Abstract
Purpose
Children with brain tumors experience cognitive late effects, often related to cranial radiation. We sought to determine differential effects of surgery and chemotherapy on brain structure and neuropsychological outcomes in children who did not receive CRT.
Methods
Twenty-eight children with a history of posterior fossa tumor (17 treated with surgery, 11 treated with surgery and chemotherapy) underwent neuroimaging and neuropsychological assessment a mean of 4.5 (surgery group) to 9 years (surgery + chemotherapy group) post-treatment, along with 18 healthy sibling controls. Psychometric measures assessed IQ, language, executive functions, processing speed, memory, and social-emotional functioning. Group differences and correlations between diffusion tensor imaging findings and psychometric scores were examined.
Results
Z-score mapping demonstrated fractional anisotropy (FA) values were ≥ 2 standard deviations lower in white matter tracts, prefrontal cortex gray matter, hippocampus, thalamus, basal ganglia, and pons between patient groups, indicating microstructural damage associated with chemotherapy. Patients scored lower than controls on visuoconstructional reasoning and memory (p ≤0.02). Lower FA in the uncinate fasciculus (R −0.82 to −0.91) and higher FA in the thalamus (R 0.73 to 0.91) associated with higher IQ scores, and higher FA in the thalamus associated with higher scores on spatial working memory (R 0.82).
Conclusions
Posterior fossa brain tumor treated with surgery and chemotherapy affects brain microstructure and neuropsychological functioning years into survivorship, with spatial processes the most vulnerable. Biomarkers indicating cellular changes in the thalamus, hippocampus, pons, prefrontal cortex and white matter tracts associate with lower psychometric scores.
Keywords: pediatric brain tumor, neuroimaging, diffusion tensor imaging, neuropsychology, survivors of childhood cancer
Introduction
Central nervous system tumors are the most common cause of cancer death in children age 0-14 years in the United States1. Treatment regimens of surgery, chemotherapy and irradiation are based upon risk including tumor molecular genetics, location, pathology, and age at diagnosis2. Cranial irradiation (CRT) is a factor in neuropsychological deficits in pediatric cancer survivors ranging from a global loss of IQ points to memory and attention deficits3,4. Evidence from large cohorts, such as the Childhood Cancer Survivor Study5 and Children’s Oncology Group, supports decreasing CRT dose or elimination when feasible6. Children under 6 years may be treated with irradiation-sparing intensive chemotherapy regimens when possible without compromising survival 7-10, as cognitive and social-emotional effects appear mitigated with this approach11-15. Late effects of systemic and CNS-directed chemotherapy on the developing brain in the absence of CRT are less well-defined, but in children with leukemia, include deficits in attention, executive functions, visual processing and visual motor deficits5,16-19.
Treatment induces neurotoxicity through oxidative stress and inflammation triggered by irradiation and/or chemotherapy3,20. Cell membrane breakdown and death, demyelination, and loss of blood-brain barrier integrity contribute to edema and a cycle of continuing injury3. Magnetic resonance imaging (MRI) illustrates extent of damage and injury subtypes in vivo. Diffusion tensor imaging (DTI), characterizing three-dimensional water diffusion as a function of spatial location, provides indices of mean diffusivity (MD) and fractional anisotropy (FA) indices. MD reflects cell size, shape, integrity, and molecular motion across tissues21,22. High MD may indicate edema or loss of axons and demyelination.22,23. Comparatively low FA represents loss of microstructural integrity, particularly in myelinated regions, reduced tissue organization21,24, and axonal damage25.
Rueckriegel et al. (2010) demonstrated that children with low-grade brain tumors treated with surgery showed decreased FA in the white matter (WM) tract skeleton, though to a lesser extent than those treated with surgery, irradiation and chemotherapy26. Similar populations treated with surgery and chemotherapy showed deficits in processing speed, visual sustained attention27 and visual working memory28, indicating that brain tumor presence, surgical resection and chemotherapy results in WM disruption. However, few such neuroimaging studies exist, and neuropsychological outcomes remain unclear.
In a pilot study, we investigated brain injury in childhood brain tumor survivors treated with surgery and chemotherapy (n=7) compared to healthy controls (n=9) using DTI29,30. Higher MD values indicated significant changes in the thalamus, pons, basal ganglia, and mammillary bodies in patients compared to controls29. Due to sample size, it could not be determined if this pattern of injury was due to tumor, surgery, chemotherapy, or a combination of the three. The purpose of the current study was to differentiate effects of surgery alone and surgery followed by chemotherapy on brain microstructure and neuropsychological function in pediatric posterior fossa tumor survivors. Our a priori hypotheses were 1) neuroimaging would indicate a pattern of injury to subcortical and brainstem structures in children treated with surgery and chemotherapy (S+C) compared to children treated with surgery (S) and healthy controls (HC), 2) children in the S+C group would score lower than those in the S and HC groups on neuropsychological outcomes, and 3) injury to pons, hippocampus, basal ganglia and thalamus would correlate with poorer neuropsychological outcomes.
Methods
A cross-sectional comparative design was used with participants completing MRI with DTI and neuropsychological assessment. Patient groups included children at least 12 months from last treatment for a posterior fossa brain tumor, currently 6-17 years old, treated with either surgery (S group) or surgery and chemotherapy (S+C group). The study was approved by the Institutional Review Board. Potential subjects were identified from the neuro-oncology database and clinic lists. Parents were approached in clinic or contacted by mail or phone. At the time of patient enrollment, parents were invited to enroll healthy 6-17 year old siblings as controls. Parents and participants were required to speak and read either English or Spanish.
Exclusion criteria for all participants included metal in the body precluding MRI, preterm birth, neurodevelopmental disability, and traumatic brain injury. Controls had to be able to undergo MRI without sedation. Patients with recurrent tumor or residual disease outside the posterior fossa were excluded, as were those with a history of posterior fossa syndrome, since related deficits, including lower IQ, working memory and processing speed31 are significant confounding variables.
All data were stored in REDCap v6.14.232,33.
Imaging Data and Preprocessing
Three-dimensional T1-weighted images were obtained on a 3.0T Philips Achieva scanner with voxel size of 1.0 × 1.0 × 1.0 mm3 with parameters: TR 9.9 ms; TE 4.6 ms; 240 × 231 matrix; FOV 24 cm. DWI imaging sequence was acquired with parameters: 70 axial slices (2 mm thick), FOV = 256 mm x 256 mm x 140 mm, TR/TE 8657/86 ms, no gap, with a 128×126 acquisition matrix, 28 gradient directions collected with b-value=1500.
