Abstract
Complete blood cell counts, including differentials, are widely available and change on aging. Peripheral blood cell counts outside the normal range have previously been associated with increased mortality rates and a number of comorbid conditions. However, data about the association between blood cell count abnormalities, other than anemia, and health-related quality of life (HRQoL) are scarce. We investigated the association between abnormalities in (differential) blood cell counts and HRQoL in 143 191 community-dwelling individuals from the prospective population-based Lifelines cohort. HRQoL was measured using the RAND 36-Item Health Survey. Logistic regression analyses were used to determine the effect of blood cell count abnormalities on the odds of having a lower score than an age- and sex-specific reference value for each domain. Leukocytosis, neutrophilia, and a high neutrophil to lymphocyte ratio were associated with impaired HRQoL across multiple domains, both for younger and older (≥60 years) individuals. Using multivariable models, we confirmed that these associations were independent of the potential confounding factors obesity, smoking, alcohol use, number of medications (as a measure of comorbidity), anemia, and mean corpuscular volume. The impact on HRQoL was most pronounced for high neutrophil levels. Further, high white blood cell counts proved to be a better marker for inferior HRQoL as compared to elevated high-sensitivity C-reactive protein levels. Decreased HRQoL in several domains was also observed for individuals with monocytosis, lymphocytosis, and thrombocytosis. Taken together, the present study demonstrates an association between inflammatory and myeloid-skewed blood cell counts and inferior HRQoL in community-dwelling individuals.
Introduction
A complete blood cell count is one of the most commonly used laboratory tests, and is regarded essential in the diagnostic process of a broad variety of clinical conditions. Most electronic counters provide additional information about differential white blood cell (WBC) counts, including neutrophils, lymphocytes, monocytes, eosinophils, and basophils.1 Hemoglobin concentration and platelet count gradually decrease with advancing age, and laboratory results outside the normal reference range are more common in older individuals. Additionally, the aging hematopoietic system is known for skewing toward the myeloid lineage, whereas lymphocyte subsets gradually decrease with age.2-8
Among the peripheral blood cell count abnormalities, anemia is extensively studied and well known for its association with increased morbidity and mortality. Moreover, older community-dwelling individuals with anemia experience inferior health-related quality of life (HRQoL).9 Multiple studies have reported on the association between other blood cell count abnormalities and mortality, both in the general population and in the context of acute or chronic medical disorders. Increased all-cause mortality rates were detected for increased levels of total WBC count, neutrophils, monocytes, basophils, lower levels of lymphocytes, and higher neutrophil to lymphocyte ratios (NLR).10-15 Platelet counts were associated with mortality in an U-shaped manner.16,17 In contrast, data about the association between blood cell count abnormalities, other than anemia, and HRQoL are scarce.
In this study, we aimed to assess the association between HRQoL and abnormalities in peripheral (differential) blood cell counts in a large population-based cohort.
Materials and methods
Lifelines cohort
This study was performed using data from the Lifelines cohort, a multidisciplinary prospective population-based cohort study examining in a unique 3-generation design the health and health-related behaviors of 167 729 persons living in the north of the Netherlands. It employs a broad range of investigative procedures in assessing the biomedical, sociodemographic, behavioral, physical, and psychological factors which contribute to the health and disease of the general population, with a special focus on multimorbidity and complex genetics. Recruitment of participants was performed between December 2006 and December 2013 using different strategies (invited through general practitioner, via participating family members or self-registered). The study protocol was approved by the medical ethics review committee of the University Medical Center Groningen and conducted in accordance with the Declaration of Helsinki. All participants provided written informed consent. The Lifelines study population is broadly representative for the population in the north of the Netherlands.18-20 For the present study, we included all participants ≥18 years.
Clinical examination, questionnaires, and sample processing
After signed informed consent was received, participants received a baseline questionnaire and an invitation to a comprehensive health assessment at the Lifelines outpatient clinic. All subjects completed a self-administered questionnaire on medical history, past and current diseases, use of medication, and health behavior. Medication use was verified by a certified research assistant, and scored by anatomical therapeutic chemical code. Smoking status was defined as nonsmoker, former smoker, and current smoker.21 Alcohol intake was based on self-report and classified into ordered groups based on daily alcohol intake: 0 drinks/d (nondrinker), ≤1 drink/d (light drinker), >1-2 drinks/d (moderate drinker), and >2 drinks/d (heavy drinker).22 Body weight was measured without shoes to the nearest 0.1 kg. Body mass index (BMI) was calculated by dividing weight in kilograms by the squared height in meters (kg/m2). Peripheral blood samples of participants were drawn after an overnight fast and processed at 4°C under tightly controlled and monitored conditions. For all routine clinical chemistry assays, samples were directly transported to the University Medical Center Groningen.
Definition of blood cell count abnormalities
Total and differential WBC counts, hemoglobin concentration, mean corpuscular volume (MCV), and platelet counts were determined using routine procedures on a XE2100-system (Sysmex, Kobe, Japan). Local laboratory reference intervals were used to define leukopenia (<4.0 × 109/L), leukocytosis (>10.0 × 109/L), thrombocytopenia (<150 × 109/L), thrombocytosis (>400 × 109/L), neutropenia (<1.8 × 109/L), neutrophilia (>7.0 × 109/L), lymphopenia (<0.8 × 109/L), lymphocytosis (>3.2 × 109/L), monocytopenia (<0.3 × 109/L), monocytosis (>0.9 × 109/L), basophilia (>0.2 × 109/L), and eosinophilia (>0.4 × 109/L). The NLR was calculated by dividing the absolute neutrophil count by the lymphocyte count. Anemia was defined according to World Health Organization criteria: hemoglobin concentration <13.0 g/dL in men and <12.0 g/dL in women. High-sensitivity C-reactive protein (hs-CRP) was initially measured with CardioPhase hsCRP (Siemens, BNII, Marburg, Germany) and after 2012 on a Roche Modular P chemistry analyzer (Roche, Basel, Switzerland). Elevated hs-CRP was defined as >10 mg/L.
