Abstract
Objectives Sinonasal adenocarcinoma (AC) is a potentially curable disease despite being an aggressive malignancy. Long-term survival can be achieved with early diagnosis and adequate multidisciplinary treatment. Our goal was to evaluate outcomes for patients with AC treated at our institution.
Design In a population-based consecutive prospective cohort, we conducted an analysis of all patients treated for surface epithelial AC between 1995 and 2018.
Results Twenty patients were included, and follow-up was 100%. The mean follow-up time was 89 months for the entire cohort (112 months for patients with no evidence of disease). Intestinal-type AC was found in 65%, whereas nonintestinal-type AC was found in 35% of all cases; 75% had stage T3/4 disease. Tumor grade was intermediate/high in 65%. Eighteen patients underwent treatment with curative intent (craniofacial resection [CFR] in 61%, transfacial approach in 39%, adjuvant radiotherapy in 89%), achieving negative margins in 56% of cases. Overall survival (OS) rates were 90, 68, and 54% after 2, 5, and 10 years of follow-up, respectively, and the corresponding disease-specific survival (DSS) rates were 90, 73, and 58%. Age over 60 years, tumor with a maxillary origin, and microscopic bone invasion were negative prognostic factors. Radical CFR was correlated with better OS and DSS.
Conclusion The high probability of achieving radicality with CFR, the low complication rate, the acceptable toxicity of modern irradiation modalities, and the promising survival rates indicate that this strategy might be considered a safe and an effective option for treating patients with very advanced sinonasal AC.
Keywords: Sinonasal Adenocarcinoma, craniofacial resection, adjuvant radiotherapy, survival
Introduction
Sinonasal carcinomas are uncommon neoplasms that account for approximately 3 to 5% of all upper respiratory tract malignancies. 1 2 3 Adenocarcinoma (AC) represents the third most common malignancy in the sinonasal tract after squamous cell carcinoma (SCC) and adenoid cystic carcinoma (AdCC) and account for approximately 15% of all sinonasal cancers. 1
ACs affect predominantly male patients and occur most frequently in the ethmoid sinuses, but they can also originate in other sites of the nasal cavity (maxillary sinus in <10%). 4 5 6 It has been hypothesized that this distribution may reflect the deposition of carcinogens in the middle meatus. However, it has been suggested, based on endoscopic findings, that many, if not all, ACs arise in the olfactory cleft. 7
Primary ACs of the sinonasal tract may originate from respiratory surface epithelium or the underlying seromucinous glands and are divided into two main types: s alivary-type AC and nonsalivary-type AC ( Fig. 1 ). The salivary-type ACs arise from the seromucinous glands and surface epithelium of the nasal cavity and paranasal sinuses. They comprise 5 to 10% of sinonasal ACs and are usually well-defined myoepithelial neoplasms, which closely resemble their salivary counterparts. AdCC is the most common salivary-type carcinoma and the second most common sinonasal malignancy overall after SCCs, and it represents 10 to 18% of all sinonasal malignancies. 6 8 9 The 5-year survival rates range from 40 to 60% in the literature, with poorest results in AdCC. 6 8 10
Fig. 1.
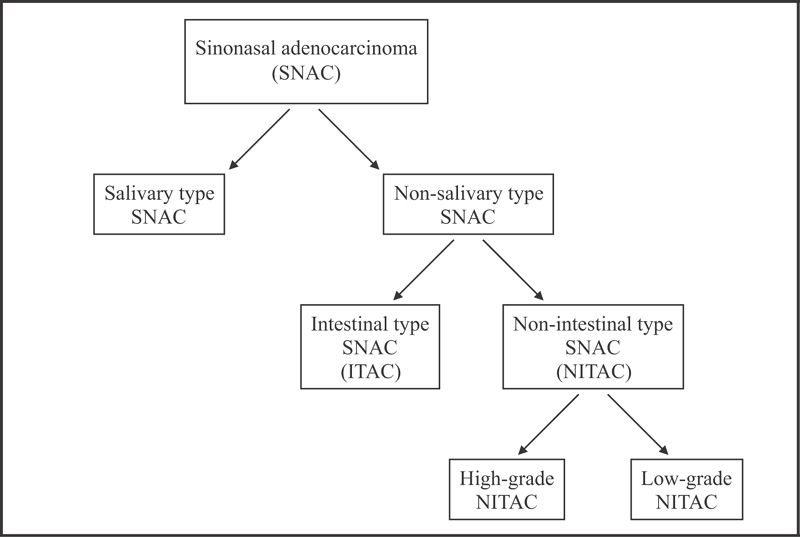
Histological types of primary adenocarcinomas of the sinonasal tract.
The nonsalivary-type ACs, also called surface epithelial, are further separated into intestinal (ITAC) and nonintestinal subtypes (NITAC). 6 11 12 13 ITACs are the second most common type of sinonasal ACs after AdCC and are generally aggressive with a local recurrence rate of up to 50%, lymphatic spread in 10%, and a distant metastasis rate of 20%. 5 Tumors in this group resemble intestinal epithelium and often arise in the ethmoid sinus. They are aggressive malignancies and may spread to adjacent structures including the orbit, the pterygopalatine fossa, the infratemporal fossa, and the cranial cavity. 6 ITACs are further subdivided into five categories according to Barnes: papillary, colonic, solid, mucinous, and mixed types. 11 The histological subtypes have been found to correlate with clinical behavior, for example, well-differentiated papillary ITACs have an indolent course, but patients with solid and mucinous ITACs have an untoward outcome. 11 12 14 A remarkable association has been identified between long-term exposure to wood dust and the occurrence of ITAC; workers with occupational exposure to hardwood dust may show incidences 1,000 times those of the general population. 15 16 17 18 This kind of exposure has been observed in around 20% of reported cases. 18 19 Also, occupational exposure to dust in the shoe and leather industry 20 and in textile manufacture, as well as exposure to chromium and nickel, has been suspected. The carcinogenic compounds have not been identified, but a possible etiological role for tannins has been discussed. 14
NITACs are of presumed seromucinous gland origin, have marked morphological heterogeneity, can arise anywhere in the sinonasal tract, and are divided into high- and low-grade types. 13 High-grade NITACs are rare malignancies of the sinonasal tract and are frequently found in the maxillary sinus. These tumors have heterogeneous features that may overlap with those of other malignancies of this area, often leading to difficulties during histopathological diagnosis. High-grade NITACs have a very poor prognosis, with a 3-year survival rate of approximately 30%. 1 5 6 21
Low-grade NITACs are uncommon (∼13% of sinonasal ACs) and occur mostly in the ethmoid sinus, the nasal cavity, and the maxillary sinuses. 6 These carcinomas have no known association with environmental carcinogens. The disease is usually localized, but local recurrences are possible. Metastasis is unusual, and death due to disease is rare. The overall prognosis of the patients is favorable, and 5-year survival rates up to 85% are reported in the literature. 5 6 22 23 24
The paranasal sinuses are anatomically complex and quite “clinically silent,” allowing a tumor to grow to a significant size before symptoms and signs develop. Therefore, at the time of diagnosis, most patients present with advanced stage disease and have extensive involvement of adjacent sites, such as the orbit, skull base, and the central nervous system, leading to difficulties in the management of ACs. 25 26 27 28 29 30 Many patients with AC of lower stages can be effectively treated with radiotherapy (XRT). However, surgical excision followed by XRT is the favored choice of treatment worldwide. 5 Open craniofacial resection (CFR) is often warranted in cases of involvement of the cribriform plate or dura mater, and in many cases, adjuvant XRT is needed because of the advanced stage of the disease at diagnosis. 31
There is a scarcity of prospectively collected data addressing management options and treatment outcomes because of the rarity of this disease. The goal of this population-based study was to evaluate the management of patients with surface epithelial (nonsalivary-type) AC treated at Oslo University Hospital (OUH) in Norway from 1995 to 2018 and to evaluate our results in light of the international literature.