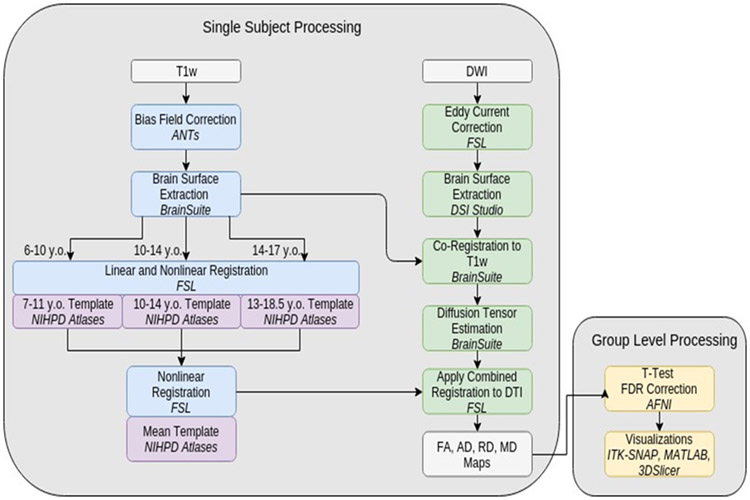
T1 images were bias field corrected using ANTs’25 N4 BFC26 tool and manually skull-stripped in Brainsuite 1627. Twelve-point linear registration was done using FSL’s28-30 FLIRT31,32 tool and nonlinear registration to the McConnell Brain Imaging Centres (MBIC) natural pediatric template for children33,34 with FSL’s FNIRT tool. The overall template space is an average of three age-appropriate MBIC templates. DW images were corrected with FSL19 for eddy current and subject motion. Resulting DWIs were skull-stripped in DSI Studio. Brainsuite’s BDP Pipeline co-registered DWI images to preprocessed T1w images, then registered to the MBIC mean template space by applying combined transformations from the T1w image’s registration pipeline (Figure 1).
Figure 1. Image processing.
Details of single subject and group level image processing Abbreviations: AFNI, Analysis of Functional NeuroImages; ANTs, Advanced Neuroimaging Tools; DWI, diffusion-weighted imaging; FSL, FMRIB Software Library; NIHPD, NIH Pediatric MRI Data Repository
Neuropsychological Assessment.
Participants completed a battery of well-validated measures (i.e., Wechsler scales, NEPSY II Memory for Designs, California Verbal Learning Test, Children’s Version, Receptive/Expressive One-Word Picture Vocabulary Tests, NIH Toolbox, and Achenbach Child Behavior Checklist) commonly used to assess domains potentially affected by posterior fossa brain tumors including intelligence, processing speed, memory, executive functions, language, and psychosocial functioning (Table 1). Assessments were performed by a board-certified pediatric neuropsychologist or by doctoral trainees under her supervision.
TABLE 1.
Neuropsychological Measures
| Area | Assessment | Method |
|---|---|---|
| General Intelligence | Full Scale Intelligence Quotient | Wechsler Abbreviated Scale of Intelligence, Second Edition (WASI-II) |
| Verbal Comprehension Index | ||
| Perceptual Reasoning Index | ||
| Vocabulary | ||
| Similarities | ||
| Block Design | ||
| Matrix Reasoning | ||
| Processing Speed | Coding | Wechsler Intelligence Scale for Children, Fourth Edition /Wechsler Adult Intelligence Scale, 4th Edition |
| Symbol Search | ||
| Pattern Comparison Processing Speed | HealthMeasures NIH Toolbox (NIH-T) | |
| Memory | List A Long-Delay Free Recall | California Verbal Learning Test, Children’s Version (CVLT®-C) |
| Memory for Designs Content & Spatial (MD) | NEPSY-II | |
| Memory for Designs Delayed Content & Spatial (MDD) | ||
| Executive Functions | Flanker Inhibitory Control & Attention | NIH-T |
| Dimensional Change Card Sort | ||
| Digit Span | Wechsler Intelligence Scale for Children, Fourth Edition Integrated (WISC-IV) | |
| Spatial Span | ||
| List Sorting Working Memory | NIH-T | |
| Behavioral Regulation Index | Behavior Rating Inventory of Executive Functions (BRIEF®) parent report questionnaire | |
| Metacognition Index | ||
| Global Executive Composite | ||
| NeuroQOL PED Cognitive Function | HealthMeasures Neuro-QOL | |
| Language | Expressive One-Word Picture Vocabulary Test | EOWPVT-4 |
| Receptive One-Word Picture Vocabulary Test | ROWPVT-4 | |
| Behavior/Mood | PED Depression | HealthMeasures Neuro-QOL |
| PED Interaction with Peers | ||
| PED Fatigue | ||
| Internalizing Problems | Achenbach Child Behavior Checklist (CBCL) parent report questionnaire | |
| Externalizing Problems | ||
| Total Problems |
Statistical Methods
Whole-brain voxel-wise analysis was performed in AFNI34 using 3dttest++ to generate pairwise z score maps. We masked the cerebellum from image analysis due to our focus on structures outside the surgical resection. Age at time of scan was a regression covariate. False Discovery Rate (FDR) was applied at p ≤0.05 to voxel-wise analyses. Within AFNI’s graphical user interface output, z-maps were thresholded to show only voxels where FA had z scores ≥ 2 SD from mean. Cluster maps were exported to ITK-SNAP35 where mean and SD of FA values in each cluster were extracted. Anatomical labels are in concordance with an MRI atlas36.
Post hoc analyses
Using AFNI’s 3dttest++, whole brain voxel T statistic maps were created for each DTI metric using group level analysis in three pairwise analyses between S, S+C, and HC groups. AFNI’s 3dcalc was then used to convert each T statistic map into a Z score map. Z maps were filtered to display regions of the brain where FA was ∣Z∣ > 2, indicating areas with ≥ 2 SD difference between treatment groups and then between each treatment group and HCs, using a Q-value of 0.05 (hypothesis 1). White matter clusters with 100 or more surviving contiguous voxels are reported. In the thalamus, pons and basal ganglia, we decreased the number of contiguous voxels required to 30 or more to allow analysis of areas specified in our hypotheses, since those structures are generally smaller in size than WM regions and allowed further analysis of FA findings in regions identified in a priori hypothesis (thalamus, basal ganglia, hippocampus).
The χ2 test and t-test examined differences in patient demographics and medical history among groups and neuropsychological scores were compared using linear regression. ANOVA compared scores in patients to HCs with age at diagnosis as a covariate (hypothesis 2). Analyses were performed in Stata37 using 2-sided tests. FDR was controlled by applying the Benjamini Hochberg procedure38 (Q-value 0.012) with significance set at p ≤ 0.02.
Pearson correlation analysis using SPSS v.25.039 assessed the relationship of FA in the 8 clusters that significantly differed between groups to neuropsychological scores, controlling for age and time off treatment in patient groups (hypothesis 3).
