Abstract
Objective Most pituitary adenomas are of soft consistency and can be resected during surgery with routine suction instruments. However, fibrous adenomas may require more aggressive techniques. The ability to predict consistency on magnetic resonance imaging (MRI) would improve preoperative preparation and may have implications on the extent of resection.
Design A retrospective review of MRI and tumor histology of 50 consecutive patients who underwent endoscopic endonasal resection for nonfunctional adenomas was performed.
Methods An intensity ratio was calculated based on quantitative MRI signal intensity of the adenoma and pons. Intraoperatively, a sequentially graded technique required for resection ranged from suction (R1) for softer tumors, curettes (R2) for tumors with intermediate consistency, and aspirators and/or other microinstruments (R3) for firmer tumors. Fibrotic content was determined from histologic collagen percentage, and rates of gross total resection (GTR) were calculated from postoperative imaging. Statistical analyses were performed to determine if resection classification could be predicted by intensity ratio or collagen percentage, calculate ratio of cut-off points for clinical use, and assess for correlation between intensity ratios and collagen percentage.
Results Tumors with ratios < 1.6 on the T2-weighted coronal image and collagen content > 5.3% were likely to have required a more aggressive resection technique. Statistically significant lower rates of GTR and higher rates of perioperative complications were seen with such tumors.
Conclusion Preoperative MRI analyses can be helpful but not definitive in predicting adenoma consistency. Fibrous adenomas, associated with higher collagen content, are more difficult to resect and have higher rates of subtotal resection.
Keywords: Endoscopic, pituitary adenoma, transsphenoidal, tumor consistency
Introduction
Pituitary adenomas (PAs) account for up to 16.7% of all intracranial tumors and rank as the third most common brain tumor. 1 2 PAs can present with a variety of symptoms due to both mass effect on surrounding neurovascular structures or due to altered pituitary secretory function. 1 The majority of PAs are of soft consistency and can be effectively resected via the endoscopic endonasal approach. However, it has been estimated that up to 10% may be fibrous and require more complex operative resection techniques. 1 3 4 To optimize the resection, it is necessary to use the appropriate equipment. Having the ability to predict tumor consistency prior to the operation may allow surgeons to improve surgical planning and guidance for patients, which may allow the operating room staff to better prepare for the operation, and may lead to realistic and specific patient outcomes with regards to tumor residual and perioperative complications.
Magnetic resonance imaging (MRI), particularly the T2-weighted and fluid attenuated inversion recovery (FLAIR) sequences, has been suggested to help distinguish varying intensities that correlate with fibrotic consistency and collagen content upon pathologic diagnosis, thus helping to predict tumor consistency. Most prior studies agree that high collagen content corresponds to a more fibrotic tumor; however, there is a debate regarding whether high collagen content can be predicted from preoperative MRI analysis. 4 5 The nature of the correlation between MRI analysis and tumor consistency has also been mixed in previous studies and no definitive conclusion exists. 3 4 6 7
In our study, we compared the signal intensity of the PA to that of the pons. In a previous study, the signal intensity of the pons was found to be relatively homogenous and stable on axial MRI and was less disturbed by magnetic susceptibility artifacts, making it a reliable reference for intensity. 1 On T2-weighted and FLAIR sequence images, fluid appears hyperintense or bright and should reflect a softer tumor consistency that would usually allow an easier resection. 3 We hypothesized that there would be a consistent correlation between signal intensity of the tumor in comparison to that of the pons and its intraoperative consistency. We predicted that more fibrous tumors (based on its intraoperative consistency) would have higher collagen content on histological examination, appear more hypointense on T2-weighted and FLAIR sequence images, and have a lower gross total resection (GTR) rate.
Materials and Methods
Patient Data
A retrospective review of 50 consecutive patients who underwent initial resection of nonfunctioning PAs via an endoscopic endonasal (transsphenoidal) approach by the same neurosurgeon and otolaryngologist team, between January 2014 and August 2017, was performed. Institutional Review Board approval was obtained prior to beginning the study. The diagnosis was histologically confirmed in each case.