Materials and Method
Clinical Setting
OUH is a tertiary referral, comprehensive cancer center with a catchment area of approximately 3 million inhabitants (56% of the entire Norwegian population). In addition, our institution accepts referrals from other health regions in Norway.
Patient Cohort
Our prospective database for brain tumors and the pathology registry of head and neck cancers were searched to identify patients eligible for this study. Inclusion criteria were histologically verified surface epithelial (nonsalivary-type) ACs and treatment at OUH from 1995 to date. The medical records of patients were also reviewed retrospectively to identify the study parameters not included in the database records.
Tumor-Related Variables
A histopathological diagnosis of AC was made by a consultant pathologist at presentation. All cases were formally reexamined by a dedicated head and neck pathologist, reclassified into correct histological subtypes, and evaluated for nerve, vessel, and bone invasion. Staging of tumors was based on the TNM staging system of the American Joint Committee on Cancer for a maxillary sinus or ethmoid sinus/nasal cavity cancers. 32 Tumor size, orbital, dural and/or cerebral infiltration was determined from radiographical images at diagnosis and completed with intraoperative registrations. The quality of the surgical margins was also retrospectively scrutinized.
Treatment Variables
According to the tumor-specific variables (i.e., tumor size and location, presence or absence of metastases to lymph nodes and/or distant metastasis) and the patient-specific variables (i.e., age, general condition, complications, mental condition), a personalized treatment plan was made for each patient after consultation with the multidisciplinary team comprising a head and neck surgeon, an oncologist, a neurosurgeon, a pathologist, a radiologist, and an ophthalmologist, if required.
The surgical technique was patient-specific and tailored based on the location of the tumor and the proximity to vital structures. In general, for resections with curative intent, gross tumor resections were performed in an en bloc fashion, if possible. In cases of a median or paramedian localization with invasion of the nasal cavity, hard palate and/or maxillary sinus, or primary localization in the medial maxillary sinus or nasal cavity, a lateral rhinotomy (LR), a modified midfacial degloving (MFD), or an endoscopic sinus surgery (ESS) technique was used to provide better visualization.
The Weber–Fergusson incision modified by Zange and Schuchardt 33 was used in selected cases to perform total hemimaxillectomy in particularly large tumors. The lamina papyracea was resected for tumors extending to the lateral ethmoidal wall, whereas the periorbita was resected in cases of invasion. Tumors infiltrating the orbital fat were treated with orbital exenteration. If an invasion of the anterior skull base was suspected, an additional bicoronal approach was performed, offering the possibility of a transcranial–transfacial resection of the tumor (i.e., CFR). The bony skull base (cribriform plate and fovea ethmoidalis) was resected for tumors involving the bony skull base, and dural resections were performed for tumors with skull base erosion. Additional brain tissue from the frontal lobe was resected in cases with limited brain involvement, as needed, to achieve negative surgical margins.
The reconstruction of the resected tissues was performed according to size and staging. Small defects were closed by local flaps or buccal fat pad, whereas larger defects were closed by either pedicled temporalis flaps or microvascular flaps. In many cases, an obturator prosthesis was applied to improve patient comfort and to facilitate clinical follow-up of the resection cavity. Closed reconstruction was offered in selected cases, when the risk of recurrence was considered low. Reconstruction of anterior skull base defects was performed using a two-layer closure of the dura and skull base. Duroplasty was performed using avascular grafts, and from 1998 onward, skull base reconstruction was performed using vascularized pericranial flaps. 26 Surgical treatment was deemed adequate if resection margins were negative according to a surgeon and pathologist joint assessment.
Statistical Analysis
The main end points of this study were overall survival (OS) and disease-specific survival (DSS). Follow-up time was calculated from the date of primary treatment to either death, with or without disease, or last known status. Event-time distributions were approximated using the Kaplan–Meier estimator, 34 and the log-rank test was used to test for any significant differences between the survival curves. 35 Prognostic factors for OS and DSS were identified using the Cox proportional hazards regression model. 36 Whether or not the observed proportions for a categorical variable differed from the hypothesized proportions was determined using the chi-square test or Fisher's exact test, as appropriate. 37 The level of statistical significance was set at p = 0.05. Descriptive statistics were reported as a mean with a 95% confidence interval (CI) or a median with a range, as appropriate. Statistical analysis was conducted using SPSS version 22 (SPSS Inc., Chicago, Illinois, United States).
Results
Clinical Findings
The medical records and pathological specimens of 25 identified patients were reviewed. Five patients were excluded after histopathology review (AdCC in three cases, carcinoma ex pleomorphic adenoma in one case, and malignant ameloblastoma in one case). Finally, 20 patients were found eligible for inclusion in this study. The sex distribution showed a clear male predominance with 15 (75%) male and 5 (25%) female patients. All of the patients were of Caucasian descent. Nine (45%) patients had possible occupational hazard present in their history; there was long-term exposure to hardwood dust in six, to chromium and nickel in two, and to tannins in one. The mean age at diagnosis was 57.5 years (range: 25–81 years; 95% CI: 50.0–65.1 years). The peak incidence of disease in our cohort occurred in the eighth decade of life. Patient characteristics are summarized in Table 1 .
