Abstract
Background Due to the diverse histopathologic features and variable survival rates seen in sinonasal undifferentiated carcinoma (SNUC), it is likely that this diagnostic entity is comprised of a heterogonous group of morphologically undifferentiated tumors. As advancements in molecular testing have led to a better understanding of tumor biology, it has become increasingly evident that SNUC may actually encompass several tumor subtypes with different clinical behavior. As a result, it is also likely that all SNUC patients cannot be treated in the same fashion. Recent investigations have identified loss of the tumor suppressor SMARCB1 (INI1) expression in a subset of undifferentiated sinonasal tumors and extrasinonasal tumors and, studies have suggested that this genetic aberration may be a poor prognostic marker. The objective of this study was to identify differential expression of SMARCB1 in SNUC and to analyze and compare the survival outcomes in SNUC patients with and without SMARCB1 expression.
Methods All cases of undifferentiated or poorly differentiated neoplasms of the sinonasal tract treated between 2007 and 2018 at a single tertiary care institution were selected. All cases of SNUC were tested for SMARCB1 status by immunohistochemistry (IHC). Clinical parameters were analyzed using Student's t -test and Fischer's test. Kaplan–Meier methods were used to estimate survival durations, while comparison between both the subgroups was done using the log-rank test. Statistical analysis was performed with the use of SPSS software, Version 25 (IBM, New York, NY, United States).
Results Fourteen cases of SNUC were identified. Approximately two-thirds (64%; n = 9) of patients were male and the majority (79%; n = 11) were between fifth to seventh decade. Skull base and orbital invasion were seen in 79% ( n = 11) and 93% ( n = 13) of cases, respectively. Fifty-seven percent of tumors ( n = 8) retained SMARCB1 expression by IHC (SR-SNUC), while the remaining 43% ( n = 6) showed loss of SMARCB1 expression and, thus, were considered as SMARCB1 -deficient (SD-SNUC). Although clinicopathological features and treatment modalities were similar, SD-SNUC showed poorer (OS: p = 0.07; disease free survival [DFS]: p = 0.02) overall survival (OS) and DFS on Kaplan–Meier curves. Additionally, SD-SNUC showed higher recurrence (75 vs. 17%) and mortality (67 vs. 14%) (hazard rate = 8.562; p = 0.05) rates. Both OS (28.82 ± 31.15 vs. 53.24 ± 37.50) and DFS durations (10.62 ± 10.26 vs. 43.79 ± 40.97) were consistently worse for SD-SNUC. Five-year survival probabilities were lower for SD-SNUC (0.33 vs. 0.85).
Conclusion SNUC represents a heterogeneous group of undifferentiated sinonasal malignancies. Based on the status of SMARCB1 expression, the two subgroups SD-SNUC and SR-SNUC appear to represent distinct clinical entities, with loss of SMARCB1 expression conferring an overall worse prognosis.
Keywords: SMARCB1, INI-1, undifferentiated, sinonasal, survival, cancer
Introduction
Sinonasal undifferentiated carcinoma (SNUC) is defined by the World Health Organization (WHO) as a highly aggressive carcinoma lacking squamous or glandular features. 1 Extension to extrasinonasal sites, such as the orbit and skull base, is common. Treatment generally involves multimodality therapy using both surgical and nonsurgical (chemotherapy and/or radiation) therapies. 2 3 Irrespective of treatment, however, SNUC has one of the worst prognoses among all sinonasal malignancies with a high risk of recurrence and extremely poor survival. 3 4 5 6 7 8 9 10 11
Five-year survival rates of SNUC over the past decade reveal widely variable rates ranging from 6 to 75%. 10 11 12 This significant variability suggests that SNUC likely represent a heterogeneous group of tumors with distinct behavior and aggressiveness.
Loss of SMARCB1 (INI-1, BAF47, or hSNF5), a tumor suppressor gene, is a genetic aberration that has been recently described in various undifferentiated sinonasal and extra sinonasal malignancies including rhabdoid tumors, epithelioid sarcomas, renal medullary carcinomas, etc. 13 14 15 16 17 18 Although SMARCB1 gene loss appears to confer poor prognosis to these tumors, 19 20 21 there is paucity of data on the effect of this genetic aberration on survival in SNUC population. The objectives of this study were, first, to examine the differential expression of SMARCB1 in the SNUC population and, second, to analyze whether this differential expression is associated with different survival outcomes.
Methodology
Institutional review board approval was obtained from Thomas Jefferson University Hospital.
Case Selection
All cases of malignant tumors occurring in the sinonasal tract which were diagnosed between 2007 and 2018 at a single tertiary care center were identified. Among these cases, patients with the following descriptions in their respective pathology reports were selected: “poorly differentiated”, “undifferentiated”, or “high grade, not otherwise specified.” Fifty-eight cases were identified according to the aforementioned criteria. Of them, 14 cases were SNUC and had adequate tissue available for testing. Full details of case selection criteria are shown in Fig. 1 .
Fig. 1.
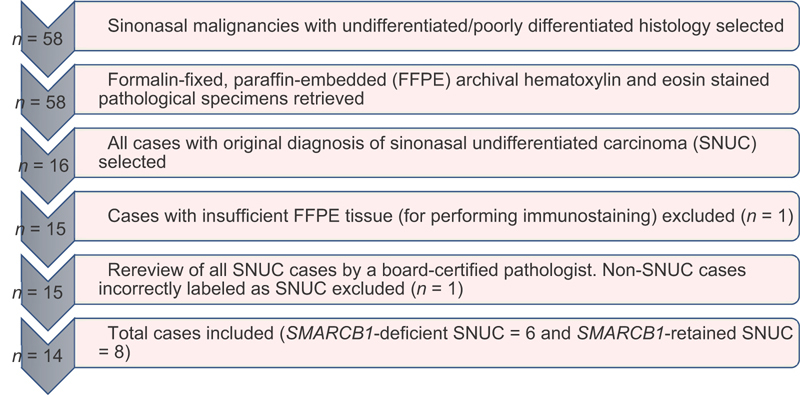
Flowchart depicting selection of cases for the study.