Clinical Setting
During surgery, tumor consistency was prospectively classified on a scale from 1 to 3 by the primary neurosurgeon based on the surgical tools utilized. Resection classification 1 (R1) required only the two-suction technique, resection classification 2 (R2) required suction and ringed curettes, and resection classification 3 (R3) additionally required microinstruments, sharp dissection, and/or an ultrasonic aspirators for tumor resection. Similar classification criteria have been used in previous studies and are clinically intuitive to the operating surgeon. 8 9 10 11
Imaging
MRI of every PA was performed preoperatively, and a blinded neuroradiologist evaluated each image with no knowledge of the tumor's intraoperative consistency or surgical resection classification score. Due to intrinsic variability in the MRI machine, it was necessary to quantitatively standardize each patient's imaging. This was done by calculating a ratio that compared the average intensity of the adenoma to that of the pons due to its homogeneity and stability on imaging. 11 On MRI analysis, a selection tool was used to select a region of interest (ROI) from two-dimensional slices of the adenoma and pons, respectively, that was most representative of the structure's overall intensity. The computer would then calculate an average intensity for the area selected ( Fig. 1 ).
Fig. 1.
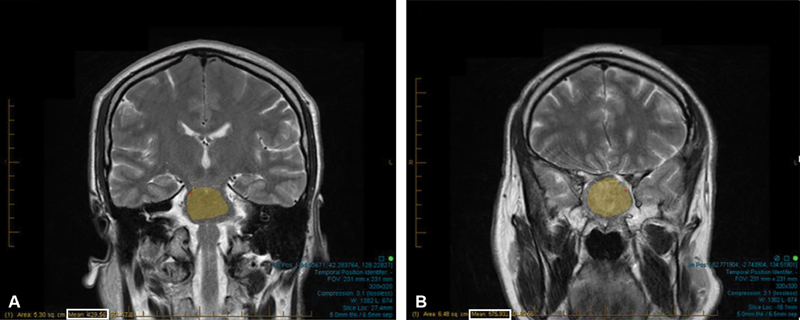
Free-hand selection tool used to select a region of interest (ROI). ( A ) ROI in pons on T2-coronal sequence. ( B ) ROI in PA on T2-coronal sequence. Maximum area was selected to ensure accurate intensity values (white box). Adenoma:pons calculation: ratio = mean intensity for ROI within PA (A; white box)/mean intensity value for ROI within pons (B; white box). Ratio = 429.56/575.93 = 0.7459.
Using these average intensity values, a ratio was calculated comparing the signal intensity of the adenoma to the pons for each patient's images. An example of these ratio calculations is given in Fig. 1 . A high ratio indicates that the adenoma is hyperintense to the ROI of the pons. If our hypothesis is true, then more fibrous tumors will have a lower ratio on T2 and FLAIR sequence imaging due to less fluid content and higher collagen content.
Calculation of Collagen Content
Formalin-fixed, paraffin-embedded pituitary specimens were stained using Masson's trichrome stain and scanned with a WSI Aperio CS2 (Leica Microsystems, Wetzlar, Germany) slide scanner using a ×20 objective. High-resolution images were exported into National Institute of Health (NIH) ImageJ program (National Institutes of Health, Bethesda, Maryland), 12 where color thresholding was applied to select fibrotic areas (i.e., blue pixels; Fig. 2 ). The area of fibrosis in each tissue section was divided by the total area of the tissue section to obtain the total fibrotic area as a percentage of total tissue section area.
Fig. 2.
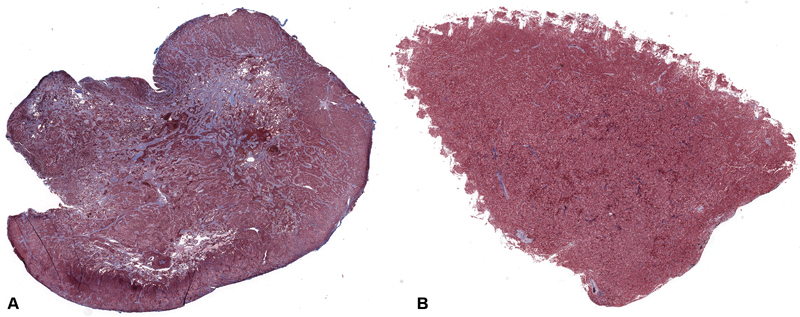
Histological slides from two different patients. Each specimen was stained w/ Masson's trichrome stain, which stains collagen blue. ( A ) It is from a PA with a high collagen percentage (31.04%). ( B ) It is from a PA with low collagen percentage (2.940%).PA, ituitary adenoma.