Results
Demographics
131 children were potentially eligible. After excluding those treated with CRT, turning 18 during recruitment, with PFS or severe developmental delay, or no longer followed at the institution, there were 48 patients. Of those, 5 families declined participation and 15 families didn’t respond to mail/telephone invitations, for a participation rate of 58%. The 20 who did not participate compared to the 28 who did were not significantly different in current age, age at diagnosis or years since treatment, but insurance status suggested a larger percentage were of higher socioeconomic status than in the final sample. However, the sample was representative of that the institution serves.
Seventeen children in the S group, 11 in the S+C group and 18 HCs participated in the study. Demographic data are presented in Table 2. Although age at study was not different between groups, age at diagnosis was, thus the distribution of current ages between patient groups varied with the S group skewed toward younger ages and the S+C group skewed toward older ages and longer time from treatment. There was also a difference between diagnoses, as most patients in the S group had pilocytic astrocytoma and most in the S+C group had medulloblastoma (p=0.0001), which was expected related to assigned treatment. For all 46 participants, there were no group differences in preschool attendance (p=0.62), receiving special education (p=0.43), family history of developmental delays, learning disabilities, psychiatric or medical illnesses (p=0.06, p=0.31, p=0.18, p=0.08, respectively). The S+C group were treated with carboplatin, etoposide and thiotepa (with 82% receiving each of these drugs), vincristine and cyclophosphamide (73%), cisplatin (64%), methotrexate (36%), temozolomide (27%), and dasatinib, lenalidomide, or irinotecan (9%).
TABLE 2.
Patient demographics by treatment group compared to controls
| Variable | Treatment group | p-value | ||||
|---|---|---|---|---|---|---|
| S (n = 17) |
S+C (n = 11) |
HC (n = 18) |
Total (n = 46) |
|||
| Age – mean (SD) | 10.76 (4.02) | 12.18 (3.52) | 10.56 (2.23) | 11.02 (3.25) | 0.41 | |
| Age at Diagnosis – mean (SD) | 6.02 (3.57) | 3.52 (3.29) | NA | 5.04 (3.63) | 0.07 | |
| Sex | Male | 4 (24%) | 6 (55%) | 10 (56%) | 20 (43%) | 0.11 |
| Female | 13 (76%) | 5 (45%) | 8 (38%) | 26 (53%) | ||
| Handedness | Right | 14 (82%) | 10 (91%) | 17 (94%) | 41 (89%) | 0.78 |
| Left | 3 (18%) | 1 (9%) | 1 (5%) | 5 (11%) | ||
| Patient Race/ethnicity | White | 15 (88%) | 10 (91%) | 16 (89%) | 41 (89%) | Race – 0.84 |
| Non-Hispanic | 7 (41%) | 3 (27%) | 10 (63%) | 20 (43%) | Ethnicity – 0.11 | |
| Hispanic/Latino | 9 (53%) | 8 (73%) | 6 (33%) | 23 (50%) | ||
| Black/African American | 1 (6%) | 0 (0%) | 0 (0%) | 1 (2%) | ||
| Asian | 1 (6%) | 1 (9%) | 2 (11%) | 4 (9%) | ||
| Diagnosis | medulloblastoma | 0 (0%) | 7 (64%) | NA | 7 (25%) | 0.0001 |
| ependymoma | 0 (0%) | 1 (9%) | 1 (4%) | |||
| Astrocytoma | 2 (12%) | 0 (0%) | 2 (7%) | |||
| Pilocytic Astrocytoma | 14 (82%) | 2 (18%) | 16 (57%) | |||
| Other | 1 (6%) | 1 (9%) | 2 (7%) | |||
| Time Off Treatment (years) – mean (SD) | 4.47 (3.20) | 8.09 (4.71) | NA | 5.89 (4.19) | 0.02 | |
| Region of tumor location | Midline | 6 (35%) | 7 (64%) | NA | 13 (46%) | 0.18 |
| Left | 4 (24%) | 3 (27%) | 7 (25%) | |||
| Right | 7 (41%) | 1 (9%) | 8 (29%) | |||
| Hydrocephalus at diagnosis | Yes | 10 (59%) | 7 (64%) | NA | 17 (61%) | 0.75 |
| No | 5 (29%) | 2 (18%) | 7 (25%) | |||
| Unknown | 2 (12%) | 2 (18%) | 4 (14%) | |||
| Hydrocephalus severity | Mild | 3 (30%) | 2 (29%) | NA | 5 (29%) | 0.87 |
| Moderate | 6 (60%) | 3 (43%) | 9 (53%) | |||
| Severe | 0 (0%) | 1 (14%) | 1 (6%) | |||
| Unknown | 1 (10%) | 1 (14%) | 2 (12%) | |||
| VP shunt | Yes | 1 (6%) | 6 (55%) | NA | 7 (25%) | 0.002 |
| No | 16 (94%) | 5 (45%) | 21 (75%) | |||
| Bilingual | No | 10 (59%) | 4 (36%) | 10 (56%) | 24 (52%) | 0.82 |
| Yes | 5 (29%) | 5 (45%) | 6 (33%) | 16 (35%) | ||
| Missing | 2 (12%) | 2 (18%) | 2 (11%) | 6 (13%) | ||
| Annual Income | ≤ $39,999 | 6 (35%) | 4 (36%) | 7 (39%) | 17 (37%) | 0.80 |
| $40,000-$79,999 | 1 (6%) | 1 (9%) | 2 (11%) | 4 (9%) | ||
| ≥ $80,000 | 7 (41%) | 3 (27%) | 8 (44%) | 18 (39%) | ||
| Missing | 3 (18%) | 3 (27%) | 1 (6%) | 7 (15%) | ||
| Did mother attend college? | No | 7 (41%) | 5 (45%) | 8 (44%) | 20 (43%) | 0.12 |
| Yes - BS | 1 (6%) | 4 (36%) | 2 (11%) | 7 (15%) | ||
| Yes - Grad | 6 (35%) | 1 (9%) | 8 (44%) | 15 (33%) | ||
| Missing | 3 (18%) | 1 (9%) | 0 (0%) | 4 (9%) | ||
| Did father attend college? | No | 6 (35%) | 5 (45%) | 3 (17%) | 14 (30%) | 0.11 |
| Yes - BS | 4 (24%) | 4 (36%) | 9 (50%) | 17 (37%) | ||
| Yes - Grad | 3 (18%) | 1 (9%) | 6 (33%) | 10 (22%) | ||
| Missing | 4 (24%) | 1 (9%) | 0 (0%) | 5 (11%) | ||
Bold font indicates significant p-value (p ≤0.05)
Neuroimaging
There were no significant group differences in regional DTI after correction for multiple comparisons with the false discovery rate (FDR) procedure, permutation analysis and cluster analysis. Despite comparable mean ages of subjects in each group, age-related effects dominated the analysis. Therefore, we partialed out participant age as a regression covariate.
