Abstract
Background:
To describe and contrast the brain development and outcome among very preterm infants, that were and were not exposed to surgery requiring general anesthesia prior to term equivalent age (TEA)..
Methods:
Preterm infants born ≤30 weeks’ gestation that did (n=25) and did not (n=59) have surgery requiring general anesthesia during the preterm period were studied. At TEA, infants had MRI scans performed with measures of brain tissue volumes, cortical surface area, gyrification index and white matter microstructure. Neurodevelopmental follow-up with the Bayley Scales of Infant and Toddler Development, 3rd Edition was undertaken at two years corrected age. Multivariate models, adjusted for clinical and social risk factors, were used to compare the groups.
Results:
After controlling for clinical and social variables, preterm infants exposed to surgical anesthesia demonstrated decreased relative white matter volumes at TEA, and lower cognitive and motor composite scores at two year follow-up. Those with longer surgical exposure demonstrated the greatest decrease in white matter volumes and lower cognitive and motor outcomes at age two years.
Conclusion:
Very preterm infants who required surgery during the preterm period had lower white mater volumes at TEA and worse neurodevelopemental outcome at age 2 years.
Introduction
There is concern that exposure to surgery and anesthesia during early childhood may be associated with heightened risk for developmental delays (1–3). Animal models exposed to surgical anesthetics show increased apoptosis of neurons and oligodendrocytes, decreased neurogenesis and neuronal differentiation, and reduced axonal growth (4, 5). However, several recent prospective studies have demonstrated that not all anesthetic exposure in early childhood results in developmental delay (6, 7). Rather, these delays may be associated with higher cumulative anesthetic exposure and/or exposure during periods of increased neurobiological vulnerability (8).
The fetal and infant brain undergoes exponential growth during the last trimester of pregnancy (9). Animal data has demonstrated increased vulnerability to anesthetic-associated injury during these periods of increased synaptogenesis and growth (8). For infants born very premature, this rapid brain growth occurs in the ex-utero environment, and during a period of increased risk of surgical anesthetic exposure due to the typical co-morbidities of prematurity. Indeed, prior studies have demonstrated that preterm infants who undergo surgery during the neonatal period have an increased risk for altered brain growth and impaired cognitive development,(3, 10–12). These studies hypothesized that anesthetic exposure may have mediated some of the negative impacts, however they were unable to provide details on the type and length of anesthesia exposure during the surgical procedures limiting further analysis and interpretation.
The aim of this study was to compare the brain development and outcome among very preterm infants, that were and were not exposed to surgery requiring general anesthesia prior to term equivalent age. This was achieved by analyzing the association between both any exposure, and duration of exposure, to surgical anesthesia on quantitative MRI metrics from scans performed at term equivalent age and standardized neurodevelopmental assessments at 2 years corrected age.
Methods
Participants
Very preterm infants were recruited from a level III Neonatal Intensive Care Unit (NICU) in St. Louis, Missouri, between 2007 and 2010 into a prospective longitudinal observational cohort study of VPT infants undergoing MRI at TEA, and long-term neuro-developmental outcome. Parental informed consent was obtained for each subject prior to participation in the study. The current study represents a retrospective secondary analysis of this larger cohort study. The study was approved by the Washington University Human Research Protection Office. Infants born ≤30 weeks gestational age were eligible for inclusion. Infants with confounding conditions that may have independently resulted in abnormalities on cerebral MRI were excluded from this analysis, including chromosomal abnormalities, congenital anomalies, or proven congenital infections.
Demographic and clinical information was prospectively collected from the infant and maternal medical record. Information regarding anesthetic exposure during the NICU stay was retrospectively gathered from the medical chart. This included type of surgery, type of anesthetic, duration of surgery and postmenstrual age (PMA) at surgery. Data on cumulative anesthetic dose was not available, therefore the total surgical duration was used as a surrogate for this. For analysis, total surgical duration was dichotomized above/below the median split.
The peri-operative medical records were retrospectively reviewed to identify any intra-operative physiological instability that could have confounded the results. Specific details that were sought included-review of the anesthetic and operative notes for documentation of any critical event (chest compressions, hypoxic event, documented hypotension, or treatment for hypotension (inotropes or fluid boluses)). Additionally the peri-operative vitals flow charts were reviewed-data collected included minimum recorded systolic, diastolic and mean blood pressure, highest and lowest heart rate, any episode of heart rate less than 90 bpm (and duration thereof), lowest recorded oxygen saturation, and any period with oxygen saturation less than 85% (and duration thereof). Lastly, the laboratory records were reviewed, and all intra-operative, and post-operative (on day of surgery) blood gases were extracted and reviewed for signs of metabolic acidosis during the peri-operative period.
Magnetic Resonance Imaging
Imaging Protocol
Infants were imaged without sedation while asleep or resting quietly after feeding at term equivalent age (35–43 weeks PMA) (13). Images were acquired on a 3T Tim Trio system (Siemens, Erlangen, Germany). Structural images included a T1-weighted sagittal, magnetization-prepared rapid gradient echo sequence (repetition time (TR)=1550 ms, inversion time (T1)=1,100 ms, echo time (TE)=3.05ms, flip angle=15°, 1×1×1.25mm3 voxels) and a T2-weighted (T2W) fast spin echo sequence (TR=8210 ms, TE=161 ms, 1×1×1 mm3 voxels). The diffusion weighted sequence acquisition parameters included 128 mm FoV, TR=13300 ms, TE=112 ms, 1266 Hz/Px bandwidth, voxel size 1.2×1.2×1.2 mm3 and 31–48b amplitudes and directions ranging from 0–1200 s/mm2. Qualitative interpretation of the MR images was undertaken using a systematic scoring for global injury and growth impairment as previously described.(14) A score of 8 or higher on the Kidokoro Global brain abnormality score was considered a moderate or severe cerebral injury.
