Abstract
Background The endoscopic endonasal approach (EEA) has become increasingly used for resection of skull base tumors in the sellar and suprasellar regions. A nasoseptal flap (NSF) is routinely used for anterior skull base reconstruction; however, there are numerous additional allografts and autografts being used in conjunction with the NSF. The role of perioperative cerebrospinal fluid (CSF) diversion is also unclear.
Objective This study was aimed to analyze success of high-flow CSF leak repair during EEA procedures without use of CSF diversion through lumbar drainage.
Methods A retrospective chart review of patients who had intraoperative high-flow CSF leak during EEA procedures at our institution between January 2013 and December 2017 was performed. CSF leaks were repaired with use of a fascia lata button graft and nasoseptal flap, without use of perioperative lumbar drains.
Results A total of 38 patients were identified (10 male, 28 female). Patient BMIs ranged from 19.7 to 49 kg/m 2 (median = 31 kg/m 2 ), with 18 patients meeting criteria for obesity (BMI > 30 kg/m 2 ) and 12 patients overweight (25 kg/m 2 < BMI < 29.9 kg/m 2 ). There was no incidence of postoperative CSF leak.
Conclusion In our experience, the nasoseptal flap used in conjunction with the fascia lata button graft is a safe, effective and robust combination for cranial base reconstruction with high-flow intraoperative CSF leaks, without need for lumbar drains.
Keywords: high-flow cerebrospinal fluid leak, endoscopic endonasal approach, fascia lata button graft, nasoseptal flap, pituitary adenoma, meningioma, craniopharyngioma
Introduction
Over the past several decades, the transsphenoidal endoscopic endonasal approach (EEA) has evolved dramatically and is now a common surgical technique to access sellar and suprasellar regions. 1 2 3 4 A characteristic of some of these procedures is an intraoperative high-flow cerebrospinal fluid (CSF) leak, created by opening of the cisterns above the sellar diaphragm, with or without opening of the third ventricle. A grading scale for CSF leak was created by Esposito et al, and defined a high-flow leak (class 3) as one involving a large diaphragmatic or dural defect. 5 In these cases, a robust reconstruction is required to prevent postoperative CSF leakage. Autologous pedicled mucoperichondrial nasoseptal flaps (NSF) have become the mainstay for endonasal cranial base reconstruction, but typically multilayered repair is required, including a primary dural repair. 6 7 8 9 10 11 There are many options for the latter, including a “button” graft, which has been previously described as consisting of two pieces of autologous fascia lata sutured together in the center. 7 Lumbar drains are commonly used for diversion of CSF flow during reconstruction, but carry risks including infection, lumbar radiculitis, and increased hospital length of stay. 9 12
In this paper, we present a case series of patients who underwent endoscopic endonasal resection of suprasellar tumors and reconstruction consisting of an autologous fascia lata “button” graft for primary dural repair and an NSF for definitive cranial base repair, without use of an intraoperative or postoperative lumbar drain. All patients had intraoperative high-flow CSF leak due to opening of the suprasellar cisterns with or without opening of the third ventricles. Demographic analysis and postoperative complications are presented.
Methods
A retrospective chart review was conducted of consecutive patients at our institution from January 2013 to December 2017 who underwent extended endoscopic endonasal cranial base surgery to the supradiaphragmatic space with opening of the suprasellar cisterns with or without opening of the third ventricle. Demographic data, BMI, pathology, and complications were reviewed. All patients had a high-flow intraoperative CSF leak, and all underwent reconstruction of the defect with a “button” fascia lata inlay-onlay graft, NSF, and polyethylene glycol glue. Lumbar drains were not used.