Statistical Analysis
Descriptive statistics were calculated to describe patient demographics. Medians and interquartile ranges (IQRs) were calculated for each adenoma/pons ratio and fibrosis percentage by tumor grade, and the statistical significance of differences were assessed using Kruskal–Wallis tests. Separate univariable logistic regression models considered each ratio as an independent variable to predict R3 PAs, and the optimal cut-off point was determined using Youden's J statistic. Estimates of sensitivity, specificity, positive predictive value, and negative predictive value of these cut-off points were calculated with exact confidence intervals (CI). Spearman's rho was used to assess rank correlation of ratios and fibrosis percentage. A linear trend for gross total resection rate with resection classification was assessed using the Cochran–Armitage test for trend. Typical perioperative complications and imaging characteristics (suprasellar extension and cavernous sinus invasion) were compared by GTR rate and resection classification using Pearson's Chi-square or Fisher's exact test as appropriate. Analyses were performed using SAS 9.4 (SAS Institute, Cary, North Carolina, United States).
Results
A total of 50 patients underwent resection of nonfunctioning PAs via an endoscopic endonasal approach between January 2014 and August 2017 by the same neurosurgery/otolaryngology team. There were 40 males and 10 females enrolled. The average age was 62 years old (±standard deviation [SD]). Of the 50 PAs, 22 (44%) were classified as R1, 19 (38%) as R2, and 9 (18%) as R3 resections. Every patient had at least one form of valid imaging; however, due to incomplete imaging, 22 patients had a valid Coronal T2-weighted image, 37 had a valid Axial T2-weighted image, and 40 had a valid FLAIR sequence image. Our analysis only compared patients with similar imaging studies to one another. For example, data from coronal T2-weighted images were only analyzed against data from other coronal T2-weighted images and not from that of axial T2-weighted images.
Patients' adenoma/pons intensity ratios and tumor resection classification scores were compared in Table 1 . The distributions of ratios for coronal view, axial view, or flair modality did not differ by tumor grade.
Table 1. Adenoma/pons ratio scores by tumor grade.
| Grade 1 n = 22 |
Grade 2 n = 19 |
Grade 3 n = 9 |
p -Value | |
|---|---|---|---|---|
| Adenoma/pons ratio (95% confidence interval) | ||||
| Coronal view | 1.73 (1.67–1.85) | 1.66 (1.40–1.96) | 1.39 (1.35–1.49) | 0.20 |
| Axial view | 1.76 (1.66–2.01) | 1.72 (1.37–1.95) | 1.66 (1.50–1.81) | 0.55 |
| Flair modality | 1.46 (1.32–1.53) | 1.36 (1.29–1.51) | 1.41 (1.26–1.47) | 0.45 |
| Fibrosis percentage | 4.57 (2.19–9.56) | 2.53 (1.31–4.77) | 9.91 (7.57–31.04) | 0.019 |
We then looked for a ratio cut-off point that could be used to rule out an R3 adenoma; for this analysis, we combined the R1 and R2 adenomas into one category since they are clinically similar in their intraoperative resection difficulty. Using Youden's J statistic, we determined that a ratio cut-off point of 1.6 maximized sensitivity (100%) and specificity (63.2%) and we applied it to our T2 Coronal images. We were able to discriminate reasonably well between patients who have R3 adenomas and those who have R1 and R2 tumors ( Table 2 ). Additional details regarding an appropriate cut-point, as well as sensitivity and specificity measures are detailed in Table 2 . Rates of GTR were assessed on imaging obtained 1 day after surgery. A blinded neuroradiologist assessed the imaging. Overall GTR rate was 86%. There was statistical significance for the rate of GTR based on the technique used intraoperatively (100% for R1, 89.5% for R2, and 44.4% for R3; p -trend < 0.001; Table 3 ). Results of additional analyses looking into complication rate and imaging characteristics as they correlate with resection classification and rate of GTR may be found in Tables 4 5 6 7 and are further discussed below.
Table 2. Sensitivity, specificity, positive predictive value, and negative predictive value of cut-off points.
| Sensitivity | Specificity | PPV | NPV | |
|---|---|---|---|---|
| Estimate, %, (95% confidence interval) | ||||
| Coronal view ≤ 1.6 | 100.0 (29.2–100.0) | 63.2 (38.4–83.7) | 30.0 (6.7–65.3) | 100.0 (73.5–100.0) |
| (38.4–83.7) | ||||
| Fibrosis percentage > 5.3 | 85.7 (42.1–99.6) | 72.2 (46.5–90.3) | 54.5 (23.4–83.3) | 92.9 (66.1–99.8) |
| (66.1–99.8) | ||||
Abbreviations: NPV, negative predictive value; PPV, positive predictive value.
