Abstract
Potential in vivo applications of RNA interference (RNAi) require suppression of various off-target activities. Herein we report that replacement of a single phosphate linkage between the first and second nucleosides of the passenger strand with an amide linkage almost completely abolished its undesired activity and restored the desired activity of guide strands that had been compromised by unfavorable amide modifications. Molecular modelling suggested that the observed effect was most likely due to suppressed loading of the amide-modified strand into Ago2 caused by inability of amide to adopt the conformation required for the backbone twist that docks the first nucleotide of the guide strand in the MID domain of Ago2. Eliminating off-target activity of the passenger strand will be important for improving therapeutic potential of RNAi.
RNA interference (RNAi) is an extremely valuable tool in functional genomics and molecular biology.1 In a typical RNAi experiment, synthetic short RNA duplexes (small interfering RNA, siRNA) are delivered into cells, where they are taken up by the RNA-induced silencing complex (RISC) that features Argonaute 2 (Ago2) as the principal protein. Ago2 uses one of the siRNA strands, the so-called guide strand, to identify and cleave the target mRNA complementary to the guide strand. Ideally, the other strand of siRNA, the so-called passenger strand, is discarded during loading of siRNA into Ago2. In practice, the passenger strand may also be loaded into Ago2, thus competing with loading of the guide strand. This could reduce the effective guide strand activity, and may cause off-target effects by knocking down unintended mRNA targets complementary to the passenger strand. In this communication, we report that replacement of the phosphate linkage between the first and second nucleosides of the passenger strand with an amide linkage almost completely abolished its undesired functionality. Suppression of the passenger strand activity rescued the activity of guide strands that had been compromised by unfavorable amide modifications.