Table 1. Demographic, pathological, and prior treatment information.
| Variables | Total |
|---|---|
| Eligible patients, n (%) | 20 (100) |
| Age, mean (SD) | 58 (16) |
| Sex, n (%) | |
| Male | 15 (75) |
| Female | 5 (25) |
| Occupational hazard, n (%) | |
| Wood dust | 6 (30) |
| Chromium and nickel | 2 (10) |
| Tannins | 1 (5) |
| Presenting symptom, n (%) | |
| Nasal stenosis | 13 (65) |
| Epistaxis | 2 (10) |
| Localized pain | 2 (10) |
| Painless swelling | 1 (5) |
| Reduced vision | 1 (5) |
| Distant metastasis | 1 (5) |
| Histology, n (%) | |
| ITAC | 13 (65) |
| NITAC, low grade | 4 (20) |
| NITAC, high grade | 3 (15) |
| Grade of differentiation, n (%) | |
| Low | 7 (35) |
| Intermediate | 8 (40) |
| High | 5 (25) |
| T stage | |
| T4 | 11 (55) |
| T3 | 4 (20) |
| T2 | 3 (15) |
| T1 | 2 (10) |
| Tumor size (mm), mean (SD) | 37 (11) |
| Effect on adjacent anatomical structures, n (%) | |
| Orbita | 8 (40) |
| Meninges | 5 (25) |
| Brain | 1 (5) |
| Bone (microscopic invasion) | 10 (50) |
| Nerve (microscopic invasion) | 5 (25) |
| Vessel (microscopic invasion) | 3 (15) |
Abbreviations: ITAC, intestinal adenocarcinoma; NITAC, nonintestinal adenocarcinoma; SD, standard deviation.
Nasal stenosis was the most common presenting symptom and was observed in 65% of all cases followed by epistaxis, local pain (two cases each), reduced vision, and swelling (one case each), whereas one case was diagnosed because of a distant metastasis originating from the disease. Presenting symptoms were predating primary diagnosis by a mean of 9.4 months (range: 1–24; 95% CI: 5.7–13.2).
Tumor Characteristics
The tumor originated from the ethmoid sinus in 17 (85%) and the maxillary sinus in 3 (15%) cases. The mean tumor size was 3.7 cm (a median of 3.8 cm; 95% CI: 3.1–4.4). Orbital involvement was observed in eight (40%), dural involvement in five (25%), and brain invasion in one (5%). Eleven (55%) patients had T4 disease at the time of diagnosis, whereas 4 (20%) patients had T3, three (15%) had T2, and two (10%) had T1 disease. No patients presented with positive lymph node status, whereas one patient had distant metastases (M2) at the time of diagnosis.
Histopathology showed ITAC in 13 (65%), low-grade NITAC in 4 (20%), and high-grade NITAC in 3 (15%) cases. The grade of overall tumor differentiation regardless of histopathological subclassification was high in five (25%), intermediate in eight (40%), and low in seven (35%) cases. Microscopic bone invasion (lamina cribrosa) was present in 10 (50%), nerve invasion in 5 (25%), and vessel invasion in 3 (15%) cases. Multifocal histology was present in four (20%) cases; remarkably, all of these patients had occupational exposure over time. Tumor characteristics are summarized in Table 1 .
Treatment
Treatment details are summarized in Fig. 2 and Table 2 . Eighteen (90%) patients were selected for surgical treatment with curative intent after multidisciplinary evaluation, whereas surgery was intentionally omitted in two (10%) patients due to extensive comorbidity and distant metastases present at the time of diagnosis in one case each. These patients underwent nonsurgical oncological treatment only.
Fig. 2.
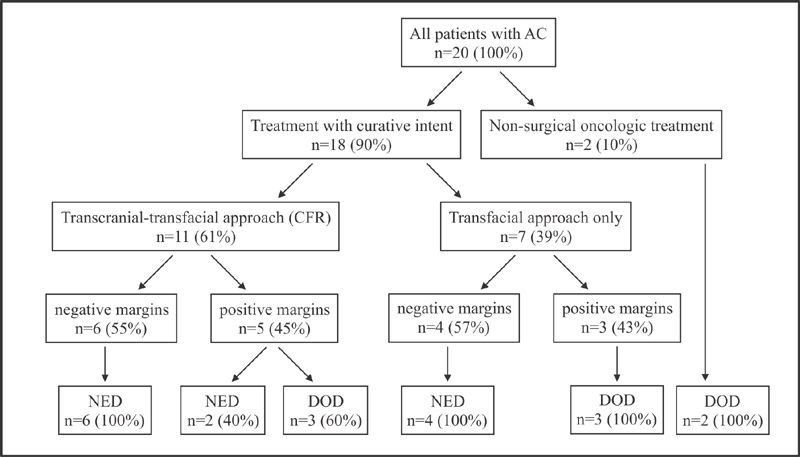
Treatment details with final status at the time of last follow-up (percentages are shown as a portion of the relevant subcohort).
Table 2. Treatment details.
| Treatment type | No. of patients (%) |
|---|---|
| Treatment with curative intent | 18 |
| Craniofacial resection | |
| Bifrontal craniotomy + lateral rhinotomy | 9 (50) |
| Bifrontal craniotomy + midfacial degloving | 2 (11) |
| Transfacial resection | |
| Lateral rhinotomy | 4 (22) |
| Endoscopic sinus surgery | 3 (17) |
| Treatment protocol | |
| Surgery + adjuvant XRT | 16 (89) |
| Surgery only | 2 (11) |
| Surgical margins | |
| Negative | 10 (56) |
| Positive | 8 (44) |
| Nonsurgical oncological treatment | 2 |
| XRT + ChT | 2 (100) |
| Complications | |
| Osteonecrosis | 1 (5) |
| Meningitis | 1 (5) |
Abbreviations: ChT, chemotherapy; XRT, radiotherapy.
Eleven (61%) patients underwent open CFR, using LR in nine (50%) and MFD in two (11) cases as the transfacial approach. Seven (39%) patients underwent tumor resection using the transfacial approach only, with LR in 4 (22%) and ESS in 3 (17%) cases.
Negative surgical margins were achieved in 10 (56%) cases. Tumor cells were found in, or close to, the resection margins in 8 (44%) cases.
All patients undergoing surgical resection underwent adjuvant XRT (50–70 Gy) postoperatively, except two patients presenting with T1 disease. Oncological treatment was administered in accordance with the guidelines of the Danish Head and Neck Cancer Group (DAHANCA). 38 39
Complications related directly to surgical treatment were registered in two cases (meningitis and osteonecrosis).
Outcomes
The outcomes of the entire study cohort are summarized in Table 3 . We obtained 100% follow-up. The mean follow-up time of the entire cohort was 89 months (range: 1–239 months; median: 71.9 months; 95% CI: 53.6–123.9) as of June 15, 2018 (date of final follow-up). The mean follow-up time of patients with no evidence of disease (NED) was 112 months (range: 5–239 months; median: 102.8 months; 95% CI: 61–162.5). Importantly, none of the patients were lost to follow-up.
