Abstract
Lymphedema is a chronic inflammatory condition that results from damage to the lymphatic system. Lymphedema is classified as either primary or secondary, the former being caused by a malformation of lymph vessels or nodes, and the latter resulting from trauma, chronic lymphatic system overload, or the sequelae of cancer treatments. In the present article, we focus on secondary cancer-related lymphedema (crl), a potential survivorship treatment-related effect. Treatments for breast, gynecologic, prostate, and head-and-neck cancers, and melanoma and other skin cancers are most frequently associated with crl.
The incidence of crl varies widely based on cancer location and treatment modalities, with estimates ranging from 5% to 83% in various cancers. Given the lack of a universal definition and diagnostic criteria, the prevalence of crl is difficult to ascertain; current estimates suggest that more than 300,000 Canadians are affected by crl.
Here, we present an overview of crl, divided into 5 subtopics: lymphedema risk factors; early identification and intervention; diagnosis and staging; management, with emphasis on the volume reduction and maintenance phases, plus patient support and education; and clinical pearls to help providers integrate knowledge about crl into their practice.
Keywords: Cancer-related lymphedema, lymphatic therapy, cancer survivorship, patient self-management, general practitioners in oncology
INTRODUCTION
Cancer-related lymphedema (crl) is one of the potential survivorship treatment-related effects that can occur within weeks, months, or years after treatment completion1. Treatments for breast, gynecologic, prostate, and head-and-neck cancers, and for melanoma and other skin cancers, are most frequently associated with crl.
The incidence of crl varies widely based on cancer site and treatment modalities, with estimates ranging from 5% to 83% in various cancers2–5. Given the lack of a universal definition and diagnostic criteria, the prevalence of crl is difficult to ascertain6; current estimates suggest that more than 300,000 Canadians are affected by crl7.
In this article, we present an overview of crl, divided into 5 subtopics: lymphedema risk factors; early identification and intervention; diagnosis and staging; management, with an emphasis on the reduction and maintenance phases, plus patient support and education; and clinical pearls whose integration into practice all providers can benefit from.
DISCUSSION
Risk Factors for the Development of CRL
Risk factors for breast cancer–related lymphedema have been the most studied to date6. Axillary lymph node dissection, an extensive number of local lymph nodes removed, and axillary radiation therapy are the main treatment-associated risk factors for the development of crl in the population with breast cancer2. Overweight and obesity have also been identified as risk factors for the development of lymphedema in those individuals2. The same risk factors are also likely associated with upper- and lower-limb lymphedema secondary to cancers other than breast cancer6. Increasing evidence suggests that the number of lymph nodes dissected for gynecologic cancers (≥28), regardless of location and extent of dissection, is also an important risk factor for the development of lower-limb lymphedema8. Underlying medical conditions and genetic predisposition related to mutations in specific genes have also been linked with an increased risk of developing secondary lymphedema9,10. Table I further outlines risk factors for upper, trunk, and lower lymphedema.
TABLE I.
Lymphedema risk factors
| Lymphedema site | |
|---|---|
| Upper limb or trunk | Lower limb |
|
|
Early Identification and Intervention
Early identification of crl is key, because it provides an opportunity for prompt intervention when the condition is at an early and potentially reversible stage1,11,12. Providers involved in the care of patients with cancer should be familiar with the risk factors for lymphedema, common early signs and symptoms of the condition, and local lymphedema resources for prompt referral when crl is suspected or identified12,13.
Screening questionnaires completed by patients in the waiting room can be effective for early crl identification without adding any significant burden to typically hectic oncology outpatient settings14,15. Before cancer treatment initiation, baseline measurement of body weight or body mass index and of both the at-risk and contralateral limbs, preferably by a trained lymphedema professional, can also facilitate early crl identification1,11,12. Moreover, patients and caregivers should be provided with crl educational resources and guidance to promptly notify their provider should any of the signs or symptoms suggestive of crl onset arise12,13.
Early signs and symptoms of crl include reports of clothes or jewelry feeling constrictive—for example, tighter wristwatch, clothing sleeve, or footwear. Other symptoms include heaviness or fullness of the affected limb or region (that is, face, neck, pelvic area, genitals)16. Reports of intermittent swelling of the cancer-treated area are also pathognomonic of lymphedema1,16. Mild swelling and minimal or no soft-tissue fibrosis are the most common findings of early-stage lymphedema on physical examination. Those findings, although potentially perceived as minor, are not to be overlooked, given that early identification and intervention might prevent progression to a more advanced lymphedema stage17. Early-stage crl management is more conservative in nature and could therefore limit the psychosocial, physical, and financial burdens associated with this chronic condition11. Thus, identification of one or more of the foregoing signs and symptoms in at-risk patients warrants a prompt referral to a qualified lymphedema professional.