In a recent study on siRNAs with amide-modified guide strands,2 we made an intriguing observation that AM1 amide linkages (Figure 1) were unexpectedly well tolerated at most positions, except between the first and second nucleosides of the guide strand. The blue, black and green siRNA sequences (for color coding, see Figure 1) are targeting PPIB gene and were selected in our previous study to allow a systematic replacement of a single phosphate with an AM1 linkage throughout the guide strand. Another interesting observation was that the activities of blue siRNA sequences (Figure 1) were consistently higher than the activity of black RNA sequences when the modification was placed at the same position (e.g., G2 and G3 vs. G2* and G3*). In fact, the activity of the amide-modified black guide strands was surprisingly low compared to other siRNA sequences.
Figure 1.
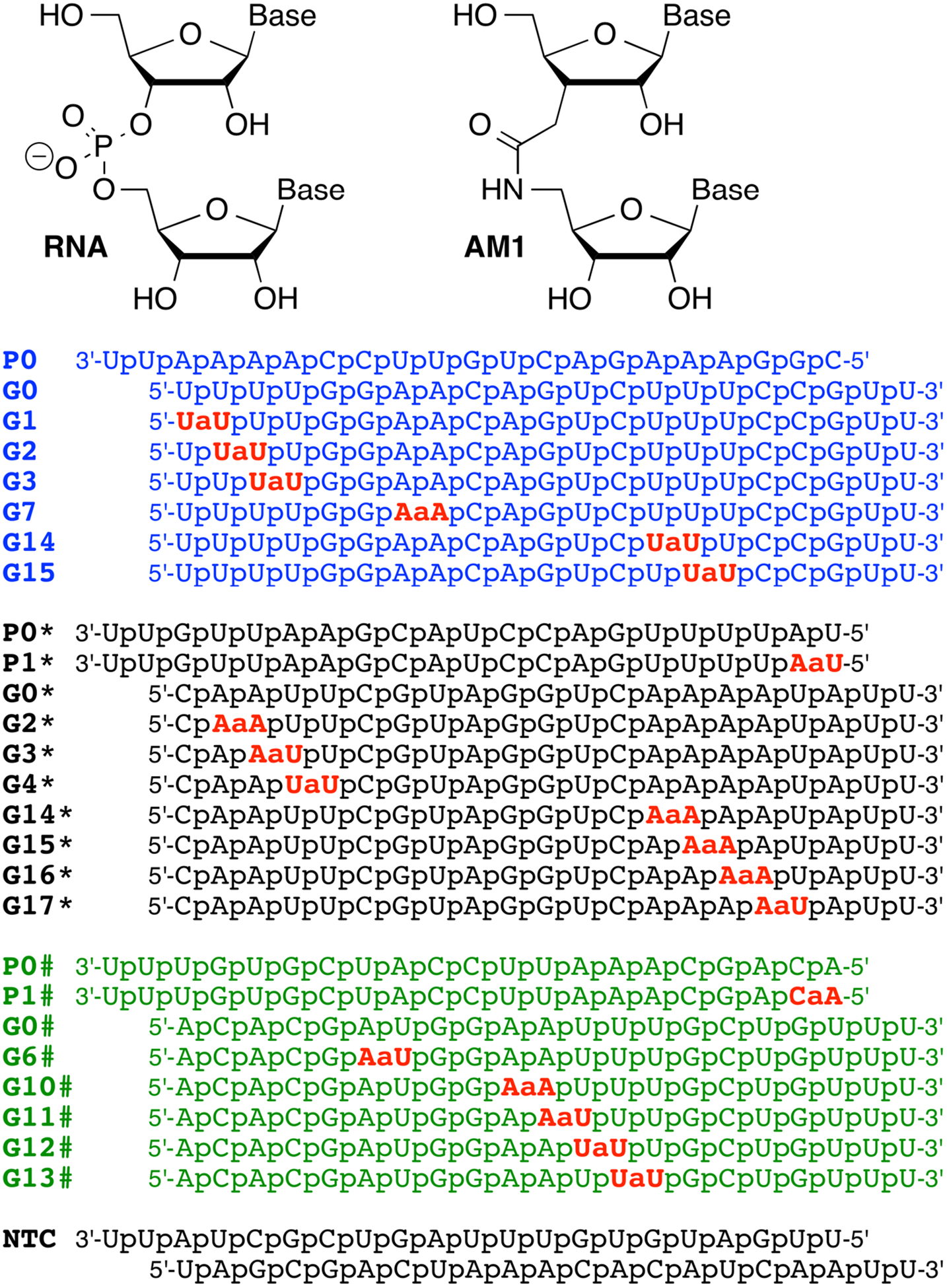
Sequences of amide-modified siRNA guide (GN) and passenger (PN) strands. N denotes the position number of the internucleoside phosphodiester linkage that has been replaced with an amide; 0 denotes the unmodified strands; * and # denotes the numbering in black and green sequences, respectively. NTC = non-targeting control siRNA.
During the loading of siRNA, Ago2 selects the strand whose 5´-end is less strongly paired to its complement as the guide strand.3 In siRNA duplexes the strands starting with a U-A or A-U base pair at the 5´-end are preferably loaded as the guide strands, while the strands starting with a C-G or G-C base pair at the 5´-end are disfavored.3 The fact that the blue sequence starts with a U-A pair and the black sequence with a C-G pair (blue and black refers to color coding in Figure 1) led us to hypothesize that the unexpected differences in activity may be caused by diminished loading of amide-modified guide strands into Ago2. To test this hypothesis, we utilized a dual luciferase assay with recognition elements that monitored the individual activity of either the guide strand or the passenger strand in HeLa cells (for experimental details, see Supporting Information).