Health-related quality of life
HRQoL was assessed using the RAND 36-Item Health Survey, which is a commonly used generic questionnaire consisting of 36 questions related to own health status covering 8 domains.23 A weighted scoring and summation of selected items results in a score between 0 and 100 for each domain, with a higher score reflecting a better HRQoL. Since HRQoL scores are not normally distributed, we defined a sex- and age-specific cut-off value at the 25th percentile for each domain. Participants with a score lower than this value were considered to have an abnormally low score for that respective domain, as described previously.9,24,25
Statistical analysis
Data are presented as mean ± standard deviation, median (interquartile range) or percentage. Between-group differences were evaluated using 1-way ANOVA, Kruskal-Wallis test, or χ2 test, as appropriate. Logistic regression was used to determine the effect of blood cell count abnormalities on the odds of having a lower score than the (age- and sex-specific) value for each HRQoL domain. To account for the effect of health behavior, comorbid conditions, and different types of anemia on HRQoL, multivariable models were adjusted for smoking status,26 alcohol consumption,27 BMI,24number of medications used28 (as a general measure of comorbidity), presence of anemia and MCV.9 Additional multivariable models were constructed to include hs-CRP, when available. Odds ratios are reported with 95% confidence interval. For all analyses, the normal range for the respective blood cell count was used as a reference. For the NLR, individuals in the lowest (1st) and highest (5th) quintile were compared with individuals in the 2nd to 4th quintiles. In subgroup analyses, individuals <60 and ≥60 years were evaluated separately. Statistical analyses were performed using IBM SPSS software, version 23.0 (SPSS Inc., Chicago, IL). A P value <0.05 was considered statistically significant.
Results
Relevant baseline characteristics for the cohort with available total WBC and platelet count (n = 143 191) and the cohort with complete differential WBC count (n = 140 890) are shown in Table 1. The overall prevalence of blood cell count abnormalities is shown in Figure 1. The prevalence of monocytopenia was the highest (6.9%), whereas basophilia was very rarely detected (0.009%) (Figure 1). The distribution of the blood cell counts and relevant baseline characteristics for individuals with and without blood cell count abnormalities are shown in Supplementary Figures S1, S2, and Table S1, http://links.lww.com/HS/A120.
Table 1.
Baseline Characteristics for Participants With Peripheral Blood Cell Counts Available.
| Available Platelet and Total WBC Count (n = 143 191) | Available Complete Differential WBC Count (n = 140 890) | |
|---|---|---|
| Male sex (%) | 41.6 | 41.5 |
| Age (y) | 44.4 ± 12.8 | 44.4 ± 12.7 |
| BMI (kg/m2) | 26.0 ± 4.3 | 26.0 ± 4.3 |
| Smoking | ||
| Current (%) | 20.9 | 20.5 |
| Former (%) | 32.9 | 33.0 |
| Never (%) | 46.2 | 46.5 |
| Number of medications used | 1 (0–2) | 1 (0–2) |
| Alcohol use | ||
| Nondrinker (%) | 21.4 | 21.4 |
| Light drinker (%) | 50.1 | 50.2 |
| Moderate drinker (%) | 20.4 | 20.4 |
| Heavy drinker (%) | 8.1 | 8.1 |
| Anemia (%) | 4.1 | 4.1 |
| MCV (fL) | 89.9 ± 4.2 | 89.8 ± 4.2 |
| Recruitment | ||
| General practitioner (%) | 53.8 | 53.7 |
| Family (%) | 32.1 | 32.1 |
| Self-registered (%) | 14.2 | 14.2 |
| WBC (109/L) | 6.1 ± 1.8 | |
| Platelets (109/L) | 250 ± 57 | |
| Neutrophils (109/L) | 3.32 ± 1.23 | |
| Lymphocytes (109/L) | 2.02 ± 0.59 | |
| Monocytes (109/L) | 0.48 ± 0.15 | |
| Basophils (109/L) | 0.03 ± 0.02 | |
| Eosinophils (109/L) | 0.18 ± 0.13 | |
| NLR | 1.75 ± 0.78 |
Data are given as mean ± SD, median (IQR) when not normally distributed, or percentage.
BMI = body mass index; IQR = interquartile range; MCV = mean corpuscular volume; NLR = neutrophil to lymphocyte ratio; SD = standard deviation; WBC = white blood cell.
Figure 1.
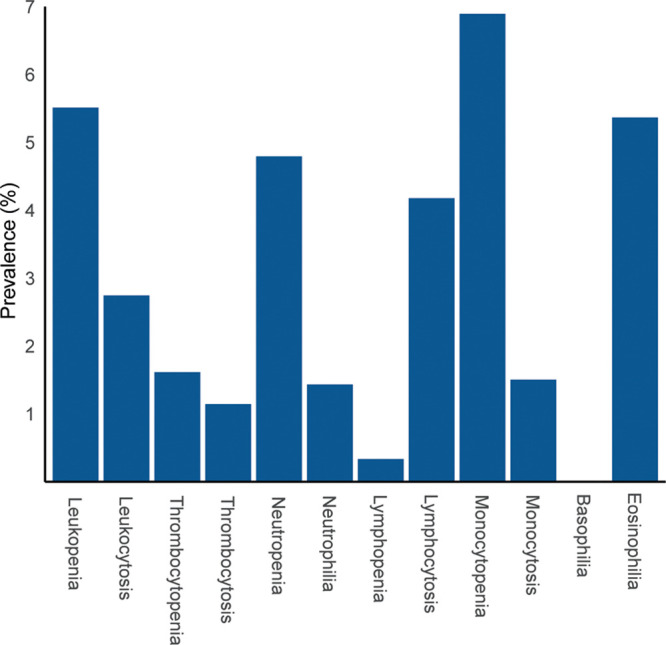
Prevalence of blood cell count abnormalities among evaluable individuals. The definitions of blood cell count abnormalities were based on local laboratory reference intervals, as indicated in the methods section.