Table 3. Rate of gross total resection (GTR) compared with resection classification.
| Resection classification | R1 | R2 | R3 |
|---|---|---|---|
| Number of resections | 22 | 19 | 9 |
| Number of GTRs | 22 | 17 | 4 |
| % of GTR | 100 | 89.5 | 44.4 |
Table 4. Imaging characteristics compared with rate of GTR.
| GTR (43) | Non-GTR (7) | p -Value | |
|---|---|---|---|
| n (%) | |||
| Cavernous sinus invasion | |||
| Yes | 2 (4.7) | 2 (28.6) | 0.09 |
| No | 41 (95.3) | 5 (71.4) | |
| Suprasellar extension (> 3 cm) | |||
| Yes | 4 (9.3) | 7 (100.0) | < 0.001 |
| No | 39 (90.7) | 0 (0.0) | |
Abbreviations: GTR, gross total resection.
Table 5. Imaging characteristics compared with resection classification.
| R1 (22) | R2 (19) | R3 (9) | p -Value | |
|---|---|---|---|---|
| n (%) | ||||
| Cavernous sinus invasion | ||||
| Yes | 0 (0.0) | 2 (10.5) | 2 (22.2) | 0.06 |
| No | 22 (100.0) | 17 (89.5) | 7 (77.8) | |
| Suprasellar extension (> 3 cm) | ||||
| Yes | 5 (22.7) | 4 (21.1) | 2 (22.2) | 0.99 |
| No | 17 (77.3) | 15 (78.9) | 7 (77.8) | |
Table 6. Complication rate compared with rate of GTR.
| GTR (43) | Non-GTR (7) | p -Value | |
|---|---|---|---|
| n (%) | |||
| Hypopituitarism | 1 (2.3) | 0 (0.0) | < 0.001 |
| Intraoperative CSF leak | 8 (18.6) | 7 (100.0) | |
| Postoperative CSF leak | 0 (0.0) | 0 (0.0) | |
| Transient DI | 3 | 0 (0.0) | |
| Permanent DI | 1 (2.3) | 0 (0.0) | |
| No complication | 30 (69.8) | 0 (0.0) | |
Abbreviations: CSF, cerebrospinal fluid; DI, diabetes insipidus; GTR, gross total resection.
Table 7. Complication rate compared with resection classification.
| R1 (22) | R2 (19) | R3 (9) | p -Value | |
|---|---|---|---|---|
| n (%) | ||||
| Hypopituitarism | 0 (0.0) | 0 (0.0) | 1 (11.1) | < 0.001 |
| Intraoperative CSF leak | 4 (18.2) | 6 (31.6) | 5 (55.6) | |
| Transient DI | 0 (0.0) | 1 (5.3) | 2 (22.2) | |
| Permanent DI | 0 (0.0) | 0 (0.0) | 1 (11.1) | |
| No complication | 18 (81.8) | 12 (63.2) | 0 (0.0) | |
Abbreviations: CSF, cerebrospinal fluid; DI, diabetes insipidus.
Histology Findings
The collagen percentage of PA specimens from different patients ranged from < 1% of total tissue area to over 80%. There was a statistically significant difference in the collagen percentage of R1, R2, and R3 adenomas ( p = 0.019), with highest fibrosis percentage in R3 (median: 9.91; IQR: 7.57–31.04; Table 1 ).
We determined that collagen percentage had a negative correlation with intensity ratios for all MRI sequences and views assessed (with coronal view having the strongest association: rho ( ρ ) = -0.44). However, based on the 95% CI, these associations were not statistically significant ( Table 8 ).
Table 8. Spearman's rho with 95% confidence intervals for adenoma/pons ratios and fibrosis percentage.
| Coronal view | Axial view | Flair modality | |
|---|---|---|---|
| Coronal view | |||
| Axial view | 0.91 (0.75–0.97) | ||
| Flair modality | 0.51 (0.05–0.79) | 0.49 (0.19–0.71) | |
| Fibrosis percentage | −0.44 (−0.86 to 0.31) | −0.04 (−0.45 to 0.38) | −0.06 (−0.45 to 0.35) |
Using Youden's J statistic to determine a clinical cut-off point, we determined that a cut-off point of collagen percentage > 5.3% was optimal to predict the intraoperative classification score for our sample, with sensitivity of 85.7% (95% CI: 42.1–99.6%) and specificity of 72.2% (46.5–90.3%; Table 2 ).
