Abstract
The advent of personalized therapy for non-small-cell lung carcinoma (nsclc) has improved patient outcomes. Selection of appropriate targeted therapy for patients with nsclc now involves testing for multiple biomarkers, including EGFR. EGFR mutation status is required to optimally treat patients with nsclc, and thus timely and accurate biomarker testing is necessary. However, in Canada, there are currently no standardized processes or methods in place to ensure consistent testing implementation. That lack creates challenges in ensuring that all appropriate biomarkers are tested for each patient and that the medical oncologist receives the results for making informed treatment decisions in a timely way.
An expert multidisciplinary working group was convened to create consensus recommendations about biomarker testing in advanced nsclc in Canada, with a primary focus on EGFR testing. Recognizing that there are biomarkers beyond EGFR that require timely identification, the expert multidisciplinary working group considered EGFR testing in the broader context of integration into complex lung biomarker testing. Primarily, the panel of experts recommends that all patients with nonsquamous nsclc, regardless of stage, should undergo comprehensive reflex biomarker testing at diagnosis with targeted next-generation sequencing. The panel also considered the EGFR testing algorithm and the challenges associated with the pre-analytic, analytic, and post-analytic elements of testing. Strategies for funding testing by reducing silos of single biomarker testing for EGFR and for optimally implementing the recommendations presented here and educating oncology professionals about them are also discussed.
Keywords: Biomarker testing, comprehensive biomarker testing, non-small-cell lung cancer, nsclc, targeted therapy, EGFR T790M, epidermal growth factor receptor, egfr
INTRODUCTION
Lung cancer is the most commonly diagnosed cancer in Canada, with approximately 29,800 new cases expected in 2020. It is the leading cause of cancer death for both men and women. Approximately 70% of lung cancers are diagnosed at a late stage (stage iii or iv), leading to poor 5-year survival rates of 19%1.
Non-small-cell lung cancer (nsclc), the most common type, is a model for the application of precision medicine to clinical oncology. The standard of care, which used to be platinum doublet chemotherapy, has shifted to personalized therapy based on histology and biomarker testing results. Genetic alterations that are oncogenic drivers are found in a higher percentage of lung adenocarcinomas than of squamous cell carcinomas or other histologic subtypes, and receiving matched targeted therapies for the genomic alterations improves response, progression-free survival, quality of life, and in many cases, overall survival2.
The first targetable mutations to be identified in nsclc were sensitizing mutations in the EGFR (epidermal growth factor receptor) gene. Those mutations are found in 10%–20% of patients with nonsquamous nsclc in Canada overall3, although they are more common in patients of Asian ethnicity, with an incidence of up to 50%4,5. Five tyrosine kinase inhibitors (tkis) have been approved by Health Canada for the treatment of lung cancer with sensitizing EGFR mutations: 2 first-generation tkis, erlotinib and gefitinib; 2 second-generation tkis, afatinib and dacomitinib; and 1 third-generation tki, osimertinib. Compared with chemotherapy, erlotinib and gefitinib demonstrate greater efficacy for first-line treatment in terms of objective response rate and progression-free survival6,7; however, progression-free survival is improved with second-generation tkis compared with first-generation tkis, although at the cost of greater toxicity8–10. Compared with first-generation egfr tkis, the third-generation tki osimertinib has shown an overall survival benefit in the first-line setting11. Thus, to be able to improve outcomes in patients with EGFR-mutated nsclc, the first step is to identify the mutation.
Testing for EGFR mutations as the standard of care for patients with advanced nsclc began in 2010 in Canada as a single-gene test to guide patient selection for therapy with an egfr tki12,13. However, recent technological advancements in genomic testing have enabled the identification of many new driver mutations that play a role in the pathogenesis of nsclc and are amenable to various targeted therapies. Molecular testing targets now include genetic alterations in EGFR, ALK, ROS1, NTRK, and BRAF; additional emerging actionable targets include alterations of KRAS, ERBB2, MET, RET, and NRG1. Tumour cell expression of PD-L1 by immunohistochemistry is an additional biomarker that predicts response to PD-1 immune checkpoint inhibitors in nsclc14,15. Given the growing number of actionable targets, it is no longer appropriate to consider EGFR testing in isolation; it is critical to interpret the results of EGFR testing in the context of all biomarkers required to guide optimal treatment selection for patients.
Molecular testing is an essential part of the diagnostic workup for patients with advanced nsclc, with a focus on patients having the nonsquamous disease subtype. Reliable processes and methods are required to ensure that the oncologist receives a timely, comprehensive, and accurate report to guide patient treatment16, and yet Canada has no currently established national strategy to ensure universal access to biomarker testing17. Challenges in biomarker testing for nsclc in Canada include initiation of testing for all patients eligible for targeted therapies, tissue quality and availability for testing, test turnaround time (tat), standardized reporting, return of results to clinicians and patients, and lack of provincial or territorial funding. To address those issues for EGFR-mutated lung cancer within the Canadian context, a national expert multidisciplinary working group considered the biomarker testing algorithm—as well as the pre-analytic, analytic, and post-analytic challenges related to lung cancer biomarker testing—and developed consensus recommendations to improve those processes. Optimal implementation of, and education about, the resulting recommendations were also discussed.
METHODS
An expert multidisciplinary working group was formed to develop recommendations for appropriate biomarker testing to identify and treat EGFR-mutated lung cancer. The group had pan-Canadian representation and included medical oncologists, pathologists, a clinical laboratory scientist, and a clinician educator. A targeted literature review was performed to identify relevant literature about the topics that the group identified as essential for optimizing biomarker testing in EGFR-mutated lung cancer, including reflex testing, biomarker testing algorithms, liquid biopsy, and tat. The expert multidisciplinary working group convened in person to discuss draft recommendations and to reach consensus.
CONSENSUS RECOMMENDATIONS FROM THE EXPERT MULTIDISCIPLINARY WORKING GROUP
Treatment of EGFR-Mutated NSCLC
The purpose of biomarker testing, including EGFR, in nsclc is to guide personalized therapy for patients. Testing recommendations should therefore align with current treatment algorithms. Thus, the expert multidisciplinary working group began by reviewing a recently published Canadian consensus treatment algorithm for systemic therapy in advanced EGFR-mutated nsclc14. The expert multidisciplinary working group endorsed the algorithm and recommendations summarized in the next subsection and used those recommendations as the context for determining optimal biomarker testing practices.