Effect of abnormal WBC and platelet counts on HRQoL
First, we evaluated whether abnormalities in total WBC and platelet counts were associated with HRQoL at younger and older age. A significantly larger proportion of individuals with leukocytosis had a score below the age- and sex-specific value, for all (<60 years) or 6 out of 8 (≥60 years) domains (all except mental health and bodily pain), as compared to individuals with a WBC in the normal range. In contrast, individuals with leukopenia were less likely to have a score below the age- and sex-specific value in 7 (<60 years, all except role limitations due to physical functioning) or 3 (≥60 years, physical functioning, vitality, and general health) domains (Figure 2A, B). For individuals <60 years with thrombocytopenia, HRQoL was slightly increased in 2 domains, whereas HRQoL was reduced for individuals with thrombocytosis in 6 (<60 years, all except social functioning and mental health) or 3 (≥60 years, role limitations due to physical functioning, bodily pain, and general health) domains (Figure 2C, D). Being a possible explanation for reactive elevated platelet counts, concurrent anemia was observed in 300 of 1640 (18.3%) individuals with thrombocytosis. Absolute scores per domain for the different subgroups are given in Supplementary Table S2, http://links.lww.com/HS/A120.
Figure 2.
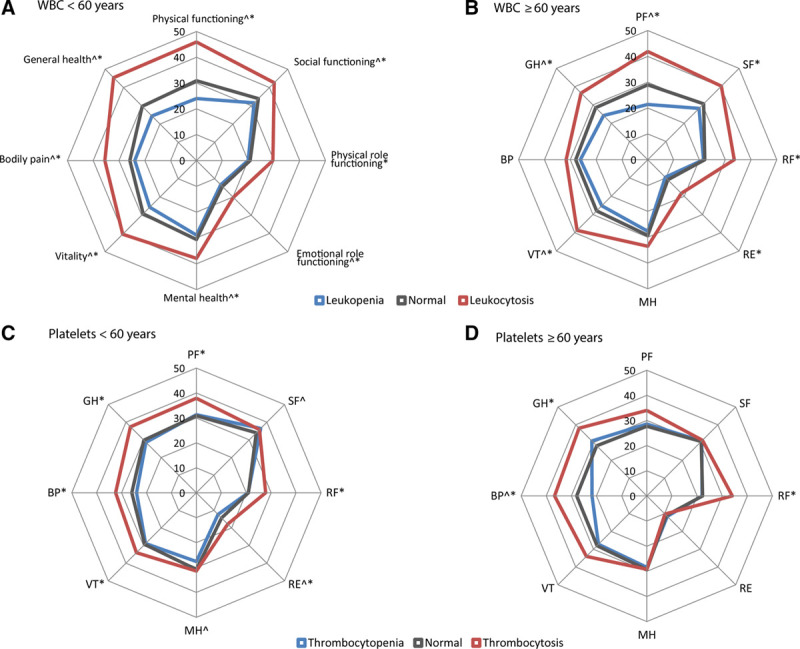
Percentage of individuals, stratified according to age group and blood cell count abnormality, with a score below the age- and sex-specific cut-off values for the different domains of the RAND-36 health survey. ^A significant difference in the proportion having a score below the age- and sex-specific cut-off value in individuals with leukopenia or thrombocytopenia as compared to individuals with a normal blood cell count. *A significant difference in the proportion having a score below the age- and sex-specific cut-off value in individuals with leukocytosis or thrombocytosis as compared to individuals with a normal blood cell count. WBC = white blood cell.
Multivariable logistic regression was performed to adjust the observed associations for BMI, smoking status, alcohol use, number of medications used, presence of anemia, and MCV (Figure 3, Supplementary Figure 3, http://links.lww.com/HS/A120). Leukocytosis remained strongly associated with a decreased HRQoL across all 8 domains, independent of these potential confounders. For leukopenia, an increase in HRQoL was observed for 2 domains (physical functioning and general health; Figure 3A). Thrombocytopenia remained independently associated with an increased HRQoL in 3 domains (role limitations due to emotional functioning, mental health, and bodily pain) in the multivariable model. For thrombocytosis, the association with decreased HRQoL was sustained for 2 domains (role limitations due to physical functioning and general health; Figure 3B).
Figure 3.
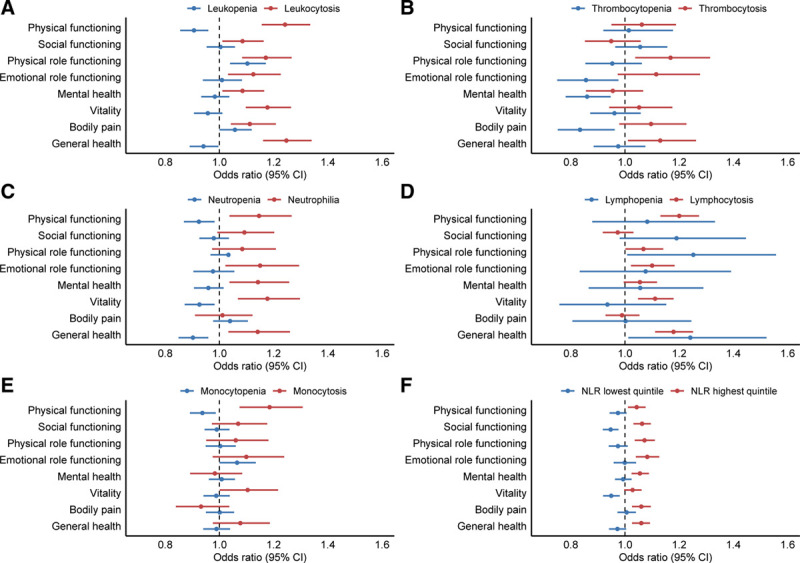
Forest plots demonstrating the odds ratios for having a lower score than the (age- and sex-specific) 25th percentile cut-off per HRQoL subscale, according to blood cell count abnormality. Logistic regression analyses included body mass index, smoking status, alcohol use, number of medications, presence of anemia and mean corpuscular volume as covariates. A blood cell count in the normal range was used as the reference group. For the NLR, the 2nd to 4th quintile was used as a reference. Circles indicate odds ratios for each cohort, with horizontal lines corresponding to 95% CI. CI = confidence interval; HRQoL = health-related quality of life; NLR = neutrophil to lymphocyte ratio.