Immunohistochemistry
Formalin-fixed, paraffin-embedded (FFPE) specimens were retrieved for selected cases and 4-µm-thick sections were cut from paraffin blocks using a fully automated system (“Benchmark XT System”, Ventana Medical Systems Inc., Arizona, United States). These cut sections were then mounted on coated slides (Matsunami Glass Ind. Ltd, Japan), deparaffinized in xylene, and rehydrated in descending grades (100 and 70%) of ethanol. Immunohistochemistry (IHC) was performed using the SMARCB1 (INI1) antibody (MRQ-27, 1:50, Zytomed).
Evaluation of the IHC staining results was performed as described. 22 Based on the IHC staining patterns, three staining grades were defined as follows: intact (strong nuclear staining in malignant cells), deficient (completely unstained nuclei in malignant cells), and reduced (very weak but still noticeable nuclear staining in malignant cells) in comparison to strong staining of normal background cells. 21 22 Strong homogeneous nuclear staining in the background (in inflammatory cells, stromal fibroblasts, vascular endothelial cells, and/or normal epithelial cells) served as an internal control and was considered a prerequisite for IHC interpretation. Only unequivocal staining of the nuclei in viable tumor tissue (away from necrotic areas) was analyzed. Cases with absent or very weak staining in the normal background cells ( n = 2) underwent repeat IHC testing with subsequent interpretable results.
Cases were divided into two subgroups, SMARCB1 -retained SNUC (SR-SNUC) and SMARCB1 -deficient SNUC (SD-SNUC).
Statistical Analysis
Survival durations were calculated for only those patients who underwent treatment with curative intent ( n = 13). Patients who underwent palliative treatment ( n = 1) and those who did not complete treatment were excluded from analysis.
Results are presented as of July 11, 2018. Descriptive statistics (mean, median, standard deviation [SD], and confidence intervals [CI]) are provided wherever relevant to summarize patient characteristics and outcomes. Student's t -test (compare continuous variables) and Fisher's exact test or Chi-square test (compare categorical variables) were used as relevant. The Cox Proportional Hazard Model was used to quantify association between mortality and SMARCB1 gene expression. Kaplan–Meier methods were used to estimate survival durations, while comparison between both the subgroups was done using the log-rank test. A two-tailed p -value of 0.05 was considered statistically significant and all limits reported are provided for 95% CI. Statistical analysis was performed with the use of SPSS software, Version 25 (IBM, New York, NY, United States).
Effect Size Calculation
Considering SNUC is a rare sinonasal malignancy, 1 the sample size for most studies is low. As a result, minor changes in sample size may significantly shift the p -value. 23 24 Therefore, effect size, which is independent of sample size, was calculated (using Cohen's “d”) and results were expressed as small, medium, or large based on conventional guidelines. 25
Results
A. SMARCB1 nuclear expression by IHC:
Fourteen patients were included in the study. Forty-three percent ( n = 6) of SNUC cases showed complete loss of nuclear expression of SMARCB1 in the tumor cells ( Fig. 2 ). None of the cases demonstrated a reduced or mixed pattern of IHC staining.
Fig. 2.
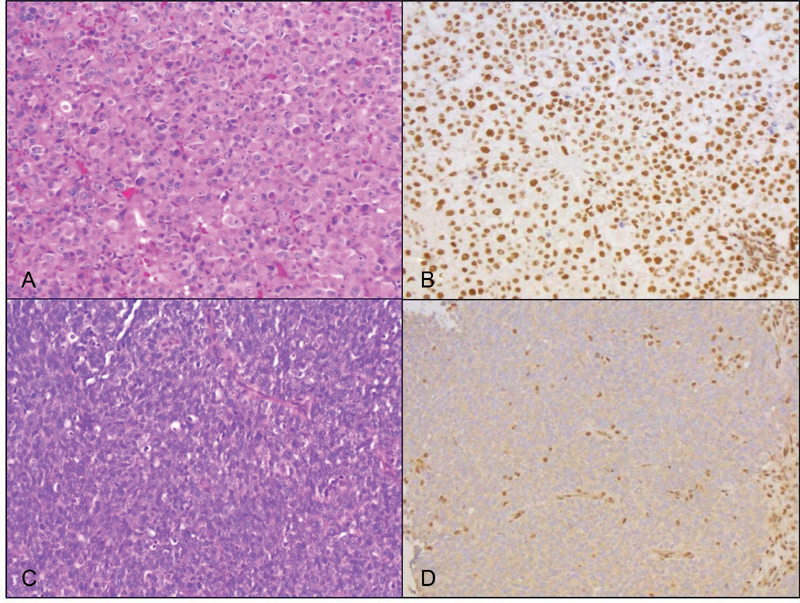
Histology of one SR-SNUC case and one SD-SNUC case. Hematoxylin and eosin (H&E) stained section of the SR-SNUC case demonstrates squamoid morphology characterized by tumor cells with abundant eosinophilic cytoplasm and distinct cell borders (A, ×20); IHC for SMARCB1 shows strong nuclear staining in the tumor cells (B, ×20). The SD-SNUC case shows basaloid morphology characterized by tumor cells with high nuclear to cytoplasmic ratio (C, H&E, ×20); SMARCB1 nuclear expression is lost in the tumor cells and retained in the background nonneoplastic inflammatory and stromal cells (D, ×20). SD-SNUC, SMARCB1 -deficient sinonasal undifferentiated carcinoma; SR-SNUC, SMARCB1 -retained SNUC.