Surgical Technique
Patients were laid supine on the operating table. Early in the series, patients were placed in a three-pin head holder fixation for the purposes of neuronavigation registration, while later, mask registration without rigid head fixation was utilized (Stryker, Freiburg, Germany). A 0-degree endoscope was introduced into the right naris and inferior and middle turbinates were outfractured and lateralized. Posterior ethmoidectomies were performed to expose the anterior face of the sphenoid sinus and a wide sphenoidotomy was done. Care was taken not to injure the vascular pedicle to the nasal septal flap within mucosa inferior to the natural ostium of the sphenoid sinus. This was similarly performed through the left naris with a slightly smaller sphenoidotomy from the natural ostium to the nasal cavity roof to allow for passage of the endoscope and visualization throughout the procedure. The NSF was harvested as described elsewhere previously on the more spacious side and where the septum was straighter. 13 Once the mucosa was elevated off the sphenoid rostrum, the rostrum and the intrasphenoid septations were drilled down using a 2-mm diamond drill. In each case, the cranial base osteotomies and dural opening were performed to appropriately address the associated pathology. An intraoperative high-flow CSF leak was created due to the opening of the cisterns. The tumor was then resected.
For reconstruction, an autologous fascia lata graft was harvested through a lateral thigh incision. After measuring the skull base defect, a fascia lata “button graft” was then created in a bilayer fashion by suturing an onlay and inlay portion together, as previously described by Luginbuhl et al. 7 This graft was then placed within the dural defect so that the inlay portion was placed intradurally and the onlay portion was over the top of the dural opening. It was adequately positioned in this bilayered “button” fashion until no visible egress of CSF was observed. Then, the autologous vascularized pedicled mucoperichondrial NSF was rotated on its pedicle and used to cover the cranial base defect ( Fig. 1 ). Surgicel (Johnson & Johnson, New Jersey, United States) was used at the borders of the flap to prevent flap migration, followed by DuraSeal (Medtronic, PLC, Minneapolis, MN, United States), and absorbable packing (Nasopore, Stryker, Kalamazoo, MI, United States) to support the flap. Finally, silicone splints were placed along the nasal septum.
Fig. 1.
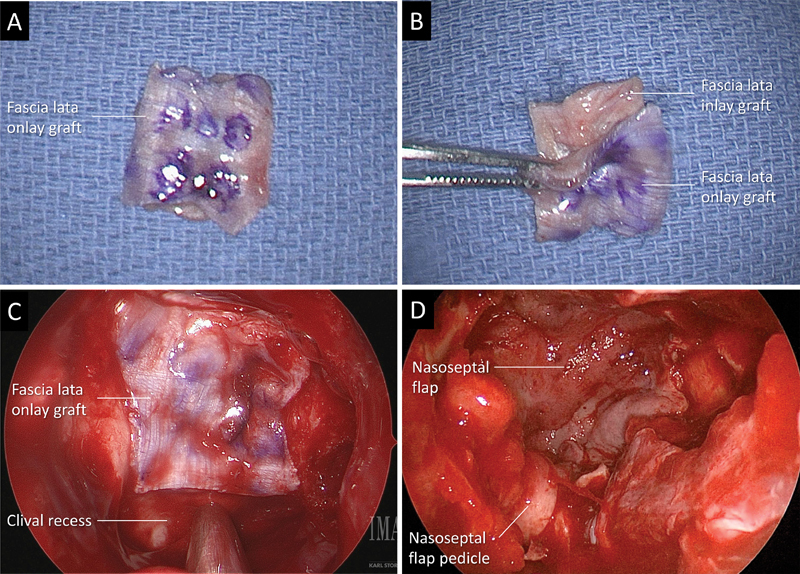
( A ) Fascia lata button graft. Observe the outer layer is marked to facilitate the differentiation between the two layers during the endoscopic placement of the graft. ( B ) The inlay graft is smaller than the onlay and both are sutured together in the center. ( C and D ) Intraoperative endoscopic picture obtained with a 0-degree endoscope during resection of a craniopharyngioma. ( C ) Observe the onlay graft covering completely the defect and still bone around it to allow bone contact to the nasoseptal flap. ( D ) Nasoseptal flap covering the entire defect and the facia lata graft. Note that the flap is much larger than the graft which allows great contact to the bone.