Table 3. Outcomes of the study.
| Survival function | Cumulative survival (%) | p -Value | ||
|---|---|---|---|---|
| 2 y | 5 y | 10 y | ||
| Pretreatment factors | ||||
| Age | ||||
| ≤60 y | 100 | 100 | 100 | |
| >60 y | 80 | 47 | 16 | 0.004 |
| Sex | ||||
| Female | 100 | 100 | 100 | |
| Male | 87 | 62 | 44 | 0.075 |
| Tumor origin | ||||
| Ethmoidal sinus | 88 | 81 | 65 | |
| Maxillary sinus | 100 | 0 | 0 | 0.022 |
| Histology | ||||
| Nonintestinal-type AC | 100 | 83 | 83 | |
| Intestinal-type AC | 85 | 67 | 48 | 0.162 |
| Grade of tumor differentiation | ||||
| High | 100 | 100 | 100 | |
| Low | 87 | 64 | 44 | 0.078 |
| Microscopic bone invasion | ||||
| No | 100 | 100 | 83 | |
| Yes | 80 | 47 | 35 | 0.041 |
| Treatment with curative intent | ||||
| OS | 94 | 76 | 61 | |
| DSS | 94 | 82 | 65 | |
| Surgical approach | ||||
| Transcranial + transfacial (CFR) | 100 | 100 | 79 | |
| Transfacial only (LR/ESS) | 86 | 46 | 46 | 0.019 |
| Surgical margins | ||||
| Negative | 100 | 100 | 100 | |
| Positive | 88 | 63 | 38 | 0.005 |
| Local recurrence | ||||
| No | 91 | 91 | 91 | |
| Yes | 86 | 67 | 34 | 0.022 |
| Recurrence-free survival | 75 | 55 | 55 | |
| Surgical margins | ||||
| Negative | 78 | 78 | 78 | |
| Positive | 71 | 28 | 28 | 0.107 |
| Surgical approach | ||||
| Transcranial + transfacial (CFR) | 91 | 61 | 61 | |
| Transfacial only (LR/ESS) | 40 | 40 | 40 | 0.184 |
| Nonsurgical oncological treatment | ||||
| Overall and disease-specific survival | 50 | 0 | 0 | |
Abbreviations: AC, adenocarcinoma; CFR, craniofacial resection; ChT, chemotherapy; DSS, disease-specific survival; ESS, endoscopic sinus surgery; LR, lateral rhinotomy; OS, overall survival; XRT, radiotherapy.
Note: Boldface signifies statistically significant values.
The OS rates in the entire cohort were 90, 68, and 54% after 2, 5 and 10 years, respectively, and the corresponding DSS rates were 90, 73, and 58%.
Age at diagnosis over 60 years ( p -value = 0.004), tumor origin from the maxillary sinus ( p -value = 0.022), and microscopic bone invasion ( p -value = 0.041) were associated with significantly dismal outcome.
We found no significant correlations between survival and previous occupational exposure; dural, orbital, or brain invasion; tumor size; tumor stage; microscopic nerve or vessel invasion; or multifocal pathology.
Outcomes after Treatment with Curative Intent
Of all 18 patients, 10 (56%) are still alive in this cohort, whereas 8 patients deceased, of which 6 died because of their disease and 2 because of other reasons. All patients who underwent surgical treatment with negative margins ( n = 10) were alive with NED at the final follow-up. The longest follow-up time was 20 years. In contrast, six out of eight patients with positive surgical margins deceased because of their disease, whereas two patients are still alive with NED (after 127 and 157 months of follow-up, respectively).
The OS rates were 94% at 2 years, 76% at 5 years, and 61% at 10 years of follow-up, and the corresponding DSS rates were 94, 82, and 65%, respectively.
DSS was 100% at 20 years of follow-up when negative surgical margins were achieved compared with 38% at 10 years of follow-up ( p < 0.005; Fig. 3 ). Combined transcranial–transfacial approach (CFR) was significantly correlated with better DSS compared with transfacial approach (LR/ESS) only ( p = 0.019; Fig. 4 ). Interestingly, all patients treated with surgery in the last decade ( n = 7), regardless of surgical approach (two CFR, two LR, two ESS), had negative surgical margins and have NED.
Fig. 3.
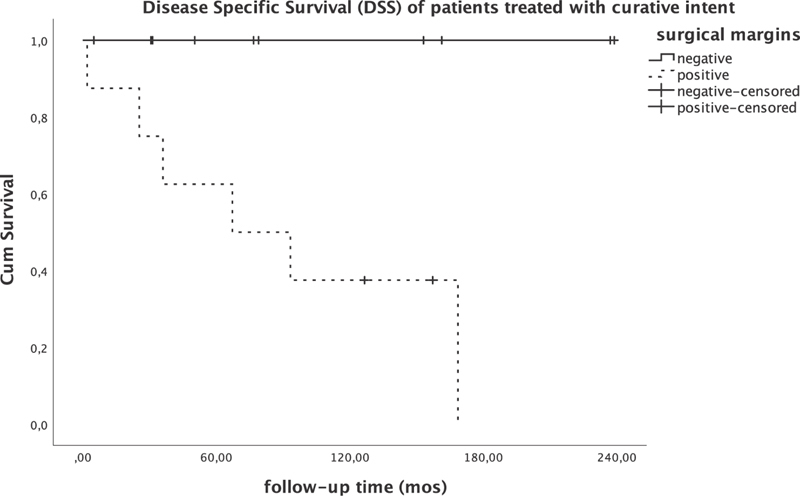
Disease-specific survival of patients undergoing treatment with curative intent.
Fig. 4.
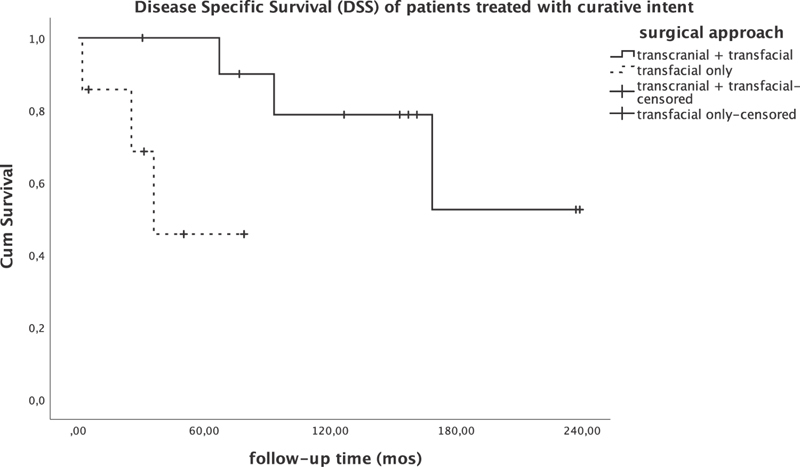
Disease-specific survival of patients undergoing treatment with curative intent.
A total of seven patients suffered recurrences, of whom five underwent surgery with positive margins. Recurrence-free survival (RFS) was 75% at 2 years, and 55% at 5 years, and 10 years of follow-up. Negative surgical margins and combined transcranial–transfacial approach were correlated with better RFS compared with positive margins and transfacial approach only (78 vs. 29% and 61 vs. 40% at 10 years of follow-up, respectively), but these correlations did not reach statistical significance ( p = 0.107 and 0.184, respectively), probably due to low cohort size. Local recurrence correlated significantly with inferior DSS ( = 0.022).