Diagnosis of Lymphedema
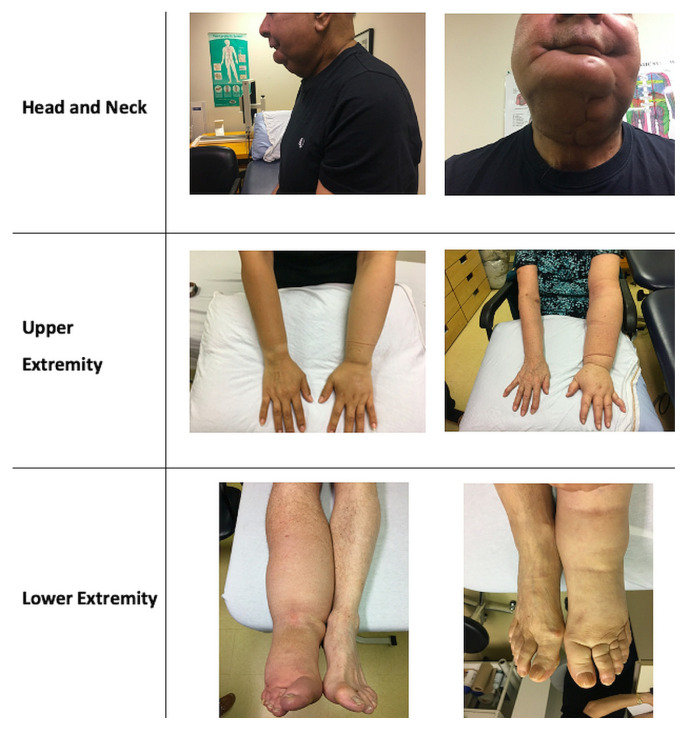
Given the lack of standardized diagnostic criteria, diagnosis of lymphedema is based primarily on presentation and examination findings6,11,12 (Figure 1). Assessment of localized regional or limb swelling typically involves a general medical assessment to rule out other causes of swelling, followed by a focused lymphedema assessment.
FIGURE 1.
Examples of cancer-related lymphedema.
General Medical Assessment
Primary care and oncology providers alike should screen and assess for potential causes of swelling other than crl12. Swelling secondary to crl can coexist with swelling from other comorbid conditions. Causes of unilateral limb swelling include deep vein thrombosis, post-thrombotic syndrome, arthritis, orthopedic or trauma surgeries, and—most importantly—active cancer or recurrence. Causes of bilateral lower limb swelling include obesity, chronic venous insufficiency, major organ insufficiency or failure (such as congestive heart failure and renal or liver dysfunction), and iatrogenic causes (side effects of medications such as steroids, nonsteroidal anti-inflammatories, and calcium channel blockers)11,12. Once non-crl causes have been assessed and appropriately addressed, referral to a qualified lymphedema professional for a more targeted assessment of the crl component should not be delayed.
Focused CRL Assessment
Assessment of crl is typically performed by a certified lymphedema professional. A history focuses on the type and extent of potential lymphatic damage secondary to cancer treatments (surgery, radiation, chemotherapy), previous trauma (fractures, musculoskeletal injuries, previous surgeries), and past history of infections (cellulitis) and thrombotic events. Current medications, psychosocial screening, and determination of functional status are also core elements of the assessment. Targeted physical evaluation includes measurement of body weight or body mass index and examination of the affected limb or area, including for the presence or absence of pitting and skin changes. Lastly, circumference measurements at various anatomic landmarks of both the affected and the unaffected limb are performed, and a formula is used to determine the difference in limb volume. Physical findings in head-and-neck crl can also be documented by measuring specific face and neck landmarks. Severity of lymphedema is determined based on the International Society of Lymphology classification (Table II)18. The crl measurements are repeated at follow-up visits to evaluate management response and evolution of the condition.
TABLE II.