Effect of abnormal differential WBC counts on HRQoL
Next, we evaluated the associations between the differential WBC count and HRQoL. Consistent with the associations observed for leukocytosis, neutrophilia (all 8 domains), lymphocytosis (all 8 domains), and monocytosis (all 8 domains) were all significantly associated with decreased HRQoL (Figure 4A–C). A significantly increased HRQoL was observed for individuals with neutropenia (all 8 domains) and monocytopenia (7 domains, all except role limitations due to emotional functioning) (Figure 4A, C). For individuals with lymphopenia, significantly decreased HRQoL was observed for 3 domains (social functioning, role limitations due to physical functioning, and general health; Figure 4B). Basophilia, hampered by low numbers, was not associated with HRQoL (Figure 4D). Individuals with eosinophilia had a slightly lower HRQoL in 5 domains (physical functioning, social functioning, role limitations due to physical functioning, vitality, and general health; Figure 4E). No major differences were observed when evaluating individuals <60 and ≥60 years separately (Supplementary Figure 4, http://links.lww.com/HS/A120). Absolute scores per domain for the different subgroups are given in Supplementary Table S2, http://links.lww.com/HS/A120.
Figure 4.
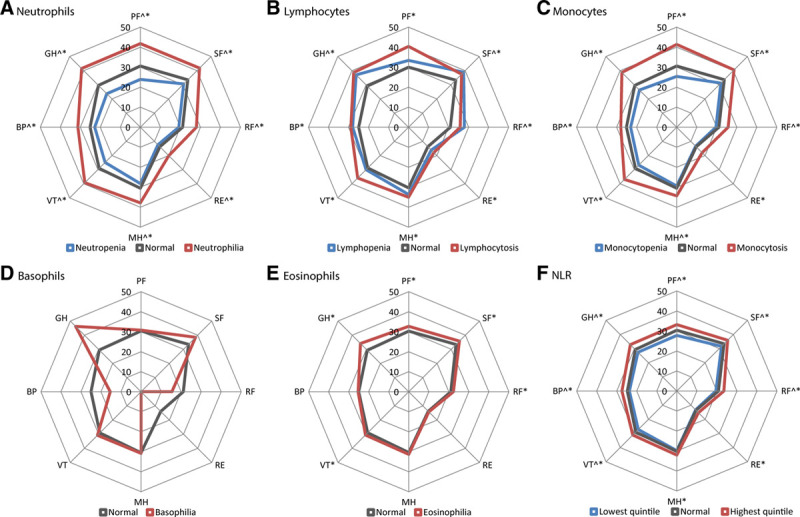
Percentage of individuals with a score below the age- and sex-specific cut-off value for the different domains of the RAND-36 health survey, according to differential blood cell count abnormality. ^A significant difference in the proportion having a score below the age- and sex-specific cut-off value in individuals with neutropenia, lymphopenia, monocytopenia, or lowest NLR quintile, as compared to individuals with a normal blood cell count. *A significant difference in the proportion having a score below the age- and sex-specific cut-off value in individuals with neutrophilia, lymphocytosis, monocytosis, basophilia, eosinophilia, or highest NLR quintile, as compared to individuals with a normal blood cell count. BP = bodily pain; GH = general health; MH = mental health; NLR = neutrophil to lymphocyte ratio; PF = physical functioning; RE = emotional role functioning; RF = physical role functioning; SF = social functioning; VT = vitality.
The reported associations were again evaluated in a multivariable logistic regression model, with adjustment for BMI, smoking status, alcohol use, number of medications used, presence of anemia, and MCV (Figure 3; Supplementary Figure S3, http://links.lww.com/HS/A120). Neutrophilia and lymphocytosis independently associated with a decreased HRQoL in 5 (all except social functioning, role limitations due to physical functioning and bodily pain), respectively 6 (all except social functioning and bodily pain) domains (Figure 3C, D). For individuals with monocytosis, HRQoL was affected in 2 domains (physical functioning and vitality) in the multivariable model (Figure 3E). The associations between neutropenia and improved HRQoL scores were retained for 3 domains (physical functioning, vitality, and general health; Figure 3C). For lymphopenia, the association with inferior HRQoL was retained for 2 domains (role limitations due to physical functioning and general health; Figure 3D). After adjustment for confounders, monocytopenia was significantly associated with increased HRQoL in 1 domain (physical functioning; Figure 3E).
When evaluating the differential WBC count, the NLR may be used as a measure for the relative myeloid contribution (“myeloid skewing”). A high NLR (represented by the highest quintile) was significantly associated with a lower HRQoL in all 8 domains (Figure 4F), and this effect was observed for both younger (<60 years) and older individuals (≥60 years; Supplementary Figure S4, http://links.lww.com/HS/A120). In multivariable logistic regression analyses, we were able to confirm this association between high NLR and inferior HRQoL in 7 domains (all except vitality; Figure 3F).
Peripheral blood cell counts and hs-CRP as a marker for systemic inflammation and effect on HRQoL
Clearly, the effects on HRQoL are most pronounced for individuals with increased inflammatory blood cell counts. We questioned whether these associations were independent of hs-CRP levels, being another marker of systemic inflammation. Hs-CRP levels were available for a subset of individuals with evaluable total and differential WBC counts (n = 55 342 and n = 54 383, respectively). Multivariable models were constructed with additional adjustment for hs-CRP, demonstrating that the association between leukocytosis and HRQoL was independent of hs-CRP in 4 domains (physical functioning, role limitations due to physical functioning, vitality, and general health). For the NLR, the association was independent of hs-CRP in 6 domains (all except role limitations due to physical functioning and vitality; Table 2).
Table 2.