Discussion
Pituitary neoplasms account for an estimated 25% of all surgical resections for central nervous system (CNS) tumors. 2 In addition, as a subset of these neoplasms are subclinical, their incidence and prevalence may be underestimated. 13 It is estimated that 30% of individuals between the ages of 50 and 60 years have incidentalomas. 13 14 Symptomatic adenomas can present with a wide range of symptoms due to either mass effect on surrounding structures or altered secretory effects. The most common indications for surgery for nonfunctional adenomas include radiographic growth, mass effect on the optic chiasm, oculomotor palsy, or apoplexy.
Generally, the transsphenoidal approach is the preferred method for pituitary neoplasms but transcranial surgery can be indicated in patients harboring adenomas with prominent extrasellar extension, extensive fibrosis, failed previous transsphenoidal surgery, insufficient decompression, ectactic carotid arteries, aneurysms, or severe sinus infections. 13 While a T1-weighted scan with and without gadolinium will allow proper visualization of optic nerves, optic chiasm, carotid arteries, cavernous sinuses, and surrounding soft tissue, T2 imaging has been suggested to provide information on the fibrotic nature of each adenoma as a measure of water content. FLAIR imaging has also been widely used to identify CNS abnormalities due to its ability to reduce the CSF signal and magnetic susceptibility artifact. 15
As is the case with all surgical procedures, there are limitations to the endoscopic endonasal approach and various factors can determine the extent of resection including tumor size, invasiveness into surrounding structures, and consistency. Highly fibrous tumors can be difficult to resect due to the limited space to maneuver surgical tools and may be more suitable for a transcranial approach. 7 The ability to anticipate a fibrous tumor based on preoperative MRI analysis may allow surgeons to more confidently guide patients with more specific rates of resection, better prepare the surgical team for the anticipated challenges from surgery, and increase the likelihood of a GTR through expected anticipation, leading to better patient outcomes. Since it is believed that residual tumor is the likely cause of regrowth, having a GTR will likely result in a decreased chance of recurrence. 8 16
Previous studies have looked for a correlation between adenoma consistency and radiologic imaging. The results have been inconclusive. Some studies indicate that a lower adenoma signal (hypointense) on T2-weighted images corresponds with a more fibrous adenoma. Others indicate that an isointense adenoma signal is indicative of a more fibrous adenoma, 1 3 4 while further research found no correlation between MRI and tumor consistency. 5 7 9 17 18 Fig. 3 shows examples of a relative hyperintense tumor compared with a hypointense tumor.
Fig. 3.
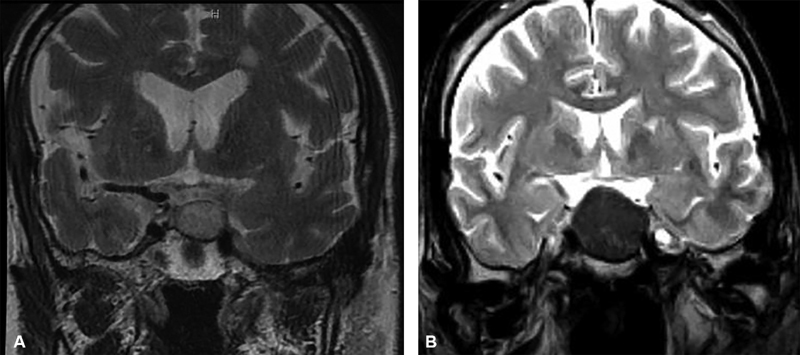
( A ) T2-coronal view of a hyperintense pituitary adenoma and ( B ) hypointense pituitary adenoma.