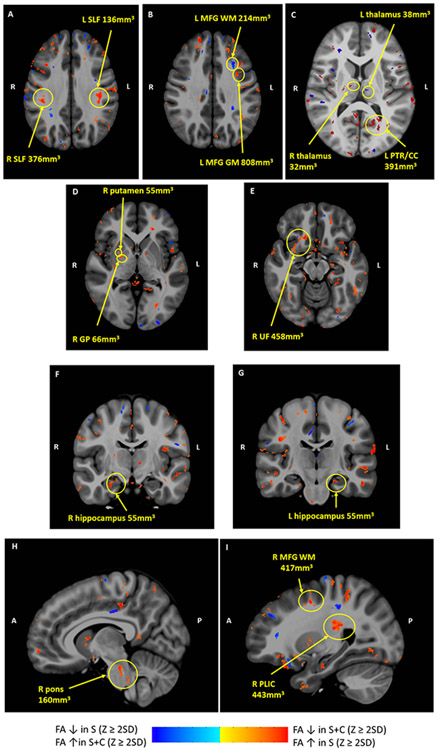
Areas of significantly different FA clusters by z masks between patient groups (S vs S+C) are shown in Figure 2. FA was higher in the S group in several regions, including the superior longitudinal (SLF) and uncinate fasciculi (UF), posterior thalamic radiation (PTR)/splenium of corpus callosum, and posterior limb of the internal capsule (PLIC), as well as in the right pons, gray and white matter of the prefrontal cortex (PFC), bilateral hippocampi, right globus pallidus and putamen, and right thalamus. FA was lower in the S group in the left thalamus.
Figure 2. Areas of FA differences between patient groups in composite axial and sagittal diffusion tensor images.
Red clusters indicate areas where FA z scores were ≥ 2SD lower in the S+C group than the S group; blue clusters indicate where FA z scores were ≥ 2SD lower in the S group than the S+C group. R and L indicate Right and Left on axial images; A and P indicate Anterior and Posterior. Cluster size is noted for each structure. A FA in bilateral SLF higher in S than S+C; B FA in prefrontal WM lower in S than S+C, FA in prefrontal GM higher in S than S+C; C FA in R thalamus higher in S than S+C, FA in L thalamus lower in S than S+C, FA in L PTR/CC higher in S than S+C; D FA in R putamen and GP higher in S than S+C; E FA in R UF higher in S than S+C; F FA higher in R hippocampus in S than S+C G F FA higher in L hippocampus in S than S+C; H FA higher in R pons in S than S+C; I FA higher in R PLIC and prefrontal WM in S than S+C. Abbreviations: CC, corpus callosum; GM, gray matter; GP, globus pallidus, MFG, middle frontal gyrus; PLIC, posterior limb of internal capsule; PTR, posterior thalamic radiation; SLF, superior longitudinal fasciculus; UF, uncinate fasciculus; WM, white matter
Areas of significantly different FA clusters between each patient group and HCs are shown in Supplemental Figures 1 and 2 supporting findings between patient groups, demonstrating higher FA in the pons, hippocampus and thalamus in controls than in S patients (Supp Fig 1). FA in controls was higher than S+C patients in the hippocampus, putamen, PLIC, and inferior fronto-occipital fasciculus (Supp Fig 2). FA was lower in controls in the thalamus and SLF than in both patient groups.
Neuropsychological Assessment
No monolingual Spanish-speaking children participated. Children completed the Children’s Oncology Group Language Preference Survey. The single bilingual participant who was not English dominant for testing was assessed by a bilingual examiner providing instructions in both English and Spanish and completed bilingual versions of Receptive and Expressive One-Word Picture Vocabulary tests.
There were no significant differences between patient groups in neuropsychological performance. When factoring in HCs, there was no difference in FSIQ (96.50, 96.80, 102.81), though HCs performed higher on Block Design, a subtest within FSIQ (p=0.01) (Supplemental Table 1). Controls also performed higher than patients on immediate design recall and delayed recall of designs and their spatial locations on Memory for Designs (p=0.02, 0.005, respectively). Distribution of scores are shown in Supplemental Figure 3. Significant group differences on other tasks with a motor component (i.e., Spatial Span, Coding, Symbol Search, Dimensional Change Card Sort, Flanker Inhibitory Control, and Pattern Comparison Processing Speed) were not found. Children in the S group and HCs scored lower (p= 0.02) than those in the S+C group on self-reported NeuroQOL Pediatric Cognitive function, though both groups placed within the norm. Scores on depression, fatigue and interacting with peers were not different between groups; however, parent questionnaires indicated clinical concerns regarding executive functions and social-emotional functioning more frequently for patients (Supplemental Table 2). The primary difference between groups was in the Externalizing Problems Index of the CBCL, where 36% of children in S, 20% of children in S+C, and no controls had parent-proxy scores in the clinical range.
Neuroimaging & Neuropsychological Assessment
Pearson correlations determined relationships between 8 clusters of FA significance in Figure 2 and psychometric scores in children with brain tumors, controlling for age and time off treatment (Table 3). Higher FA in the right thalamus correlated with higher scores on spatial working memory, Perceptual Reasoning, Block Design, FSIQ, and Similarities in the S+C group. Higher FA in the right pons correlated with lower scores on memory in the S+C group. Children in the S+C group demonstrated inverse correlations between FA in the UF and FSIQ, Vocabulary, and Similarities. In the S group, higher FA in the left hippocampus and right PLIC correlated with improved scores in spatial working memory, while lower FA in prefrontal GM correlated with higher scores in spatial working memory.
TABLE 3.
Neuropsychological Assessment Scores & Fractional Anisotropy R values*
| Domain/ Test |
L MFG GM | R UF | R PLIC | R thalamus | L hippocampus | R pons |
|---|---|---|---|---|---|---|
|
General Intelligence Verbal Comprehension |
−0.84 S+C | |||||
| Perceptual Reasoning | 0.83 S+C | |||||
| Block Design | 0.91 S+C | |||||
| FSIQ | −0.91 S+C | 0.88 S+C | ||||
| Vocabulary | −0.82 S+C | |||||
| Similarities | −0.84 S+C | 0.73 S | ||||
|
Working Memory Digit Span Total |
0.98 S | |||||
| Spatial Span Total | 0.82 S+C | |||||
| Spatial Span Forward | −0.98 S | 0.98 S | ||||
|
Memory Memory for Designs Delayed Total |
−0.87 S+C | |||||
|
Language Expressive One Word Vocabulary Test |
−0.83 S+C |
p < 0.01
GM, gray matter; MFG, middle frontal gyrus; PLIC, posterior limb of internal capsule; UF, uncinate fasciculus
The sole significant correlation in HCs was between spatial working memory and left thalamus FA (R=0.63, p=0.01).