Two patients suffered distant metastases (lung and bone) 2 and 11 years after their primary treatment, respectively. Both of these patients subsequently died from their disease.
Outcomes after Nonsurgical Oncological Treatment
Both patients in this group received chemoradiotherapy but died of their disease, with neither of them surviving more than 34 months after diagnosis.
Discussion
Sinonasal AC is a potentially curable disease despite being an aggressive malignancy with a poor natural history. Sinonasal tumors often have innocuous symptoms, thus leading to delayed diagnosis. 40 41 42 Late diagnosis explains the high frequency of advanced stage tumors (T3–4); 75% of tumors were T3 and T4 in our series. The percentages of the different clinical signs in this study are consistent with published data. 31 40 43 44 45 46 Nasal obstruction, epistaxis, and, in many cases, visible nasal polyp upon clinical investigation are the main symptoms.
There was a clear correlation between inferior DSS and male sex ( p = 0.075), intestinal type of tumor differentiation (ITAC; p = 0.162), and low grade of tumor differentiation ( p = 0.078), but these correlations did not reach statistical significance probably due to low cohort size.
The involvement of key structures such as the anterior skull base (especially the dura mater and the brain), the orbital apex, the cavernous sinus, and the infratemporal fossa is recognized in the literature to be a factor influencing survival. 31 40 These data could not be confirmed in this study. The absence of statistically significant results could be caused by the effect of generally more aggressive surgical treatment in this cohort, resulting in a higher rate of radicality or small size of the series.
Nodal involvement at the time of initial diagnosis affects approximately 10% of reported cases. 47 Distant metastases are rare in this histological type and at this location. 44 There were no patients presenting with nodal involvement, and only 5% of all patients had distant metastasis at the time of diagnosis. Due to the small number of events in this series, it was not possible to highlight the influence of these factors.
Wood dust as a carcinogen was identified in 1995 by the International Agency for Research on Cancer. 48 Other recognized occupational risk factors are leather, tannin, and nickel. 49 50 51 International literature reports on proportions of patients with wood dust exposure ranging between 12.5 and 96.4%. 31 40 43 44 The GETTEC (Groupe d'etude des Tumeurs de la Tête et du Cou) study found that 84.7% of 418 patients treated for AC were woodworkers; however, it is also described that this proportion is usually higher in European populations (in particular the French population) than in other countries. 52 The mechanism of carcinogenesis is thought to be influenced by the duration and degree of exposure and the type of wood. 53 The mechanism of carcinogenesis is not completely understood; however, several molecular pathways influencing pathogenesis have been identified, for example, TP53 mutation, CYP1A1 codon 461 polymorphism, GSTM1 null genotype, and various epidermal growth factor receptor (EGFR) expression patterns. 54 55 56 This series report on possible occupational hazard in 45% of all patients, wood dust in 30%, in accordance with the literature. The GETTEC study has also suggested that the prognosis of AC in woodworkers was better than that in nonwoodworkers, although this correlation could not be observed in our cohort.
Nasal obstruction, epistaxis, and, in many cases, visible nasal polyp upon clinical investigation are the main symptoms.
The involvement of key structures such as the anterior skull base (especially the dura mater and the brain), the orbital apex, the cavernous sinus, and the infratemporal fossa is recognized in the literature to be a factor influencing survival. 31 40 These data could not be confirmed in this study, and the absence of statistically significant results is probably due to the small size of series.
There are no randomized clinical trials to date to guide the treatment of patients with AC, and the management of the disease is based on observational studies with limited numbers of patients due to the rarity of the disease. Many studies on AC often include patients with different origins, different stages, or histological subtypes pooled from several institutions. 40 Furthermore, ACs are so rare that it is unlikely that the impact of multimodal treatment would ever be analyzed in a randomized prospective fashion, even within the framework of a multi-institutional study.
There are no trials comparing surgery alone with other treatment regimens. However, in pooled series of varying types of sinonasal malignancies, surgery is more beneficial than other techniques. 57
Although clear evidence to support the use of XRT in sinonasal AC is difficult to obtain, local control rates of combined treatment strategies for advanced cases are comparable with less advanced cases with surgery alone, suggesting a positive role for postoperative XRT. 40 58 59 60 Most data concerning XRT derive from retrospective series, and there is understandable selection bias as patients treated with XRT alone are more likely to have locally advanced incompletely resectable tumors and are not comparable with those treated with surgery alone, as reported in this study as well. XRT can be avoided in low-stage (T1–T3) tumors when resection margins are wide, and it should also be avoided for small tumors with limited extension far from the high-risk structures (orbit, cribriform plate, meninges, cavernous sinus, internal carotid artery). 31 58 In addition, XRT is insufficient when macroscopic excision is incomplete. 61
The single most important factor influencing long-term survival of patients with AC is radical complete surgical resection of the tumor. There is a current debate in AC management about the most appropriate surgical approach. Open (external) surgical procedures have over a long time been considered as the mainstay of treatment, but these approaches are often criticized for higher morbidity. However, critiques often refer to old articles, thereby disregarding the advancements made in this type of surgery over the past two decades. There is a recent trend toward endoscopic resection as a primary treatment of AC, as with other sinonasal malignancies, and several authors have reported series of endoscopically resected tumors with comparatively good outcomes. 62 63 64 65 66 67 68 There is, however, a constant bias in these studies toward the smaller, lower-staged tumors being more suitable for endoscopic resection.
The International Head and Neck Scientific Group evaluated the evidence for treatment strategies in sinonasal AC, concluding that the ethos to the surgical strategy is to use whichever approach that gains access to remove the whole tumor with curative intent. 58 Whatever the surgical technique, the bilateral resection of the ethmoid is of paramount importance to minimize the possibility of the appearance of subsequent primary tumors, as in ACs, there is histological evidence on the existence of tumor nests in healthy mucosa of areas far from the tumor. 69
Five-year OS rates vary between 21.2 and 78% in the international literature. 40 42 43 44 70 71 According to published data, adjuvant XRT is used in 38 to 100% of cases. 31 40 42 43 45 70 71 72 Five-year OS after treatment with surgery was 76% in this series, the next highest rate ever published after Dulguerov et al. 42 Adjuvant XRT was administered in 89% of all patients. All patients undergoing surgery with negative margins are still NED. The proportion of patients with NED after long-term follow-up is 73% after surgery with the combined transcranial–transfacial approach (CFR) compared with 57%, when only the transfacial approach was used. There was only one complication (meningitis) reported after open surgical approach, and there was no perioperative mortality.
Study Limitations and Strengths
A weakness of this study is that it is based on observational data. Our cohort included patients treated over two decades. Thus, it was subject to the impact of improvements in radiological, surgical, XRT, and chemotherapy techniques.