Volumetric and Surface-Based Processing
Volumetry was performed using the Advanced Normalization Tools (ANTs) software package (http://www.picsl.upenn.edu/ANTS), followed by manual correction using ITK-Snap software tools (15). Total Tissue Volume (TTV) was calculated for each scan by summing the volume of white, cortical gray, deep nuclear gray matter and the cerebellum. Semi-automated cortical segmentation was based on the LIGASE method followed by manual editing in Caret (16). Surface-based morphometry was performed (17). Cortical Surface Area (CSA) was calculated using the mid-thickness surface for both the left and right hemispheres. Gyrification Index (GI), the ratio of the area of the mid-thickness cortical surface area to the surface area of the cerebral hull, was also calculated for both right and left hemispheres.
Diffusion Tensor Imaging Processing
Tract Based Spatial Statistics (TBSS) was used to perform voxel-wise analysis of whole brain DTI data (18). Fractional anisotropy (FA) maps were generated from each subject’s motion-corrected, aligned, and averaged DTI data set using FMRIB’s Diffusion Toolbox (FDT), and FMRIB’s Brain Extraction Tool (BET) for skull stripping. These FA maps were used to develop a target template for registration. A mean FA image was calculated and used to produce the mean FA skeleton, which represented the center of white matter tracts. The FA images generated were projected onto the skeleton and set at a threshold of FA>0.20 for voxel-wise cross-subject statistics, comparing those that did and did not have anesthesia exposure during the neonatal period, adjusting for PMA at scan.
Neurodevelopmental Outcome
Subjects were targeted for assessment at 18 months to 2 years corrected age (mean chronological age at assessment was 24.5 months, standard deviation 3.3 months) by a blinded psychometrician using the Bayley Scales of Infant and Toddler Development, 3rd Edition (Bayley-III). Outcomes included Bayley-III cognitive, language and motor composite scores. Additionally, parents completed a medical history for the follow-up visit, indicating if the child had undergone any surgery between NICU discharge and follow-up.
Clinial and Social Risk Scores
Premature infants are exposed to a multitude of risk factors for neurodevelopmental impairment. To account for these risk factors, a clinical propensity score and a social risk index were calculated. The clinical propensity score developed included the following risk factors; gestational age, birth weight z-score, gender, duration of ventilation in hours, presence PDA requiring treatment, and confirmed sepsis. The Sociodemographic Risk Index developed included the following risk factors; maternal education, race, public health insurance, maternal age at birth and single-parent household. We utilized these composite measures as controlling for multiple covariates would not have been appropriate using conventional multivariable methods due to the small number of case events.
Data Analysis
Data were analyzed using SPSS version 23. Normally distributed data were reported as mean values with standard deviation, and comparisons performed using independent t-test. Non-parametric data were reported as median values with inter-quartile range (IQR) or range as specified in the text, and comparisons performed using the Mann-Whitney U test or Kruskal-Wallis H Test, as appropriate. The Chi Squared test was used when comparing proportions.
The clinical propensity score was calculated using a conditional forward stepwise logistic regression (p(in)=0.3, p(out)=0.15). Variables entered into the regression model were chosen as they are known risk factors for neuro-developmental impairment in VPT infants, and included variables that differed between the surgical and non-surgical population. To develop the model the identified clinical risk factors were entered as continuous variables (gestational age at birth and birthweight z-score) or transformed into dichotomous variables representing child exposure to at-risk (1) or no-risk (0) factors based on: a) clinical cut-offs, or b) the lowest/highest tertile of the sample distribution. The following variables were entered into the model: gestational age at birth, sex (male=1/female=0), medical or surgical intervention for PDA (yes=1/no=0), birthweight z-score, length of mechanical ventilation (at or above upper quartile=1/below upper quartile=0), and culture-positive sepsis (yes=1/no=0). Given the distribution of the continuous propensity score, it was dichotomized at or below 0.25. (Supplemental Figure S1 (online)).
The Sociodemographic Risk Index (range 0–5) was calculated using five maternal factors (absent=0, present=1). Factors of interest included: no high school diploma or GED, African-American, public health insurance, young maternal age at birth (≤18 years), and single-parent household. To impute missing data, the mean of the remaining components was substituted for the missing one(s) in calculating the sum. The social risk index was not calculated for individuals missing greater than 50% of the required factors within the respective domains (19–21).
Surgical anesthetic exposure was assessed as: 1) exposure versus no exposure and 2) total surgical duration (cumulative time for all surgical procedures performed). Linear mixed models were developed to explore the impact of the surgical anesthetic exposure on tissue volumes, CSA, GI and Bayley-III composite scores. For tissue volumes, separate models were developed for both absolute tissue volumes and relative tissue volumes (the relative tissue volumes were calculated as the tissue sub-type expressed as a percentage of the total tissue volume). Models of the absolute volumes explored the effect of surgery requiring anesthesia on the growth of brain tissue types in isolation, while analysis of relative volumes explored if the exposure had a greater effect on the growth of a particular tissue type relative to the infant’s other brain tissues (22).