Data available on request from authors
Discussion
This study demonstrated FA differences indicating microstructural injury in brain areas, many distant from the tumor site, in children treated with surgery and chemotherapy compared to those treated with surgery only, accompanied by minor differences in cognitive performance. FA differences were found in WM association pathways connecting temporal lobes with orbitofrontal and prefrontal cortices (UF)40,41, and those connecting parietal lobes to prefrontal cortices (SLF)41; in the PLIC, PTR/CC; pons and hippocampi; basal ganglia and thalami; and the PFC. These findings both confirm and expand upon results of our pilot study, where MD differences indicated injury to subcortical GM and pons in children treated with S+C for brain tumors29,30, also in the absence of major cognitive deficits30.
Lower FA in certain brain structures of children in the S+C group may indicate increased vulnerability of healthy tissue to chemotherapy apart from tumor or surgery effects. White matter in the PFC and association pathways continues to develop past adolescence42 and structures such as the UF develop later in life43, possibly rendering these tracts more vulnerable to neurotoxicity when injured earlier in development. Children in the S+C group were younger at diagnosis than children in the S group.
Lower mean FA in WM compared to HCs is common to many neuropathological conditions, including multiple sclerosis44, stroke45, epilepsy46, Alzheimer’s disease47, and brain tumors including meningiomas, low-grade gliomas, and glioblastoma multiforme48 indicating demyelination, edema, and/or inflammation49. It is a marker of WM damage, but nonspecific as to exact underlying pathology.
Other pediatric brain tumor studies found decreased mean FA in WM areas identified in our study, including the UF43,50, internal capsule, PTR/splenium of CC, SLF51, and frontal WM52. However, unlike this study, most did not control for tumor location and included children treated heterogeneously with combinations of surgery, CRT, and/or chemotherapy.
The additional neuronal damage in patients who received chemotherapy was expected, as all patients in the S+C group received chemotherapeutic agents known to cross the BBB (methotrexate, cisplatin, thiotepa, and temozolomide)20 and cause neurotoxicity53. While direct effects of chemotherapy in the developing brain are not fully understood, hypothesized mechanisms include neurotoxic injury to cerebral parenchyma, induction of a secondary inflammatory response, microvascular injury, indirect chemical toxicity, increased oxidative stress, altered neurotransmitter levels20, DNA damage, decreased neurogenesis, and shortening of telomeres53. These disruptions interfere with normal myelination, synaptogenesis, and pruning, all of which could contribute to our findings.
The S+C group demonstrated inverse correlations between FA in the UF and general intelligence. One would not expect that lower FA in WM, indicative of myelin disruption, would correlate with higher intelligence scores. One explanation is that FA in the UF is lower than expected where the UF crosses the SLF54, as crossing fibers cause the diffusion tensor to become more spherical or oblate55, reducing FA even in the presence of intact myelinated fiber tracts. Our methods did not allow determination of whether the cluster of significance was within these crossing fibers.
While FA is most commonly measured in WM, we found decreased FA in the right thalamus and increased FA in the left thalamus in the S+C group compared to the S group. This contrary finding may be due to location of the FA clusters in different thalamic nuclei (right ventrolateral nucleus and left medial thalamic nucleus), although imaging resolution did not allow us to confirm location in each subject. The medial thalamic nucleus is located much closer to the lateral ventricle than the ventrolateral nucleus, and as such may have been injured from hydrocephalus, which was more prevalent in the S+C group. High FA in GM may be a biomarker of neuronal injury in patients with chemotherapy-induced toxicity. The organization of GM, primarily made up of cell bodies, unmyelinated axons, dendrites and synapses, is less directionally organized than WM, making interpretation of DTI findings less clear56. Increased FA in GM was associated with gliosis in an animal model of traumatic brain injury57. Similar patterns of increased FA in GM are noted in chronic subdural hematoma58, where a compression-dependent increase in FA in the caudate and putamen decreased following evacuation of hematomas, suggesting increased FA in GM could indicate damage caused by tissue compression. These studies provide evidence that increased FA in the left thalamus could be a result of gliosis, damage to surrounding white matter, and/or compression of tissues from hydrocephalus.
Decreased FA in the basal ganglia, thalamus, and pons supports our earlier findings of elevated MD in these areas in children with brain tumors treated with surgery and chemotherapy30, as both directional indices represent microstructural injury. In 5 cognitive domains assessed (IQ, language, executive functions, processing speed, and memory), group differences were visual-spatial, suggesting greater sensitivity of these measures to broad CNS dysfunction59, commonly reported in neurologic conditions such as congenital hydrocephalus60.
Block Design, a visuoconstructional task requiring one to analyze part-whole relationships to copy two-dimensional patterns, is sensitive to CNS dysfunction59. Patient groups scored significantly lower than the HC group, though all performed within normal limits. This encouraging finding suggests that although children in the S+C group were younger at diagnosis, often a risk factor for poorer outcomes, and had intensive treatment, there were more years from diagnosis in which to, at least theoretically, develop neural plasticity. On a spatial memory task, patients performed lower than controls on learning designs and on delayed recall of both designs and locations, with those who received chemotherapy showing an additional, though non-significant, reduction in performance. This suggests patients had difficulty encoding, a process involving the hippocampus and WM connections between frontal and parietal regions via the thalamus. It could also reflect breakdown in connections involved in consolidating memory into long-term storage, a process thought to be mediated by medial temporal and diencephalic structures, as well as specific unimodal and heteromodal cortices or in retrieving it through activity in regions of neocortex without need for medial temporal or medial diencephalic involvement61.
Finally, it is notable that the S+C group performed similarly to the S group on neuropsychological assessment, but scored higher on QOL, even compared to controls. This further supports the theory that these children are relatively functionally intact due to neuroplasticity related to treatment at a younger age.
Limitations
As is common in pediatric brain tumor research, our sample size is relatively small, making definitive conclusions difficult, and participants had a range of cognitive abilities from impaired to superior, making aggregate data less representative. While only diagnosis and age at diagnosis differed between patient groups, patients were not specifically matched by sex, age, language, handedness or tumor location/laterality.
Considerations/Conclusions
Few prior neuroimaging studies include children with brain tumors treated with chemotherapy without CRT. We found clear patterns of brain injury in children with posterior fossa tumors treated with surgery with or without chemotherapy.
While most patients performed within normal limits on neuropsychological assessment, they performed lower than healthy controls on visual-constructional reasoning and spatial memory. Earlier neurological injury in children in the S+C group could allow for reorganization/compensation with other parts of the attention network with frontal or parietal aspects of attention playing a more important role in performance. Although not tested in this study, this is an important focus for future studies.