International Society of Lymphology (ISL) lymphedema staging19
| ISL stage | Description |
|---|---|
| 0 | A subclinical state in which swelling is not evident despite impaired lymph transport. This stage may continue for months or years before edema becomes evident. |
| I | Early-onset lymphedema, in which there is accumulation of tissue fluid that subsides with limb elevation. The edema might be pitting at this stage. |
| II | Limb elevation alone rarely reduces swelling, and pitting is manifest |
| Late-stage II | Pitting might or might not be present, because tissue fibrosis is more evident. |
| III | The tissue is hard (fibrotic), and pitting is absent. Skin changes such as thickening, hyperpigmentation, increased skin folds, fat deposits, and warty overgrowths develop. |
Management
Lymphedema is a chronic and incurable condition, requiring a lifelong management regimen20. A holistic and interdisciplinary approach is critical for effective management, which ideally includes a physician and a lymphedema therapist. To date, decongestive lymphatic therapy (dlt) is considered the “gold standard” of lymphedema management. The goals of dlt13,20,21 are to
■ educate patients about crl and its progression.
■ provide risk reduction strategies.
■ reduce limb volume and fibrosis levels.
■ minimize the risk of infection (cellulitis).
■ restore functional mobility and activities of daily living.
■ improve the cosmetic appearance of the limb and the patient’s quality of life.
■ teach self-care to promote independence in crl management.
There are two phases to dlt: volume reduction and maintenance11,22.
The goal of the reduction phase is to decrease the size of the lymphedema-affected limb, aiming to attain the same size as the unaffected limb if possible. Reduction is accomplished with multi-layer short-stretch inelastic bandages (worn for between 24 and 72 hours, and then reapplied), manual lymph drainage, pristine skin care, and exercises to enhance venous and lymphatic flow. Velcro devices can be used as an alternative bandaging for reduction in patients with comorbid conditions that render bandage application difficult. The reduction phase typically takes a few weeks depending on the volume reduction required.
The aim of the maintenance phase is to preserve the volume reduction once limb size is reduced. Maintenance uses daytime prescription compression garments of a specific class, ranging from 15 mmHg to 50 mmHg based on the site and the crl severity, preferably to be worn on a daily basis. Other garment types, such as those for head and neck crl, are available for nighttime use. Garments are available in standard and custom-made sizes. Garment selection is also determined by the patient’s ability to don and doff garments, thereby encouraging optimal adherence11,22. Patient education is a cornerstone of long-term maintenance. It includes counselling about healthy lifestyle (weight management, exercise to enhance lymphatic and venous flows and to minimize long-term functional impairments) and strategies for risk reduction, including an optimal skin care regimen to reduce cellulitis risk, and self-performed manual lymphatic drainage when appropriate. Psychosocial concerns must also be screened for and appropriately addressed.
The development of crl after treatment might indicate active cancer or cancer recurrence. Malignant lymphedema can occur as a result of active malignancy causing lymphatic dysfunction or systemic dissemination through the lymphatic system (or both)23. Modified dlt with lower compression can still be applied in such patients, working in conjunction with oncologists or palliative care providers for symptom management. Notably, advances in technology have also led to the development of surgical options for crl management, including lymphatic bypass techniques and vascularized lymph node transfers24. Despite those advances, nonsurgical management of crl currently remains the first-line approach24.
Clinical Pearls for Providers
Oncology providers can benefit from integrating the following clinical pearls into their practice.
First, identifying local lymphedema-certified professionals and patient resources is a worthy time investment. Lymphedema associations across Canada are particularly helpful in identifying certified therapists for patient referrals and in pointing to useful patient resources (Table III).
TABLE III.
Lymphedema associations and additional resources
| Associations |
|
|
| Newfoundland and Labrador: https://lymphnl.com/ |
|
|
| Nova Scotia: https://lymphedemanovascotia.com/ |
|
|
| Quebec: https://en.infolympho.ca/ |
|
|
| Ontario: https://www.lymphontario.ca/ |
|
|
| Manitoba: https://www.lymphmanitoba.ca/ |
|
|
| Saskatchewan: https://www.sasklymph.ca/ |
|
|
| Alberta: https://albertalymphedema.com/ |
|
|
| British Columbia: https://bclymph.org/ |
| Additional resources |
|
|
| Canadian Lymphedema Framework: https://canadalymph.ca/ |
|
|
| U.S. National Comprehensive Cancer Network: https://www.nccn.org/professionals/physician_gls/pdf/survivorship.pdf |
|
|
| U.S. National Lymphedema Network: https://lymphnet.org/ |
Second, initiation of crl management should not be delayed. In settings of resource scarcity, or when delay in assessment by a lymphedema-certified professional is foreseeable, patient education and initiation of dlt with the simple provision of a prescription for a compression garment can safely be instituted in most early-stage cases. Lymphedema professionals can provide prompt guidance to cancer care providers in writing such prescriptions, pending initial consultation. Contraindications to compression therapy should be excluded before treatment11,12,25 (Table IV).
TABLE IV.