Results
Patient characteristics: A total of 38 patients were studied, 34 adult and 4 pediatric. Among the adult patients, 27 were female and seven were male, while one of the pediatric patients was female and three were male. All of the patients had high-flow intraoperative CSF leak due to opening of the suprasellar cisterns. Body mass index (BMI) of adult patients ranged from 19.7 to 49 kg/m 2 (median = 31 kg/m 2 ), with an average BMI of 32.2 kg/m 2 which is classified as obese. Eighteen patients were obese (BMI > 30 kg/m 2 ) and 12 patients were overweight (25 kg/m 2 < BMI < 29.9 kg/m 2 ). Four of the adult patients were of normal weight (18.5 kg/m 2 < BMI < 25 kg/m 2 ). Also, 83% of the obese patients were female (15/18), while only 17% were male (3/18). Adult patient ages ranged from 31 to 89 years, with the median age for adults being 53 years old and 65% (22/34) of patients being over 50 years old. The majority of the pediatric patients (three-fourths) were adolescents from age 13 to 15 years, while one patient was 3 years old. The average BMI for the pediatric patients was 16.2 kg/m 2 , with two out of four patients being underweight for their age group (BMI for age < 5th percentile), and two patients within the normal weight range (BMI for age between 5th and 85th percentile). The average length of hospital stay of all patients was 5.1 days, with most patients (66%) being discharged from 0 to 5 days postoperative, and 34% of patients requiring a stay from 7 to 12 days postoperative ( Table 1 ).
Table 1. Patient demographics.
| Total number of patients studied ( n ) | 38 |
|---|---|
| Mean age (y) | 50.3 |
| Male | 10 |
| Female | 28 |
| Underweight (BMI < 18.5 kg/m 2 ) | 2 a |
| Normal weight (BMI < 25 kg/m 2 ) | 6 b |
| Overweight (25 kg/m 2 < BMI < 29.9 kg/m 2 ) | 12 |
| Obese (BMI > 30 kg/m 2 ) | 18 |
| Mean length of hospital stay (d) | 5.1 |
| Patients discharged 0–5 days postoperative | 25 |
| Patients discharged 6–17 days postoperative | 13 |
Abbreviation: BMI, body mass index.
Patients were pediatric.
Included two pediatric patients.
Histopathological diagnoses: Postsurgical histopathological diagnoses consisted of the following: 12 craniopharyngiomas, 10 meningiomas, five pituitary adenomas, two arachnoid cysts, two Rathke's cleft cysts, two CSF leaks due to prior pituitary tumor resection at outside hospitals, one spontaneous high-flow CSF leak, one astrocytoma, one granulomatous hypophysitis, one hypothalamic hamartoma, and one case of metastatic adenocarcinoma from the lung. In 14 patients (37%), intraoperative opening of the third ventricle and subsequent communication of the ventricular system with the cranial base defect was required to treat the underlying pathology ( Table 2 ).
Table 2. Histopathological diagnoses ( n = 38) .
| Craniopharyngiomas | 12 |
| Meningiomas | 10 |
| Pituitary adenomas | 5 |
| Arachnoid cyst | 2 |
| Rathke's cleft cyst | 2 |
| CSF leak due to prior pituitary tumor resection at outside hospital | 2 |
| Spontaneous CSF leak | 1 |
| Astrocytoma | 1 |
| Granulomatous hypophysitis | 1 |
| Hypothalamic hamartoma | 1 |
| Metastatic lung adenocarcinoma | 1 |
| Total | 38 |
Abbreviation: CSF, cerebrospinal fluid.
Postoperative complications: Postoperative complications occurred in 26% of patients (10/38), ranging from one major complication of intra-axial brain abscess, to minor complications, such as electrolyte disturbances and postoperative diabetes insipidus. One patient developed a postoperative intra-axial brain abscess in an area of previous pial invasion after the meningioma resection and required reoperation. This patient had no CSF leak in the first postoperative period nor at the time of the second operation, which was also performed endonasally. The minor complications included five cases of postoperative diabetes insipidus, one instance of hypernatremia, two of hyponatremia, one incidence of postoperative anosmia, and one case of supraventricular tachycardia. There were no postoperative CSF leaks, or development of meningitis in any of these patients ( Table 3 ).