Study strengths were the setting, design, and follow-up duration (long term). The data were restricted to one health center only, reducing the possible confounding effect of differences in access to the health care service. Thus, the selection bias that is inherently present in a larger multicenter study was seemingly avoided. Only end points that were verifiable were used with respect to the data quality. Lastly, 100% follow-up was obtained.
Acknowledgments
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of Interest On behalf of all authors, the corresponding author states that there is no conflict of interest.
Ethical Approval
This study was approved by the data protection official at OUH ( ePhorte 2015–5042 ). All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This study does not contain any studies with animals performed by any of the authors.
Informed Consent
For this type of study, formal consent is not required.
References
- 1.European Rhinologic Society Advisory Board on Endoscopic Techniques in the Management of Nose, Paranasal Sinus and Skull Base Tumours . Lund V J, Stammberger H, Nicolai P. European position paper on endoscopic management of tumours of the nose, paranasal sinuses and skull base. Rhinol Suppl. 2010;22:1–143. [PubMed] [Google Scholar]
- 2.Myers L L, Nussenbaum B, Bradford C R, Teknos T N, Esclamado R M, Wolf G T. Paranasal sinus malignancies: an 18-year single institution experience. Laryngoscope. 2002;112(11):1964–1969. doi: 10.1097/00005537-200211000-00010. [DOI] [PubMed] [Google Scholar]
- 3.Waldron J, Witterick I. Paranasal sinus cancer: caveats and controversies. World J Surg. 2003;27(07):849–855. doi: 10.1007/s00268-003-7111-8. [DOI] [PubMed] [Google Scholar]
- 4.Llorente J L, Pérez-Escuredo J, Alvarez-Marcos C, Suárez C, Hermsen M. Genetic and clinical aspects of wood dust related intestinal-type sinonasal adenocarcinoma: a review. Eur Arch Otorhinolaryngol. 2009;266(01):1–7. doi: 10.1007/s00405-008-0749-y. [DOI] [PubMed] [Google Scholar]
- 5.Haerle S K, Gullane P J, Witterick I J, Zweifel C, Gentili F. Sinonasal carcinomas: epidemiology, pathology, and management. Neurosurg Clin N Am. 2013;24(01):39–49. doi: 10.1016/j.nec.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 6.Leivo I. sinonasal adenocarcinoma: update on classification, immunophenotype and molecular features. Head Neck Pathol. 2016;10(01):68–74. doi: 10.1007/s12105-016-0694-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jankowski R, Georgel T, Vignaud J M. Endoscopic surgery reveals that woodworkers' adenocarcinomas originate in the olfactory cleft. Rhinology. 2007;45(04):308–314. [PubMed] [Google Scholar]
- 8.Thompson L D, Penner C, Ho N J. Sinonasal tract and nasopharyngeal adenoid cystic carcinoma: a clinicopathologic and immunophenotypic study of 86 cases. Head Neck Pathol. 2014;8(01):88–109. doi: 10.1007/s12105-013-0487-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bhaijee F, Carron J, Bell D. Low-grade nonintestinal sinonasal adenocarcinoma: a diagnosis of exclusion. Ann Diagn Pathol. 2011;15(03):181–184. doi: 10.1016/j.anndiagpath.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 10.Batsakis J G, Rice D H, Solomon A R. The pathology of head and neck tumors: squamous and mucous-gland carcinomas of the nasal cavity, paranasal sinuses, and larynx, part 6. Head Neck Surg. 1980;2(06):497–508. doi: 10.1002/hed.2890020610. [DOI] [PubMed] [Google Scholar]
- 11.Barnes L. Intestinal-type adenocarcinoma of the nasal cavity and paranasal sinuses. Am J Surg Pathol. 1986;10(03):192–202. doi: 10.1097/00000478-198603000-00006. [DOI] [PubMed] [Google Scholar]
- 12.Kleinsasser O, Schroeder H G. Adenocarcinomas of the inner nose after exposure to wood dust. Morphological findings and relationships between histopathology and clinical behavior in 79 cases. Arch Otorhinolaryngol. 1988;245(01):1–15. doi: 10.1007/BF00463541. [DOI] [PubMed] [Google Scholar]
- 13.Franchi A, Santucci A, Wenig B M. Lyon: IARC Press; 2005. Adenocarcinoma. World Health Organization Classification of Tumours, Pathology and Genetics, Head and Neck Tumours. [Google Scholar]
- 14.Franchi A, Miligi L, Palomba A, Giovannetti L, Santucci M. Sinonasal carcinomas: recent advances in molecular and phenotypic characterization and their clinical implications. Crit Rev Oncol Hematol. 2011;79(03):265–277. doi: 10.1016/j.critrevonc.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 15.Acheson E D, Hadfield E H, Macbeth R G.Carcinoma of the nasal cavity and accessory sinuses in woodworkers Lancet 19671(7485):311–312. [DOI] [PubMed] [Google Scholar]
- 16.Imbus H R, Dyson W L. A review of nasal cancer in furniture manufacturing and woodworking in North Carolina, the United States, and other countries. J Occup Med. 1987;29(09):734–740. [PubMed] [Google Scholar]
- 17.Ironside P, Matthews J. Adenocarcinoma of the nose and paranasal sinuses in woodworkers in the state of Victoria, Australia. Cancer. 1975;36(03):1115–1124. doi: 10.1002/1097-0142(197509)36:3<1115::aid-cncr2820360342>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 18.Leclerc A, Luce D, Demers P A. Sinonasal cancer and occupation. Results from the reanalysis of twelve case-control studies. Am J Ind Med. 1997;31(02):153–165. doi: 10.1002/(sici)1097-0274(199702)31:2<153::aid-ajim4>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 19.Moran C A, Wenig B M, Mullick F G. Primary adenocarcinoma of the nasal cavity and paranasal sinuses. Ear Nose Throat J. 1991;70(12):821–828. [PubMed] [Google Scholar]
- 20.IARC Working Group on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. International Agency for Research on Cancer . Lyon: International Agency for Research on Cancer; 1982. Chemicals, Industrial Processes, and Industries Associated with Cancer in Humans: IARC Monographs. Volumes 1–29. [Google Scholar]