Amide-modified guide and passenger strands (Figure 1) were synthesized as previously reported.2, 4 Consistent with our previous study,2 all amide-modified blue guide strands where highly active except G1 (dark blue bars in Figure 2A) in silencing the luciferase expression. The unmodified passenger strand of the blue sequence showed somewhat lower, but still appreciable activity (light blue bars in Figure 2A), consistent with the notion that Ago2 can load either the passenger strand or the guide strand. As a proof of principal, siRNA with the matching sequence was custom synthesized using the commercially available ON-TARGETplus (OTP; Dharmacon) modification with the passenger strand having two 2´-OMe modification at positions 1 and 2, and the guide strand having one 2’-OMe modification a position 2,5 and as expected, the undesired passenger strand activity was reduced (Figure 2A). Most interestingly, the activity of the passenger strand was enhanced when paired with the amide-modified G1 guide strand. In fact, the silencing profile for G1/P0 - diminished guide strand activity, accompanied by increased passenger activity - was reversed compared to all other duplexes.
Figure 2.
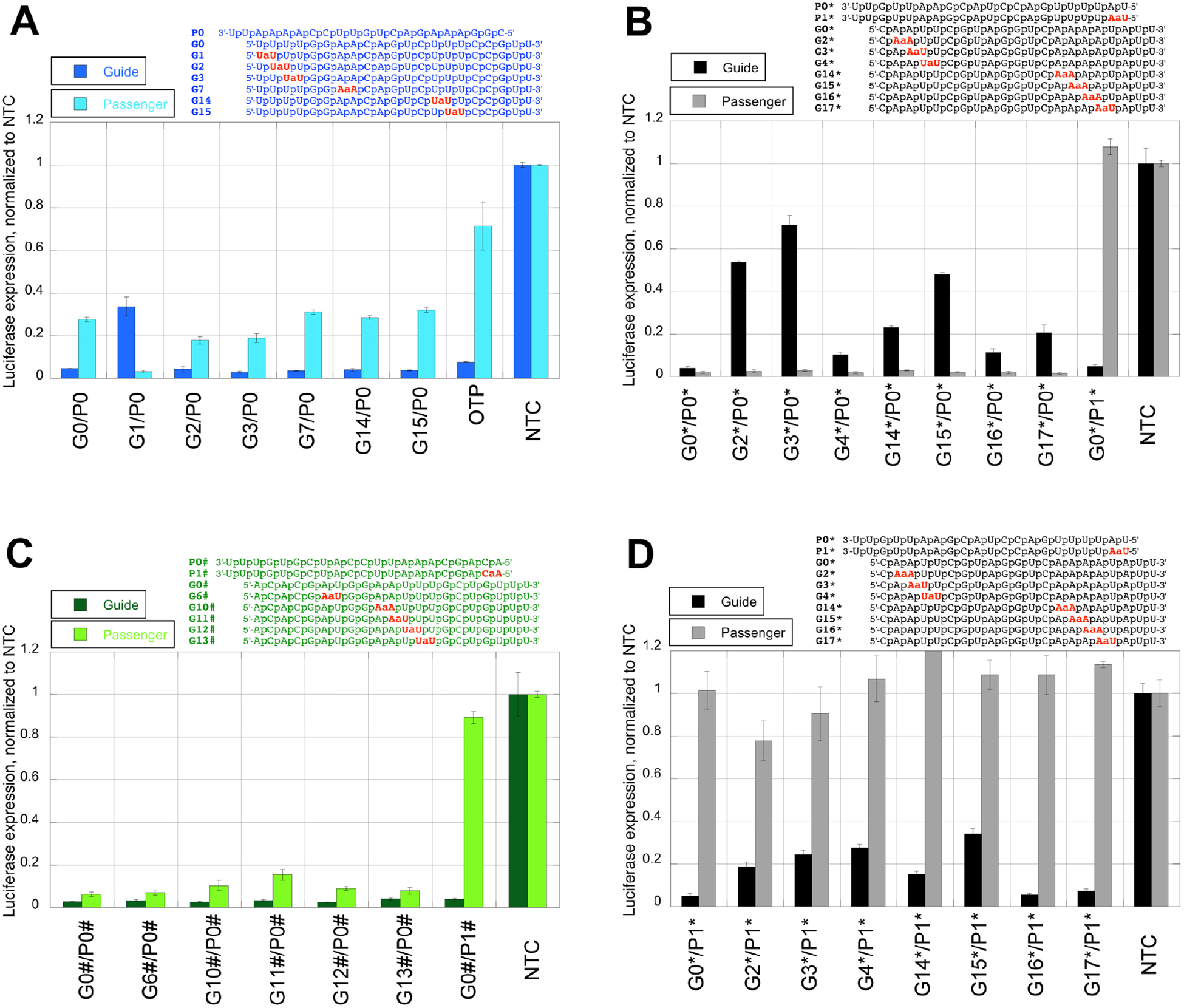
Comparison of silencing activity of the amide-modified siRNAs monitored separately for two different target RNAs, one complementary to the guide strand (darker colored bars) and one complementary to the passenger strand (lighter colored bars). (A) blue siRNA guide strands (GN) duplexed with the unmodified passenger strand (P0) or the ON-TARGETplus (OTP) modifications; (B) black siRNA guide strands (GN*) duplexed with the unmodified passenger strand (P0*) and the unmodified guide strand (G0*) duplexed with the amide-modified passenger strand (P1*); (C) green siRNA guide strands (GN#) duplexed with the unmodified passenger strand (P0#) and the unmodified guide strand (G0#) duplexed with the modified passenger strand (P1#); and (D) black siRNA guide strands (GN*) duplexed with the amide-modified passenger strand (P1*).