Table 3. Postoperative complications in 38 patients studied.
| Major | |
| Intraaxial brain abscess | 1 |
| Minor | |
| Diabetes insipidus | 5 |
| Hypernatremia | 1 |
| Hyponatremia | 2 |
| Anosmia | 1 |
| Supraventricular tachycardia | 1 |
| CSF Leak | 0 |
| Total number of postoperative complications | 10 |
Abbreviation: CSF, cerebrospinal fluid.
Discussion
Extended endonasal endoscopic cranial base surgery has proven highly effective and has reduced the occurrence of complications after removal of parasellar tumors. 2 14 However, postoperative CSF leak remains a concern after EEA procedures. When considering management and prevention of postoperative CSF leaks after endonasal surgery, it is important to consider obesity. Elevated body mass index (BMI) is known to be associated with high intracranial pressure, and it is thought that this may lead to spontaneous CSF rhinorrhea in obese patients. 15 16 Given the rising rates of obesity in the United States, it is of significant concern whether this correlation could play a role in the incidence of postoperative CSF leaks. Dlouhy et al found that elevated BMI is likely to affect success of transsphenoidal surgery by causing postoperative CSF leak, and recommended that patients with high BMIs be closely monitored for this and other complications. 17 In our study, the vast majority of our adult patients (30/34) were either overweight or obese, but no patients had postoperative CSF leak. Despite this, we do believe that this is an important consideration and if there is any question as to the adequacy of a cranial base repair in an obese patient, we always favor the most robust reconstruction possible.
Though the rise of EEA techniques has increased efficacy of parasellar tumor resection and a decrease in postoperative complications compared with the open approach, some concerns remain. 2 For example, postoperative CSF leak is still a concern if reconstruction is inadequate due to opening of the cisterns with or without opening of the third ventricle. Many options have been utilized for reconstruction, such as vascularized flaps, lumbar drains, free grafts, and absorbable materials and glues. 11 18 19 Each of these techniques has its own benefits and risks regarding efficacy of reconstruction. Avascular autologous grafts may lead to prolonged healing of both the repair and the donor sites. 18 The autologous vascularized mucoperichondrial NSF is regarded as one of the most effective techniques for reconstruction of dural defects after endoscopic cranial base surgery. 1 13 20 21 22 23 24 NSF usage has led to a decreased incidence of CSF leaks when compared with autologous avascular grafts and lumbar drain insertions, especially in high-flow CSF leak. 21 23 Zanation et al observed a postoperative CSF leak rate of 5.7% in a cohort of 70 patients who received an NSF for reconstruction after intradural lesion resection, but noted that other risk factors, such as age of patients, size of dural defect, and whether or not the patients received radiation therapy afterwards were correlated in the incidence of postoperative CSF leak. 25 Horridge et al observed an incidence of 3% postoperative CSF leaks with usage of the NSF when compared with 12.5% with other grafts, and concluded that the use of the NSF alone decreased morbidity and length of hospital stay. 21 Horiguchi et al had a cohort of 32 patients (with 11 patients receiving fascia lata graft and 21 patients receiving NSF with balloon catheter) and found that CSF leaks occurred near twice more in patients with only a fascia lata graft (3/11 patients) compared with patients with only NSFs (2/21 patients). 20