Factors underlying vulnerability or resilience in the face of neurotoxic therapies merit further evaluation to determine effects of genetics and environment. Future studies involving multiple institutions enabling larger sample sizes over multiple time points are necessary to pinpoint periods of neuronal vulnerability amenable to intensive interventions.
Our findings illustrate that treatment of pediatric posterior fossa brain tumors results in long-term alterations to gray and white matter microstructure, and that more pronounced differences are seen when chemotherapy is used in addition to surgical intervention.
Supplementary Material
Supplemental Figure 3. Histograms of score distributions on assessments significantly different between groups with test scores on the X axis and frequency of participant scores on the Y axis. A Block Design T scores for Groups S, S+C, and HC; B Memory for Designs content scaled scores for Group S, S+C, and HC; C Memory for Designs Delayed content scaled scores for Group S, S+C, and HC; D Memory for Designs Delayed spatial scaled scores for Group S, S+C, and HC
Supplemental Figure 2. Areas of FA differences between S+C and healthy control groups in composite axial and sagittal diffusion tensor images Red clusters indicate areas where FA z scores were ≥ 2SD lower in the S+C group than the control group; blue clusters indicate where FA z scores were ≥ 2SD lower in the control group than the S+C group. R and L indicate Right and Left on axial images; A and P indicate Anterior and Posterior. Cluster size is noted for each structure. A FA in L SLF higher in S+C than controls; B FA in R IFOF higher in controls than S+C; C FA in R putamen and L PLIC higher in controls than S+C, FA in R and L thalamus lower in controls than S+C; D FA in R hippocampus higher in controls than S+C. Abbreviations: IFOF, inferior fronto-occipital fasciculus; PLIC, posterior limb of internal capsule; SLF, superior longitudinal fasciculus
Supplemental Figure 1. Areas of FA differences between S+C and healthy control groups in composite axial and sagittal diffusion tensor images Red clusters indicate areas where FA z scores were ≥ 2SD lower in the S group than controls; blue clusters indicate where FA z scores were ≥ 2SD lower in the controls than the S group. R and L indicate Right and Left on axial images; A and P indicate Anterior and Posterior. Cluster size is noted for each structure. A FA in L SLF higher in S than controls; B FA in L putamen higher in S than controls; C FA in R thalamus higher in controls than S; D FA in L pons higher in controls than S; E FA in R hippocampus higher in controls than S. Abbreviations: SLF, superior longitudinal fasciculus
Acknowledgements:
this study was funded by K23NR014902 and Children’s Hospital Los Angeles Hemonc/BMT Division Research Funds; and supported by The Saban Research Institute (TSRI) of Children’s Hospital Los Angeles; and USC CTSI REDCap UL1TR001855. This study was also funded in part by the SC CTSI, which is part of the Clinical and Translational Science Awards (CTSA), a national network funded through the National Center for Advancing Translational Sciences (NCATS) at the NIH (Grant Number UL1TR000130). Under the mandate of “Translating Science into Solutions for Better Health,” SC CTSI provides a wide range of services, funding, and education for researchers and promotes online collaboration tools such as USC Health Sciences Profiles. Dr. Lepore is funded by R01EB025031 and TSRI 000013228. Dr. Rajagopalan is funded by K01 HL153942 and TSRI 00011096. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
The authors would like to thank Drs. Ki Moore and Bradley Peterson, who were mentors for this study; and Christine Obioha and Francesca Trane, who assisted with data entry.
Abbreviations Key
- AFNI
Analysis of Functional Neuroimages
- CC
Corpus callosum
- CRT
Cranial radiation therapy
- DTI
Diffusion tensor imaging
- FA
Fractional anisotropy
- GM
Gray matter
- MD
Mean diffusivity
- MFG
Middle frontal gyrus
- MRI
Magnetic resonance imaging
- PFC
Prefrontal cortex
- PLIC
Posterior limb of internal capsule
- PTR
Posterior thalamic radiation
- SLF
Superior longitudinal fasciculus
- UF
Uncinate fasciculus
- WM
White matter
Footnotes
The results of this study were presented in part at the 6th Annual Pediatric Medical Student Research Forum in Orlando, FL in August 2019; and preliminary results were accepted as a poster presentation at ASPHO in Ft. Worth, TX in May 2020 (meeting cancelled due to pandemic), published at http://aspho.org/meetings/conference/2020-conference/2020-accepted-papers-and-posters#d, and at the Society for Neuro-oncology, meeting online in November 2020.
The authors have no conflicts of interest to disclose.
References
- 1.Ostrom QT, de Blank PM, Kruchko C, et al. Alex's Lemonade Stand Foundation infant and childhood primary brain and central nervous system tumors diagnosed in the United States in 2007-2011. Neuro Oncol. 2015:x1–x36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pollack IF. Multidisciplinary management of childhood brain tumors: a review of outcomes, recent advances, and challenges. Journal of neurosurgery Pediatrics. 2011;8(2):135–148. [DOI] [PubMed] [Google Scholar]
- 3.Kim JH, Brown SL, Jenrow KA, Ryu S. Mechanisms of radiation-induced brain toxicity and implications for future clinical trials. J Neurooncol. 2008;87:279–286. [DOI] [PubMed] [Google Scholar]
- 4.Turner CD, Rey-Casserly C, Liptak CC, Chordas C. Late effects of therapy for pediatric brain tumor survivors. J Child Neurol. 2009;24(11):1455–1463. [DOI] [PubMed] [Google Scholar]
- 5.Anderson FS, Kunin-Batson AS. Neurocognitive late effects of chemotherapy in children: The past 10 years of research on brain structure and function. Pediatric Blood & Cancer. 2009;52(2):159–164. [DOI] [PubMed] [Google Scholar]
- 6.Packer RJ, Goldwein JL, Nicholson HS, al E. Treatment of children with medulloblastoma with reduced-dose cranio-spinal radiation and adjuvant chemotherapy: a Children’s Cancer Group study. J Clin Oncol. 1999;17:2127–2136. [DOI] [PubMed] [Google Scholar]
- 7.Gardner S, Finlay J. High-dose chemotherapy with autologous stem cell rescue for the treatment of children with brain tumors. 2001.