The Canadian guidelines consider specific EGFR mutations and the presence of central nervous system metastases for treatment decisions. To summarize, based on the phase iii flaura trial, osimertinib is the preferred first-line treatment for patients with advanced nsclc harbouring common EGFR mutations (exon 19 deletion or exon 21 L858R mutation) and for patients with the de novo EGFR T790M mutation, because of improved overall survival and a favourable toxicity profile compared with first-generation egfr tkis12. Because of its activity in the central nervous system, osimertinib is also recommended for patients with brain metastases or leptomeningeal disease12,18,19. First- and second-generation tkis are options for patients with common EGFR mutations when osimertinib is not available or not tolerated. For patients with uncommon EGFR sensitizing mutations, afatinib is recommended as first-line therapy based on the lux-Lung 3 and 6 trials20, which included patients harbouring uncommon EGFR mutations such as G719X, L861Q, and S768I. For patients with exon 20 insertions, a heterogeneous group of alterations that are generally resistant to currently available egfr tkis, the preferred treatment is a platinum doublet chemotherapy regimen or enrolment in a clinical trial.
Biomarker results also guide treatment selection after progression on egfr tkis. Based on data from the aura3 clinical trial, patients who receive a first- or second-generation tki in the first line and who develop the EGFR T790M resistance mutation should receive osimertinib21. For patients who develop resistance to tkis and who do not have the T790M mutation, the preferred treatment is a platinum doublet chemotherapy regimen.
Recommendations for Biomarker Testing
Identifying the Presence or Absence of EGFR Mutations
■ EGFR mutation testing should be performed as part of a comprehensive panel that includes the current standard-of-care biomarkers as summarized by international guidelines, including the College of American Pathologists, the International Association for the Study of Lung Cancer, and the Association for Molecular Pathology12.
■ To avoid delay in treatment initiation, single-gene testing for EGFR could, at the discretion of the treating oncologist, be performed first in patients with advanced nsclc who are at imminent risk of clinical deterioration while awaiting comprehensive testing results.
■ Comprehensive reflex biomarker testing, including EGFR, is recommended for all patients diagnosed with nonsquamous nsclc regardless of disease stage and should be initiated by the pathologist at the time of initial diagnosis. Biomarker test results should be reported without delay, but should also be compiled by the pathologist and listed sequentially in a single comprehensive lung biomarker report, regardless of the type of testing [immunohistochemistry, fluorescence in situ hybridization, next-generation sequencing (ngs), etc.]. The PD-L1 biomarker report should state that PD-L1 results should be interpreted in the context of other biomarker results.
■ When tissue biopsy harbours scarce tumour cells, when time for a tissue biopsy is too lengthy, or when invasive procedures for tissue procurement are contraindicated, liquid biopsy for the detection of activating EGFR mutations and other biomarkers is recommended at baseline, if available. A positive liquid biopsy result for an actionable biomarker alteration should be regarded as a true positive. A negative liquid biopsy result should be confirmed using a tissue biopsy.
■ Optimal treatment decision-making requires results from biomarker testing that assesses actionable driver mutations; systemic therapy should therefore not be initiated in patients with metastatic nsclc based on PD-L1 expression level until actionable driver mutation results (at a minimum, aberrations in EGFR, ALK, and ROS1) are also available, unless patient deterioration is imminent or patients require urgent initiation of therapy.
■ If a patient with advanced lung adenocarcinoma has to start systemic therapy and cannot wait for EGFR test results, it is recommended to start platinum doublet chemotherapy. The panel consensus is, if possible, to avoid checkpoint inhibitors in the first-line setting because of the risk of subsequent pneumonitis if treatment is changed to a tki after checkpoint inhibitor therapy.
■ At the time of progression after a first- or second-generation egfr tki, testing for the identification of the EGFR T790M resistance mutation should be performed. Liquid biopsy is recommended as the initial test, but tissue biopsy should be obtained when liquid biopsy is negative. The liquid biopsy or circulating tumour dna (ctdna) assay should include both EGFR T790M and common EGFR sensitizing mutations, for which identification serves as confirmation of tumour shedding18.
The expert multidisciplinary working group recommends that all patients with nsclc undergo reflex biomarker testing, wherein comprehensive biomarker testing using a tissue sample is ordered by the pathologist at the time of diagnosis, regardless of disease stage. For cases in which squamous cell histology is confirmed, but for which the clinical parameters are atypical (for example, nonsmoking patient or a peripheral lesion), it might still be relevant to request a complete biomarker assessment that includes EGFR. Liquid biopsy could be used if available. The expert multidisciplinary working group recommends that the comprehensive testing panel should incorporate, at a minimum, EGFR and biomarkers based on the current standard of care and guideline recommendations12,14,19,22,23. Current recommendations include biomarkers currently approved by Health Canada and emerging actionable nsclc targets to identify patients for clinical trials or early drug access programs, understanding the limitations of funding that might attend such testing.
Initiation of reflex testing by pathologists for confirmed nsclc optimizes sample processing by eliminating the need to retrieve archived tissue, bypasses the need (and associated delay) for oncology consultation to initiate testing24, and has been shown to increase rates of testing25. When ordered at the time of initial diagnosis, it can improve time to treatment19,24–27. Findings from one Canadian centre showed a median reduction in time to testing of 10 days (to 26 days from 36 days) when EGFR and ALK testing was requested by pathologists at the time of diagnosis regardless of clinical stage, and a significant improvement in time to optimal systemic therapy according to guidelines (24 days vs. 36 days)19. Although, in general, the expert multidisciplinary working group recommends reflex testing for all patients with nsclc regardless of stage, rare institutions at which biomarker results are consistently available to treating clinicians within 21 calendar days could consider reflex testing for advanced-stage disease only.
The expert multidisciplinary working group recommends parallel testing with a comprehensive ngs panel rather than single-gene EGFR testing, in line with current guidelines12,19,22,23. That approach makes efficient use of the sample, improves timely access to results, bypasses delays for ordering follow-up testing, and can be cost-effective if enough targets are included19. New technologies enable concurrent testing for all relevant biomarkers, at moderate additional expense compared with single-gene testing26, and overall higher assay success rates (95% vs. 71%)26. Failure of single-gene tests is largely attributed to an inadequate or insufficient sample for the full complement of an algorithm of single-gene tests26. In contrast, ngs uses input material comparable to that for a single-gene test to detect oncogenic driver mutations across multiple genes, which could be beneficial for patients because it reduces the need for, and risks associated with, rebiopsy26. Comprehensive ngs testing has been demonstrated to be feasible, with acceptable tats in line with current guidelines12,26,28.