In the black siRNA sequence, the activity of the unmodified guide strand was slightly lower than the activity of the unmodified passenger strand (G0*/P0*, Figure 2B). We also observed lower activity of the modified guide strands (black bars, Figure 2B) compared to the unmodified passenger strand (grey bars, Figure 2B), which was consistent with our previous study2 and the hypothesis of a more favorable loading of the unmodified passenger strand than the modified guide strands. Attempts at using molecular modeling of Ago2-siRNA interactions to explain why amide linkage at G2* and G3* results in weaker silencing than at G4* were inconclusive.2 Most interestingly, placing the amide linkage between the first and second nucleoside completely abolished the activity of this modified passenger strand when paired with unmodified black guide strand (G0*/P1*, Figure 2B).
Consistent with the unique intolerance of the amide linkage in G1 (Figure 2A), the beneficial effect was unique to P1*. Amide modifications at other positions close to the 5´-end of the passenger strand (P2*-P5*) did not significantly decrease the activity (Figure S13). The P1* result was not unique to the black sequence and was also observed in the green sequence where both guide and passenger strands were highly active in all duplexes, but the activity of the amide-modified passenger P1# was greatly diminished (G0#/P1# in Figure 2C). Most importantly, using P1* as the passenger strand for amide-modified black guide strands almost completely abolished the undesired passenger strand activity of this siRNA and significantly rescued the activity of the guide strands in all cases except G4* where we observed ~ 20% decrease in gene knockdown (Figure 2D). For example, addition of P1* further silenced the gene expression for G2* from 46% to 81% and for G3* from 29% to 76%, significantly enhancing the activity of these siRNAs. At this point, we do not have a good explanation for the somewhat weaker silencing of G4*/P1*; however, the beneficial effect of reduced passenger strand activity was fully manifested for this combination. As in our previous studies,2, 4 UV thermal melting experiments (Tables S2–S7) showed no correlation between RNAi activity and thermal stability of the siRNA duplexes.