- 21.Lund V J, Howard D J, Wei W I, Cheesman A D. Craniofacial resection for tumors of the nasal cavity and paranasal sinuses--a 17-year experience. Head Neck. 1998;20(02):97–105. doi: 10.1002/(sici)1097-0347(199803)20:2<97::aid-hed1>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 22.Heffner D K, Hyams V J, Hauck K W, Lingeman C. Low-grade adenocarcinoma of the nasal cavity and paranasal sinuses. Cancer. 1982;50(02):312–322. doi: 10.1002/1097-0142(19820715)50:2<312::aid-cncr2820500225>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 23.Lund V J. Malignancy of the nose and sinuses. Epidemiological and aetiological considerations. Rhinology. 1991;29(01):57–68. [PubMed] [Google Scholar]
- 24.Lund V J, Harrison D F.Craniofacial resection for tumors of the nasal cavity and paranasal sinuses Am J Surg 1988156(3 Pt 1):187–190. [DOI] [PubMed] [Google Scholar]
- 25.König M, Osnes T, Jebsen P, Evensen J F, Meling T R. Olfactory neuroblastoma: a single-center experience. Neurosurg Rev. 2018;41(01):323–331. doi: 10.1007/s10143-017-0859-3. [DOI] [PubMed] [Google Scholar]
- 26.König M, Osnes T, Jebsen P, Meling T R. Craniofacial resection of malignant tumors of the anterior skull base: a case series and a systematic review. Acta Neurochir (Wien) 2018;160(12):2339–2348. doi: 10.1007/s00701-018-3716-4. [DOI] [PubMed] [Google Scholar]
- 27.König M, Osnes T A, Lobmaier I. Multimodal treatment of craniofacial osteosarcoma with high-grade histology. A single-center experience over 35 years. Neurosurg Rev. 2017;40(03):449–460. doi: 10.1007/s10143-016-0802-z. [DOI] [PubMed] [Google Scholar]
- 28.Katz T S, Mendenhall W M, Morris C G, Amdur R J, Hinerman R W, Villaret D B. Malignant tumors of the nasal cavity and paranasal sinuses. Head Neck. 2002;24(09):821–829. doi: 10.1002/hed.10143. [DOI] [PubMed] [Google Scholar]
- 29.Bridgeman A M, Murphy M J, Sizeland A, Wiesenfeld D. Midfacial tumours: a review of 72 cases. Br J Oral Maxillofac Surg. 2000;38(02):94–103. doi: 10.1054/bjom.1998.0150. [DOI] [PubMed] [Google Scholar]
- 30.Jun B C, Song S W, Park C S, Lee D H, Cho K J, Cho J H. The analysis of maxillary sinus aeration according to aging process; volume assessment by 3-dimensional reconstruction by high-resolutional CT scanning. Otolaryngol Head Neck Surg. 2005;132(03):429–434. doi: 10.1016/j.otohns.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 31.de Gabory L, Maunoury A, Maurice-Tison S. Long-term single-center results of management of ethmoid adenocarcinoma: 95 patients over 28 years. Ann Surg Oncol. 2010;17(04):1127–1134. doi: 10.1245/s10434-010-0933-3. [DOI] [PubMed] [Google Scholar]
- 32.Amin M B, Greene F L, Edge S B. The Eighth Edition AJCC Cancer Staging Manual: continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J Clin. 2017;67(02):93–99. doi: 10.3322/caac.21388. [DOI] [PubMed] [Google Scholar]
- 33.Zange J, Schuchardt K. Leipzig: Thieme; 1950. Rhinologische und plastiche Operationen auf Grenzgebieten mit der Ophthalmologie und Chirurgie; p. 1302. [Google Scholar]
- 34.Kaplan E, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 35.Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep. 1966;50(03):163–170. [PubMed] [Google Scholar]
- 36.Cox D. Regression models and life tables. J R Stat Soc B. 1972;34:187–220. [Google Scholar]
- 37.Fisher R A. On the interpretation of X2 from contingency tables, and the calculation of P. J R Stat Soc. 1922;85(01):87–94. [Google Scholar]
- 38.Danish Head and Neck Cancer Group (DAHANCA) https://www.dahanca.dk/. Accessed February 1, 2018
- 39.European Organization for Research and Treatment of Cancer Trial 22931 . Bernier J, Domenge C, Ozsahin M. Postoperative irradiation with or without concomitant chemotherapy for locally advanced head and neck cancer. N Engl J Med. 2004;350(19):1945–1952. doi: 10.1056/NEJMoa032641. [DOI] [PubMed] [Google Scholar]
- 40.GETTEC Study Group . Choussy O, Ferron C, Védrine P O. Adenocarcinoma of ethmoid: a GETTEC retrospective multicenter study of 418 cases. Laryngoscope. 2008;118(03):437–443. doi: 10.1097/MLG.0b013e31815b48e3. [DOI] [PubMed] [Google Scholar]
- 41.Dulguerov P, Allal A S. Nasal and paranasal sinus carcinoma: how can we continue to make progress? Curr Opin Otolaryngol Head Neck Surg. 2006;14(02):67–72. doi: 10.1097/01.moo.0000193177.62074.fd. [DOI] [PubMed] [Google Scholar]
- 42.Dulguerov P, Jacobsen M S, Allal A S, Lehmann W, Calcaterra T. Nasal and paranasal sinus carcinoma: are we making progress? A series of 220 patients and a systematic review. Cancer. 2001;92(12):3012–3029. doi: 10.1002/1097-0142(20011215)92:12<3012::aid-cncr10131>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 43.Orvidas L J, Lewis J E, Weaver A L, Bagniewski S M, Olsen K D. Adenocarcinoma of the nose and paranasal sinuses: a retrospective study of diagnosis, histologic characteristics, and outcomes in 24 patients. Head Neck. 2005;27(05):370–375. doi: 10.1002/hed.20168. [DOI] [PubMed] [Google Scholar]
- 44.Michel J, Radulesco T, Penicaud M, Mancini J, Dessi P. Sinonasal adenocarcinoma: clinical outcomes and predictive factors. Int J Oral Maxillofac Surg. 2017;46(04):422–427. doi: 10.1016/j.ijom.2016.11.018. [DOI] [PubMed] [Google Scholar]
- 45.Gras-Cabrerizo J R, Montserrat-Gili J R, León-Vintró X, Massegur-Solench H, de Vega J M, Virós-Porcuna D. Treatment results for ethmoid sinus carcinoma. J Laryngol Otol. 2009;123(10):1120–1124. doi: 10.1017/S0022215109990752. [DOI] [PubMed] [Google Scholar]
- 46.Bhayani M K, Yilmaz T, Sweeney A. Sinonasal adenocarcinoma: a 16-year experience at a single institution. Head Neck. 2014;36(10):1490–1496. doi: 10.1002/hed.23485. [DOI] [PubMed] [Google Scholar]
- 47.Cantù G, Bimbi G, Miceli R. Lymph node metastases in malignant tumors of the paranasal sinuses: prognostic value and treatment. Arch Otolaryngol Head Neck Surg. 2008;134(02):170–177. doi: 10.1001/archoto.2007.30. [DOI] [PubMed] [Google Scholar]