The expert multidisciplinary working group recommends that clinically actionable biomarker results should be reported immediately as they become available, and that the pathologist should also compile lung biomarker results, including EGFR, to be summarized in a comprehensive final synoptic report. Thus, the medical oncologist and the patient are provided with the information required to make informed treatment decisions. For patients with metastatic nsclc, treatment should not be based on PD-L1 expression levels alone, but rather considered in the context of actionable driver mutation results, including EGFR and ALK results at a minimum, to prevent suboptimal immunotherapy treatment of patients with actionable driver mutations. Approximately 27% of patients with PD-L1 expression also have actionable genomic targets29. In addition, PD-L1 expression should not be used as a biomarker to determine eligibility for checkpoint inhibitor therapy in patients with certain actionable alterations, including EGFR, ALK, and ROS130.
Rapid results might be urgently needed in certain clinical scenarios—for example, if there is a risk of clinical deterioration in patients with advanced nonsquamous nsclc. In such cases, single-gene testing for EGFR might be appropriate while awaiting comprehensive testing results and could lead to avoidance of unacceptable delay in treatment decision-making or treatment initiation24. If a patient has to start systemic therapy and cannot wait for biomarker testing, the expert multidisciplinary working group recommends starting platinum doublet chemotherapy without an immune checkpoint inhibitor and tailoring treatment once biomarker results are available, because of the risk of pneumonitis in patients receiving tkis after checkpoint inhibitor therapy30. The maximum acceptable time to wait for biomarker results for each individual patient should be at the clinician’s discretion and be based on the risks of waiting (for example, patient deterioration or missed treatment opportunities). Clinicians should follow patients closely for signs of deterioration while awaiting results.
At disease progression after a first- or second-generation egfr tki, the expert multidisciplinary working group recommends broad comprehensive molecular testing, including for the EGFR T790M mutation, using a new biopsy. The rationale is to identify emerging resistance mechanisms for which clinical trial options might be available. Liquid biopsy is preferred because it reduces the inherent risks and discomfort of tissue biopsy and is more cost-effective for follow-up testing and monitoring31,32. High pairwise concordance has been demonstrated for liquid biopsy and matched tissue for EGFR mutational analysis in resistance settings31,33–37. Circulating biomarkers have an advantage, in that they are theoretically more likely to reflect the intratumoural heterogeneity in actively growing metastatic tumours that might be missed by single-site tissue biopsies31,32,38,39. Typically, ctdna represents less than 0.5% of total circulating free dna in a liquid biopsy31. Moreover, shedding of ctdna differs between tumours and can be lower in patients whose disease is not progressing or who are responding to therapy, potentially leading to false negative results31. The EGFR testing should therefore, where possible, include testing for activating EGFR mutations that would serve as controls to confirm tumour shedding and the presence of adequate ctdna in the sample. A tissue biopsy is recommended when a liquid biopsy does not detect T790M; the tissue biopsy should also evaluate the specimen for small-cell transformation. In situations in which a patient cannot wait for a sequential approach, tests on both sample types can be performed simultaneously40.
Turnaround Time
■ Lung cancer working groups should consider local contextual factors and define a target tat for biomarker results, starting when a diagnosis of nsclc is issued until when the report is received by the treating oncologist.
■ The total tat from ordering EGFR testing to reporting the results and making the report available to the ordering physician should not exceed 21 calendar days.
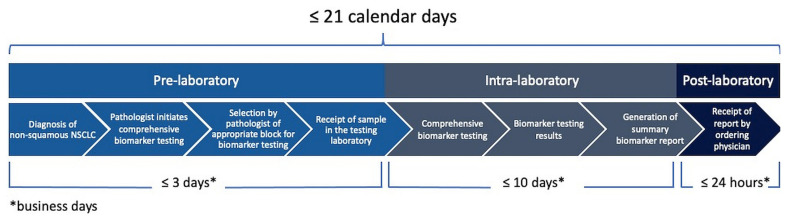
■ The pre-laboratory tat, from test request to sample receipt by the testing laboratory, should not exceed 3 business days. The intra-laboratory tat, from sample receipt to generation of the report, should not exceed 10 business days. The post-laboratory tat, from result reporting to report receipt by the ordering physician, should be less than 24 hours.
A rapid tat for biomarker testing results in nsclc is extremely important for timely treatment initiation. In a study at one Canadian centre, biomarker testing results were available for only 21% of patients at the time of first consultation with a medical oncologist24. Patients with biomarker test results available had a mean time to treatment initiation of 16 days, compared with 29 days for those whose biomarker test results were not available at that time. In some cases, lack of timely biomarker test results led to unnecessary initiation of chemotherapy: one study reported that 19% of patients with EGFR- and ALK-positive disease had initiated first-line chemotherapy before biomarker results were available24.
Current guidelines recommend a 10-day tat from sample receipt in the laboratory to generation of the report (intra-laboratory tat)12. The expert multidisciplinary working group recommends that tat in the context of reflex testing should be defined from when biomarker tests are ordered at the time of diagnosis of nsclc to when the treating clinician receives the report. The impact of lengthy administrative aspects of EGFR testing is significant and influences the ability of oncologists to obtain results and make treatment decisions for patients. To ensure timely access to testing year-round, even during periods with multiple holidays, the expert multidisciplinary working group recommends a maximum of 21 calendar days from ordering the EGFR testing and comprehensive biomarker panel to receiving the report. However, particularly when testing is not performed in-house, breaking the overall tat into its component parts can be useful, because it allows for the identification of processes whose efficiency has to improve. The intra-laboratory tat typically has the least variability; in contrast, archival specimen handling and transportation to the testing laboratory are highly variable41. In addition, there is a need for more efficient release of results from testing laboratories to the medical record41. A recent Canadian study found that implementing in-house testing was associated with a median reduction of 2 months in biomarker report tat, demonstrating that, when tests are sent to an outside laboratory, the pre-laboratory and post-laboratory processes can significantly increase the overall time for the oncologist to receive results42. The expert multidisciplinary working group therefore recommends that the pre-laboratory tat should not exceed 3 business days, the intra-laboratory tat should not exceed 10 business days (in line with current guidelines12), and the post-laboratory tat should be less than 24 hours (Figure 1). Taking into account weekends, holiday periods, and other resource constraints, the full tat should be less than 21 calendar days.