Compression: contraindications and precautions
| Contraindications | Precautions |
|---|---|
|
|
Lastly, the use of diuretics is not recommended12. In the context of lymphedema, diuretics reduce swelling by removing water excess, leaving proteins in the soft tissues. As the efficacy of the diuretic decreases, the proteins draw water back to the affected area, which can lead to re-accumulation of lymphedema volume, increased fibrosis, and secondary local inflammation26. Diuretic use should therefore be reserved for select cases in which coexisting conditions might warrant their use—for example, congestive heart failure or a palliative care situation. Consultation with appropriate specialists is recommended for those particular cases27.
SUMMARY
Cancer-related lymphedema can occur at any point in the cancer care trajectory, from the time of cancer diagnosis, through active treatment, into rehabilitation and survivorship, and in the palliative care context. Early detection and patient education are key to effective crl management, because advanced stages are associated with worse consequences for a patient’s functional status and overall quality of life13. Referral to certified lymphedema therapists should not be delayed when crl is suspected or identified. The current paucity of robust clinical evidence remains an issue, and continued advocacy and research in this cancer survivorship field are therefore crucial.
Key Points.
■ All cancer care professionals should be aware of the risk factors for the development of lymphedema.
■ Early crl detection and management can potentially prevent progression to more advanced stages of the condition, thereby reducing the negative psychosocial and functional consequences for patients.
■ Prompt referral to lymphedema-certified professionals is key to ensuring optimal patient education and dlt for crl management.
ACKNOWLEDGMENTS
The authors are grateful to Tristan Williams for invaluable help in literature overview preparation, and for formatting and final preparation of the manuscript.
Footnotes
This series is brought to you in partnership with the Canadian Association of General Practitioners in Oncology.
REFERENCES
- 1.Denlinger CS, Sanft T, Moslehi JJ, et al. nccn guidelines insights: survivorship, version 2.2020: featured updates to the nccn guidelines. JNCCN. 2020;18:1016–23. doi: 10.6004/jnccn.2020.0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.DiSipio T, Rye S, Newman B, Hayes S. Incidence of unilateral arm lymphoedema after breast cancer: a systematic review and meta-analysis. Lancet Oncol. 2013;14:500–15. doi: 10.1016/S1470-2045(13)70076-7. [DOI] [PubMed] [Google Scholar]
- 3.Hayes SC, Janda M, Ward LC, et al. Lymphedema following gynecological cancer: results from a prospective, longitudinal cohort study on prevalence, incidence and risk factors. Gynecol Oncol. 2017;146:623–9. doi: 10.1016/j.ygyno.2017.06.004. [DOI] [PubMed] [Google Scholar]
- 4.Gjorup CA, Dahlstrøm K, Hendel HW, et al. Health-related quality of life in melanoma-related limb lymphedema and associated risk factors. Eur J Cancer. 2017;85:122–32. doi: 10.1016/j.ejca.2017.07.052. [DOI] [PubMed] [Google Scholar]
- 5.Ridner SH, Dietrich MS, Niermann K, Cmelak A, Mannion K, Murphy B. A prospective study of the lymphedema and fibrosis continuum in patients with head and neck cancer. Lymphat Res Biol. 2016;14:198–205. doi: 10.1089/lrb.2016.0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rockson SG, Keeley V, Kilbreath S, Szuba A, Towers A. Cancer-associated secondary lymphoedema. Nat Rev Dis Primers. 2019;5:22. doi: 10.1038/s41572-019-0072-5. [DOI] [PubMed] [Google Scholar]
- 7.Canadian Lymphedema Framework. What is it? [Web page] Toronto, ON: Canadian Lymphedema Framework; 2015. [Available at: https://canadalymph.ca/what-is-it; cited 21 August 2020] [Google Scholar]
- 8.Kunitake T, Kakuma T, Ushijima K. Risk factors for lower limb lymphedema in gynecologic cancer patients after initial treatment. Int J Clin Oncol. 2020;25:963–71. doi: 10.1007/s10147-019-01608-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Asdourian MS, Skolny MN, Brunelle C, Seward CE, Salama L, Taghian AG. Precautions for breast cancer-related lymphoedema: risk from air travel, ipsilateral arm blood pressure measurements, skin puncture, extreme temperatures, and cellulitis. Lancet Oncol. 2016;17:e392–405. doi: 10.1016/S1470-2045(16)30204-2. [DOI] [PubMed] [Google Scholar]