- 48.Wood dust. IARC Monogr Eval Carcinog Risks Hum. 1995;62:35–215. [PMC free article] [PubMed] [Google Scholar]
- 49.Schwaab G, Julieron M, Janot F. Epidemiology of cancers of the nasal cavities and paranasal sinuses [in Italian] Neurochirurgie. 1997;43(02):61–63. [PubMed] [Google Scholar]
- 50.Brinton L A, Blot W J, Becker J A. A case-control study of cancers of the nasal cavity and paranasal sinuses. Am J Epidemiol. 1984;119(06):896–906. doi: 10.1093/oxfordjournals.aje.a113812. [DOI] [PubMed] [Google Scholar]
- 51.Bonneterre V, Deschamps E, Persoons R. Sino-nasal cancer and exposure to leather dust. Occup Med (Lond) 2007;57(06):438–443. doi: 10.1093/occmed/kqm050. [DOI] [PubMed] [Google Scholar]
- 52.Demers P A, Kogevinas M, Boffetta P. Wood dust and sino-nasal cancer: pooled reanalysis of twelve case-control studies. Am J Ind Med. 1995;28(02):151–166. doi: 10.1002/ajim.4700280202. [DOI] [PubMed] [Google Scholar]
- 53.Kauppinen T, Vincent R, Liukkonen T. Occupational exposure to inhalable wood dust in the member states of the European Union. Ann Occup Hyg. 2006;50(06):549–561. doi: 10.1093/annhyg/mel013. [DOI] [PubMed] [Google Scholar]
- 54.Holmila R, Bornholdt J, Heikkilä P. Mutations in TP53 tumor suppressor gene in wood dust-related sinonasal cancer. Int J Cancer. 2010;127(03):578–588. doi: 10.1002/ijc.25064. [DOI] [PubMed] [Google Scholar]
- 55.Cantu G, Solero C L, Mariani L. Intestinal type adenocarcinoma of the ethmoid sinus in wood and leather workers: a retrospective study of 153 cases. Head Neck. 2011;33(04):535–542. doi: 10.1002/hed.21485. [DOI] [PubMed] [Google Scholar]
- 56.Projetti F, Durand K, Chaunavel A. Epidermal growth factor receptor expression and KRAS and BRAF mutations: study of 39 sinonasal intestinal-type adenocarcinomas. Hum Pathol. 2013;44(10):2116–2125. doi: 10.1016/j.humpath.2013.03.019. [DOI] [PubMed] [Google Scholar]
- 57.Airoldi M, Garzaro M, Valente G. Clinical and biological prognostic factors in 179 cases with sinonasal carcinoma treated in the Italian Piedmont region. Oncology. 2009;76(04):262–269. doi: 10.1159/000206140. [DOI] [PubMed] [Google Scholar]
- 58.Lund V J, Chisholm E J, Takes R P. Evidence for treatment strategies in sinonasal adenocarcinoma. Head Neck. 2012;34(08):1168–1178. doi: 10.1002/hed.21770. [DOI] [PubMed] [Google Scholar]
- 59.Podboj J, Smid L. Endoscopic surgery with curative intent for malignant tumors of the nose and paranasal sinuses. Eur J Surg Oncol. 2007;33(09):1081–1086. doi: 10.1016/j.ejso.2007.01.016. [DOI] [PubMed] [Google Scholar]
- 60.Meccariello G, Deganello A, Choussy O. Endoscopic nasal versus open approach for the management of sinonasal adenocarcinoma: a pooled-analysis of 1826 patients. Head Neck. 2016;38 01:E2267–E2274. doi: 10.1002/hed.24182. [DOI] [PubMed] [Google Scholar]
- 61.Moreau J J, Bessede J P, Heurtebise F. Adenocarcinoma of the ethmoid sinus in woodworkers. Retrospective study of 25 cases [in French] Neurochirurgie. 1997;43(02):111–117. [PubMed] [Google Scholar]
- 62.Bogaerts S, Vander Poorten V, Nuyts S, Van den Bogaert W, Jorissen M. Results of endoscopic resection followed by radiotherapy for primarily diagnosed adenocarcinomas of the paranasal sinuses. Head Neck. 2008;30(06):728–736. doi: 10.1002/hed.20771. [DOI] [PubMed] [Google Scholar]
- 63.Goffart Y, Jorissen M, Daele J. Minimally invasive endoscopic management of malignant sinonasal tumours. Acta Otorhinolaryngol Belg. 2000;54(02):221–232. [PubMed] [Google Scholar]
- 64.Jardeleza C, Seiberling K, Floreani S, Wormald P J. Surgical outcomes of endoscopic management of adenocarcinoma of the sinonasal cavity. Rhinology. 2009;47(04):354–361. doi: 10.4193/Rhin08.222. [DOI] [PubMed] [Google Scholar]
- 65.Lund V, Howard D J, Wei W I. Endoscopic resection of malignant tumors of the nose and sinuses. Am J Rhinol. 2007;21(01):89–94. doi: 10.2500/ajr.2007.21.2957. [DOI] [PubMed] [Google Scholar]
- 66.Nicolai P, Battaglia P, Bignami M. Endoscopic surgery for malignant tumors of the sinonasal tract and adjacent skull base: a 10-year experience. Am J Rhinol. 2008;22(03):308–316. doi: 10.2500/ajr.2008.22.3170. [DOI] [PubMed] [Google Scholar]
- 67.Van Gerven L, Jorissen M, Nuyts S, Hermans R, Vander Poorten V. Long-term follow-up of 44 patients with adenocarcinoma of the nasal cavity and sinuses primarily treated with endoscopic resection followed by radiotherapy. Head Neck. 2011;33(06):898–904. doi: 10.1002/hed.21556. [DOI] [PubMed] [Google Scholar]
- 68.Hanna E, DeMonte F, Ibrahim S, Roberts D, Levine N, Kupferman M. Endoscopic resection of sinonasal cancers with and without craniotomy: oncologic results. Arch Otolaryngol Head Neck Surg. 2009;135(12):1219–1224. doi: 10.1001/archoto.2009.173. [DOI] [PubMed] [Google Scholar]
- 69.Bussi M, Gervasio C F, Riontino E. Study of ethmoidal mucosa in a population at occupational high risk of sinonasal adenocarcinoma. Acta Otolaryngol. 2002;122(02):197–201. doi: 10.1080/00016480252814225. [DOI] [PubMed] [Google Scholar]
- 70.McKay S P, Shibuya T Y, Armstrong W B. Cell carcinoma of the paranasal sinuses and skull base. Am J Otolaryngol. 2007;28(05):294–301. doi: 10.1016/j.amjoto.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 71.Vergez S, du Mayne M D, Coste A. Multicenter study to assess endoscopic resection of 159 sinonasal adenocarcinomas. Ann Surg Oncol. 2014;21(04):1384–1390. doi: 10.1245/s10434-013-3385-8. [DOI] [PubMed] [Google Scholar]
- 72.EUROCARE Working Group . Gatta G, Botta L, Sánchez M J, Anderson L A, Pierannunzio D, Licitra L. Prognoses and improvement for head and neck cancers diagnosed in Europe in early 2000s: the EUROCARE-5 population-based study. Eur J Cancer. 2015;51(15):2130–2143. doi: 10.1016/j.ejca.2015.07.043. [DOI] [PubMed] [Google Scholar]