FIGURE 1.
Turnaround time from ordering EGFR testing to receipt of report by the ordering physician. Total turnaround time should not exceed 21 calendar days. NSCLC = non-small-cell lung cancer.
Pre-analytic Considerations
■ All pathology reports in nsclc should include, in the diagnostic comment, information about the tumour content and identification of best tumour block or blocks for further testing. Diagnostic reports in nsclc should specify the best tumour block, the total number of viable neoplastic cells, and the percentage of viable neoplastic cells among all viable nucleated cells in the slide.
■ Basic training for assessing and reporting tissue adequacy parameters for biomarker testing should be provided to all anatomic pathologists, regardless of their place of practice.
■ Laboratories should, whenever possible, avoid using decalcification. When necessary, use of molecular-friendly decalcification methods, such as edta, is recommended.
■ Any cytology sample with adequate cell block tumour cellularity can be used for molecular testing. However, immunohistochemistry-based biomarkers usually require formalin-fixed cytology samples.
■ Requisitions of samples obtained upon progression for the investigation of resistance mechanisms should state the reason for tissue procurement and mention current and previous treatments.
■ To preserve the specimen for molecular testing, additional samples obtained in the context of resistance mechanisms assessment should not be re-evaluated extensively with immunohistochemistry stains.
■ Specimens should be sent through a courier that uses a digital tracking system, so as to track specimens from the referring laboratory to the reference laboratory.
Pre-analytic considerations, including sample collection and handling upon receipt into the laboratory and prior to testing, are important to ensure that the best specimen is selected for testing43. The International Organization for Standardization’s 15189 standard is one of the most cited guidelines for molecular diagnostics43 and specifies that laboratories should provide information about acceptable specimen types and volumes, tat, specimen transportation requirements, rejection criteria, and factors affecting test performance and interpretation, and should allow for access to laboratory personnel with expertise in providing clinical advice on test ordering and interpretation.
The expert multidisciplinary working group considered the foregoing elements in the setting of nonsquamous nsclc and highlighted essential pre-analytic elements in that setting. Those elements include recommendations about sample procurement, fixation, transportation, and processing. Pathologists should become familiar with tumour requirements for specific biomarker tests in nsclc and subsequently be trained in the process of selecting the tumour tissue block best-suited for testing. To provide immediate feedback on tissue quality, real-time assessment of tissue quality at the time of procedures, such as rapid on-site evaluation, should be performed where possible. Radiology–pathology, respirology–pathology, or surgery–pathology correlation rounds are encouraged as ongoing education for tissue providers and as a way to improve the quality of tissue samples obtained by various techniques. Cytology samples with adequate cell block tumour cellularity should be used for testing, with results comparable to those obtained with histology samples12. Specimen adequacy parameters should be assessed by all pathologists. If a local laboratory is unsure of the specimen adequacy parameters for specific tests and methods, the specimen should still be sent to the testing laboratory. An effort should be made to educate community pathologists to assess parameters of sample adequacy for testing44.
Laboratories should follow standard operating procedures to control tissue storage and handling, including fixation method and ischemia and fixation times, and should avoid the use of decalcification methods that will preclude molecular testing. Acid decalcification should be avoided, because it extensively fragments dna and renders samples unsuitable for molecular testing12,32,45.
Previous treatments can alter tumour gene expression. For example, EGFR resistance mutations are associated with nsclc progression after first- or second-line treatment with an egfr tki16. Samples obtained upon progression to investigate resistance mechanisms should therefore clearly state on the requisition the reason for the biopsy and should list previous treatments to assist pathologists in deciding on appropriate use of ancillary testing. Clear communication on requisitions for treatment resistance also helps to ensure that the specimen is not re-evaluated unnecessarily with immunohistochemistry stains. Each specimen should be collected so as to assure a short ischemia time, should be submitted to the laboratory in an appropriate fixative, and should be properly fixed per current guidelines. Specimen transportation to the reference laboratory should be digitally recorded and tracked to ensure timely and accurate tracking of specimens between laboratories.
Analytic Considerations
■ A targeted ngs panel, including EGFR, is preferred over single-gene testing. Copy number variations and gene rearrangements should also be considered for comprehensive gene analysis. Single-gene testing for EGFR T790M could be appropriate in the context of treatment resistance.
EGFR testing can be performed by ngs using tumour tissue or blood. It is beyond the scope of this guideline to review the analytic characteristics of the various ngs platforms. The expert multidisciplinary working group considers that each laboratory should choose the method of testing best suited to their clinical practice and in accordance with the samples to be tested, the local technical and professional expertise, and access to equipment. The advantage of screening all samples for all EGFR mutations is the detection of all known and novel mutations46. The disadvantages are the potential for lower sensitivity than could be achievable with targeted methods and a labour-intensive process requiring extensive expertise and longer tat. Targeted EGFR testing takes less time and uses fewer resources, generally has higher sensitivity for detection of mutations, and uses a technology that is fairly widely available46. Its disadvantage, however, is that rare mutations are not detected, and reagents might be more costly than those for other screening methods. The expert disciplinary working group therefore recommends a combination of EGFR screening methods and targeted EGFR testing, on tissue and plasma, which could be adapted by the pathologist to each clinical scenario as detailed in other parts of this guideline.
Post-analytic Considerations
■ The biomarker report should use both standard Human Genome Variation Society and commonly recognized nomenclature.
■ The biomarker report should not make recommendations for or against the use of specific therapies, but could comment on expected drug sensitivities based on the biomarker results.
A primary purpose of the molecular pathology report is to provide testing results to the medical oncologist in a manner that allows the oncologist to easily ascertain the information required to select therapy for the patient, while also communicating the limitations of the testing results. Existing reporting guidelines should be followed when reporting biomarker testing results in nsclc13,16,32,47. Additionally, as stated earlier, the pathologist should summarize the results of all biomarker testing, including EGFR, in a comprehensive report. The summarized results allow the medical oncologist to find, in one location, all the information needed to make treatment decisions. Although existing provincial accreditation programs require results to be communicated according to Human Genome Variation Society nomenclature47, the expert multidisciplinary working group recommends that the report also use commonly recognized nomenclature for ease of interpretation by the medical oncologist. For example, the commonly recognized name EGFR T790M should be used in the report as should the Human Genome Variation Society nomenclature NC_000007.14:g.55181378C>T. In addition, specific therapies should not be recommended in the report, but expected sensitivities can be noted based on biomarker results.