B. Comparison of pretreatment and treatment related characteristics:
Sixty-four percent ( n = 9) of patients were male and 79% ( n = 11) were within the fifth and seventh decade. Fifty percent ( n = 7) of patients showed bilateral sinonasal tract involvement, 79% ( n = 11) had skull base invasion and 93% ( n = 13) had orbital invasion. More than two-thirds of patients (71%; n = 10) presented with TNM stage-IV disease.
Comparison of SR-SNUC and SD-SNUC on the basis of pretreatment- and treatment-related characteristics was performed. Pretreatment data (demographics, functional status, and comorbidity index) were found to be comparable between the groups ( Table 1 ). There were no statistically significant differences in growth pattern, cytomorphology, presence of necrosis, and mitotic rate between the SR-SNUC and SD-SNUC groups ( Tables 2 and 3 ). Site of origin, extent and stage of tumor were comparable between the two subgroups ( Table 2 ). Of note, the SD-SNUCs showed diverse cytomorphology (squamoid, basaloid, and plasmacytoid/rhabdoid, n = 2 each). On analyzing various treatment-related variables, it was noted that both SR-SNUC and SD-SNUC received comparable treatment ( Table 4 ).
Table 1. Comparison of demographic details and preoperative comorbidity status between SR-SNUC and SD-SNUC.
| Variable | SR-SNUC | SD-SNUC | p -Value |
|---|---|---|---|
| Age (in y) | |||
| Mean | 52.88 | 56.50 | 0.59 |
| Standard deviation | 10.60 | 14.52 | |
| Gender | |||
| Males | 05 | 04 | 1.000 |
| Females | 03 | 02 | |
| Race | |||
| Caucasian | 06 | 03 | 0.74 |
| African American | 01 | 02 | |
| Asian | 01 | 01 | |
| Smoking history | |||
| Ever | 04 | 04 | 1.000 0.58 (including UK) |
| Never | 02 | 02 | |
| UK | 02 | 00 | |
| Alcohol intake history | |||
| Ever | 03 | 03 | 1.000 |
| Never | 03 | 03 | 0.6 (including UK) |
| UK | 02 | 00 | |
| Preoperative Charlson's comorbidity index | |||
| Score 2 | 06 | 05 | 0.99 |
| Score 3 | 01 | 01 | |
| Score 4 | 01 | 00 | |
| Preoperative ECOG functional status | |||
| 0 | 01 | 00 | 0.99 0.86 (including UK) |
| 1 | 03 | 03 | |
| 2 | 00 | 01 | |
| UK | 04 | 02 | |
Abbreviations: ECOG, Eastern Cooperative Oncology Group; SD-SNUC, SMARCB1 -deficient sinonasal undifferentiated carcinoma; SR-SNUC, SMARCB1 -retained SNUC; UK, unknown.
Table 2. Comparison of pathological characteristics between SR-SNUC and SD-SNUC.
| Variable | SR-SNUC | SD-SNUC | p -Value |
|---|---|---|---|
| Primary site | |||
| PNS alone | 04 | 06 | 0.08 |
| Both nasal cavity and PNS | 04 | 00 | |
| Laterality | |||
| Unilateral | 03 | 04 | 0.59 |
| Bilateral | 05 | 02 | |
| Orbital involvement | |||
| Present | 07 | 06 | 1.000 |
| Absent | 01 | 00 | |
| Skull base involvement | |||
| Present | 05 | 06 | 0.2 |
| Absent | 03 | 00 | |
| T stage | |||
| T3 | 01 | 00 | 1.000 1.000 (including UK) |
| T4a | 04 | 04 | |
| T4b | 02 | 02 | |
| UK | 01 | 00 | |
| N stage | |||
| N 0 | 06 | 05 | 1.000 0.99 (Including UK) |
| N 2 | 01 | 01 | |
| Unknown | 01 | 00 | |
| M stage | |||
| M 0 | 01 | 01 | 1.000 0.99 (Including UK) |
| M 1 | 06 | 05 | |
| UK | 01 | 00 | |
| Overall TNM stage | |||
| Stage III | 01 | 00 | 1.000 1.000 (including UK) |
| Stage IVA | 03 | 03 | |
| Stage IVB | 02 | 02 | |
| UK | 02 | 01 | |
| Growth pattern | |||
| Trabecular | 05 | 04 | 0.74 |
| Sheet-like | 03 | 01 | |
| Papillary | 00 | 01 | |
| Cytomorphology | |||
| Basaloid | 04 | 02 | 1.000 |
| Squamoid | 02 | 02 | |
| Plasmacytoid/rhabdoid | 02 | 02 | |
| Necrosis | |||
| Absent | 00 | 02 | 0.3 |
| Focal | 05 | 03 | |
| Diffuse | 03 | 01 | |
| Mitotic count | |||
| Mean | 26.50 | 22.17 | 0.6 |
| Standard deviation | 16.87 | 11.67 | |
Abbreviations: PNS, paranasal sinus; SD-SNUC, SMARCB1 -deficient sinonasal undifferentiated carcinoma; SR-SNUC, SMARCB1 -retained SNUC; TNM, tumor node metastasis; UK, unknown.
