capturing patients who might not have been included in clinical trials. We found relatively high hus values among patients with ALK+ which was maintained over time when disease remained stable.
Footnotes
CONFLICT OF INTEREST DISCLOSURES
We have read and understood Current Oncology’s policy on disclosing conflicts of interest, and we declare the following interests: GL has received honoraria or research funding from Novartis, Pfizer, Roche, and Takeda. AGS is a consultant or advisor, has received honoraria, or has participated in clinical trials with AstraZeneca, Amgen, Merck, Pfizer, Bayer, Genentech–Roche, KisoJi Biotechnology, Bristol Myers Squibb, Tesaro, Spectrum, and GlaxoSmithKline. The remaining authors have no conflicts to disclose.
REFERENCES
- 1.Soda M, Choi YL, Enomoto M, et al. Identification of the transforming EML4–ALK fusion gene in non-small-cell lung cancer. Nature. 2007;448:561–6. doi: 10.1038/nature05945. [DOI] [PubMed] [Google Scholar]
- 2.Chia PL, Mitchell P, Dobrovic A, John T. Prevalence and natural history of ALK-positive non-small-cell lung cancer and the clinical impact of targeted therapy with alk inhibitors. Clin Epidemiol. 2014;6:423–32. doi: 10.2147/CLEP.S69718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fallet V, Cadranel J, Doubre H, et al. Prospective screening for ALK: clinical features and outcome according to ALK status. Eur J Cancer. 2014;50:1239–46. doi: 10.1016/j.ejca.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 4.Doebele RC, Lu X, Sumey C, et al. Oncogene status predicts patterns of metastatic spread in treatment-naive non-small-cell lung cancer. Cancer. 2012;118:4502–11. doi: 10.1002/cncr.27409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shaw AT, Kim DW, Nakagawa K, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med. 2013;368:2385–94. doi: 10.1056/NEJMoa1214886. [DOI] [PubMed] [Google Scholar]
- 6.Solomon BJ, Mok T, Kim DW, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med. 2014;371:2167–77. doi: 10.1056/NEJMoa1408440. [DOI] [PubMed] [Google Scholar]
- 7.Doebele RC, Pilling AB, Aisner DL, et al. Mechanisms of resistance to crizotinib in patients with ALK gene rearranged non-small-cell lung cancer. Clin Cancer Res. 2012;18:1472–82. doi: 10.1158/1078-0432.CCR-11-2906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Katayama R, Shaw AT, Khan TM, et al. Mechanisms of acquired crizotinib resistance in ALK-rearranged lung cancers. Sci Transl Med. 2012;4:120ra17. doi: 10.1126/scitranslmed.3003316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crinò L, Ahn MJ, De Marinis F, et al. Multicenter phase ii study of whole-body and intracranial activity with ceritinib in patients with ALK-rearranged non-small-cell lung cancer previously treated with chemotherapy and crizotinib: results from ascend-2. J Clin Oncol. 2016;34:2866–73. doi: 10.1200/JCO.2015.65.5936. [DOI] [PubMed] [Google Scholar]
- 10.Kim DW, Mehra R, Tan DSW, et al. Activity and safety of ceritinib in patients with ALK-rearranged non-small-cell lung cancer (ascend-1): updated results from the multicentre, open-label, phase 1 trial. Lancet Oncol. 2016;17:452–63. doi: 10.1016/S1470-2045(15)00614-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shaw AT, Kim TM, Crinò L, et al. Ceritinib versus chemotherapy in patients with ALK-rearranged non-small-cell lung cancer previously given chemotherapy and crizotinib (ascend-5): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2017;18:874–86. doi: 10.1016/S1470-2045(17)30339-X. [DOI] [PubMed] [Google Scholar]
- 12.Kim DW, Tiseo M, Ahn MJ, et al. Brigatinib in patients with crizotinib-refractory anaplastic lymphoma kinase–positive non-small-cell lung cancer: a randomized, multicenter phase ii trial. J Clin Oncol. 2017;35:2490–8. doi: 10.1200/JCO.2016.71.5904. [DOI] [PubMed] [Google Scholar]
- 13.Shaw AT, Gandhi L, Gadgeel S, et al. Alectinib in ALK-positive, crizotinib-resistant, non-small-cell lung cancer: a single-group, multicentre, phase 2 trial. Lancet Oncol. 2016;17:234–42. doi: 10.1016/S1470-2045(15)00488-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Soria JC, Tan DSW, Chiari R, et al. First-line ceritinib versus platinum-based chemotherapy in advanced ALK-rearranged non-small-cell lung cancer (ascend-4): a randomised, open-label, phase 3 study. Lancet. 2017;389:917–29. doi: 10.1016/S0140-6736(17)30123-X. [DOI] [PubMed] [Google Scholar]
- 15.Peters S, Camidge DR, Shaw AT, et al. Alectinib versus crizotinib in untreated ALK-positive non-small-cell lung cancer. N Engl J Med. 2017;377:829–38. doi: 10.1056/NEJMoa1704795. [DOI] [PubMed] [Google Scholar]
- 16.Camidge DR, Kim HR, Ahn MJ, et al. Brigatinib versus crizotinib in ALK-positive non-small-cell lung cancer. N Engl J Med. 2018;379:2027–39. doi: 10.1056/NEJMoa1810171. [DOI] [PubMed] [Google Scholar]
- 17.Turchetti G, Spadoni E, Geisler E. Health technology assessment. IEEE Eng Med Biol Mag. 2010;29:70–6. doi: 10.1109/MEMB.2010.936553. [DOI] [PubMed] [Google Scholar]
- 18.Whitehead SJ, Ali S. Health outcomes in economic evaluation: the qaly and utilities. Br Med Bull. 2010;96:5–21. doi: 10.1093/bmb/ldq033. [DOI] [PubMed] [Google Scholar]