Funding for Biomarker Testing in EGFR-Mutated NSCLC
Although funding for EGFR testing does exist in Canada, the expert multidisciplinary working group recommends comprehensive biomarker testing to optimally perform genomic profiling in nsclc, including identifying the presence or absence of EGFR mutations, and to allow for timely treatment. Funding is a major challenge for comprehensive biomarker testing in Canada; it is often reserved to single-gene testing. Effective biomarker testing requires from provincial reimbursement bodies not just funding for the test itself, but also funding for other aspects of testing such as human resources, infrastructure, digital tracking of specimens, and bioinformatics. A recently published Canadian guideline on the use of ngs assays in oncology identified an unmet need in funding for ngs infrastructure, local expertise, methodologic validation, and test implementation48. Although the clinical utility of concurrent testing for a panel of biomarkers is recognized, funding for comprehensive biomarker testing is not yet standard in Canada; the expert multidisciplinary working group therefore recommends that provincial reimbursement bodies should support comprehensive testing rather than single-gene assays.
Optimal Biomarker Testing Implementation
■ Translating guidelines into clinical practice requires a multidisciplinary effort, adapting to provincial and local contexts.
Despite publication of practice guidelines, large gaps between the recommended care and the care that patients receive often remain49. Barriers to guideline implementation include not just physician factors such as lack of knowledge of guidelines, but also external factors such as resource and organizational constraints50.
Although education about new recommendations is required, education is not sufficient for implementation, which also requires teamwork at an institutional level to develop context-specific solutions. Such a systemic and collaborative approach was used at one hospital in Canada to support process improvement throughout the lung cancer diagnostic pathway. That effort ultimately resulted in a decrease of 48% in the time from referral to initial treatment51. Recognizing that a collaborative approach is key to implementation of the recommendations in the present guideline, the expert multidisciplinary working group recommends review of the guideline recommendations by a multidisciplinary team at each institution and conduct of a local needs assessment to identify the needs of all target groups and stakeholders. Such an approach ensures that the perspectives of health care professionals involved in various aspects of lung cancer diagnosis, biomarker testing, and treatment will be considered in the implementation of the guidelines. That “community of practice” should be the focus of education and implementation strategies, in which social interaction will facilitate behavioural change, the identification of contextual constraints, and the proposal of solutions that take into account the local care setting.
Effective education about these guidelines also requires interprofessional collaboration. The expert multidisciplinary working group recommends that the design and delivery of educational meetings should be interprofessional, practice-based, focused on outcomes that are relevant to the professional practice of each health care discipline, and be underpinned by the “communities of practice” learning theory52,53. Factors that might increase the effectiveness of educational meetings include strategies to increase attendance by various health care professionals; using mixed interactive and didactic formats; and having multiple, spaced, and longer exposures54–57. Faculty development is critical to prepare and support facilitators in the delivery of interprofessional education, and educators should be consulted to design effective educational interventions56,57.
Accurate and timely biomarker testing is multifactorial: education and implementation strategies should extend beyond the medical facets of testing to include coordination of care, tissue procurement and handover, requisition and report design, clear workflow within and between services, automatic information exchange between electronic health systems, and improved communication, with fast feedback loops between health care practitioners. In addition, education has to be extended beyond physicians to include nurse navigators, clerks, and laboratory technologists. The implementation strategies should include decision-support systems and standardized orders. There is a need to approach implementation with a continuing quality improvement lens, to perform robust program evaluation, to measure processes and products, and to monitor critical quality indicators so as to guide future educational interventions for individuals and the team.
SUMMARY
Comprehensive reflex biomarker testing for patients with confirmed nonsquamous nsclc is important for the selection of appropriate therapies. To guide optimal patient care, each biomarker result, including EGFR, should be interpreted in the context of the full set of biomarker data. Lung Cancer Canada’s Policy on Molecular Testing in Lung Cancer, published in 2014, highlighted the need for national policy standards and a sustainable public funding model for lung cancer molecular testing so that all patients across Canada are treated in a timely fashion. The organization also recommended that, in Canada, reflex testing be performed at diagnosis58. That effort was followed by Canadian guidelines for the standardization of biomarker testing in patients with advanced nsclc14 and best-practice recommendations for EGFR T790M testing in lung cancer in Canada59. More recently, recommendations about the systemic treatment of EGFR-positive nsclc were published60. Our recommendations align with those guidelines in that they encourage both comprehensive reflex molecular testing for all patients with nonsquamous nsclc at diagnosis to rapidly identify patients with EGFR-mutated nsclc, and molecular testing for resistance mutations during treatment. Here, we have made recommendations for EGFR mutation testing and focused on breaking silos of single-gene testing with the use of more comprehensive panels. Recommendations for the optimization of the pre-analytic, analytic, and post-analytic elements of testing are also provided to complement existing guidelines in the setting of nsclc. The importance of standardizing those elements and the impact that such standardization can have on the timeliness and accuracy of the biomarker report and the time to treatment is highlighted. Review of the present guideline by a multidisciplinary team is important to ensure that perspectives from all relevant stakeholders are considered for the successful local implementation of its recommendations.
ACKNOWLEDGMENTS
The authors thank AstraZeneca Canada for supporting the working group meeting and Precision Rx-Dx for assistance with the working group. The authors acknowledge Philippa Bridge-Cook phd and Ciara Airey phd of Precision Rx-Dx for medical writing support that was funded by AstraZeneca in accordance with the version 3 of the Good Publication Practice guidelines (https://www.ismpp.org/gpp3).