Table 3. Comparison of immunostaining characteristics between SR-SNUC and SD-SNUC.
| Variable | SR-SNUC | SD-SNUC | p -Value |
|---|---|---|---|
| Pancytokeratin | |||
| Absent | 01 | 01 | 0.99 |
| Focal | 01 | 00 | |
| Diffuse | 06 | 05 | |
| P40 | |||
| Absent | 01 | 01 | 0.19 |
| Focal | 00 | 03 | |
| Diffuse | 01 | 00 | |
| Not available | 06 | 02 | |
| P63 | |||
| Absent | 02 | 01 | 1.000 |
| Focal | 00 | 01 | |
| Diffuse | 00 | 01 | |
| Not available | 06 | 03 | |
| Synaptophysin | |||
| Absent | 04 | 06 | 1.000 |
| Focal | 00 | 00 | |
| Diffuse | 00 | 00 | |
| Not available | 04 | 00 | |
| Chromogranin | |||
| Absent | 05 | 06 | 1.000 |
| Focal | 00 | 00 | |
| Diffuse | 00 | 00 | |
| Not available | 03 | 00 | |
| S-100 | |||
| Absent | 05 | 06 | 1.000 |
| Focal | 00 | 00 | |
| Diffuse | 00 | 00 | |
| Not available | 03 | 00 | |
| P16 | |||
| Absent | 01 | 01 | 0.6 |
| Focal | 00 | 01 | |
| Diffuse | 02 | 00 | |
| Not available | 05 | 04 | |
Abbreviations: SD-SNUC, SMARCB1 -deficient sinonasal undifferentiated carcinoma; SR-SNUC, SMARCB1 -retained SNUC.
Table 4. Comparison of treatment details between SR-SNUC and SD-SNUC a .
| Variable | SR-SNUC | SD-SNUC | p -Value |
|---|---|---|---|
| Treatment lag (from diagnosis to initiation of treatment), d | |||
| Mean | 48.50 | 52.67 | 0.46 |
| Standard deviation | 31.13 | 34.72 | |
| Treatment modality | |||
| Surgery ± adjuvant chemo radiation | 06 | 02 | 0.1 |
| Neoadjuvant chemotherapy + concurrent chemoradiation | 01 | 04 | |
| Surgical approach | |||
| Endoscopic | 05 | 01 | 0.46 |
| Open | 00 | 01 | |
| Combined | 01 | 00 | |
| Oncological clearance following surgical treatment | |||
| Total clearance | 01 | 02 | 0.21 |
| Microscopic disease present | 04 | 00 | |
| Gross disease present | 01 | 00 | |
| Type of radiation therapy | |||
| VMAT | 03 | 03 | 0.46 0.23 (including UK) |
| IMRT | 03 | 00 | |
| UK | 01 | 03 | |
| Radiation dose (in cGy) | |||
| Mean | 61.56 | 66.67 | 0.43 |
| Standard deviation | 06.88 | 05.77 | |
| Interruption during radiation therapy (d) | |||
| Mean | 4.25 | 1.33 | 0.05 |
| Standard deviation | 2.50 | 0.58 | |
Abbreviations: cGy, centigray; IMRT, intensity-modulated radiation therapy; SD-SNUC, SMARCB1 -deficient sinonasal undifferentiated carcinoma; SR-SNUC, SMARCB1 -retained SNUC; UK, unknown; VMAT, volumetric modulated arc therapy.
excluding one case of palliative treatment in SR-SNUC which was treated with radiotherapy.
C. Comparison of posttreatment characteristics:
Following completion of treatment, patients were under surveillance for a mean duration of 41.97 ± 35.61 months. Posttreatment variables analyzed included recurrence and mortality. Overall recurrence rate was 40% and overall mortality was 38%. On analyzing the sub group-specific data, it was noted that SD-SNUC had both higher recurrence (75 vs. 17%) and higher mortality rates (67 vs. 14%; hazard rate = 8.562; p = 0.05). The pattern of recurrent disease was distinct between both the groups. Patients with SR-SNUC developed local recurrence ( n = 1), while patients with SD-SNUC developed all three patterns of recurrent disease–local ( n = 1), regional ( n = 1), and metastatic ( n = 1). Metastatic disease was noted in adrenal, portocaval, and mediastinal lymph nodes. One of the SD-SNUC cases recurred twice, both times in the skull base region. Time to recurrence was shorter for SR-SNUC (7.3 months [ n = 1] vs. 16.64 ± 11.54 months [mean: n = 3]). Overall, 62% of patients (86% of SR-SNUC and 33% of SD-SNUC) were alive at the time of completion of the study.
Comparison of Survival Outcomes
Kaplan–Meier survival curves showed that both overall survival (OS) and disease free survival (DFS) were consistently (OS: p = 0.07; DFS: p = 0.02) worse for SD-SNUC ( Figs. 3 and 4 ). Additionally, 5-year survival probabilities were lower for SD-SNUC (0.33 vs. 0.85; Table 5 ). The rest of the survival outcomes are shown below ( Table 6 , Figs. 5 and 6 ).
Fig. 3.
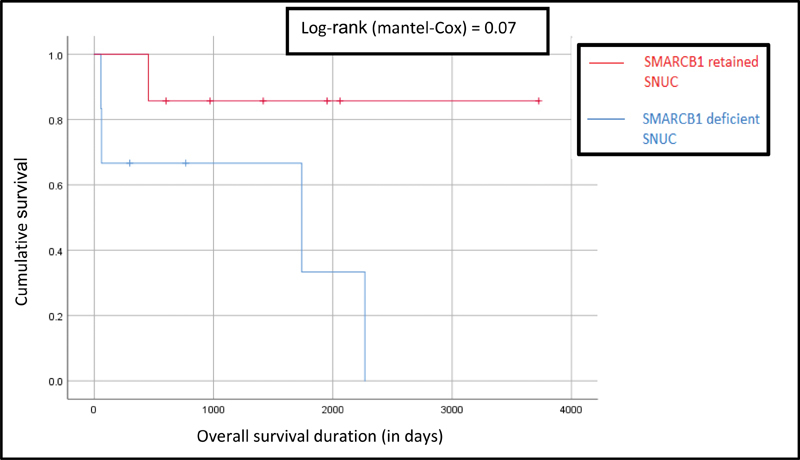
Comparison of Kaplan–Meier survival curves for overall survival between SR-SNUC and SD-SNUC. X axis denotes the duration of survival (in days) and Y axis denoted the cumulative survival. Vertical lines on each of the curves denote censored patients. SD-SNUC, SMARCB1 -deficient sinonasal undifferentiated carcinoma; SR-SNUC, SMARCB1 -retained SNUC.