Another technique that has been utilized by some physicians is the gasket-seal closure. This method of reconstruction uses autologous fascia lata held by a firm buttress support to create a cranial defect closure that is impermeable to water, and has shown considerable efficacy in preventing postoperative CSF leak. 26 When Garcia-Navarro et al utilized the gasket-seal reconstruction along with a vascularized NSF in 46 patients, their postoperative CSF leak rate was 5%. One of their patients required reconstructive surgery, while another required a lumbar drain to control the CSF leak. 27 Hu et al compared the use of a vascularized NSF + lumbar drain (18 patients) to the use of a combined technique comprising of gasket seal + NSF + lumbar drain insertion (15 patients). In the group with gasket seal + NSF + lumbar drain, a 0% rate of postoperative CSF leak was observed compared with 5% in the NSF + lumbar drain group. They concluded that the combined technique, as well as a lumbar drain was optimal to decrease the rate of postoperative CSF leak and other complications after EEA. 28
There is some debate as to the role of perioperative lumbar drainage in cases of high-flow intraoperative CSF leak. The rationale behind this is that a drain should decompress the cranial base reconstruction by diverting CSF away from the repair site to allow for improved healing. 10 However, the use of lumbar drains is not free of complications. Potential risks include infection and lumbar radiculitis. At worst, placement of a lumbar drain could possibly lead to over drainage, occurrence of extra-axial hematomas, tension pneumocephalus, or uncal herniation. 12 29 To prevent such complications, it is vital to train nursing staff to manage lumbar drains appropriately. Patients with lumbar drains are thus more likely have an increased length of inpatient stay. Some studies have shown that the risk of lumbar drain related complications may be greater than the occurrence of postoperative CSF leak. 9 12 Because of such risks, we do not utilize lumbar drains in our protocol for cranial base reconstruction, and we have not found their omission to increase our postoperative CSF leak rate.
The fascia lata “button” graft was first described by Luginbuhl et al in 2010 and has proven to be a versatile technique for decreasing postoperative CSF leak after EEA 7 . As described in the methods section, this technique consists of harvesting a graft of autologous fascia lata with an incision on the patient's lateral thigh, and then using this graft to create a bilayer “button” with an inlay and onlay portion sutured together and placed over the dural opening. This technique has been highly successful in preventing postoperative CSF leak. 7 In our series of 38 consecutive patients treated with the combination of both NSF, as well as a fascia lata “button” graft for intraoperative high-flow CSF leak, no postoperative CSF leak was observed despite treating an obese population without using perioperative lumbar drains.
Conclusion
Our data suggest that a NSF along with a button graft derived from fascia lata is a highly effective reconstruction for high-flow CSF leaks after extended endoscopic endonasal surgery to remove parasellar lesions. This technique leads to minimal postoperative CSF leak in patients regardless of patient BMI and eliminates the need for perioperative lumbar drain insertion.
Conflict of Interest None declared.
Financial Disclosure
None declared.
References
- 1.Bedrosian J C, Anand V K, Schwartz T H.The endoscopic endonasal approach to repair of iatrogenic and noniatrogenic cerebrospinal fluid leaks and encephaloceles of the anterior cranial fossa World Neurosurg 201482(6, Suppl.)S86–S94. [DOI] [PubMed] [Google Scholar]