In all models, a family ID variable was entered as a random effect accounting for sibling correlation due to the high percentage of multiple births. Models examining absolute volumes, CSA and GI included PMA at scan as a covariate. Models examining relative volumes could not adjust for PMA at scan, due to collinearity between PMA and total tissue volume. Additional covariates in all models included the clinical risk propensity score and the presence of moderate/severe cerebral injury on qualitative MRI assessment based upon the Kidokoro score (14). Models of neurodevelopmental outcome were additionally adjusted for the Sociodemographic Risk Index and any surgical anesthetic exposure following NICU discharge ascertained form parent report and clinical records in the first 2 years of life. Statistical significance was taken as p<0.05, and post-hoc analysis was performed using the least significant difference method.
Results
The study population comprised 84 children who underwent both term-equivalent MRI and developmental assessment at age 2 years (Supplemental Figure S2 (online)). Twenty-five of these children had an anesthetic exposure prior to term equivalent age (TEA) and 59 infants did not. All infants had an MRI at TEA. Infants with no surgical anesthetic exposure had an MRI slightly sooner than infants with surgical anesthetic exposure (No exposure vs. Exposure, 37 wk PMA (36–38) vs. 38 wk PMA (37–40), p=.002). The group that was exposed to anesthesia had a lower mean gestational age and birthweight (Table 1).
Table 1:
Demographic, perinatal and clinical details
| No Anesthesia (n=59) | Anesthesia (n=25) | p | |
|---|---|---|---|
| Gestational Age (wks) | 27 (26–28) | 25 (24–27) | <0.001 |
| Birthweight (g) | 1012 ± 256 | 787 ± 228 | <0.001 |
| z-score | −0.41 ± 0.96 | −0.67 ± 1.03 | 0.25 |
| Pre-natal steroids | 54 (92) | 23 (92) | 0.94 |
| Maternal Age at birth | 27 (22–33) | 29 (24–33) | 0.22 |
| Singleton | 37 (63) | 14 (56) | 0.56 |
| Male | 24 (41) | 15 (60) | 0.10 |
| Race | |||
| African-American | 21 (36) | 12 (48) | 0.28 |
| Caucasian | 33 (56) | 12 (48) | |
| Asian | 3 (5) | 1 (4) | |
| Biracial | 2 (3) | 0 | |
| Duration of Ventilation (hours) | 24 (5–96) | 696 (180–1103) | <0.001 |
| Confirmed Sepsis | 14 (24) | 14 (56) | 0.004 |
| PDA treated | 15 (25) | 18 (72) | <0.001 |
| NEC | 2 (3) | 3 (12) | 0.13 |
| Cranial Ultrasound | |||
| IVH grades 3–4 | 2 (3%) | 4 (16%) | 0.06 |
| Cystic PVL | 0 | 0 | |
| MRI abnormality score | 0.08 | ||
| Normal (0–3) | 13 (22) | 1 (4) | |
| Mild (4–7) | 24 (41) | 10 (40) | |
| Moderate (8–11) | 8 (14) | 7 (28) | |
| Severe (>12) | 7 (12) | 6 (24) | |
| Significant MRI Injury | 15 (25) | 13 (52) | 0.07 |
| Surgical and Anesthetic Data | |||
| Number of Surgeries | -- | ||
| None | 59 (100) | 0 | |
| One | 14 (56) | ||
| Two or more | 11 (44) | ||
| Total Surgical Duration (mins) | -- | 148 (63–244) | -- |
| Type of Surgery | |||
| PDA Ligation | -- | 15 (60) | -- |
| Retinopathy surgery | -- | 8 (32) | |
| Inguinal Surgery | -- | 5 (20) | -- |
| Abdominal Surgery | -- | 5 (20) | -- |
| Number of Anesthetic Agents | -- | 2 (1–3) | -- |
| Type of Anesthetic | |||
| Iso/Sevo/Des-flurane | -- | 16 (64) | -- |
| Fentanyl | -- | 20 (80) | -- |
| Propofol | -- | 9 (36) | -- |
| Ketamine | -- | 5 (20) | -- |
| Midazolam | -- | 4 (16) | -- |
| Anesthesia after discharge | 8 (14) | 11 (44) | <0.001 |
N (%), mean ± SD, median (IQR). PMA-postmenstrual age, TEA-term equivalent age, PDA-Patent Ductus Arteriosus medically or surgically treated, NEC-necrotizing enterocolitis
Among the 25 children that underwent a surgical procedure, there were 55 surgical procedures performed. Review of the peri-operative documentation found no evidence of any adverse events, or periods of significant physiological instability occurring in any infant during the peri-operative period. Two infants did have an intra-operative mean arterial pressure (MAP) that was less than 30 mmHg (an infant with cGA of 29 weeks with a MAP of 27 mmHg, and an infant with cGA 27 weeks with a MAP of 25 mmHg). However the remainder of both infant’s vitals were within normal parameters, their blood gases were not acidotic, and the clinicians present determined that they were hemodynamically stable through-out.
The median age of first surgical exposure was 27 weeks PMA (IQR: 26–32 weeks), and the median time from first surgery to MRI being performed was 11 weeks (IQR 6.5–13 wk). There was no correlation between either the PMA at which the first surgery was performed, nor the time interval between first surgery and MRI being performed, and either the absolute tissue volumes, gyrification indices or cortical surface areas (data not shown). The median duration of surgery was 148 minutes (IQR 63–244; Table 1). There was no correlation between duration of surgery and either the clinical propensity score (−.297, p=.15), nor the PMA at which surgery was performed (−.14, p=.5). Infants were stratified into never required surgery during the neonatal period (n=59), surgical duration below the median (n=12), and surgical duration above the median (n=13). Most infants received a combination of anesthetic agents, with the most common agents including a flurane-based inhaled anesthetic and fentanyl (Table 1). However, there were multiple additional agents used, reflecting a wide range in practice. Due to this variation, further analysis of the impact of the specific type of anesthetic agent was not performed.