Our results clearly show that amide linkage between the first two nucleosides (as in G1 and P1) influences the strand selection during the loading of siRNA into Ago2. The first nucleotide of the guide strand, the so-called anchor, is unique in that it does not interact with the target mRNA, but instead is docked in the MID domain of Ago2. This binding mode causes the phosphate backbone to undergo a sharp turn between the first two residues. Molecular modeling of an amide linkage at the first position using the crystal structure of an RNA guide strand from microRNA miR-20a bound to the MID domain of Ago26 (PDB ID 4F3T) revealed that the amide in its preferred conformation (yellow carbons, Figure 3) is unable to mimic a phosphodiester at that site (brown carbons, Figure 3). In A-type RNA helices, amide linkages closely track the conformation of the phosphate backbone, whereby the amide carbonyl aligns with the OP2 non-bridging phosphate oxygen.4, 7 A recent crystal structure of RNase H in complex with an amide-modified RNA paired opposite DNA revealed that the amide moiety in such an orientation is able to mimic phosphate-protein interactions.2 However, when placed between the first and second nucleosides of the guide strand (Figure 3), amide adopts a conformation that precludes stabilizing interactions with Ago2. Molecular dynamics simulations of amide at this site demonstrate the loss of stabilizing interactions (Figure S14). We propose that the loss of favorable RNA-backbone:Ago2 contacts at this site disfavors loading of amide-modified G1 guide and P1 passenger strands. The latter suppresses the unwanted activity of the passenger strand and enhances the loading and activity of the guide strands paired with amide-modified P1.
Figure 3.
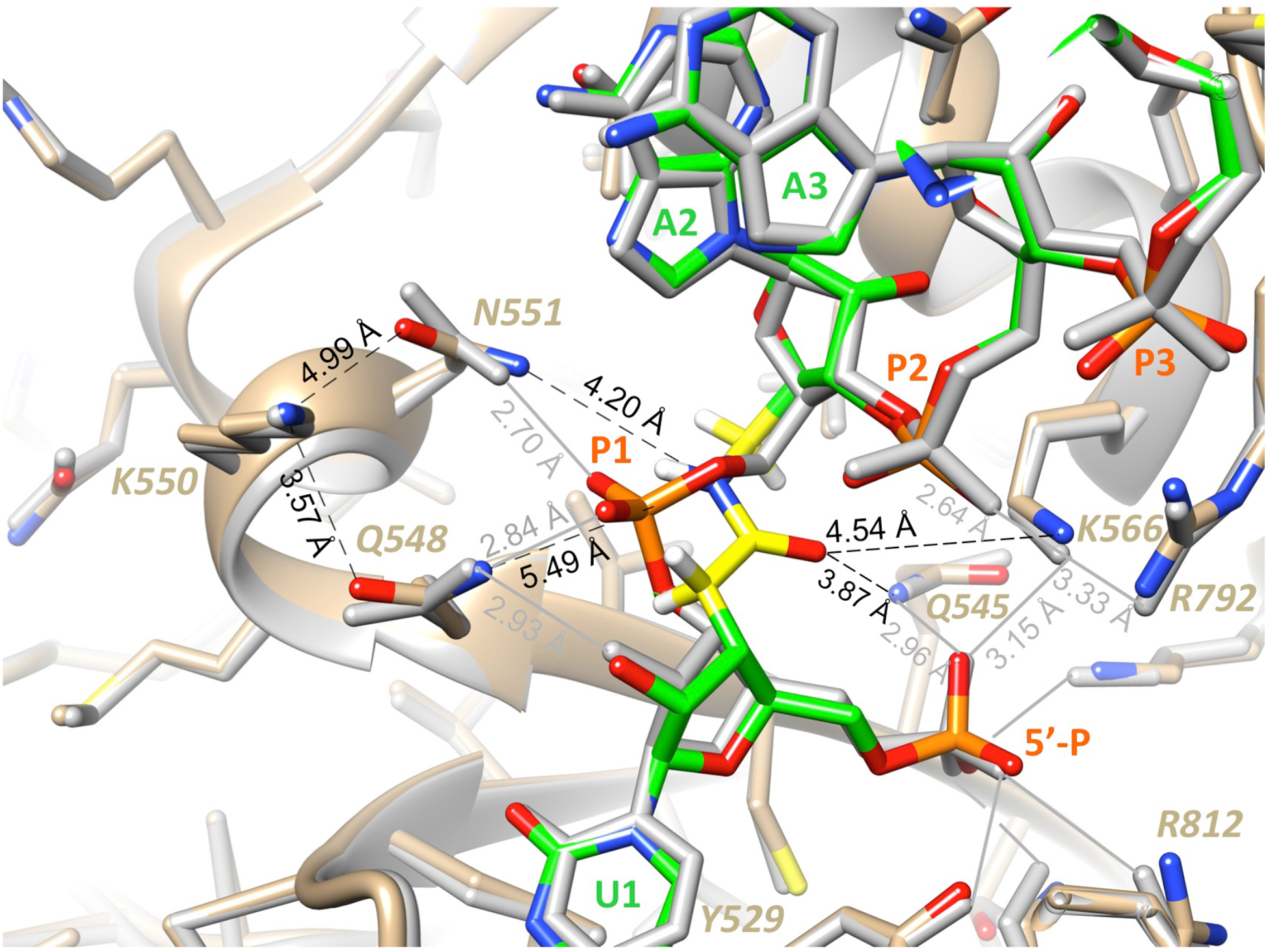
Molecular modeling illustrates the difference in conformations and interactions for phosphate and amide linkages between the first and second nucleosides of the guide strand (5´-P, P1, P2 and P3 identify the guide strand phosphates). The structure of the simulated complex with amide (highlighted with yellow carbon atoms) replacing the bridging phosphate at G1 is colored by atom and superimposed on the structure of the reference complex6 (PDB ID 4F3T; all residues are colored in gray and phosphate P1 is colored in orange (P) and red (O)). Distances between amide NH and O and selected Ago2 amino acid side chains as well as K550 and Q548 and N551 are indicated with dashed black lines and H-bonds involving 5´-P, P1 and P2 in the reference structure are indicated with solid gray lines.