Histologically, many studies have concurred that a high collagen percentage corresponds with a more fibrous PA. 1 5 10 11 One study recommended that adenomas with a collagen percentage greater than 5% be defined as a “fibrosis” adenoma. 1 19 Previous studies have also looked for a correlation between collagen percentage and radiologic image analysis. One study found no correlation between T1-weighted images and adenoma collagen percentage but found on T2-weighted imaging that a high collagen percentage was significantly correlated to a lower signal intensity. 4 They concluded that those tumors were also more firm and fibrous on resection. Another study, however, found no correlation between contrast-enhanced T1-weighted imaging and T2-weighted imaging and the collagen percentage. 5
Our study also looked for a correlation between tumor intensity on preoperative MRI and intraoperative tumor consistency. Intraoperative adenoma consistency was classified on a three-level scale based on the surgical tools used to resect, where R1 tumors required only suction, R2 tumors required suction and ringed curettes, and R3 tumors required anything else including sharp dissection, an ultrasonic aspirator, and/or Rhoton microinstruments (Integra Life Sciences, Plainsboro, New Jersey). Previous studies have dichotomized adenoma consistency (soft or firm) or used a similar three-level classification scale also based on the surgical tools used to resect. 1 3 4 6 7 Iuchi et al divided adenomas into three groups 4 : soft, which were easily resected with suction; medium, which were difficult to remove with suction; and tough, which could not be removed with suction and required bipolar electrocoagulation or sharp segmentation resection. 4
The tumors were initially graded on a three-level scale as described earlier. However, we later switched to a dichotomous scale, grouping the R1 and R2 adenomas together (R1/R2). Clinically, R1 and R2 PAs are similar with respect to their resection. R3 tumors are much more difficult to resect and require the most preparation. Because of this, the way a surgeon guides a patient through their treatment is different between softer R1/R2 PAs and firmer R3 PAs. By dichotomizing the scale and maximizing sensitivity, the surgeon is less likely to misdiagnose the consistency of a firm adenoma (R3 adenoma) and will be better able to guide the patient and be more prepared for the operation. Information on the resection classification scores and imaging data can be found in Table 9 .
Table 9. Resection classification score and imaging totals.
| Resection classification score | Coronal T2 ( n = 22) | Axial T2 ( n = 37) | Axial FLAIR ( n = 40) |
|---|---|---|---|
| R1/R2 | 19 | 29 | 31 |
| R3 | 3 | 8 | 9 |
Abbreviations: FLAIR, fluid attenuated inversion recovery; R1, resection classification 1, R2, resection classification 2; R3, resection classification 3.
Like previous studies, we used ratios to quantitatively determine the relative signal intensity of the adenoma. 8 10 14 A ratio > 1 indicated that the adenoma was more intense than the standardized ROI of the pons and, therefore, was predicted to be less fibrotic in consistency and have a lower collagen content than an adenoma with a low ratio. Different views (axial and coronal) were used from each patient to improve intratumor sampling. We did not detect any statistically significant associations between patient's intensity ratios and tumor resection classification score for any of the sequences or views assessed.
Using Youden's J statistic, we calculated an intensity ratio of cut-off point to discriminate between R3 and R1/R2 adenomas and applied it to patient imaging. We found that coronal T2-weighted images were able to discriminate reasonably well between patients who underwent R3 and R1/R2 resections. We found that neither axial T2-weighted sequences nor FLAIR sequences discriminated particularly well between patients who underwent R3 versus R1/R2 resections. A cut-off point ratio of 1.6 maximizes sensitivity (100%) and specificity (68%) in this patient sample. Using this cut-off point, the negative predictive value was maximized (100%). It is unclear why coronal T2-weighted images, but not axial T2-weighted images, were able to discriminate between resection classifications. One possible explanation may involve the typical growth pattern of PAs. Monsalves et al demonstrated that out of 153 patients with PAs, 123 patients (80.4%) demonstrated predominant growth in the superior direction defined as extension into the suprasellar cistern and/or compression of the optic chiasm as observed in the coronal plane. 20 A tendency for PAs to grow superiorly may allow a greater surface area to be analyzed on MRI when viewed in the vertical plane as seen with a coronal view but not an axial view. A greater surface area for analysis will be more representative of the entire tumor and increase the likelihood of detecting certain characteristics (such as fibrosis) within the tumor.
Our study also looked for a correlation between histologic collagen percentage and intraoperative tumor consistency. The collagen percentage of PA specimens from different patients ranged from < 1% of total tissue area to over 80%. Using a Kruskal–Wallis test, we were able to detect a statistically significant difference in collagen percentage of R1, R2, and R3 adenomas, meaning that histologic analysis of adenomas is effective at retrospectively predicting the intraoperative difficulty of resection. We used Youden's J statistic to calculate a cut-off point of collagen percentage to discriminate between R3 and R1/R2 adenomas and found that a cut-off point of 5.3% maximizes both sensitivity (85.7%) and specificity (72.2%; Table 2 ). Based on this analysis, PAs with collagen below 5.3% were unlikely to have been intraoperatively classified as R3 adenomas.
Our study also looked for a correlation between collagen percentage and adenoma/pons intensity ratios. Using Spearman's rho analysis, we determined that collagen percentage is negatively correlated to the intensity ratios, such that as collagen percentage decreases, the intensity ratio should be expected to increase. The coronal T2-weighted images had the strongest negative association. This would support our hypothesis that a PA with less-collagen content should have higher fluid content and should appear hyperintense on MRI and would also agree with the histologic findings of previous studies. However, based on the 95% CI, this conclusion is not statistically significant ( Table 3 ).