Fig. 4.
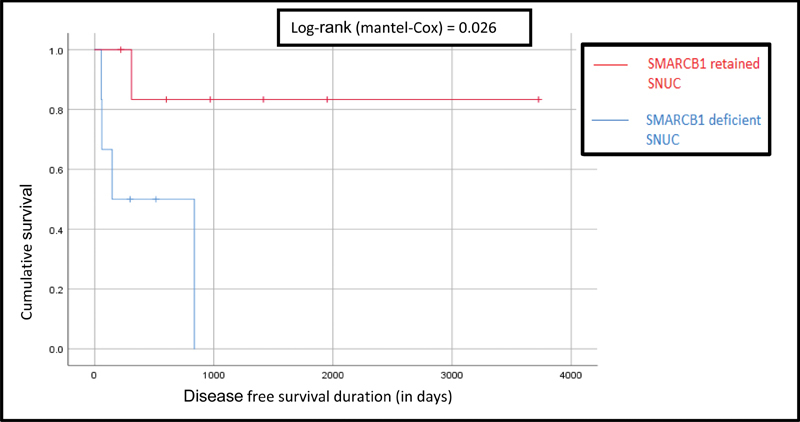
Comparison of Kaplan–Meier survival curves for disease free progression survival between SR-SNUC and SD-SNUC. X axis denotes the duration of survival (in days) and Y axis denoted the cumulative survival. Vertical lines on each of the curves denote censored patients. SD-SNUC, SMARCB1 -deficient sinonasal undifferentiated carcinoma; SR-SNUC, SMARCB1 -retained SNUC.
Table 5. Comparison of 5-year survival probabilities (SP) between SR-SNUC and SD-SNUC.
| Time period (y) | SP of SR-SNUC (95% limits) | SP of SD-SNUC (95% limits) |
|---|---|---|
| 1 | 1.00 (0.56–1.00) | 0.66 (0.24–0.94) |
| 2 | 0.85 (0.42–0.99) | 0.66 (0.24–0.94) |
| 3 | 0.85 (0.42–0.99) | 0.66 (0.24–0.94) |
| 4 | 0.85 (0.42–0.99) | 0.66 (0.24–0.94) |
| 5 | 0.85 (0.42–0.99) | 0.33 (0.05–0.75) |
Abbreviations: SD-SNUC, SMARCB1 -deficient sinonasal undifferentiated carcinoma; SR-SNUC, SMARCB1 -retained SNUC.
Table 6. Comparison of survival durations between SR-SNUC and SD-SNUC.
| SR-SNUC | SD-SNUC | p -Value | Effect size index (Cohen's “d”) | |
|---|---|---|---|---|
| Overall survival duration (mo) | ||||
| Mean | 53.24 | 28.82 | 0.23 | 0.7 (medium) |
| Standard deviation | 37.50 | 31.15 | ||
| Disease free survival duration (mo) | ||||
| Mean | 43.79 | 10.62 | 0.08 | 1.07 (large) |
| Standard deviation | 40.97 | 10.26 | ||
Abbreviations: SD-SNUC, SMARCB1-deficient sinonasal undifferentiated carcinoma; SR-SNUC, SMARCB1-retained SNUC.
Fig. 5.
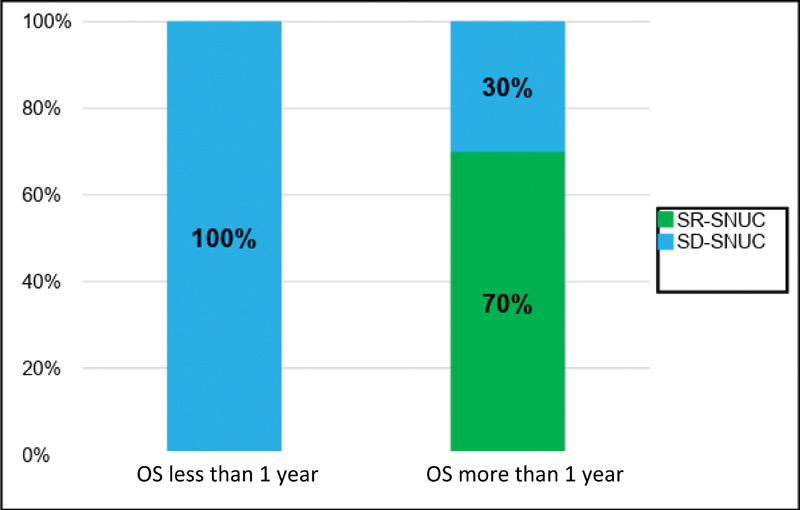
Comparison of overall survival less than and more than 1 year between SR-SNUC and SD-SNUC. X axis denotes the duration of DFS and Y axis denoted the proportion of patients. Values inside each of these boxes represent respective proportion of patients. OS, overall survival; SD-SNUC, SMARCB1 -deficient sinonasal undifferentiated carcinoma; SR-SNUC, SMARCB1 -retained SNUC.