Surgery requiring General Anesthesia during the preterm period and quantitative MRI measures at TEA
Linear mixed models demonstrated that surgical anesthesia exposure during the preterm period was associated with a decrease in the relative white matter volume (p=.007) and an increase in the relative cortical gray matter volume (p=.007) (Table 2). There was no difference in other relative tissue volumes, any of the absolute tissue volumes, cerebellar volume, GI or CSA between those that were and were not exposed (Table 2).
Table 2:
Association between Surgical Anesthesia and Volumetric and Cortical Surface Measures at TEA
| No Surgery | Surgery | No Surgery | Surgery | |||||
|---|---|---|---|---|---|---|---|---|
| Tissue Volumes (cm3) | Absolute Volumes | p1 | p2 | Relative Volumes (%) | p1 | p3 | ||
| White Matter | 133.3 (1.8) | 126.3 (2.0) | 0.056 | 0.19 | 48.1 (3.2) | 45.6 (8.0) | 0.001 | 0.007 |
| Cortical Gray Matter | 102.8 (1.8) | 112.7 (2.1) | 0.003 | 0.478 | 37.4 (3.1) | 40.5 (4.0) | 0.001 | 0.007 |
| DNGM | 22.9 (0.3) | 22.0 (0.4) | 0.14 | 0.056 | 8.3 (.6) | 8.0 (.7) | 0.153 | .081 |
| Cerebellum | 16.9 (0.4) | 16.6 (0.5) | 0.125 | 0.156 | 6.3 (.7) | 5.9 (.8) | 0.029 | 0.626 |
| Total Tissue | 279.9 (5.1) | 270.1 (6.1) | 0.945 | 0.260 | ||||
| Gyrification Index | ||||||||
| Right Hemisphere | 1.81 (0.02) | 1.76 (0.02) | 0.804 | 0.061 | ||||
| Left Hemisphere | 1.77 (0.02) | 1.75 (0.02) | 0.598 | 0.533 | ||||
| Cortical Surface Area (cm2) | ||||||||
| Right Hemisphere | 251.0 (5.1) | 249.1 (5.9) | 0.543 | 0.815 | ||||
| Left Hemisphere | 247.4 (4.9) | 246.6 (5.9) | 0.517 | 0.928 | ||||
Absolute volumes- estimated marginal means (SD), relative volumes - % [tissue type/TTV](SD)
p1- Unadjusted
p2- Adjusted for pma, propensity score, and MRI brain injury
p3-Adjusted for propensity score, and MRI brain injury
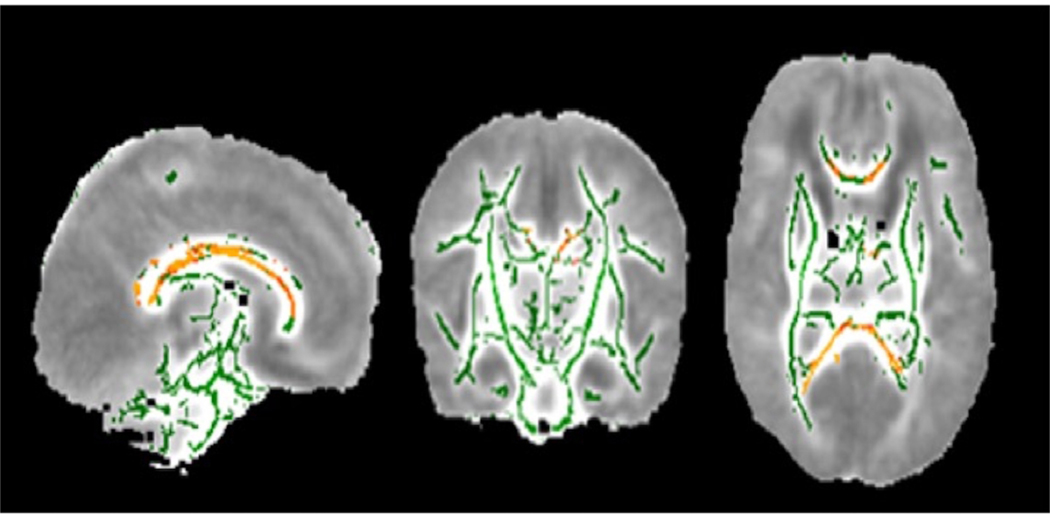
TBSS analysis demonstrated significantly lower FA values in the corpus callosum and cingulum bundle infants exposed to surgery during the preterm period compared to infants that were not exposed (p<.05) (Figure 1).
Figure 1: Tract-based spatial statistics (TBSS) results comparing infants that were and were not exposed to anesthesia during the neonatal period.
Yellow and Red tracts indicate decreased Fractional Anisotropy (FA) in anesthesia exposed infants in cingulate gyrus and corpus callosum. Green tracts indicate no difference in FA. (p <.05)
Duration of Exposure
Linear mixed models showed decreased relative white matter volumes were associated with a longer duration of surgery (p=.002) (Table 3). On post-hoc analysis, the difference in relative white matter volumes remained significant between those that had surgical duration above the median and both those that had no surgery (p=.001) and those below the median surgical duration (p=.02), respectively. Differences in relative white matter volumes between infants with shorter surgical duration and those that had no surgery did not reach statistical significance (p=.58).