- 8.Marachelian A, Butturini A, Finlay J. Myeloablative chemotherapy with autologous hematopoietic progenitor cell rescue for childhood central nervous system tumors. Bone Marrow Transplantation. 2008;41:167–172. [DOI] [PubMed] [Google Scholar]
- 9.Grill J, Sainte-Rose C, Jouvet A, al. E. Treatment of medulloblastoma with postoperative chemotherapy alone: an SFOP prospective trial in young children. Lancet Oncol. 2005;6:573–580. [DOI] [PubMed] [Google Scholar]
- 10.Rutkowski S, Bode U, Deinlein F, et al. Treatment of early childhood medulloblastoma by postoperative chemotherapy alone. N Engl J Med. 2005;352(10):978–986. [DOI] [PubMed] [Google Scholar]
- 11.Sands SA, van Gorp WG, Finlay JL. Pilot neuropsychological findings from a treatment regimen consisting of intensive chemotherapy and bone marrow rescue for young children with newly diagnosed malignant brain tumors. Child’s Nervous System. 1998;14:587–589. [DOI] [PubMed] [Google Scholar]
- 12.Sands SA, Oberg JA, Gardner SL, Whiteley JA, Glade-Bender JL, Finlay JL. Neuropsychological functioning of children treated with intensive chemotherapy followed by myeloablative consolidation chemotherapy and autologous hematopoietic cell rescue for newly diagnosed CNS tumors: an analysis of the Head Start II survivors. Pediatr Blood Cancer. 2010;54:429–436. [DOI] [PubMed] [Google Scholar]
- 13.Sands SA, Pasichow KP, Weiss R, et al. Quality of life and behavioral follow-up study of Head Start I pediatric brain tumor survivors. J Neuro-Oncol. 2011;101:287–295. [DOI] [PubMed] [Google Scholar]
- 14.Fay-McClymont TB, Ploetz DM, Mabbott DJ, al. E. Long-term neuropsychological follow-up of young children with medulloblastoma treated with sequential high-dose chemotherapy and irradiation sparing approach. J Neuro-Oncol. 2017;133:119–128. [DOI] [PubMed] [Google Scholar]
- 15.O’Neil SH, Whitaker AM, Kayser K, et al. Neuropsychological outcomes on HeadStart III: a prospective, multi-institutional clinical trial for young children diagnosed with malignant brain tumors. Neuro-Oncol Practice. 2020. [DOI] [PMC free article] [PubMed]
- 16.Beaulieu C, Genschaft M, Huebner T, et al. Impact of Chemotherapy for Childhood Leukemia on Brain Morphology and Function. PLoS ONE. 2013;8(11):e78599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kesler SR, Tanaka H, Koovakkattu D. Cognitive reserve and brain volumes in pediatric acute lymphoblastic leukemia. Brain Imaging and Behavior. 2010;4(3–4):256–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reddick WE, Shan ZY, Glass JO, et al. Smaller white-matter volumes are associated with larger deficits in attention and learning among long-term survivors of acute lymphoblastic leukemia. Cancer. 2006;106(4):941–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Robinson KE, Livesay KL, Campbell LK, et al. Working memory in survivors of childhood acute lymphocytic Leukemia: Functional neuroimaging analyses. Pediatric Blood & Cancer. 2009;54(4):585–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Myers JS, Pierce J, Pazdernik T. Neurotoxicology of chemotherapy in relation to cytokine release, the blood-brain barrier, and cognitive impairment. Oncology Nursing Forum. 2008;35(6):916–920. [DOI] [PubMed] [Google Scholar]
- 21.Cercignani M, Inglese M, Pagani E, Comi G, Filippi M. Mean diffusivity and fractional anisotropy histograms of patients with multiple sclerosis. Am J Neuroradiol. 2001;22:952–958. [PMC free article] [PubMed] [Google Scholar]
- 22.Kumar R, Macey PM, Woo MA, Alger JR, Harper RM. Elevated mean diffusivity in widespread brain regions in congenital central hypoventilation syndrome. J Magn Reson Imaging. 2006;24(6):1252–1258. [DOI] [PubMed] [Google Scholar]
- 23.Iannucci G, Rovaris M, Giacomotti L, Comi G, Filippi M. Correlation of multiple sclerosis measures derived from T2-weighted, T1-weighted, magnetization transfer, and diffusion tensor MR imaging. Am J Neuroradiol. 2001;22:1462–1467. [PMC free article] [PubMed] [Google Scholar]
- 24.Mukherjee P, Miller JH, Shimony JS, et al. Diffusion-tensor MR imaging of gray and white matter development during normal human brain maturation. Am J Neuroradiol. 2002;23:1445–1456. [PMC free article] [PubMed] [Google Scholar]
- 25.Mac Donald CL, Dikranian K, Song SK, Bayly PV, Holtzman DM, Brody DL. Detection of traumatic axonal injury with diffusion tensor imaging in a mouse model of traumatic brain injury. Exp Neurol. 2007;205(1):116–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rueckriegel SM, Driever PH, Blankenburg F, Ludemann L, Henze G, Bruhn H. Differences in supratentorial damage of white matter in pediatric survivors of posterior fossa tumors with and without adjuvant treatment as detected by magnetic resonance diffusion tensor imaging. Int J Radiat Oncol Biol Phys. 2010;76(3):859–866. [DOI] [PubMed] [Google Scholar]
- 27.Aarsen FK, Paquier PF, Arts WF, et al. Cognitive deficits and predictors 3 years after diagnosis of a pilocytic astrocytoma in childhood. J Clin Oncol. 2009;27(21):3626–3632. [DOI] [PubMed] [Google Scholar]
- 28.Peterson RK, Tabori U, Bouffet E, et al. Predictors of neuropsychological late effects and white matter correlates in children treated for a brain tumor without radiation therapy. Pediatr Blood Cancer. 2019. [DOI] [PubMed]
- 29.Baron Nelson M, Macey PM, Harper RM, et al. Structural brain alterations in children an average of 5 years after surgery and chemotherapy for brain tumors. J Neurooncol. 2014;119(2):317–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baron Nelson M, Compton P, Macey PM, et al. Diffusion Tensor Imaging and Neurobehavioral Outcome in Children With Brain Tumors Treated With Chemotherapy. Journal of Pediatric Oncology Nursing. 2016;33(2):119–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schreiber JE, Palmer SL, Conklin HM, et al. Posterior fossa syndrome and long-term neuropsychological outcomes among children treated for medulloblastoma on a multi-institutional prospective study. Neuro Oncol. 2017;19(12):1673–1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harris PA, Taylor R, Minor BL, et al. The REDCap consortium: building an international community of software partners. J Biomed Inform. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. [DOI] [PubMed] [Google Scholar]
- 35.Yushkevich PA, Piven J, Hazlett HC, et al. User=guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. Neuroimage. 2006;31(3):1116–1128. [DOI] [PubMed] [Google Scholar]
- 36.Oishi K, Faria A, Van Zijl PCM, Mori S. MRI Atlas of Human White Matter. Second ed. Boston, MA: Elsevier; 2011. [Google Scholar]
- 37.Statistical Software: Release 14 [computer program]. College Station, TX: StataCorp LP; 2015. [Google Scholar]
- 38.Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: a practical and powerful approach to multiple testing. J Royal Stat Soc. 1995;57(1):289–300. [Google Scholar]