Fig. 6.
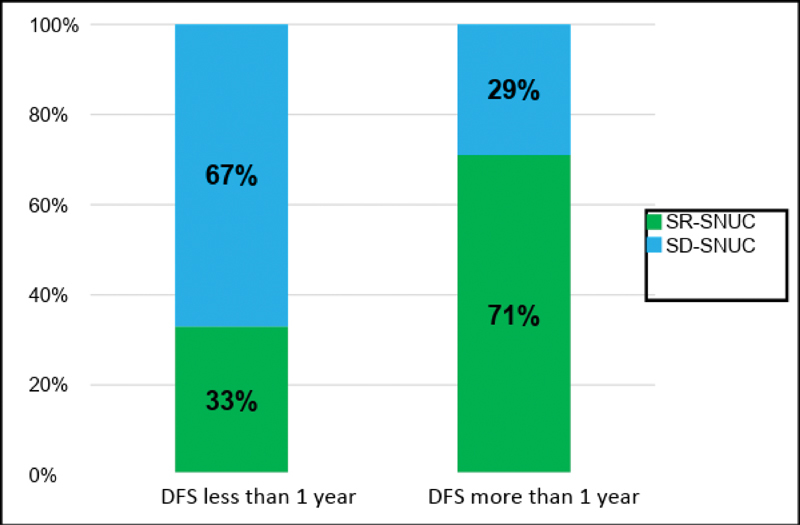
Comparison of disease free survival less than and more than one year between SR-SNUC and SD-SNUC. X axis denotes the duration of DFS and Y axis denoted the proportion of patients. Values inside each of these boxes represent respective proportion of patients. DFS, disease free survival; SD-SNUC, SMARCB1 -deficient sinonasal undifferentiated carcinoma; SR-SNUC, SMARCB1 -retained SNUC.
Discussion
A pathologic diagnosis of exclusion, SNUC represents a diverse group of highly aggressive malignancies that frequently invade the skull base, dura or brain (62–64%). 26 Classically, SNUC presents as a rapidly growing mass in the sinonasal tract with aggressive clinical behavior. Males are more frequently affected and most commonly present in their sixth decade. 6 27 The typical histological appearance includes sheets and trabeculae of cytologically malignant tumor cells with frequent mitoses and necrosis (WHO). Both SR-SNUC and SD-SNUC in our study showed variable growth patterns (trabecular, sheet-like, and papillary) and cytomorphology (squamoid, rhabdoid, and basaloid). A recent study of 39 SMARCB1 -deficient sinonasal carcinomas, which included 10 cases originally diagnosed as SNUC, likewise showed diverse histomorphologic findings in these tumors. 21
Studies conducted in the past few years (2010–2018) have demonstrated that 5-year survival rates for SNUC vary significantly, ranging from 6 to 75%. 10 11 12 This variability demonstrates that SNUC is not a homogenous group of tumors and, therefore, these cases may not all respond equally to the same treatments. There is a clear need to subtype tumors in an effort to better stratify them by behavior and varying aggressiveness in order to individualize treatment. However, attempts to do so remain challenging and are limited by the fact that SNUC has significant variations with respect to histopathological and immunohistochemical features.
In this study, we propose a new classification system for all cases of SNUC based on the expression of SMARCB1 by IHC. The SMARCB1 gene is a tumor suppressor gene found on chromosome 22q11.2 and is a highly conserved core subunit of SWF/SNF complex responsible for regulation of cell differentiation, cell cycle control, and apoptosis. 28 29 30 Recent studies have identified loss of SMARCB1 expression in poorly differentiated sinonasal malignancies and have suggested its potential to be a poor prognostic marker. 19 20 31 Of note, similar genetic aberrations have also been identified not only in sinonasal tumors 31 32 33 34 but also nonsinonasal 13 14 15 16 17 18 malignancies.
Furthermore, irrespective of the site of origin, genetic aberrations in SMARCB1 expression appear to confer a poor prognosis with high-recurrence rates 19 20 and short-survival durations. 31 For example, SMARCB1 deficient gastrointestinal carcinomas have a poorer prognosis when compared to those tumors that express SMARCB1 , with 1-year mortality of over 80% in the SMARCB1 -deficient subtype. 31 Similarly, the results of our study suggest that loss of SMARCB1 expression may be associated with poor prognosis in SNUC. In the SD-SNUC group, 1-year mortality rate following treatment was over 50%, while it was 0% for the SR-SNUC group
This stark contrast in prognosis between the two subgroups based on SMARCB1 expression is could be the basis for pathological subtyping of SNUC patients. In addition to subtyping, SMARCB1 loss could potentially serve as a basis for a novel therapeutic model for SNUC. Newer treatment strategies for nonsinonasal SMARCB1 deficient malignancies that are currently under clinical trials include targeted therapies using EZH2 inhibitors, histone deacetylase inhibitors, and CDK4 inhibitors. 35 Based on similar genetic aberration, it is possible that these agents may prove to be beneficial in treating SMARCB1 deficient sinonasal malignancies including SD-SNUC.
The results of our study, including subgroup analysis, demonstrate that the poor prognosis observed in SMARCB1 deficient SNUC is in accordance with shorter survival and frequent recurrences noted in nonsinonasal SMARCB1 deficient tumors described in the current literature. All survival outcomes, especially those related to disease progression, were consistently poorer for SD-SNUC (Kaplan-Meier curve; p = 0.02). Considering the fact that both these subgroups shared similar pretreatment and treatment related variables, this stark difference in prognosis may be attributed to differential expression of SMARCB1 . This is the first study of its kind to report differential expression of SMARCB1 in SNUC and to analyze the survival outcomes of SNUC based on this genetic aberration.