Table 3:
Effect of Surgical Duration on Volumetric and Cortical Surface Measures
| Surgical Duration | Surgical Duration | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No Surgery | Below Median | Above Median | p1 | p2 | No Surgery | Below Median | Above Median | p1 | p2 | |
| Tissue Volumes (cm3) | Absolute Volumes | Relative Volumes (%) | ||||||||
| White Matter | 133.2 (1.7) | 130.9 (3.0) | 121.5 (2.7) | 0.112 | 0.290 | 48.1 (3.2) | 47.0 (2.4) | 44.2 (3.7) | 0.001 | 0.002 |
| Cortical Gray Matter | 104.9 (1.7) | 107.8 (2.9) | 117.3 (2.9) | <0.001 | 0.128 | 37.4 (3.1) | 38.6 (2.7) | 42.3 (4.3) | <0.001 | 0.001 |
| DNGM | 22.9 (0.3) | 22.4 (0.6) | 21.5 (0.6) | 0.281 | 0.161 | 8.3 (.6) | 8.4 (0.7) | 7.7 (0.7) | 0.042 | 0.028 |
| Cerebellum | 16.9 (0.4) | 17.1 (0.7) | 16.2 (0.7) | 0.260 | 0.241 | 6.3 (.7) | 5.9 (.8) | 5.8 (1.0) | 0.076 | 0.732 |
| Total Tissue | 279.8 (5.1) | 263.2 (8.2) | 278.8 (9.2) | 0.062 | 0.244 | |||||
| Gyrification Index | ||||||||||
| Right Hemisphere | 1.82 (0.02) | 1.79 (0.04) | 1.73 (0.03) | 0.457 | 0.077 | |||||
| Left Hemisphere | 1.77 (0.19) | 1.79 (0.03) | 1.71 (0.03) | 0.481 | 0.228 | |||||
| Cortical Surface Area (cm2) | ||||||||||
| Right Hemisphere | 250.9 (5.1) | 240.6 (9.2) | 256.3 (8.5) | 0.011 | 0.484 | |||||
| Left Hemisphere | 247.0 (4.9) | 240.8 (8.6) | 252.6 (8.5) | 0.011 | 0.669 | |||||
Absolute volumes- estimated marginal means (SD), relative volumes - % [tissue type/TTV](SD)
p1- Unadjusted
p2- Adjusted for pma, propensity score, and MRI brain injury
p3- Adjusted for propensity score, and MRI brain injury
Relative cortical gray matter volumes demonstrated the opposite pattern, showing a positive linear relationship to the duration of surgery (Table 3). Further analysis of the cortical gray matter volumes showed the differences remained significant between those that had surgical duration above the median duration and both those that had no surgery (p<0.001), and those below the median surgical duration (p=0.008), respectively. There was again no difference in relative cortical gray matter volume between those that had no surgery and those whose surgical duration was less than the median duration (p=0.58).
Lastly there was a difference in the relative deep nuclear grey matter volumes, with decreased volumes associated with longer surgical duration (p=.028). Post-hoc analysis demonstrated that this effect was mediated by prolonged surgical exposure. Infants’ who had a surgical duration above the median had decreased relative volumes compared to those that had no surgical exposure (p=.013) and those with a surgical duration below the median (p=.04). There was no difference between those with no surgical exposure and those with surgical duration below the median (p=.9).
Surgery requiring General Anesthesia Anesthesia during the preterm period and neurodevelopmental outcomes at 2 years
The mean cognitive composite score on the Bayley-III at age two years in the cohort was 86.3 (SD ±9.3), language composite score was 88.8 (±11.2), and motor composite score was 83.9 (±10.8). Multivariable models adjusting for the clinical propensity score, cerebral injury, and sociodemographic risk index were developed to explore the effect of exposure to surgery requiring anesthesia on neurodevelopmental outcome. These models demonstrated surgical exposure during the preterm period was associated with lower cognitive (p=.002) and motor composite scores (p=.005), with a trend toward lower language composite scores (p=.09) (Table 4).