Because GTR has been associated with a lower rate of tumor recurrence, we also looked for a correlation between resection classification and rate of GTR. We found a statistically significant correlation between resection classification and rate of GTR, with decreasing GTR rate with increasing resection classification. However, cavernous sinus invasion and suprasellar extension are factors that are known to affect the extent of PA resection and may be possible confounders to this finding. While we found a significant correlation between suprasellar extent > 3 cm and rate of GTR, we did not find a significant correlation between cavernous sinus invasion and rate of GTR ( Table 4 ). In addition, we did not find a significant correlation between either cavernous sinus invasion and/or suprasellar extension > 3 cm and resection classification ( Table 5 ). For this reason, we cannot definitively conclude that PAs that require more complicated resection techniques are less likely to achieve GTR, as GTR may be affected by cavernous sinus invasion or extensive suprasellar extension. However, we can conclude that tumors with suprasellar extension > 3 cm were less likely to attain GTR than tumors with cavernous sinus invasion and that neither imaging characteristic was definitively predictive of the required resection technique.
In addition, we compared the rate of relevant perioperative complications (including hypopituitarism, CSF leak, and diabetes insipidus [DI]) of GTR tumors to perioperative complications of non-GTR tumors. We found a statistically significant positive correlation between overall complication rate and rate of GTR ( Table 6 ). We also assessed for a relationship between resection classification and rate of perioperative complications. We found a statistically significant positive correlation between overall complication rate and resection classification ( Table 7 ). Due to small sample size, we deemed an analysis investigating rates of each individual complication not to be robust enough; however, this may be explored in future studies. These findings can have implications for perioperative management and can help individualize patient's needs for more aggressive postoperative follow-up. There are limitations to this study. There were only nine patients who required a R3 resection, and the findings here including cut-off points for predicting R3 should be confirmed in other patient populations and study sites. Additionally, tumors were not always homogeneous in consistency which may have contributed to the intra-grade variability of the tumor's MRI intensity. We attempted to account for this by selecting an ROI that was most representative of overall tumor intensity. Finally, not all patients had the exact same images due to interhospital and intrahospital variability.
Conclusion
PAs are predominantly of soft consistency, but more fibrous PAs can be more difficult to resect when encountered, have higher rates of subtotal resection, and are histologically associated with higher collagen content. Using T2-weighted coronal MR imaging as a screening tool can give the surgeon a general prediction of the PA's fibrous consistency. PAs with adenoma/pons cut-off point ratios > 1.6 are unlikely to be fibrous in consistency and are unlikely to require aggressive resection techniques and instruments. However, usage of this ratio should not be overstated, and its results should not be considered diagnostic.
Conflict of Interest None declared.
Disclosure
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
Previous Presentations
Partial results were delivered in oral presentation form at the North American Skull Base Society Annual Meeting in San Diego, California, United States, in February 2018.
References
- 1.Wei L, Lin S A, Fan K, Xiao D, Hong J, Wang S. Relationship between pituitary adenoma texture and collagen content revealed by comparative study of MRI and pathology analysis. Int J Clin Exp Med. 2015;8(08):12898–12905. [PMC free article] [PubMed] [Google Scholar]
- 2.Ezzat S, Asa S L, Couldwell W T. The prevalence of pituitary adenomas: a systematic review. Cancer. 2004;101(03):613–619. doi: 10.1002/cncr.20412. [DOI] [PubMed] [Google Scholar]