We acknowledge the limitations of this study, especially those related to retrospective study design including incomplete and inconsistent data in medical records. Additionally low-sample size, owing to rarity of the tumor is also recognized. Future investigations, however, may be similarly hampered by the low incidence of SNUC and, therefore, a deeper investigation of the genetic signature of these samples is advised. It is necessary to better understand this genetic aberration by evaluating whether differential gene expression is responsible for variations in clinical tumor behavior and aggressiveness, and, furthermore, to elucidate whether it may represent a viable basis for tumor subtyping and stratification of patients for targeted therapy.
Conclusion
SNUC represent a heterogeneous group of undifferentiated sinonasal malignancies. This study demonstrates that tumors with and without SMARCB1 expression by IHC have marked differences in survival and, therefore, may represent distinct clinical entities. We propose that SMARCB1 expression may represent a viable option to subtype morphologically undifferentiated sinonasal tumors in an effort to develop more individualized treatment protocols.
Conflict of Interest None.
Financial Disclosure
None.
References
- 1.Frierson H F. Zurich, Switzerland: Lyon: IARC Press; 2005. Sinonasal undifferentiated carcinoma; p. 19. [Google Scholar]
- 2.Rischin D, Porceddu S, Peters L, Martin J, Corry J, Weih L. Promising results with chemoradiation in patients with sinonasal undifferentiated carcinoma. Head Neck. 2004;26(05):435–441. doi: 10.1002/hed.10396. [DOI] [PubMed] [Google Scholar]
- 3.Ejaz A, Wenig B M. Sinonasal undifferentiated carcinoma: clinical and pathologic features and a discussion on classification, cellular differentiation, and differential diagnosis. Adv Anat Pathol. 2005;12(03):134–143. doi: 10.1097/01.pap.0000163958.29032.56. [DOI] [PubMed] [Google Scholar]
- 4.Frierson H F, Jr, Mills S E, Fechner R E, Taxy J B, Levine P A. Sinonasal undifferentiated carcinoma. An aggressive neoplasm derived from schneiderian epithelium and distinct from olfactory neuroblastoma. Am J Surg Pathol. 1986;10(11):771–779. [PubMed] [Google Scholar]
- 5.Righi P D, Francis F, Aron B S, Weitzner S, Wilson K M, Gluckman J. Sinonasal undifferentiated carcinoma: a 10-year experience. Am J Otolaryngol. 1996;17(03):167–171. doi: 10.1016/s0196-0709(96)90055-1. [DOI] [PubMed] [Google Scholar]
- 6.Cerilli L A, Holst V A, Brandwein M S, Stoler M H, Mills S E. Sinonasal undifferentiated carcinoma: immunohistochemical profile and lack of EBV association. Am J Surg Pathol. 2001;25(02):156–163. doi: 10.1097/00000478-200102000-00003. [DOI] [PubMed] [Google Scholar]
- 7.Musy P Y, Reibel J F, Levine P A.Sinonasal undifferentiated carcinoma: the search for a better outcome Laryngoscope 2002112(8, Pt. 1):1450–1455. [DOI] [PubMed] [Google Scholar]
- 8.Kramer D, Durham J S, Sheehan F. Sinonasal undifferentiated carcinoma: case series and systemic review of the literature. J Otolaryngol. 2004;33(01):32–36. doi: 10.2310/7070.2004.00032. [DOI] [PubMed] [Google Scholar]
- 9.Kim B S, Vongtama R, Juillard G. Sinonasal undifferentiated carcinoma: case series and literature review. Am J Otolaryngol. 2004;25(03):162–166. doi: 10.1016/j.amjoto.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 10.Lin E M, Sparano A, Spalding A. Sinonasal undifferentiated carcinoma: a 13-year experience at a single institution. Skull Base. 2010;20(02):61–67. doi: 10.1055/s-0029-1236165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu C C, Dziegielewski P T, McGaw W T, Seikaly H. Sinonasal undifferentiated carcinoma (SNUC): the Alberta experience and literature review. J Otolaryngol Head Neck Surg. 2013;42:2. doi: 10.1186/1916-0216-42-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khan M N, Konuthula N, Parasher A. Treatment modalities in sinonasal undifferentiated carcinoma: an analysis from the national cancer database. Int Forum Allergy Rhinol. 2017;7(02):205–210. doi: 10.1002/alr.21861. [DOI] [PubMed] [Google Scholar]
- 13.Betz B L, Strobeck M W, Reisman D N, Knudsen E S, Weissman B E. Re-expression of hSNF5/INI1/BAF47 in pediatric tumor cells leads to G1 arrest associated with induction of p16ink4a and activation of RB. Oncogene. 2002;21(34):5193–5203. doi: 10.1038/sj.onc.1205706. [DOI] [PubMed] [Google Scholar]
- 14.Folpe A L, Schoolmeester J K, McCluggage W G. SMARCB1-deficient Vulvar Neoplasms: A Clinicopathologic, Immunohistochemical, and Molecular Genetic Study of 14 Cases. Am J Surg Pathol. 2015;39(06):836–849. doi: 10.1097/PAS.0000000000000397. [DOI] [PubMed] [Google Scholar]