Table 4:
Association between Surgical Anesthesia and 2 Year Neurodevelopmental Outcome
| Bayley-III | No Anesthesia | Anesthesia | p |
|---|---|---|---|
| Cognitive Composite | 89.4 (2.1) | 75.6 (3.9) | 0.002 |
| Language Composite | 89.4 (2.6) | 80.1 (4.9) | 0.09 |
| Motor Composite | 86.7 (2.3) | 73.5 (4.0) | 0.005 |
Estimated Marginal Means and Standard Error from Linear Mixed Models for 2 Year Follow-Up adjusted for propensity score, moderate/severe MRI abnormality, anesthetic exposure post NICU discharge and Sociodemographic Risk index
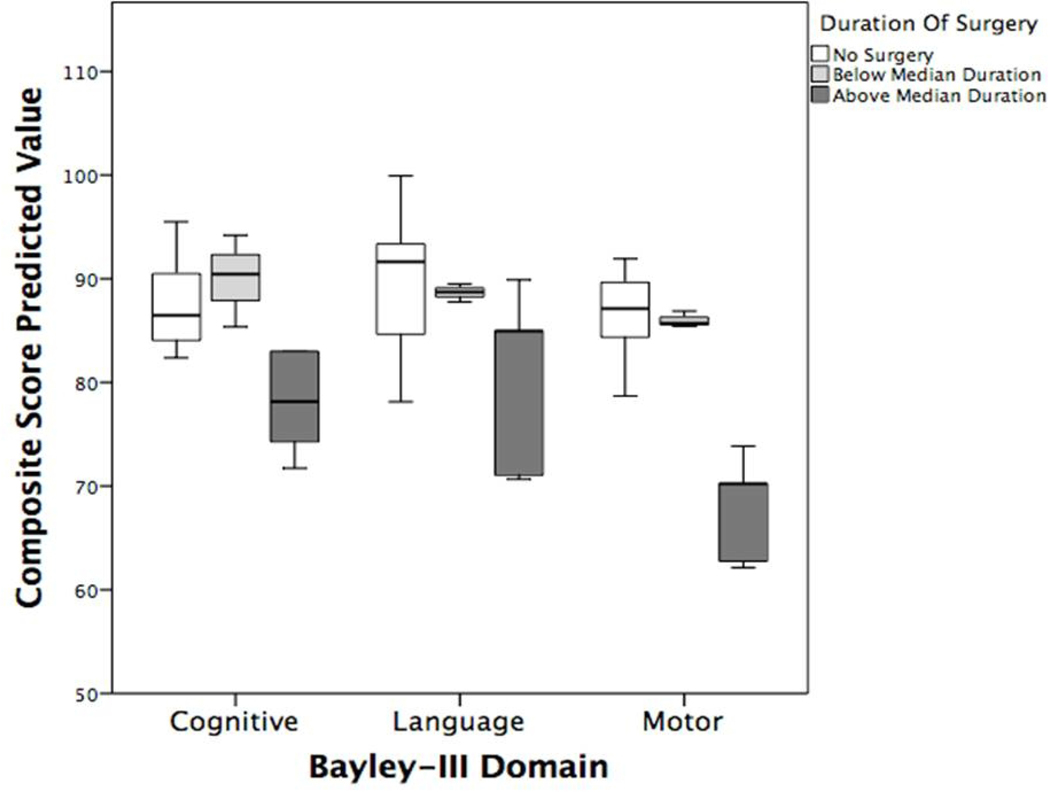
Stratifying by duration of surgery, there was an association between longer surgical duration and lower cognitive (p=.001) and motor composite scores (p=.001), but not language scores (p=.2). Post-hoc analysis demonstrated the differences in cognitive scores remained significant only for infants with longer surgical duration compared to no exposure (p=.001). Post-hoc analysis of the motor composite scores found infants with longer surgical duration had lower scores than both those with no exposure (p=.001) or infants with shorter surgical duration (p=.017) (Figure 2).
Figure 2: Association between duration of surgery and age 2 neurodevelopmental outcomes.
Boxplots showing no anesthetic exposure in neonatal period (white), surgical duration less than median duration (grey), and surgical duration greater than the median duration (black).
To assess if either the reduced relative white matter or increased cortical gray matter on MRI at TEA explained the neurodevelopmental findings, the analysis was rerun adding these variables as covariates in the mixed models. Addition of these quantitative MRI metrics did not significantly alter the results; the cognitive composite score and motor composite score remained lower in those that were exposed to anesthesia (p=.005, and p=.004; respectively), and continued to be negatively associated with duration of exposure (p=.008, and p=.001).
Discussion
This study described an association between exposure to surgery requiring general anesthesia during the preterm period with both alterations in brain development at TEA and cognitive and motor deficits at age two years. Infants with longer exposures had the greatest relative changes in white and gray matter volumes, and lowest cognitive and motor scores during early childhood. This association with the brain tissue volumes was only significant when comparing relative volumes. This indicates a range in the vulnerability of brain tissue types, with the white and cortical gray matter demonstrating increased susceptibility. Interestingly, controlling for the MRI findings, surgical anesthetic exposure continued to be associated with cognitive and motor deficits at age two. This suggests that the adverse outcome observed is not fully explained by current neuroimaging methods of delineating cerebral injury or altered volumes at TEA, but rather a more complex alteration in neural development.
The infants who required surgery had a lower gestational age, and higher incidence of the complications of prematurity than those who did not require surgical intervention. This is to be expected, however, these factors could potentially confound the results and therefore needed to be controlled for in the analysis. To achieve this we included a clinical propensity score, presence of MRI injury and socio-demographic risk index as covariates in our models. These incorporated the baseline risks prior to, and independent of, surgery into the models. Controlling for these variables the impact of the exposure remained significant. Additionally there was no correlation between the duration of surgery and either the propensity score or social risk index. Despite this, infants with the longest surgical duration demonstrated the greatest alteration in brain volumes, and worst neuro-developmental outcome. This further suggests that the altered brain volumes and neuro-developmental outcome we described were associated with the period of surgery itself.
There has been increasing concern about the exposure of the developing brain to both surgery and anesthesia over the past decade. Animal models have demonstrated the potential neurotoxic effect of a range of anesthetic agents on the developing brain (5, 23–26). The exact mechanism of injury is likely multi-modal (24),(27),32−34, and appears to be selective to the immature brain (27),(28). In particular the immature oligodendrocyte appears to be sensitive to the direct neurotoxic effects of anesthetics (5, 29). In the preterm infant the immature oligodendrocyte is known to be exquisitely vulnerable to inflammatory mediated injury, likely compounding the injury further (30–32). A reduction in mature oligodendrocytes, either through direct neurotoxic effects, or secondary to pro-inflammatory injury, would be consistent with the reductions in white matter volumes and alterations in white matter microstructure in the cingulum and corpus callosum demonstrated in the current study.