- 39.IBM SPSS Statistics for Windows, Version 25.0 [computer program]. Armonk, NY: IBM Corp; 2017. [Google Scholar]
- 40.Von Der Heide RJ, Skipper LM, Klobusicky E, Olson IR. Dissecting the uncinate fasciculus: disorders, controversies and a hypothesis. Brain. 2013;136(6):1692–1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schmahmann JD, Pandya DN, Wang R, et al. Association fibre pathways of the brain: parallel observations from diffusion spectrum imaging and autoradiography. Brain. 2007;130(630–653). [DOI] [PubMed] [Google Scholar]
- 42.Sowell ER, Thompson PM, Leonard CM, Welcome SE, Kan E, Toga AW. Longitudinal mapping of cortical thickness and brain growth in normal children. J Neurosci. 2004;24(38):8223–8231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Riggs L, Bouffet E, Laughlin S, et al. Changes to Memory Structures in Children Treated for Posterior Fossa Tumors. Journal of the International Neuropsychological Society. 2014;20(02):168–180. [DOI] [PubMed] [Google Scholar]
- 44.Bammer R, Augustin M, Strasser-Fuchs S, et al. Magnetic resonance diffusion tensor imaging for characterizing diffuse and focal white matter abnormalities in multiple sclerosis. Magn Reson Imaging in Medicine. 2000;44(4):583–591. [DOI] [PubMed] [Google Scholar]
- 45.Werring DJ, Toosy AT, Clark CA, et al. Diffusion tensor imaging can detect and quantify corticospinal tract degeneration after stroke. J Neurol Neurosurg Psychiatry. 2000;69(2):269–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dumas de la Roque A, Oppenheim C, Chassoux F, et al. Diffusion tensor imaging of partial intractable epilepsy. European Radiology. 2005;15:279–285. [DOI] [PubMed] [Google Scholar]
- 47.Fellgiebel A, Wille P, Muller MJ, et al. Ultrastructural hippocampal and white matter alterations in mild cognitive impairment: a diffusion tensor imaging study. Dementia and Geriatric Cognitive Disorders. 2004;18(1):101–108. [DOI] [PubMed] [Google Scholar]
- 48.Lu S, Ahn D, Johnson G, Law M, Zagzag D, Grossman RI. Diffusion-tensor MR imaging of intracranial neoplasia and associated peritumoral edema: introduction of the tumor infiltration index. Radiology. 2004;232(1):221–228. [DOI] [PubMed] [Google Scholar]
- 49.Assaf Y, Pasternak O. Diffusion tensor imaging (DTI)-based white matter mapping in brain research: a review. J Mol Neurosci. 2008;34(1):51–61. [DOI] [PubMed] [Google Scholar]
- 50.Aleksonis HA, Wier R, Pearson MM, et al. Associations among diffusion tensor imaging and neurocognitive function in survivors of pediatric brain tumor: a pilot study. Appl Neuropsychol: Child. 2019. [DOI] [PubMed]
- 51.Palmer SL, Glass JO, Li Y, et al. White matter integrity is associated with cognitive processing in patients treated for a posterior fossa brain tumor. Neuro Oncol. 2012;14(9):1185–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mabbott DJ, Noseworthy MD, Bouffet E, Rockel C, Laughlin S. Diffusion tensor imaging of white matter after cranial radiation in children for medulloblastoma: correlation with IQ. Neuro Oncol. 2006:244–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chemotherapy Ikonomidou C. and the pediatric brain. Mol Cell Pediatr. 2018;5(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schmahmann JD, Pandya DN. Fiber Pathways of the Brain. New York, NY: Oxford University Press, Inc; 2006. [Google Scholar]
- 55.Tournier J The biophysics of crossing fibers In: Jones DK, ed. Diffusion MRI. Oxford University Press; 2010:467. [Google Scholar]
- 56.Baron Nelson M, O’Neil SH, Wisnowski JL, et al. Maturation of brain microstructure and metabolism associates with increased capacity for self-regulation during the transition from childhood to adolescence. J Neurosci. 2019;19(42):8362–8375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Budde MD, Janes L, Gold E, Turtzo LC, Frank JA. The contribution of gliosis to diffusion tensor anisotropy and tractography following traumatic brain injury: validation in the rat using Fourier analysis of stained tissue sections. Brain. 2011. [DOI] [PMC free article] [PubMed]
- 58.Osuka S, Matsushita A, Ishikawa E, Saotome K, Yamamoto T, al. e. Elevated diffusion anisotropy in gray matter and the degree of brain compression. J Neurosurg. 2012;117:363–371. [DOI] [PubMed] [Google Scholar]
- 59.Lezak MD, Howieson DB, Loring DW. Neuropsychological Assessment. 4th ed. Oxford: Oxford University Press; 2004. [Google Scholar]
- 60.Bigler ED. The neuropsychology of hydrocephalus. Arch Clin Neuropsychol. 1988;3(1):81–100. [PubMed] [Google Scholar]
- 61.Blumenfeld H Neuroanatomy Through Clinical Cases. Sunderland, MA: Sinauer; 2002. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 3. Histograms of score distributions on assessments significantly different between groups with test scores on the X axis and frequency of participant scores on the Y axis. A Block Design T scores for Groups S, S+C, and HC; B Memory for Designs content scaled scores for Group S, S+C, and HC; C Memory for Designs Delayed content scaled scores for Group S, S+C, and HC; D Memory for Designs Delayed spatial scaled scores for Group S, S+C, and HC
Supplemental Figure 2. Areas of FA differences between S+C and healthy control groups in composite axial and sagittal diffusion tensor images Red clusters indicate areas where FA z scores were ≥ 2SD lower in the S+C group than the control group; blue clusters indicate where FA z scores were ≥ 2SD lower in the control group than the S+C group. R and L indicate Right and Left on axial images; A and P indicate Anterior and Posterior. Cluster size is noted for each structure. A FA in L SLF higher in S+C than controls; B FA in R IFOF higher in controls than S+C; C FA in R putamen and L PLIC higher in controls than S+C, FA in R and L thalamus lower in controls than S+C; D FA in R hippocampus higher in controls than S+C. Abbreviations: IFOF, inferior fronto-occipital fasciculus; PLIC, posterior limb of internal capsule; SLF, superior longitudinal fasciculus
Supplemental Figure 1. Areas of FA differences between S+C and healthy control groups in composite axial and sagittal diffusion tensor images Red clusters indicate areas where FA z scores were ≥ 2SD lower in the S group than controls; blue clusters indicate where FA z scores were ≥ 2SD lower in the controls than the S group. R and L indicate Right and Left on axial images; A and P indicate Anterior and Posterior. Cluster size is noted for each structure. A FA in L SLF higher in S than controls; B FA in L putamen higher in S than controls; C FA in R thalamus higher in controls than S; D FA in L pons higher in controls than S; E FA in R hippocampus higher in controls than S. Abbreviations: SLF, superior longitudinal fasciculus