Footnotes
CONFLICT OF INTEREST DISCLOSURES
We have read and understood Current Oncology’s policy on disclosing conflicts of interest, and we declare the following interests: PKC reports consultancy fees and honoraria from AstraZeneca, Bristol Myers Squibb, Amgen, Roche, Novartis, Merck, Pfizer, Takeda, and emd Serono. MG reports grants and personal fees from AstraZeneca and Hoffmann–La Roche, unrestricted educational grants from AstraZeneca, Hoffmann–La Roche, Eli Lilly, Boehringer Ingelheim, Pfizer, and Merck, and nonfinancial support from Merck, outside the submitted work. SB reports personal fees from AstraZeneca, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, GlaxoSmithKline, Lilly, Merck, Novartis, Pfizer, Roche, and Takeda, outside the submitted work. NBL reports institutional research funding from AstraZeneca, Pfizer, Roche, and Merck Sharp and Dohme; and honoraria for continuing medical eduation lectures from Merck Sharp and Dohme. BM reports advisory board fees or honoraria from Boehringer Ingelheim, Pfizer, AstraZeneca, Roche, and Novartis. BSS reports personal fees from AstraZeneca during the conduct of the study; grants and personal fees from AstraZeneca, Boehringer Ingelheim, Eli Lilly, emd Serono, Novartis, and Pfizer; and personal fees from Bayer and Roche, outside the submitted work. TS reports personal fees from AstraZeneca during the conduct of the study. DNI reports personal fees from AstraZeneca during the conduct of the study; grants, personal fees, and nonfinancial support from AstraZeneca; grants and personal fees from Pfizer and Roche; nonfinancial support from Biocartis; and personal fees from Bayer, Boehringer Ingelheim, Eli Lilly, Amgen, Merck, and Novartis, outside the submitted work.
REFERENCES
- 1.Brenner DR, Weir HK, Demers AA, et al. on behalf of the Canadian Cancer Statistics Advisory Committee. Projected estimates of cancer in Canada in 2020. CMAJ. 2020;192:E199–205. doi: 10.1503/cmaj.191292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kris MG, Johnson BE, Berry LD, et al. Using multiplexed assays of oncogenic drivers in lung cancers to select targeted drugs. JAMA. 2014;311:1998–2006. doi: 10.1001/jama.2014.3741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ellis PM, Verma S, Sehdev S, Younus J, Leighl NB. Challenges to implementation of an epidermal growth factor receptor testing strategy for non-small-cell lung cancer in a publicly funded health care system. J Thorac Oncol. 2013;8:1136–41. doi: 10.1097/JTO.0b013e31829f6a43. [DOI] [PubMed] [Google Scholar]
- 4.Pao W, Miller V, Zakowski M, et al. EGF receptor gene mutations are common in lung cancers from “never smokers” and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc Natl Acad Sci U S A. 2004;101:13306–11. doi: 10.1073/pnas.0405220101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shiau CJ, Babwah JP, da Cunha Santos G, et al. Sample features associated with success rates in population-based EGFR mutation testing. J Thorac Oncol. 2014;9:947–56. doi: 10.1097/JTO.0000000000000196. [DOI] [PubMed] [Google Scholar]
- 6.Thatcher N, Chang A, Parikh P, et al. Gefitinib plus best supportive care in previously treated patients with refractory advanced non-small-cell lung cancer: results from a randomised, placebo-controlled, multicentre study (Iressa Survival Evaluation in Lung Cancer) Lancet. 2005;366:1527–37. doi: 10.1016/S0140-6736(05)67625-8. [DOI] [PubMed] [Google Scholar]
- 7.Shepherd FA, Rodrigues-Pereira J, Ciuleanu T, et al. on behalf of the National Cancer Institute of Canada Clinical Trials Group. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005;353:123–32. doi: 10.1056/NEJMoa050753. [DOI] [PubMed] [Google Scholar]
- 8.Wu YL, Cheng Y, Zhou X, et al. Dacomitinib versus gefitinib as first-line treatment for patients with EGFR-mutation–positive non-small-cell lung cancer (archer 1050): a randomised, open-label, phase 3 trial. Lancet Oncol. 2017;18:1454–66. doi: 10.1016/S1470-2045(17)30608-3. [DOI] [PubMed] [Google Scholar]
- 9.Soria JC, Ohe Y, Vansteenkiste J, et al. on behalf of the flaura investigators. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med. 2018;378:113–25. doi: 10.1056/NEJMoa1713137. [DOI] [PubMed] [Google Scholar]
- 10.Park K, Tan EH, O’Byrne K, et al. Afatinib versus gefitinib as first-line treatment of patients with EGFR mutation–positive non-small-cell lung cancer (lux-Lung 7): a phase 2B, open-label, randomised controlled trial. Lancet Oncol. 2016;17:577–89. doi: 10.1016/S1470-2045(16)30033-X. [DOI] [PubMed] [Google Scholar]
- 11.Ramalingam SS, Vansteenkiste J, Planchard D, et al. on behalf of the flaura investigators. Overall survival with osimertinib in untreated, EGFR-mutated advanced nsclc. N Engl J Med. 2020;382:41–50. doi: 10.1056/NEJMoa1913662. [DOI] [PubMed] [Google Scholar]
- 12.Lindeman NI, Cagle PT, Aisner DL, et al. Updated molecular testing guideline for the selection of lung cancer patients for treatment with targeted tyrosine kinase inhibitors: guideline from the College of American Pathologists, the International Association for the Study of Lung Cancer, and the Association for Molecular Pathology. J Mol Diagn. 2018;20:129–59. doi: 10.1016/j.jmoldx.2017.11.004. [DOI] [PubMed] [Google Scholar]
- 13.Lindeman NI, Cagle PT, Beasley MB, et al. Molecular testing guideline for selection of lung cancer patients for egfr and alk tyrosine kinase inhibitors: guideline from the College of American Pathologists, International Association for the Study of Lung Cancer, and Association for Molecular Pathology. J Mol Diagn. 2013;15:415–53. doi: 10.1016/j.jmoldx.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 14.Melosky B, Blais N, Cheema P, et al. Standardizing biomarker testing for Canadian patients with advanced lung cancer. Curr Oncol. 2018;25:73–82. doi: 10.3747/co.25.3867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gay ND, Wang Y, Beadling C, et al. Durable response to afatinib in lung adenocarcinoma harboring NRG1 gene fusions. J Thorac Oncol. 2017;12:e107–10. doi: 10.1016/j.jtho.2017.04.025. [DOI] [PubMed] [Google Scholar]