- 10.Asdourian MS, Swaroop MN, Sayegh HE, et al. Association between precautionary behaviors and breast cancer–related lymphedema in patients undergoing bilateral surgery. J Clin Oncol. 2017;35:3934–41. doi: 10.1200/JCO.2017.73.7494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zuther JE, Norton S. Lymphedema Management: The Comprehensive Guide for Practitioners. 4th ed. New York, NY: Thieme Medical Publishers; 2017. [Google Scholar]
- 12.The Lymphoedema Framework. Best Practice for the Management of Lymphoedema. International Consensus. London, U.K.: Medical Education Partnership; 2006. [Google Scholar]
- 13.Armer JM, Ostby PL, Ginex PK, et al. ons guidelines for cancer treatment–related lymphedema. Oncol Nurs Forum. 2020;47:518–38. doi: 10.1188/20.ONF.518-538. [DOI] [PubMed] [Google Scholar]
- 14.Tsai RJ, Dennis LK, Lynch CF, Snetselaar LG, Zamba GKD, Scott-Conner C. The risk of developing arm lymphedema among breast cancer survivors: a meta-analysis of treatment factors. Ann Surg Oncol. 2009;16:1959–72. doi: 10.1245/s10434-009-0452-2. [DOI] [PubMed] [Google Scholar]
- 15.Cariati M, Bains SK, Grootendorst MR, et al. Adjuvant taxanes and the development of breast cancer-related arm lymphoedema. Br J Surg. 2015;102:1071–8. doi: 10.1002/bjs.9846. [DOI] [PubMed] [Google Scholar]
- 16.Armer JM, Radina ME, Porock D, Culbertson SD. Predicting breast cancer-related lymphedema using self-reported symptoms. Nurs Res. 2003;52:370–9. doi: 10.1097/00006199-200311000-00004. [DOI] [PubMed] [Google Scholar]
- 17.Stout Gergich NL, Pfalzer LA, McGarvey C, Springer B, Gerber LH, Soballe P. Preoperative assessment enables the early diagnosis and successful treatment of lymphedema. Cancer. 2008;112:2809–19. doi: 10.1002/cncr.23494. [DOI] [PubMed] [Google Scholar]
- 18.International Lymphoedema Framework. The Management of Lymphoedema in Advanced Cancer and Oedema at the End of Life. London, U.K.: Medical Education Partnership; 2010. [Google Scholar]
- 19.Executive Committee of the International Society of Lymphology. The diagnosis and treatment of peripheral lymphedema: 2020 Consensus Document of the International Society of Lymphology. Lymphology. 2020;53:3–19. [PubMed] [Google Scholar]
- 20.Cheville AL, McGarvey CL, Petrek JA, Russo SA, Taylor ME, Thiadens SR. Lymphedema management. Semin Radiat Oncol. 2003;13:290–301. doi: 10.1016/S1053-4296(03)00035-3. [DOI] [PubMed] [Google Scholar]
- 21.Petrek JA, Pressman PI, Smith RA. Lymphedema: current issues in research and management. CA Cancer J Clin. 2000;50:292–307. doi: 10.3322/canjclin.50.5.292. [DOI] [PubMed] [Google Scholar]
- 22.Lawenda BD, Mondry TE, Johnstone PAS. Lymphedema: a primer on the identification and management of a chronic condition in oncologic treatment. CA Cancer J Clin. 2009;59:8–24. doi: 10.3322/caac.20001. [DOI] [PubMed] [Google Scholar]
- 23.Aldrich MB, Rasmussen JC, Fife CE, Shaitelman SF, Sevick-Muraca EM. The development and treatment of lymphatic dysfunction in cancer patients and survivors. Cancers (Basel) 2020;12:2280. doi: 10.3390/cancers12082280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Manrique OJ, Bustos SS, Ciudad P, et al. Overview of lymphedema for physicians and other clinicians: a review of fundamental concepts. Mayo Clin Proc. 2020 doi: 10.1016/j.mayocp.2020.01.006. [Online ahead of print]. [DOI] [PubMed] [Google Scholar]
- 25.Rabe E, Partsch H, Morrison N, et al. Risks and contraindications of medical compression treatment—a critical reappraisal. An international consensus statement. Phlebology. 2020;35:447–60. doi: 10.1177/0268355520909066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grądalski T. Limb edema in patients with advanced disease—a pilot study of compression therapy combined with diuretics. Palliat Med. 2019;13:51–6. [Google Scholar]
- 27.Bakar Y, Tuğral A. Lower extremity lymphedema management after gynecologic cancer surgery: a review of current management strategies. Ann Vasc Surg. 2017;44:442–50. doi: 10.1016/j.avsg.2017.03.197. [DOI] [PubMed] [Google Scholar]