Univariable and Multivariable Logistic Regression Analyses Assessing the Association Between Increased Inflammatory Blood Cell Counts and Health-Related Quality of Life Independent of hs-CRP Levels.
| Univariable | Multivariable (Model 1) | Multivariable (Model 2) | ||||
|---|---|---|---|---|---|---|
| Odds Ratio (95% CI) | P Value | Odds Ratio (95% CI) | P Value | Odds Ratio (95% CI) | P Value | |
| Leukocytosis | ||||||
| Physical functioning | 1.91 (1.79-2.03) | <0.001 | 1.24 (1.16-1.34) | <0.001 | 1.15 (1.02-1.26) | 0.024 |
| Social functioning | 1.46 (1.37-1.56) | <0.001 | 1.09 (1.01-1.16) | 0.023 | 1.07 (0.96-1.21) | 0.24 |
| Physical role functioning | 1.61 (1.50-1.72) | <0.001 | 1.17 (1.08-1.27) | <0.001 | 1.17 (1.03-1.33) | 0.014 |
| Emotional role functioning | 1.57 (1.45-1.70) | <0.001 | 1.13 (1.03-1.23) | 0.008 | 1.13 (0.98-1.30) | 0.087 |
| Mental health | 1.37 (1.29-1.47) | <0.001 | 1.09 (1.01-1.17) | 0.024 | 1.07 (0.95-1.20) | 0.27 |
| Vitality | 1.67 (1.54-1.76) | <0.001 | 1.18 (1.10-1.26) | <0.001 | 1.18 (1.05-1.32) | 0.007 |
| Bodily pain | 1.54 (1.44-1.64) | <0.001 | 1.11 (1.04-1.21) | 0.002 | 1.08 (0.91-1.10) | 0.21 |
| General health | 1.92 (1.80-2.05) | <0.001 | 1.25 (1.16-1.34) | <0.001 | 1.21 (1.08-1.36) | 0.002 |
| NLR highest quintile | ||||||
| Physical functioning | 1.14 (1.11-1.18) | <0.001 | 1.04 (1.01-1.08) | 0.009 | 1.06 (1.00-1.11) | 0.043 |
| Social functioning | 1.12 (1.09-1.15) | <0.001 | 1.06 (1.03-1.10) | <0.001 | 1.10 (1.05-1.16) | <0.001 |
| Physical role functioning | 1.17 (1.13-1.21) | <0.001 | 1.07 (1.04-1.10) | <0.001 | 1.04 (0.98-1.10) | 0.17 |
| Emotional role functioning | 1.15 (1.11-1.20) | <0.001 | 1.08 (1.04-1.13) | <0.001 | 1.08 (1.00-1.15) | 0.033 |
| Mental health | 1.09 (1.06-1.12) | <0.001 | 1.06 (1.02-1.09) | 0.001 | 1.07 (1.01-1.12) | 0.016 |
| Vitality | 1.10 (1.07-1.14) | <0.001 | 1.03 (1.00-1.06) | 0.078 | 1.04 (0.99-1.10) | 0.14 |
| Bodily pain | 1.15 (1.12-1.19) | <0.001 | 1.06 (1.03-1.10) | <0.001 | 1.09 (1.03-1.15) | 0.002 |
| General health | 1.16 (1.13-1.19) | <0.001 | 1.06 (1.03-1.09) | <0.001 | 1.06 (1.01-1.12) | 0.025 |
Logistic regression analyses included body mass index, smoking status, alcohol use, number of medications used, presence of anemia, and mean corpuscular volume as covariates (model 1). Model 2 was additionally adjusted for hs-CRP, when available. A WBC count in the normal range and a NLR in the 2nd to 4th quintile were used as reference group.
CI = confidence interval; hs-CRP = high-sensitivity C-reactive protein; NLR = neutrophil to lymphocyte ratio; WBC = white blood cell.
Since leukocytosis and hs-CRP appear to be independent markers of decreased HRQoL, we assessed whether there was a differential effect on HRQoL for individuals with one or both markers of inflammation. Individuals were categorized based on elevated (differential) blood cell count and/or elevated hs-CRP (>10 mg/L). As shown in Table 3, leukocytosis and elevated hs-CRP associated with inferior HRQoL as compared to normal counts for both markers. However, this effect was more pronounced for individuals with leukocytosis and normal hs-CRP as compared to individuals with elevated hs-CRP and normal WBC count in 5 domains (social functioning, role limitations due to emotional functioning, mental health, vitality, and general health). When the effects of elevated hs-CRP and high NLR were evaluated, both associated with inferior HRQoL, although the observed associations with inferior HRQoL were stronger for elevated hs-CRP for 5 domains (physical functioning, role limitations due to physical functioning, vitality, bodily pain, and general health). Overall, individuals with a combination of both inflammatory markers (leukocytosis or NLR in the highest quintile combined with elevated hs-CRP) did not experience different HRQoL as compared to individuals having one of either markers of inflammation.
Table 3.
Percentage of Individuals With a Score Below the Sex- and Age-Specific Cut-Off Values for the Different Domains of the RAND-36 Health Survey According to the Presence of Elevated Inflammatory Blood Cell Counts and/or High hs-CRP Levels.
| PF | P Value | SF | P Value | RF | P Value | RE | P Value | MH | P Value | VT | P Value | BP | P Value | GH | P Value | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WBC | 0.43 | 0.003 | 0.07 | <0.001 | <0.001 | 0.002 | 0.94 | 0.008 | ||||||||
| Normal WBC and hs-CRP (n = 49 015) | 31.1 | 33.7 | 21.3 | 14.0 | 30.6 | 30.1 | 26.1 | 29.6 | ||||||||
| Normal WBC and high hs-CRP (n = 1705) | 42.9 | 36.9 | 27.6 | 16.0 | 30.6 | 36.8 | 34.7 | 40.1 | ||||||||
| High WBC and normal hs-CRP (n = 1300) | 44.3 | 42.3 | 30.7 | 21.9 | 37.6 | 42.3 | 34.8 | 44.8 | ||||||||
| High WBC and hs-CRP (n = 300) | 48.7 | 40.0 | 31.0 | 16.0 | 35.7 | 39.0 | 34.3 | 46.7 | ||||||||
| NLR | <0.001 | 0.50 | 0.004 | 0.21 | 0.95 | 0.001 | 0.005 | <0.001 | ||||||||
| Normal NLR and hs-CRP (n = 32 094) | 30.9 | 33.4 | 21.1 | 13.7 | 30.1 | 30.0 | 24.7 | 29.5 | ||||||||
| Normal NLR and high hs-CRP (n = 901) | 43.1 | 37.4 | 27.4 | 17.1 | 32.1 | 37.8 | 32.0 | 40.6 | ||||||||
| High NLR and normal hs-CRP (n = 9862) | 33.7 | 36.3 | 23.1 | 15.5 | 32.2 | 32.2 | 27.6 | 32.5 | ||||||||
| High NLR and hs-CRP (n = 885) | 42.5 | 36.9 | 27.6 | 15.6 | 30.2 | 36.0 | 33.3 | 39.8 |
P values are given to compare individuals with elevated inflammatory blood cell counts and normal hs-CRP with individuals having normal blood cell counts and elevated hs-CRP (>10 mg/L).