- 3.Smith K A, Leever J D, Chamoun R B. Prediction of consistency of pituitary adenomas by magnetic resonance imaging. J Neurol Surg B Skull Base. 2015;76(05):340–343. doi: 10.1055/s-0035-1549005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iuchi T, Saeki N, Tanaka M, Sunami K, Yamaura A. MRI prediction of fibrous pituitary adenomas. Acta Neurochir (Wien) 1998;140(08):779–786. doi: 10.1007/s007010050179. [DOI] [PubMed] [Google Scholar]
- 5.Yamamoto J, Kakeda S, Shimajiri S. Tumor consistency of pituitary macroadenomas: predictive analysis on the basis of imaging features with contrast-enhanced 3D FIESTA at 3T. AJNR Am J Neuroradiol. 2014;35(02):297–303. doi: 10.3174/ajnr.A3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Snow R B, Johnson C E, Morgello S, Lavyne M H, Patterson R H., Jr Is magnetic resonance imaging useful in guiding the operative approach to large pituitary tumors? Neurosurgery. 1990;26(05):801–803. doi: 10.1097/00006123-199005000-00011. [DOI] [PubMed] [Google Scholar]
- 7.Bahuleyan B, Raghuram L, Rajshekhar V, Chacko A G. To assess the ability of MRI to predict consistency of pituitary macroadenomas. Br J Neurosurg. 2006;20(05):324–326. doi: 10.1080/02688690601000717. [DOI] [PubMed] [Google Scholar]
- 8.Heringer L C, de Oliveira M F, Rotta J M, Botelho R V. Effect of repeated transsphenoidal surgery in recurrent or residual pituitary adenomas: a systematic review and meta-analysis. Surg Neurol Int. 2016;7:14. doi: 10.4103/2152-7806.175896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boxerman J L, Rogg J M, Donahue J E, Machan J T, Goldman M A, Doberstein C E. Preoperative MRI evaluation of pituitary macroadenoma: imaging features predictive of successful transsphenoidal surgery. AJR Am J Roentgenol. 2010;195(03):720–728. doi: 10.2214/AJR.09.4128. [DOI] [PubMed] [Google Scholar]
- 10.Liu J K, Das K, Weiss M H, Laws E R, Jr, Couldwell W T. The history and evolution of transsphenoidal surgery. J Neurosurg. 2001;95(06):1083–1096. doi: 10.3171/jns.2001.95.6.1083. [DOI] [PubMed] [Google Scholar]
- 11.Pierallini A, Caramia F, Falcone C. Pituitary macroadenomas: preoperative evaluation of consistency with diffusion-weighted MR imaging--initial experience. Radiology. 2006;239(01):223–231. doi: 10.1148/radiol.2383042204. [DOI] [PubMed] [Google Scholar]
- 12.Schindelin J, Arganda-Carreras I, Frise E. Fiji: an open-source platform for biological-image analysis. Nat Methods. 2012;9(07):676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Theodros D, Patel M, Ruzevick J, Lim M, Bettegowda C. Pituitary adenomas: historical perspective, surgical management and future directions. CNS Oncol. 2015;4(06):411–429. doi: 10.2217/cns.15.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kane L A, Leinung M C, Scheithauer B W. Pituitary adenomas in childhood and adolescence. J Clin Endocrinol Metab. 1994;79(04):1135–1140. doi: 10.1210/jcem.79.4.7525627. [DOI] [PubMed] [Google Scholar]
- 15.De Coene B, Hajnal J V, Gatehouse P. MR of the brain using fluid-attenuated inversion recovery (FLAIR) pulse sequences. AJNR Am J Neuroradiol. 1992;13(06):1555–1564. [PMC free article] [PubMed] [Google Scholar]
- 16.Dickerman R D, Oldfield E H. Basis of persistent and recurrent Cushing disease: an analysis of findings at repeated pituitary surgery. J Neurosurg. 2002;97(06):1343–1349. doi: 10.3171/jns.2002.97.6.1343. [DOI] [PubMed] [Google Scholar]
- 17.Hagiwara A, Inoue Y, Wakasa K, Haba T, Tashiro T, Miyamoto T. Comparison of growth hormone-producing and non-growth hormone-producing pituitary adenomas: imaging characteristics and pathologic correlation. Radiology. 2003;228(02):533–538. doi: 10.1148/radiol.2282020695. [DOI] [PubMed] [Google Scholar]
- 18.Suzuki C, Maeda M, Hori K. Apparent diffusion coefficient of pituitary macroadenoma evaluated with line-scan diffusion-weighted imaging. J Neuroradiol. 2007;34(04):228–235. doi: 10.1016/j.neurad.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 19.Naganuma H, Satoh E, Nukui H.Technical considerations of transsphenoidal removal of fibrous pituitary adenomas and evaluation of collagen content and subtype in the adenomas Neurol Med Chir (Tokyo) 20024205202–212., discussion 213 [DOI] [PubMed] [Google Scholar]
- 20.Monsalves E, Larjani S, Loyola Godoy B. Growth patterns of pituitary adenomas and histopathological correlates. J Clin Endocrinol Metab. 2014;99(04):1330–1338. doi: 10.1210/jc.2013-3054. [DOI] [PubMed] [Google Scholar]