Studies looking at the associations between pediatric surgery, anesthesia and neuro-developmental outcomes have typically been performed on older children, who underwent surgery after the neonatal period (1, 2, 6, 7). However two recent studies, exclusively looked at neonatal surgery, and demonstrated an association with both cerebral injuries(12), and delayed cerebral maturation (3). Stolwijk et al reported that 75% of preterm infants (median GA at birth 34 weeks) demonstrated mild to moderate MRI injury post-operatively. In an alternate study, Moran et al. looked at the term equivalent MRI and two year neuro-developmental outcome of infants that underwent non-cardiac surgery. The median GA at birth was 37.8 weeks. Infants exposed to surgery had increased white mater injury on MRI (median age at scan was 25 days), and lower motor and language composite scores at age 2 years. Interestingly despite the short time interval between surgery and MRI (less than 4 weeks) they found that surgery was associated with significantly delayed gyral maturation. The cohorts in these studies are however different to our own, as very preterm infants were not included in their respective studies.
There is limited published data regarding anesthesia exposure in preterm infants. In a large cohort of very preterm infants (VPT), Morriss et al. reported that infants who underwent surgery during the initial hospitalization, had lower mean MDI and PDI scores at 18 to 24 months of age on the Bayley II scales (10). Interestingly, in a secondary analysis sub-dividing infants into those that required “major surgery” versus “minor surgery”, after controlling for clinical, and social covariates, only “major surgery” exposure was associated with neurodevelopmental impairment. The authors postulated that this may reflect duration of surgery and/or anesthetic exposure, as all major surgeries would have required general anesthesia, while the minor surgeries may have been performed under regional anesthesia. In a smaller cohort, Filan et al. compared the TEA brain volumes of preterm infants that underwent surgery to those that did not (11). The authors demonstrated that infants who underwent surgery during the preterm period had reductions in deep nuclear grey volumes, with increased white matter injury on TEA MRI scans. These findings are in keeping with the current study, which demonstrated reductions in relative deep nuclear grey volumes at TEA among those with the longest surgical duration. Filan et al. postulated that the alterations in brain growth described may have been associated with exposure to anesthesia in the surgical subgroup, pointing out that the original FDA warning had highlighted concerns over reduced deep nuclear gray matter volumes associated with certain anesthetic agents. Data on the actual anesthetic agent used, or level of exposure, was not available in either of these studies.
The only prior study of preterm infants that provided any data on anesthetic exposure during the preterm period was that of Gano et al (33). They reported on a cohort of infants born <33 weeks gestation who underwent surgery prior to TEA. These infants were followed to school age, and their outcome was assessed using the Wechsler Preschool and Primary Scale of Intelligence, 3rd edition. Gano reported that undergoing two or more surgical procedures prior to TEA was associated with adverse neurodevelopmental outcome with a 20-point lower full scale IQ compared to infants that had no surgical exposure. The authors also noted that a single surgical exposure was not associated with any adverse neurodevelopmental outcome. It is important to note that there were some differences between the current cohort, and that of Gano. The first is that there was a variation in which agents infants were exposed to, with only 25% of Gano’s cohort being exposed to a volatile anesthetic agent, contrasting with 60% in the current study. Second, the median surgical duration in the Gano study was 80 minutes as compared to 190 minutes in the current study. Although neither study could report on the cumulative anesthetic dose, the disparity in surgical duration between the cohorts may imply a considerable difference in the associated anesthetic exposure. It must be emphasized, however, that while there are some differences between populations and results, ultimately both the current study and that of Gano, the only studies to-date of surgical anesthetic exposure in the preterm period, both demonstrated an association between longer surgical anesthetic exposure and lower neurodevelopmental scores..
The current study also reported an increase in cortical gray matter volumes, which may appear paradoxical. One potential explanation is that these findings may reflect altered brain development in regions such as the subventricular zone, which is known to be a vulnerable region in the immature brain and is responsible for late neuronal migration (32),(34). These late migrating neurons are important contributors to cortical thickness. Moeskops et al. recently reported serial MRI scans at 30 and 40 weeks PMA on a cohort of ELBW infants (not exposed to anesthesia) (35). They found that at 30 weeks there was no correlation between cerebral atrophy and cortical indices, however, by 40 weeks a significant association had developed. Specifically, they found that increased cerebral atrophy was positively correlated with an increase of both cortical thickness and cortical grey matter volumes. The authors proposed that these counter-intuitive findings represented altered brain development secondary to a combination of injury to the premyelinating oligodendrocytes and disrupted migration of the late migrating GABAergic neurons. This, they suggested, would result in a reduced distinction between the unmyelinated white matter and cortical grey matter, leading to the increased cortical indices. The current study reported a similar pattern on TEA MRI. Therefore, it is plausible that surgical anesthetic exposure impacts the premature brain through a combination of both injury to the white matter and disruption to neuronal migration. Disruption to neuronal migration could in part explain why the differences between groups for neurodevelopmental outcome remained after controlling for the altered regional volumes on TEA MRI.