BP = bodily pain; GH = general health; hs-CRP = high-sensitivity C-reactive protein; MH = mental health; NLR = neutrophil to lymphocyte ratio; PF = physical functioning; RE = emotional role functioning; RF = physical role functioning; SF = social functioning; VT = vitality; WBC = white blood cell.
Discussion
This is the first comprehensive study to assess the association between (differential) blood cell count abnormalities and HRQoL in community-dwelling individuals. In general, our data reveal that elevated platelet and WBC counts were associated with decreased HRQoL in multiple domains. This phenomenon was most pronounced for high neutrophil levels, being the most abundant leukocyte and myeloid cell population. Notably, these associations were observed for both younger and older individuals and were independent of the potential confounding factors obesity, smoking, alcohol use, comorbidity, presence of anemia, and MCV. Overall, the observed absolute differences in HRQoL domain scores reached the 3- to 5-point difference that was previously suggested as the minimal clinically important difference.29
There is increasing evidence for low-grade inflammation being a common pathway underlying multiple conditions, including cardiovascular disease, cancer, diabetes, and various other health outcomes.30 In multiple previous population-based studies, proinflammatory cytokines (including IL-6 and TNF-α),31-36 acute-phase proteins (CRP and fibrinogen),34,35,37-42 and erythrocyte sedimentation rate43 were associated with decreased HRQoL or subjectively perceived health. Together with cytokines and acute-phase proteins, leukocytes are main components of the immune response in reaction to acute injury or illness. However, data about a possible link between cellular markers of inflammation (ie, leukocytosis, lymphocytosis, elevated NLR) and HRQoL are scarce. Jylhä et al44 demonstrated that individuals with a WBC >6.8 × 109/L experienced a lower self-rated health than individuals with a WBC ≤4.8 × 109/L in a sample of 4065 subjects ≥71 years. Furthermore, several studies demonstrated a negative association between WBC and well-being.45,46 Our results are in agreement with these findings, demonstrating a clear link between leukocytosis and inferior HRQoL. In addition, we show that leukocytosis may be a better predictor of inferior HRQoL than hs-CRP.
When evaluating the differential WBC count, the effect on HRQoL was most pronounced for neutrophils. Although traditionally considered the main actors in acute inflammatory responses, there is emerging evidence for the direct contribution of neutrophils to chronic inflammatory processes.47 In addition, remodeling of the hematopoietic system in response to inflammatory signaling results in overproduction of myeloid cells.48 Indeed, reduced HRQoL was observed for individuals with higher NLR levels, reflecting relative myeloid predominance. Thus, myeloid-skewed leukocyte counts may represent a state of chronic inflammation that is associated with HRQoL. The relation between thrombocytosis and HRQoL may have different explanations. Although anemia of iron deficiency may be underlying in a subset of individuals and was previously associated with reduced HRQoL,9 this was corrected for in multivariable analysis. Alternatively, reactive thrombocytosis may represent another phenotypic manifestation of chronic inflammation.48
A chronic state of low-grade inflammation is increasingly recognized as a hallmark of aging. So-called “inflammaging” is used to describe the age-related increase in circulating markers of inflammation, including proinflammatory cytokines.4,49 In contrast to anemia,9 the associations between inflammatory blood cell counts and HRQoL were observed both for younger and older (≥60 years) individuals. Although inflammatory markers, including skewing toward the myeloid lineage,4 increase with age, the impact of inflammatory blood cell counts on HRQoL is relevant for all ages.
Multivariable analyses were corrected for comorbidities, suggesting that the association between inflammatory blood cell counts and HRQoL is not merely a reflection of recognized underlying chronic diseases. However, we cannot exclude that underlying conditions are mediating the observed associations between inflammation and HRQoL. On the other hand, inflammation may serve as a proxy for a general state of accelerated aging that includes morbidity, frailty, and otherwise unrelated adverse health outcomes, including lower HRQoL.
It should be noted that inflammatory blood cell counts may not only be a cause of impaired HRQoL but also a consequence. Chronic (physical and/or mental) stress may induce proliferation of primitive hematopoietic progenitors, giving rise to an increase in inflammatory leukocytes, including higher numbers of neutrophils, monocytes, and lymphocytes.50
This study has some strengths and limitations. This is the first study to investigate the possible effect of (differential) blood cell count abnormalities on HRQoL. Additionally, we used information on HRQoL from a large number of participants from the general population with a wide range of age, socioeconomic status, and comorbidities. All subjects were uniformly characterized and were not aware of their blood cell count status when filling out the HRQoL questionnaire. The associations between inflammatory blood cell counts and inferior HRQoL were observed across all domains, further strengthening the observed effects. Several potential limitations should also be acknowledged. First, although the Lifelines cohort is representative of the general population in the North of the Netherlands,20 this is an ethnically homogeneous population, limiting wider applicability of the results. Since this is a cross-sectional study, the analyses do not provide information about causality. Furthermore, due to the observational nature of the study, it was not possible to exclude the role of unknown or unmeasured confounding variables. Although there is a heterogeneous collection of medical disorders which affect peripheral blood counts, we were not able to ascertain the potential underlying cause of abnormalities. Finally, we used only a single blood cell count for the analyses. As peripheral blood cell counts may fluctuate over time, multiple measurements over time may provide more accurate information.