- 16.Cree IA, Deans Z, Ligtenberg MJ, et al. on behalf of the European Society of Pathology Task Force on Quality Assurance in Molecular Pathology and the Royal College of Pathologists. Guidance for laboratories performing molecular pathology for cancer patients. J Clin Pathol. 2014;67:923–31. doi: 10.1136/jclinpath-2014-202404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sung MR, Ellis PM, Verma S, Duncan E, Leighl NB. Approach to biomarker testing: perspectives from various specialties. Curr Oncol. 2016;23:178–83. doi: 10.3747/co.23.3019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sacher AG, Komatsubara KM, Oxnard GR. Application of plasma genotyping technologies in non–small cell lung cancer: a practical review. J Thorac Oncol. 2017;12:1344–56. doi: 10.1016/j.jtho.2017.05.022. [DOI] [PubMed] [Google Scholar]
- 19.Cheema PK, Menjak IB, Winterton-Perks Z, et al. Impact of reflex EGFR/ALK testing on time to treatment of patients with advanced nonsquamous non-small-cell lung cancer. J Oncol Pract. 2017;13:e130–8. doi: 10.1200/JOP.2016.014019. [DOI] [PubMed] [Google Scholar]
- 20.Yang JCH, Sequist LV, Geater SL, et al. Clinical activity of afatinib in patients with advanced non-small-cell lung cancer harbouring uncommon EGFR mutations: a combined post-hoc analysis of lux-Lung 2, lux-Lung 3, and lux-Lung 6. Lancet Oncol. 2015;16:830–8. doi: 10.1016/S1470-2045(15)00026-1. [DOI] [PubMed] [Google Scholar]
- 21.Wu YL, Ahn MJ, Garassino MC, et al. cns efficacy of osimertinib in patients with T790M-positive advanced non-small-cell lung cancer: data from a randomized phase iii trial (aura3) J Clin Oncol. 2018;36:2702–9. doi: 10.1200/JCO.2018.77.9363. [DOI] [PubMed] [Google Scholar]
- 22.Kalemkerian GP, Narula N, Kennedy EB, et al. Molecular testing guideline for the selection of patients with lung cancer for treatment with targeted tyrosine kinase inhibitors: American Society of Clinical Oncology endorsement of the College of American Pathologists/International Association for the Study of Lung Cancer/Association for Molecular Pathology clinical practice guideline update. J Clin Oncol. 2018;36:911–19. doi: 10.1200/JCO.2017.76.7293. [DOI] [PubMed] [Google Scholar]
- 23.National Comprehensive Cancer Network (nccn) NCCN Clinical Practice Guidelines in Oncology: Non–Small Cell Lung Cancer. Ver. 2.2020. Fort Washington, PA: NCCN; 2020. [Current version available online at: https://www.nccn.org/professionals/physician_gls/pdf/nscl_blocks.pdf (free registration required); cited 17 November 2020] [Google Scholar]
- 24.Lim C, Tsao MS, Le LW, et al. Biomarker testing and time to treatment decision in patients with advanced nonsmall-cell lung cancer. Ann Oncol. 2015;26:1415–21. doi: 10.1093/annonc/mdv208. [DOI] [PubMed] [Google Scholar]
- 25.Cheema PK, Raphael S, El-Maraghi R, et al. Rate of EGFR mutation testing for patients with nonsquamous non-small-cell lung cancer with implementation of reflex testing by pathologists. Curr Oncol. 2017;24:16–22. doi: 10.3747/co.24.3266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miller TE, Yang M, Bajor D, et al. Clinical utility of reflex testing using focused next-generation sequencing for management of patients with advanced lung adenocarcinoma. J Clin Pathol. 2018;71:1108–15. doi: 10.1136/jclinpath-2018-205396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Anand K, Phung TL, Bernicker EH, Cagle PT, Olsen RJ, Thomas JS. Clinical utility of reflex ordered testing for molecular biomarkers in lung adenocarcinoma. Clin Lung Cancer. 2020;21:437–42. doi: 10.1016/j.cllc.2020.05.007. [DOI] [PubMed] [Google Scholar]
- 28.Moore DA, Balbi K, Ingham A, Arkenau HT, Bennett P. Analysis of a large cohort of non–small cell lung cancers submitted for somatic variant analysis demonstrates that targeted next-generation sequencing is fit for purpose as a molecular diagnostic assay in routine practice. J Clin Pathol. 2018;71:1001–6. doi: 10.1136/jclinpath-2018-205319. [DOI] [PubMed] [Google Scholar]
- 29.Leighl NB, Page RD, Raymond VM, et al. Clinical utility of comprehensive cell-free dna analysis to identify genomic biomarkers in patients with newly diagnosed metastatic non–small cell lung cancer. Clin Cancer Res. 2019;25:4691–700. doi: 10.1158/1078-0432.CCR-19-0624. [DOI] [PubMed] [Google Scholar]
- 30.Lisberg A, Cummings A, Goldman JW, et al. A phase ii study of pembrolizumab in EGFR-mutant, PD-L1+, tyrosine kinase inhibitor naïve patients with advanced nsclc. J Thorac Oncol. 2018;13:1138–45. doi: 10.1016/j.jtho.2018.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pisapia P, Malapelle U, Troncone G. Liquid biopsy and lung cancer. Acta Cytol. 2019;63:489–96. doi: 10.1159/000492710. [DOI] [PubMed] [Google Scholar]
- 32.Rolfo C, Mack PC, Scagliotti GV, et al. Liquid biopsy for advanced non–small cell lung cancer (nsclc): a statement paper from the iaslc. J Thorac Oncol. 2018;13:1248–68. doi: 10.1016/j.jtho.2018.05.030. [DOI] [PubMed] [Google Scholar]
- 33.Arriola E, Paredes-Lario A, Garcia-Gomez R, et al. Comparison of plasma ctdna and tissue/cytology-based techniques for the detection of EGFR mutation status in advanced nsclc: Spanish data subset from assess. Clin Transl Oncol. 2018;20:1261–7. doi: 10.1007/s12094-018-1855-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim E, Feldman R, Wistuba II. Update on EGFR mutational testing and the potential of noninvasive liquid biopsy in non-small-cell lung cancer. Clin Lung Cancer. 2018;19:105–14. doi: 10.1016/j.cllc.2017.08.001. [DOI] [PubMed] [Google Scholar]
- 35.Komiya K, Nakashima C, Nakamura T, et al. Current status and problems of T790M detection, a molecular biomarker of acquired resistance to egfr tyrosine kinase inhibitors, with liquid biopsy and re-biopsy. Anticancer Res. 2018;38:3559–66. doi: 10.21873/anticanres.12628. [DOI] [PubMed] [Google Scholar]