Similar trends were observed in a comprehensive study of ribose-modified siRNAs, where disfavored modifications in 3´-overhangs favored selection of opposing strands as the guides irrespective of the thermodynamic asymmetry of the siRNA duplexes.8 Other passenger strand modifications that disfavor its loading and reduce its activity are methylation of the terminal 5´-OH,9 2´-OMe modification at positions 1 and 25 and incorporation of the strongly destabilizing unlocked nucleic acid.10, 11 Conversely, Beal and co-workers have demonstrated that rationally designed triazole derivatives as anchor nucleobases improve siRNA potency by enhancing guide:Ago2 interactions.12, 13 Previous studies have also found that poor activity of modified guide strands can be partially rescued by combination with an optimized passenger strand.8 Taken together, previous studies and our results suggest that low activity of heavily modified guide strands may frequently be caused by poor loading into Ago2, rather than poor efficiency in target mRNA cleavage.
In conclusion, our study demonstrated that a single amide linkage placed between nucleosides 1 and 2 of the passenger strand strongly reduced the unwanted activity of that strand and concurrently improved the intended activity of the guide strand. Of note, much of the siRNA off-targeting events are a result of microRNA-like binding and knockdown of unintended gene targets by both the guide and passenger strands.14 Reducing passenger strand activity with modifications will also decrease both the complementarity and microRNA-like off-targeting. Conversely, enhancing activity of the guide strand can also increase complementarity and microRNA-like off-targeting of the guide strand. In addition to chemical modifications, it is important that siRNAs are designed with high specificity, especially considering the seed region of the siRNA. Our study opens the door to a new way of increasing siRNA specificity by significantly decreasing passenger-related off-target effects. This is imperative because off-target effects are an important roadblock and unsolved problem for therapeutic applications of RNAi.
Supplementary Material
ACKNOWLEDGMENT
This work was supported by the National Institutes of Health (R01 GM71461 to E.R.).
Footnotes
Supporting Information Available: This material is available free of charge via the Internet.