In conclusion, the present study demonstrates an association between elevated and myeloid-skewed inflammatory blood cell counts and inferior HRQoL in community-dwelling individuals. Peripheral blood cell counts are easy to obtain and inexpensive, thereby representing an interesting alternative marker for inferior health outcomes associated with chronic inflammation, including quality of life.
Sources of funding
The Lifelines Biobank initiative has been made possible by subsidy from the Dutch Ministry of Health, Welfare and Sport, the Dutch Ministry of Economic Affairs, the University Medical Center Groningen (UMCG the Netherlands), University Groningen and the Northern Provinces of the Netherlands.
Data sharing statement
The article is based on data from the Lifelines Cohort Study. Lifelines adheres to standards for data availability. The data catalogue of Lifelines is publicly accessible at www.lifelines.nl. All international researchers can obtain data at the Lifelines research office (research@lifelines.nl), for which a fee is required.
Acknowledgments
The authors acknowledge the services of the Lifelines Cohort Study, the contributing research centers delivering data to Lifelines, and all the study participants.
Disclosures
The authors declare no competing interest.
Supplementary Material
Footnotes
HJCMW and IAvZ have contributed equally to this study.
Supplemental digital content is available for this article.
References
- 1.Tefferi A, Hanson CA, Inwards DJ. How to interpret and pursue an abnormal complete blood cell count in adults. Mayo Clin Proc. 2005; 80:923–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Segal JB, Moliterno AR. Platelet counts differ by sex, ethnicity, and age in the United States. Ann Epidemiol. 2006; 16:123–130. [DOI] [PubMed] [Google Scholar]
- 3.Hsieh MM, Everhart JE, Byrd-Holt DD, et al. Prevalence of neutropenia in the U.S. population: age, sex, smoking status, and ethnic differences. Ann Intern Med. 2007; 146:486–492. [DOI] [PubMed] [Google Scholar]
- 4.Geiger H, de Haan G, Florian MC. The ageing haematopoietic stem cell compartment. Nat Rev Immunol. 2013; 13:376–389. [DOI] [PubMed] [Google Scholar]
- 5.Santimone I, Di Castelnuovo A, De Curtis A, et al. White blood cell count, sex and age are major determinants of heterogeneity of platelet indices in an adult general population: results from the MOLI-SANI project. Haematologica. 2011; 96:1180–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Biino G, Santimone I, Minelli C, et al. Age- and sex-related variations in platelet count in Italy: a proposal of reference ranges based on 40987 subjects’ data. PLoS One. 2013; 8:e54289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adeli K, Raizman JE, Chen Y, et al. Complex biological profile of hematologic markers across pediatric, adult, and geriatric ages: establishment of robust pediatric and adult reference intervals on the basis of the Canadian Health Measures Survey. Clin Chem. 2015; 61:1075–1086. [DOI] [PubMed] [Google Scholar]
- 8.Nah EH, Kim S, Cho S, et al. Complete blood count reference intervals and patterns of changes across pediatric, adult, and geriatric ages in Korea. Ann Lab Med. 2018; 38:503–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wouters HJCM, van der Klauw MM, de Witte T, et al. Association of anemia with health-related quality of life and survival: a large population-based cohort study. Haematologica. 2019; 104:468–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ruggiero C, Metter EJ, Cherubini A, et al. White blood cell count and mortality in the Baltimore Longitudinal Study of Aging. J Am Coll Cardiol. 2007; 49:1841–1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kabat GC, Kim MY, Manson JE, et al. White blood cell count and total and cause-specific mortality in the Women’s Health Initiative. Am J Epidemiol. 2017; 186:63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Welsh C, Welsh P, Mark PB, et al. Association of total and differential leukocyte counts with cardiovascular disease and mortality in the UK Biobank. Arterioscler Thromb Vasc Biol. 2018; 38:1415–1423. [DOI] [PubMed] [Google Scholar]
- 13.Horne BD, Anderson JL, John JM, et al. Which white blood cell subtypes predict increased cardiovascular risk? J Am Coll Cardiol. 2005; 45:1638–1643. [DOI] [PubMed] [Google Scholar]
- 14.Templeton AJ, McNamara MG, Šeruga B, et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst. 2014; 106:dju124. [DOI] [PubMed] [Google Scholar]
- 15.Fest J, Ruiter TR, Groot Koerkamp B, et al. The neutrophil-to-lymphocyte ratio is associated with mortality in the general population: the Rotterdam Study. Eur J Epidemiol. 2019; 34:463–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsai MT, Chen YT, Lin CH, et al. U-shaped mortality curve associated with platelet count among older people: a community-based cohort study. Blood. 2015; 126:1633–1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vinholt PJ, Hvas AM, Frederiksen H, et al. Platelet count is associated with cardiovascular disease, cancer and mortality: a population-based cohort study. Thromb Res. 2016; 148:136–142. [DOI] [PubMed] [Google Scholar]
- 18.Scholtens S, Smidt N, Swertz MA, et al. Cohort Profile: LifeLines, a three-generation cohort study and biobank. Int J Epidemiol. 2015; 44:1172–1180. [DOI] [PubMed] [Google Scholar]
- 19.Stolk RP, Rosmalen JG, Postma DS, et al. Universal risk factors for multifactorial diseases: LifeLines: a three-generation population-based study. Eur J Epidemiol. 2008; 23:67–74. [DOI] [PubMed] [Google Scholar]
- 20.Klijs B, Scholtens S, Mandemakers JJ, et al. Representativeness of the LifeLines cohort study. PLoS One. 2015; 10:e0137203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Slagter SN, van Vliet-Ostaptchouk JV, Vonk JM, et al. Associations between smoking, components of metabolic syndrome and lipoprotein particle size. BMC Med. 2013; 11:195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Slagter SN, van Vliet-Ostaptchouk JV, Vonk JM, et al. Combined effects of smoking and alcohol on metabolic syndrome: the LifeLines cohort study. PLoS One. 2014; 9:e96406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.VanderZee KI, Sanderman R, Heyink JW, et al. Psychometric qualities of the RAND 36-Item Health Survey 1.0: a multidimensional measure of general health status. Int J Behav Med. 1996; 3:104–122. [DOI] [PubMed] [Google Scholar]