- 19.Blackhall F, Kim DW, Besse B, et al. Patient-reported outcomes and quality of life in profile 1007: a randomized trial of crizotinib compared with chemotherapy in previously treated patients with ALK-positive advanced non-small-cell lung cancer. J Thorac Oncol. 2014;9:1625–33. doi: 10.1097/JTO.0000000000000318. [DOI] [PubMed] [Google Scholar]
- 20.Zhou ZY, Mutebi A, Han S, et al. Cost-effectiveness of ceritinib in previously untreated anaplastic lymphoma kinase-positive metastatic non-small-cell lung cancer in the United States. J Med Econ. 2018;21:577–86. doi: 10.1080/13696998.2018.1443111. [DOI] [PubMed] [Google Scholar]
- 21.Carlson JJ, Suh K, Orfanos P, Wong W. Cost effectiveness of alectinib vs. crizotinib in first-line anaplastic lymphoma kinase-positive advanced non-small-cell lung cancer. PharmacoEconomics. 2018;36:495–504. doi: 10.1007/s40273-018-0625-6. [DOI] [PubMed] [Google Scholar]
- 22.Ou SHI, Socinski MA, Gadgeel S, et al. Patient-reported outcomes in a phase ii, North American study of alectinib in patients with ALK-positive, crizotinib-resistant, non-small-cell lung cancer. ESMO Open. 2018;3:e000364. doi: 10.1136/esmoopen-2018-000364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ko CY, Maggard M, Livingston EH. Evaluating health utility in patients with melanoma, breast cancer, colon cancer, and lung cancer: a nationwide, population-based assessment. J Surg Res. 2003;114:1–5. doi: 10.1016/S0022-4804(03)00167-7. [DOI] [PubMed] [Google Scholar]
- 24.Trippoli S, Vaiani M, Lucioni C, Messori A. Quality of life and utility in patients with non–small cell lung cancer. Quality-of-life Study Group of the Master 2 Project in Pharmacoeconomics. PharmacoEconomics. 2001;19:855–63. doi: 10.2165/00019053-200119080-00007. [DOI] [PubMed] [Google Scholar]
- 25.Bansback N, Tsuchiya A, Brazier J, Anis A. Canadian valuation of EQ-5D health states: preliminary value set and considerations for future valuation studies. PLoS One. 2012;7:e31115. doi: 10.1371/journal.pone.0031115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Camidge DR, Bang YJ, Kwak EL, et al. Activity and safety of crizotinib in patients with ALK-positive non-small-cell lung cancer: updated results from a phase 1 study. Lancet Oncol. 2012;13:1011–19. doi: 10.1016/S1470-2045(12)70344-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu B, Yuan M, Sun Y, et al. Incidence and risk of hepatic toxicities associated with anaplastic lymphoma kinase inhibitors in the treatment of non-small-cell lung cancer: a systematic review and meta-analysis. Oncotarget. 2018;9:9480–8. doi: 10.18632/oncotarget.23840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klimek L, Bergmann KC, Biedermann T, et al. Visual analogue scales (vas): measuring instruments for the documentation of symptoms and therapy monitoring in cases of allergic rhinitis in everyday health care. Allergo J Int. 2017;26:16–24. doi: 10.1007/s40629-016-0006-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dueck AC, Mendoza TR, Mitchell SA, et al. Validity and reliability of the US National Cancer Institute’s Patient-Reported Outcomes version of the Common Terminology Criteria for Adverse Events (pro-ctcae) JAMA Oncol. 2015;1:1051–9. doi: 10.1001/jamaoncol.2015.2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bagha SM, Macedo A, Jacks LM, et al. The utility of the Edmonton Symptom Assessment System in screening for anxiety and depression: esas for distress screening. Eur J Cancer Care (Engl) 2013;22:60–9. doi: 10.1111/j.1365-2354.2012.01369.x. [DOI] [PubMed] [Google Scholar]
- 31.Akhtar-Danesh N, Finley C. Temporal trends in the incidence and relative survival of non–small cell lung cancer in Canada: a population-based study. Lung Cancer. 2015;90:8–14. doi: 10.1016/j.lungcan.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 32.Hida T, Nokihara H, Kondo M, et al. Alectinib versus crizotinib in patients with ALK-positive non-small-cell lung cancer (j-alex): an open-label, randomised phase 3 trial. Lancet. 2017;390:29–39. doi: 10.1016/S0140-6736(17)30565-2. [DOI] [PubMed] [Google Scholar]
- 33.Rangachari D, Yamaguchi N, VanderLaan PA, et al. Brain metastases in patients with EGFR-mutated or ALK-rearranged non-small-cell lung cancers. Lung Cancer. 2015;88:108–11. doi: 10.1016/j.lungcan.2015.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hurry M, Zhou ZY, Zhang J, et al. Cost-effectiveness of ceritinib in patients previously treated with crizotinib in anaplastic lymphoma kinase positive (ALK+) non-small-cell lung cancer in Canada. J Med Econ. 2016;19:936–44. doi: 10.1080/13696998.2016.1187151. [DOI] [PubMed] [Google Scholar]
- 35.Lu S, Yu Y, Fu S, Ren H. Cost-effectiveness of ALK testing and first-line crizotinib therapy for non-small-cell lung cancer in China. PLoS One. 2018;13:e0205827. doi: 10.1371/journal.pone.0205827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kawata AK, Lenderking WR, Eseyin OR, et al. Converting eortc qlq-C30 scores to utility scores in the brigatinib alta study. J Med Econ. 2019;22:924–35. doi: 10.1080/13696998.2019.1624080. [DOI] [PubMed] [Google Scholar]