Experimental details and additional data (PDF)
REFERENCES
- [1].Tripp RA and Karpilow JM (2014) Frontiers in RNAi, Vol. 1, Bentham Science. [Google Scholar]
- [2].Mutisya D, Hardcastle T, Cheruiyot SK, Pallan PS, Kennedy SD, Egli M, Kelley ML, Smith Anja van B., and Rozners E (2017) Amide linkages mimic phosphates in RNA interactions with proteins and are well tolerated in the guide strand of short interfering RNAs, Nucleic Acids Res. 45, 8142–8155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Schwarz DS, Hutvagner G, Du T, Xu Z, Aronin N, and Zamore PD (2003) Asymmetry in the assembly of the RNAi enzyme complex, Cell 115, 199–208. [DOI] [PubMed] [Google Scholar]
- [4].Mutisya D, Selvam C, Lunstad BD, Pallan PS, Haas A, Leake D, Egli M, and Rozners E (2014) Amides are excellent mimics of phosphate internucleoside linkages and are well tolerated in short interfering RNAs, Nucleic Acids Res. 42, 6542–6551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Jackson AL, Burchard J, Leake D, Reynolds A, Schelter J, Guo J, Johnson JM, Lim L, Karpilow J, Nichols K, Marshall W, Khvorova A, and Linsley PS (2006) Position-specific chemical modification of siRNAs reduces “off-target” transcript silencing, RNA 12, 1197–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Elkayam E, Kuhn Claus D, Tocilj A, Haase Astrid D, Greene Emily M, Hannon Gregory J, and Joshua-Tor L (2012) The structure of human argonaute-2 in complex with miR-20a, Cell 150, 100–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Selvam C, Thomas S, Abbott J, Kennedy SD, and Rozners E (2011) Amides as Excellent Mimics of Phosphate Linkages in RNA, Angew. Chem., Int. Ed 50, 2068–2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Bramsen JB, Laursen MB, Nielsen AF, Hansen TB, Bus C, Langkjaer N, Babu BR, Hojland T, Abramov M, Van Aerschot A, Odadzic D, Smicius R, Haas J, Andree C, Barman J, Wenska M, Srivastava P, Zhou C, Honcharenko D, Hess S, Mueller E, Bobkov GV, Mikhailov SN, Fava E, Meyer TF, Chattopadhyaya J, Zerial M, Engels JW, Herdewijn P, Wengel J, and Kjems J (2009) A large-scale chemical modification screen identifies design rules to generate siRNAs with high activity, high stability and low toxicity, Nucleic Acids Res. 37, 2867–2881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Chen PY, Weinmann L, Gaidatzis D, Pei Y, Zavolan M, Tuschl T, and Meister G (2008) Strand-specific 5’-O-methylation of siRNA duplexes controls guide strand selection and targeting specificity, RNA 14, 263–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Laursen MB, Pakula MM, Gao S, Fluiter K, Mook OR, Baas F, Langklaer N, Wengel SL, Wengel J, Kjems J, and Bramsen JB (2010) Utilization of unlocked nucleic acid (UNA) to enhance siRNA performance in vitro and in vivo, Mol. BioSyst 6, 862–870. [DOI] [PubMed] [Google Scholar]
- [11].Snead NM, Escamilla-Powers JR, Rossi JJ, and McCaffrey AP (2013) 5’ Unlocked nucleic acid modification improves siRNA targeting, Mol. Ther. Nucleic Acids 2, E103/101–E103/106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Onizuka K, Harrison JG, Ball-Jones AA, Ibarra-Soza JM, Zheng Y, Ly D, Lam W, Mac S, Tantillo DJ, and Beal PA (2013) Short Interfering RNA Guide Strand Modifiers from Computational Screening, J. Am. Chem. Soc 135, 17069–17077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Suter SR, Sheu-Gruttadauria J, Schirle NT, Valenzuela R, Ball-Jones AA, Onizuka K, MacRae IJ, and Beal PA (2016) Structure-Guided Control of siRNA Off-Target Effects, J. Am. Chem. Soc 138, 8667–8669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Burchard J, Jackson AL, Malkov V, Needham RHV, Tan Y, Bartz SR, Dai H, Sachs AB, and Linsley PS (2009) MicroRNA-like off-target transcript regulation by siRNAs is species specific, RNA 15, 308–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