- 24.Slagter SN, van Vliet-Ostaptchouk JV, van Beek AP, et al. Health-related quality of life in relation to obesity grade, type 2 diabetes, metabolic syndrome and inflammation. PLoS One. 2015; 10:e0140599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wouters HJ, van Loon HC, van der Klauw MM, et al. No effect of the Thr92Ala polymorphism of deiodinase-2 on thyroid hormone parameters, health-related quality of life, and cognitive functioning in a large population-based cohort study. Thyroid. 2017; 27:147–155. [DOI] [PubMed] [Google Scholar]
- 26.Coste J, Quinquis L, D’Almeida S, et al. Smoking and health-related quality of life in the general population. Independent relationships and large differences according to patterns and quantity of smoking and to gender. PLoS One. 2014; 9:e91562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Salonsalmi A, Rahkonen O, Lahelma E, et al. The association between alcohol drinking and self-reported mental and physical functioning: a prospective cohort study among City of Helsinki employees. BMJ Open. 2017; 7:e014368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garin N, Olaya B, Moneta MV, et al. Impact of multimorbidity on disability and quality of life in the Spanish older population. PLoS One. 2014; 9:e111498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Samsa G, Edelman D, Rothman ML, et al. Determining clinically important differences in health status measures: a general approach with illustration to the Health Utilities Index Mark II. Pharmacoeconomics. 1999; 15:141–155. [DOI] [PubMed] [Google Scholar]
- 30.Furman D, Campisi J, Verdin E, et al. Chronic inflammation in the etiology of disease across the life span. Nat Med. 2019; 25:1822–1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cohen HJ, Pieper CF, Harris T, et al. The association of plasma IL-6 levels with functional disability in community-dwelling elderly. J Gerontol A Biol Sci Med Sci. 1997; 52:M201–M208. [DOI] [PubMed] [Google Scholar]
- 32.Lekander M, Elofsson S, Neve IM, et al. Self-rated health is related to levels of circulating cytokines. Psychosom Med. 2004; 66:559–563. [DOI] [PubMed] [Google Scholar]
- 33.Undén AL, Andréasson A, Elofsson S, et al. Inflammatory cytokines, behaviour and age as determinants of self-rated health in women. Clin Sci (Lond). 2007; 112:363–373. [DOI] [PubMed] [Google Scholar]
- 34.Steptoe A, O’Donnell K, Badrick E, et al. Neuroendocrine and inflammatory factors associated with positive affect in healthy men and women: the Whitehall II study. Am J Epidemiol. 2008; 167:96–102. [DOI] [PubMed] [Google Scholar]
- 35.Christian LM, Glaser R, Porter K, et al. Poorer self-rated health is associated with elevated inflammatory markers among older adults. Psychoneuroendocrinology. 2011; 36:1495–1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Andreasson AN, Szulkin R, Undén AL, et al. Inflammation and positive affect are associated with subjective health in women of the general population. J Health Psychol. 2013; 18:311–320. [DOI] [PubMed] [Google Scholar]
- 37.Fielding R, Lam TH, Ho SY, et al. Subjective health and fibrinogen in a healthy Chinese cohort. Br J Health Psychol. 2004; 9(Pt 4):523–532. [DOI] [PubMed] [Google Scholar]
- 38.Tanno K, Ohsawa M, Onoda T, et al. Poor self-rated health is significantly associated with elevated C-reactive protein levels in women, but not in men, in the Japanese general population. J Psychosom Res. 2012; 73:225–231. [DOI] [PubMed] [Google Scholar]
- 39.Nowakowski AC. Chronic inflammation and quality of life in older adults: a cross-sectional study using biomarkers to predict emotional and relational outcomes. Health Qual Life Outcomes. 2014; 12:141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shanahan L, Bauldry S, Freeman J, et al. Self-rated health and C-reactive protein in young adults. Brain Behav Immun. 2014; 36:139–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.de Almeida Roediger M, de Fátima Nunes Marucci M, Duim EL, et al. Inflammation and quality of life in later life: findings from the health, well-being and aging study (SABE). Health Qual Life Outcomes. 2019; 17:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dinh KM, Kaspersen KA, Mikkelsen S, et al. Low-grade inflammation is negatively associated with physical Health-Related Quality of Life in healthy individuals: results from The Danish Blood Donor Study (DBDS). PLoS One. 2019; 14:e0214468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Warnoff C, Lekander M, Hemmingsson T, et al. Is poor self-rated health associated with low-grade inflammation in 43,110 late adolescent men of the general population? A cross-sectional study. BMJ Open. 2016; 6:e009440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jylhä M, Volpato S, Guralnik JM. Self-rated health showed a graded association with frequently used biomarkers in a large population sample. J Clin Epidemiol. 2006; 59:465–471. [DOI] [PubMed] [Google Scholar]
- 45.Fancourt D, Steptoe A. The longitudinal relationship between changes in wellbeing and inflammatory markers: are associations independent of depression? Brain Behav Immun. 2020; 83:146–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Steptoe A, Fancourt D. Leading a meaningful life at older ages and its relationship with social engagement, prosperity, health, biology, and time use. Proc Natl Acad Sci U S A. 2019; 116:1207–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Soehnlein O, Steffens S, Hidalgo A, et al. Neutrophils as protagonists and targets in chronic inflammation. Nat Rev Immunol. 2017; 17:248–261. [DOI] [PubMed] [Google Scholar]
- 48.Pietras EM. Inflammation: a key regulator of hematopoietic stem cell fate in health and disease. Blood. 2017; 130:1693–1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ferrucci L, Fabbri E. Inflammageing: chronic inflammation in ageing, cardiovascular disease, and frailty. Nat Rev Cardiol. 2018; 15:505–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Heidt T, Sager HB, Courties G, et al. Chronic variable stress activates hematopoietic stem cells. Nat Med. 2014; 20:754–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