- 36.Li Y, Xu Y, Wu X, He C, Liu Q, Wang F. Comprehensive analysis of EGFR T790M detection by ddpcr and arms-pcr and the effect of mutant abundance on the efficacy of osimertinib in nsclc patients. J Thorac Dis. 2019;11:3004–14. doi: 10.21037/jtd.2019.07.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mondaca S, Offin M, Borsu L, et al. Lessons learned from routine, targeted assessment of liquid biopsies for EGFR T790M resistance mutation in patients with EGFR mutant lung cancers. Acta Oncol. 2019;58:1634–9. doi: 10.1080/0284186X.2019.1645354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Del Re M, Crucitta S, Gianfilippo G, et al. Understanding the mechanisms of resistance in EGFR-positive nsclc: from tissue to liquid biopsy to guide treatment strategy. Int J Mol Sci. 2019;20:3951. doi: 10.3390/ijms20163951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thress KS, Brant R, Carr TH, et al. EGFR mutation detection in ctdna from nsclc patient plasma: a cross-platform comparison of leading technologies to support the clinical development of AZD9291. Lung Cancer. 2015;90:509–15. doi: 10.1016/j.lungcan.2015.10.004. [DOI] [PubMed] [Google Scholar]
- 40.Aggarwal C, Rolfo CD, Oxnard GR, Gray JE, Sholl LM, Gandara DR. Strategies for the successful implementation of plasma-based nsclc genotyping in clinical practice. Nat Rev Clin Oncol. 2020 doi: 10.1038/s41571-020-0423-x. [Online ahead of print]. [DOI] [PubMed] [Google Scholar]
- 41.DiStasio M, Chen Y, Rangachari D, Costa DB, Heher YK, VanderLaan PA. Molecular testing turnaround time for non–small cell lung cancer in routine clinical practice confirms feasibility of cap/iaslc/amp guideline recommendations: a single-center analysis. Clin Lung Cancer. 2017;18:e349–56. doi: 10.1016/j.cllc.2017.03.001. [DOI] [PubMed] [Google Scholar]
- 42.Matthews P, Beharry A, Nematollahi M, Bendzsak K, Irshad P, Cheema P. Clinical impact of rapid biomarker testing in non–small cell lung cancer in a community setting. Lab Invest. 2020;100(suppl 1):1802. [Google Scholar]
- 43.Payne DA, Baluchova K, Peoc’h KH, et al. Pre-examination factors affecting molecular diagnostic test results and interpretation: a case-based approach. Clin Chim Acta. 2017;467:59–69. doi: 10.1016/j.cca.2016.06.018. [DOI] [PubMed] [Google Scholar]
- 44.Lim C, Sekhon HS, Cutz JC, et al. Improving molecular testing and personalized medicine in non-small-cell lung cancer in Ontario. Curr Oncol. 2017;24:103–10. doi: 10.3747/co.24.3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mok TS, Carbone DP, Hirsch FR, editors. IASLC Atlas of EGFR Testing in Lung Cancer. Aurora, CO: International Association for the Study of Lung Cancer; 2017. [Google Scholar]
- 46.Chen M, Zhao H. Next-generation sequencing in liquid biopsy: cancer screening and early detection. Hum Genomics. 2019;13:34. doi: 10.1186/s40246-019-0220-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li MM, Datto M, Duncavage EJ, et al. Standards and guidelines for the interpretation and reporting of sequence variants in cancer: a joint consensus recommendation of the Association for Molecular Pathology, American Society of Clinical Oncology, and College of American Pathologists. J Mol Diagn. 2017;19:4–23. doi: 10.1016/j.jmoldx.2016.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yip S, Christofides A, Banerji S, et al. A Canadian guideline on the use of next-generation sequencing in oncology. Curr Oncol. 2019;26:e241–54. doi: 10.3747/co.26.4731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.United States, Institute of Medicine of the National Academies. 6. Promoting adoption of clinical practice guidelines. In: Graham R, Mancher M, Miller Wolman D, Greenfield S, Steinberg E, editors. Clinical Practice Guidelines We Can Trust. Washington, DC: National Academies Press; 2011. [DOI] [PubMed] [Google Scholar]
- 50.Fischer F, Lange K, Klose K, Greiner W, Kraemer A. Barriers and strategies in guideline implementation—a scoping review. Healthcare (Basel) 2016;4:36. doi: 10.3390/healthcare4030036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fung-Kee-Fung M, Maziak DE, Pantarotto JR, et al. Regional process redesign of lung cancer care: a learning health system pilot project. Curr Oncol. 2018;25:59–66. doi: 10.3747/co.25.3719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wenger E. Communities of Practice: Learning, Meaning, and Identity. New York, NY: Cambridge University Press; 1998. [DOI] [Google Scholar]
- 53.Hean S, Craddock D, Hammick M, Hammick M. Theoretical insights into interprofessional education: amee guide no. 62. Med Teach. 2012;34:e78–101. doi: 10.3109/0142159X.2012.650740. [DOI] [PubMed] [Google Scholar]
- 54.Cervero RM, Gaines JK. The impact of cme on physician performance and patient health outcomes: an updated synthesis of systematic reviews. J Contin Educ Health Prof. 2015;35:131–8. doi: 10.1002/chp.21290. [DOI] [PubMed] [Google Scholar]
- 55.Forsetlund L, Bjorndal A, Rashidian A, et al. Continuing education meetings and workshops: effects on professional practice and health care outcomes. Cochrane Database Syst Rev. 2009;2009:CD003030. doi: 10.1002/14651858.CD003030.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hammick M, Freeth D, Koppel I, Reeves S, Barr H. A best evidence systematic review of interprofessional education: beme guide no. 9. Med Teach. 2007;29:735–51. doi: 10.1080/01421590701682576. [DOI] [PubMed] [Google Scholar]
- 57.Reeves S, Fletcher S, Barr H, et al. A beme systematic review of the effects of interprofessional education: beme guide no. 39. Med Teach. 2016;38:656–68. doi: 10.3109/0142159X.2016.1173663. [DOI] [PubMed] [Google Scholar]
- 58.Lung Cancer Canada. Policy on Molecular Testing in Lung Cancer. Toronto, ON: Lung Cancer Canada; 2014. [Google Scholar]
- 59.Stockley T, Souza CA, Cheema PK, et al. Evidence-based best practices for EGFR T790M testing in lung cancer in Canada. Curr Oncol. 2018;25:163–9. doi: 10.3747/co.25.4044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Melosky B, Banerji S, Blais N, et al. Canadian consensus: a new systemic treatment algorithm for advanced EGFR-mutated non-small-cell lung cancer. Curr Oncol. 2020;27:e146–55. doi: 10.3747/co.27.6007. [DOI] [PMC free article] [PubMed] [Google Scholar]