- 2.Cavallo L M, Frank G, Cappabianca P. The endoscopic endonasal approach for the management of craniopharyngiomas: a series of 103 patients. J Neurosurg. 2014;121(01):100–113. doi: 10.3171/2014.3.JNS131521. [DOI] [PubMed] [Google Scholar]
- 3.Doglietto F, Prevedello D M, Jane J A, Jr., Han J, Laws E R., Jr Brief history of endoscopic transsphenoidal surgery--from Philipp Bozzini to the First World Congress of Endoscopic Skull Base Surgery. Neurosurg Focus. 2005;19(06):E3. doi: 10.3171/foc.2005.19.6.4. [DOI] [PubMed] [Google Scholar]
- 4.Kassam A, Carrau R L, Snyderman C H, Gardner P, Mintz A. Evolution of reconstructive techniques following endoscopic expanded endonasal approaches. Neurosurg Focus. 2005;19(01):E8. [PubMed] [Google Scholar]
- 5.Esposito F, Dusick J R, Fatemi N, Kelly D F.Graded repair of cranial base defects and cerebrospinal fluid leaks in transsphenoidal surgery Neurosurgery 2007600402295–303., discussion 303–304 [DOI] [PubMed] [Google Scholar]
- 6.Ahn J YKS, Kim S H.A new technique for dural suturing with fascia graft for cerebrospinal fluid leakage in transsphenoidal surgery Neurosurgery 200965(6, Suppl.)65–71, discussion 71–72. [DOI] [PubMed] [Google Scholar]
- 7.Luginbuhl A JCP, Campbell P G, Evans J, Rosen M. Endoscopic repair of high-flow cranial base defects using a bilayer button. Laryngoscope. 2010;120(05):876–880. doi: 10.1002/lary.20861. [DOI] [PubMed] [Google Scholar]
- 8.Pereira E AC, Grandidge C A, Nowak V A, Cudlip S A. Cerebrospinal fluid leaks after transsphenoidal surgery - Effect of a polyethylene glycol hydrogel dural sealant. J Clin Neurosci. 2017;44:6–10. doi: 10.1016/j.jocn.2017.06.016. [DOI] [PubMed] [Google Scholar]
- 9.Ransom E RPJ, Palmer J N, Kennedy D W, Chiu A G. Assessing risk/benefit of lumbar drain use for endoscopic skull-base surgery. Int Forum Allergy Rhinol. 2011;1(03):173–177. doi: 10.1002/alr.20026. [DOI] [PubMed] [Google Scholar]
- 10.Sade B, Mohr G, Frenkiel S.Management of intra-operative cerebrospinal fluid leak in transnasal transsphenoidal pituitary microsurgery: use of post-operative lumbar drain and sellar reconstruction without fat packing Acta Neurochir (Wien) 20061480113–18., discussion 18–19 [DOI] [PubMed] [Google Scholar]
- 11.Sigler A C, D'Anza B, Lobo B C, Woodard T D, Recinos P F, Sindwani R. Endoscopic skull base reconstruction: an evolution of materials and methods. Otolaryngol Clin North Am. 2017;50(03):643–653. doi: 10.1016/j.otc.2017.01.015. [DOI] [PubMed] [Google Scholar]
- 12.Stokken J, Recinos P F, Woodard T, Sindwani R. The utility of lumbar drains in modern endoscopic skull base surgery. Curr Opin Otolaryngol Head Neck Surg. 2015;23(01):78–82. doi: 10.1097/MOO.0000000000000119. [DOI] [PubMed] [Google Scholar]
- 13.Hadad G, Bassagasteguy L, Carrau R L. A novel reconstructive technique after endoscopic expanded endonasal approaches: vascular pedicle nasoseptal flap. Laryngoscope. 2006;116(10):1882–1886. doi: 10.1097/01.mlg.0000234933.37779.e4. [DOI] [PubMed] [Google Scholar]
- 14.Fatemi N, Dusick J R, de Paiva Neto M A, Kelly D F.The endonasal microscopic approach for pituitary adenomas and other parasellar tumors: a 10-year experience Neurosurgery 2008630402244–256., discussion 256 [DOI] [PubMed] [Google Scholar]
- 15.Ooi L YWB, Walker B R, Bodkin P A, Whittle I R. Idiopathic intracranial hypertension: can studies of obesity provide the key to understanding pathogenesis? Br J Neurosurg. 2008;22(02):187–194. doi: 10.1080/02688690701827340. [DOI] [PubMed] [Google Scholar]