- 15.Lopez-Beltran A, Cheng L, Raspollini M R, Montironi R. SMARCB1/INI1 Genetic Alterations in Renal Medullary Carcinomas. Eur Urol. 2016;69(06):1062–1064. doi: 10.1016/j.eururo.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 16.Dabek B, Kram A, Kubrak J. A rare mutation in a rare tumor--SMARCB1-deficient malignant glomus tumor. Genes Chromosomes Cancer. 2016;55(01):107–109. doi: 10.1002/gcc.22296. [DOI] [PubMed] [Google Scholar]
- 17.Johann P D, Hovestadt V, Thomas C. Cribriform neuroepithelial tumor: molecular characterization of a SMARCB1-deficient non-rhabdoid tumor with favorable long-term outcome. Brain Pathol. 2017;27(04):411–418. doi: 10.1111/bpa.12413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kohashi K, Oda Y. Oncogenic roles of SMARCB1/INI1 and its deficient tumors. Cancer Sci. 2017;108(04):547–552. doi: 10.1111/cas.13173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laco J, Chmelařová M, Vošmiková H. SMARCB1/INI1-deficient sinonasal carcinoma shows methylation of RASSF1 gene: A clinicopathological, immunohistochemical and molecular genetic study of a recently described entity. Pathol Res Pract. 2017;213(02):133–142. doi: 10.1016/j.prp.2016.10.012. [DOI] [PubMed] [Google Scholar]
- 20.Dogan S, Chute D J, Xu B. Frequent IDH2 R172 mutations in undifferentiated and poorly-differentiated sinonasal carcinomas. J Pathol. 2017;242(04):400–408. doi: 10.1002/path.4915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Agaimy A, Hartmann A, Antonescu C R. SMARCB1 (INI-1)-deficient sinonasal carcinoma: a series of 39 cases expanding the morphologic and clinicopathologic spectrum of a recently described entity. Am J Surg Pathol. 2017;41(04):458–471. doi: 10.1097/PAS.0000000000000797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Strehl J D, Wachter D L, Fiedler J. Pattern of SMARCB1 (INI1) and SMARCA4 (BRG1) in poorly differentiated endometrioid adenocarcinoma of the uterus: analysis of a series with emphasis on a novel SMARCA4-deficient dedifferentiated rhabdoid variant. Ann Diagn Pathol. 2015;19(04):198–202. doi: 10.1016/j.anndiagpath.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 23.Snyder P, Lawson S. Evaluating results using corrected and uncorrected effect size estimates. Journal of Experimental Evaluation. 1993;61(04):334–349. [Google Scholar]
- 24.Green J L, Camilli G, Elmore P B, Skukauskaiti A, Grace E. Mahwah, NJ: Erlbaum; 2006. Research synthesis: effect sizes. Handbook of Complementary Methods in Educational Research; pp. 583–603. [Google Scholar]
- 25.Sullivan G M, Feinn R. Using effect size-or why the p value is not enough. J Grad Med Educ. 2012;4(03):279–282. doi: 10.4300/JGME-D-12-00156.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morand G B, Anderegg N, Vital D. Outcome by treatment modality in sinonasal undifferentiated carcinoma (SNUC): A case-series, systematic review and meta-analysis. Oral Oncol. 2017;75:28–34. doi: 10.1016/j.oraloncology.2017.10.008. [DOI] [PubMed] [Google Scholar]
- 27.Jeng Y M, Sung M T, Fang C L. Sinonasal undifferentiated carcinoma and nasopharyngeal-type undifferentiated carcinoma: two clinically, biologically, and histopathologically distinct entities. Am J Surg Pathol. 2002;26(03):371–376. doi: 10.1097/00000478-200203000-00012. [DOI] [PubMed] [Google Scholar]
- 28.Roberts C WM, Orkin S H. The SWI/SNF complex--chromatin and cancer. Nat Rev Cancer. 2004;4(02):133–142. doi: 10.1038/nrc1273. [DOI] [PubMed] [Google Scholar]
- 29.Wilson B G, Roberts C W. SWI/SNF nucleosome remodellers and cancer. Nat Rev Cancer. 2011;11(07):481–492. doi: 10.1038/nrc3068. [DOI] [PubMed] [Google Scholar]
- 30.Masliah-Planchon J, Bièche I, Guinebretière J M, Bourdeaut F, Delattre O. SWI/SNF chromatin remodeling and human malignancies. Annu Rev Pathol. 2015;10:145–171. doi: 10.1146/annurev-pathol-012414-040445. [DOI] [PubMed] [Google Scholar]
- 31.Agaimy A, Rau T T, Hartmann A, Stoehr R. SMARCB1 (INI1)-negative rhabdoid carcinomas of the gastrointestinal tract: clinicopathologic and molecular study of a highly aggressive variant with literature review. Am J Surg Pathol. 2014;38(07):910–920. doi: 10.1097/PAS.0000000000000173. [DOI] [PubMed] [Google Scholar]
- 32.Agaimy A.The expanding family of SMARCB1(INI1)-deficient neoplasia: implications of phenotypic, biological, and molecular heterogeneity Adv Anat Pathol 2014. a2106394–410. [DOI] [PubMed] [Google Scholar]
- 33.Bishop J A, Antonescu C R, Westra W H. SMARCB1 (INI-1)-deficient carcinomas of the sinonasal tract. Am J Surg Pathol. 2014;38(09):1282–1289. doi: 10.1097/PAS.0000000000000285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bell D, Hanna E Y, Agaimy A, Weissferdt A. Reappraisal of sinonasal undifferentiated carcinoma: SMARCB1 (INI1)-deficient sinonasal carcinoma: a single-institution experience. Virchows Arch. 2015;467(06):649–656. doi: 10.1007/s00428-015-1853-1. [DOI] [PubMed] [Google Scholar]
- 35.Kalimuthu S N, Chetty R. Gene of the month: SMARCB1. J Clin Pathol. 2016;69(06):484–489. doi: 10.1136/jclinpath-2016-203650. [DOI] [PMC free article] [PubMed] [Google Scholar]