- 16.Pérez M ABO, Bialer O Y, Bruce B B, Newman N J, Biousse V. Primary spontaneous cerebrospinal fluid leaks and idiopathic intracranial hypertension. J Neuroophthalmol. 2013;33(04):330–337. doi: 10.1097/WNO.0b013e318299c292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dlouhy B JMK, Madhavan K, Clinger J D. Elevated body mass index and risk of postoperative CSF leak following transsphenoidal surgery. J Neurosurg. 2012;116(06):1311–1317. doi: 10.3171/2012.2.JNS111837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roxbury C RST, Saavedra T, Ramanathan M., Jr Layered sellar reconstruction with avascular free grafts: acceptable alternative to the nasoseptal flap for repair of low-volume intraoperative cerebrospinal fluid leak. Am J Rhinol Allergy. 2016;30(05):367–371. doi: 10.2500/ajra.2016.30.4356. [DOI] [PubMed] [Google Scholar]
- 19.Sciarretta V, Mazzatenta D, Ciarpaglini R, Pasquini E, Farneti G, Frank G. Surgical repair of persisting CSF leaks following standard or extended endoscopic transsphenoidal surgery for pituitary tumor. Minim Invasive Neurosurg. 2010;53(02):55–59. doi: 10.1055/s-0029-1246161. [DOI] [PubMed] [Google Scholar]
- 20.Horiguchi K, Murai H, Hasegawa Y, Hanazawa T, Yamakami I, Saeki N.Endoscopic endonasal skull base reconstruction using a nasal septal flap: surgical results and comparison with previous reconstructions Neurosurg Rev 20103302235–241., discussion 241 [DOI] [PubMed] [Google Scholar]
- 21.Horridge M, Jesurasa A, Olubajo F, Mirza S, Sinha S. The use of the nasoseptal flap to reduce the rate of post-operative cerebrospinal fluid leaks following endoscopic trans-sphenoidal surgery for pituitary disease. Br J Neurosurg. 2013;27(06):739–741. doi: 10.3109/02688697.2013.795525. [DOI] [PubMed] [Google Scholar]
- 22.Kassam A BTA, Thomas A, Carrau R L.Endoscopic reconstruction of the cranial base using a pedicled nasoseptal flap Neurosurgery 2008630101ONS44–ONS52., discussion ONS52–ONS53 [DOI] [PubMed] [Google Scholar]
- 23.Thorp B D, Sreenath S B, Ebert C S, Zanation A M. Endoscopic skull base reconstruction: a review and clinical case series of 152 vascularized flaps used for surgical skull base defects in the setting of intraoperative cerebrospinal fluid leak. Neurosurg Focus. 2014;37(04):E4. doi: 10.3171/2014.7.FOCUS14350. [DOI] [PubMed] [Google Scholar]
- 24.Xuejian W, Fan H, Xiaobiao Z. Endonasal endoscopic skull base multilayer reconstruction surgery with nasal pedicled mucosal flap to manage high flow CSF leakage. Turk Neurosurg. 2013;23(04):439–445. doi: 10.5137/1019-5149.JTN.6176-12.0. [DOI] [PubMed] [Google Scholar]
- 25.Zanation A M, Carrau R L, Snyderman C H. Nasoseptal flap reconstruction of high flow intraoperative cerebral spinal fluid leaks during endoscopic skull base surgery. Am J Rhinol Allergy. 2009;23(05):518–521. doi: 10.2500/ajra.2009.23.3378. [DOI] [PubMed] [Google Scholar]
- 26.Leng L Z, Brown S, Anand V K, Schwartz T H.“Gasket-seal” watertight closure in minimal-access endoscopic cranial base surgery Neurosurgery 2008620502E342–E343., discussion E343 [DOI] [PubMed] [Google Scholar]
- 27.Garcia-Navarro V, Anand V K, Schwartz T H. Gasket seal closure for extended endonasal endoscopic skull base surgery: efficacy in a large case series. World Neurosurg. 2013;80(05):563–568. doi: 10.1016/j.wneu.2011.08.034. [DOI] [PubMed] [Google Scholar]
- 28.Hu F, Gu Y, Zhang X. Combined use of a gasket seal closure and a vascularized pedicle nasoseptal flap multilayered reconstruction technique for high-flow cerebrospinal fluid leaks after endonasal endoscopic skull base surgery. World Neurosurg. 2015;83(02):181–187. doi: 10.1016/j.wneu.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 29.Ruiz-Juretschke F, Mateo-Sierra O, Iza-Vallejo B, Carrillo-Yagüe R. [Intraventricular tension pneumocephalus after transsphenoidal surgery: a case report and literature review] Neurocirugia (Astur) 2007;18(02):134–137. [PubMed] [Google Scholar]