A limitation of the current study was that while only VPT infants were included, there were significant differences between the infants that required surgery and those who did not. VPT infants of lower gestational age are at higher risk for surgery than those at a higher gestational age, but also they have a higher independent risk of having the common co-morbidities of prematurity than their older counter-parts. This is an issue that each of the studies discussed above, that investigated the association between surgery and outcome in VPT infants, have reported. Similar to the current study these previous papers reported that VPT infants requiring surgery had a significantly lower gestational age(10, 33), and smaller birth weight (10, 11), and additionally had a higher incidence of the complications of prematurity including sepsis(10, 33), BPD(10, 11, 33), and PDAs(10, 33), compared to their non-surgical populations. These are important confounding variables which, similar to previous studies, we have attempted to control for in our models. However the authors cannot exclude the possibility of unrecognized events such as subtle perturbation of physiological homeostasis during the NICU course, or during transfer to the operating room, that could also contribute to alterations in brain development and neurodevelopmental impairment. Certain surgical procedures can be safely performed in the NICU rather than in the OR (38). Such an approach could reduce the potential for unrecognized physiological disturbances by negating the need for transport, however this is not standard of care in our unit. These limitations prevent us moving beyond describing an observed association.
As the median PMA at time of surgery was 27 weeks, pre-operative imaging was confined to cranial ultrasound. Cranial ultrasound imaging is not directly comparable to MRI imaging, and therefore without pre-operative MR imaging we cannot exclude the possibility that some of the MRI injuries described were present pre-operatively. Further limitations include the retrospective nature of the analysis, a small sample size of VPT infants that underwent surgery, that could increase the risk of a type II error, and that we were unable to provide data on the use of opiates for sedation during the time in the NICU. However recent work has clearly shown that opiate exposure in the preterm infant in particular impacts the cerebellum, leading to decreased volumes at TEA (36, 37). There was no apparent effect of the surgical anesthesia on cerebellar volumes in our cohort. This would suggest that the impact of surgical anesthesia described here may be mediated by a different pathway than that found in pure opiate exposure.
In conclusion, this retrospective analysis found that VPT infants who required surgery during the preterm period had reduced white mater volumes at TEA and lower cognitive and motor composite scores at 2 years. Our results support previous publications in the field, however the underlying pathophysiology for these findings remains unclear. Further research is required to explore potential mechanisms for the associations described.
Supplementary Material
Impact:
In very preterm infants, there is an association between surgery requiring general anesthesia during the preterm period and reduced white mater volume on MRI at TEA, and lower cognitive and motor composite scores at age 2 years.
It is known that the very preterm infant’s brain undergoes rapid growth during the period corresponding to the third trimester.
The current study suggests an association between surgery requiring general anesthesia during this period and worse outcomes.
Acknowledgements:
We would like to thank Dr. Regina Triplett for her assistance in qualitative interpretation of the MR images.
Statement of Financial Support: This work was supported by the Eunice Kennedy Shriver National Institute Of Child Health & Human Development with Awards U54 HD087011 (Intellectual and Developmental Disabilities Research Center at Washington University (JSS)) and R01 HD 057098 (original WUNDER cohort grant): K02 NS089852 and UL1 TR000448.
Footnotes
Disclosure Statement: The authors have no potentical conflicts of interest, and no disclosures to report.
Category of Study: Clinical Research Study
Consent: The study was approved by the Washington University Human Research Protection Office. Parental informed consent was obtained for each subject prior to enrollment.
References
- 1.Wilder RT, et al. Early exposure to anesthesia and learning disabilities in a population-based birth cohort. Anesthesiology 2009;110:796–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Flick RP, et al. Cognitive and behavioral outcomes after early exposure to anesthesia and surgery. Pediatrics 2011;128:e1053–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moran MM, et al. Associations of Neonatal Noncardiac Surgery with Brain Structure and Neurodevelopment: A Prospective Case-Control Study. J Pediatr 2019;212:93–101.e2. [DOI] [PubMed] [Google Scholar]
- 4.Sanders RD, Hassell J, Davidson AJ, Robertson NJ, Ma D. Impact of anaesthetics and surgery on neurodevelopment: an update. Br J Anaesth 2013;110 Suppl 1:i53–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brambrink AM, et al. Isoflurane-induced apoptosis of oligodendrocytes in the neonatal primate brain. Ann Neurol 2012;72:525–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sun LS, et al. Association Between a Single General Anesthesia Exposure Before Age 36 Months and Neurocognitive Outcomes in Later Childhood. JAMA 2016;315:2312–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davidson AJ, et al. Neurodevelopmental outcome at 2 years of age after general anaesthesia and awake-regional anaesthesia in infancy (GAS): an international multicentre, randomised controlled trial. Lancet 2016;387:239–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rizzi S, Ori C, Jevtovic-Todorovic V. Timing versus duration: determinants of anesthesia-induced developmental apoptosis in the young mammalian brain. Ann N Y Acad Sci 2010;1199:43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Andescavage NN, et al. Complex Trajectories of Brain Development in the Healthy Human Fetus. Cereb Cortex 2017;27:5274–5283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Filan PM, Hunt RW, Anderson PJ, Doyle LW, Inder TE. Neurologic outcomes in very preterm infants undergoing surgery. J Pediatr 2012;160:409–14. [DOI] [PubMed] [Google Scholar]
- 11.Morriss FH Jr., et al. Surgery and neurodevelopmental outcome of very low-birth-weight infants. JAMA Pediatr 2014;168:746–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stolwijk LJ, et al. Neonatal Surgery for Noncardiac Congenital Anomalies: Neonates at Risk of Brain Injury. J Pediatr 2017;182:335–41.e1. [DOI] [PubMed] [Google Scholar]
- 13.Mathur AM, Neil JJ, McKinstry RC, Inder TE. Transport, monitoring, and successful brain MR imaging in unsedated neonates. Pediatr Radiol 2008;38:260–4. [DOI] [PubMed] [Google Scholar]
- 14.Kidokoro H, Neil JJ, Inder TE. New MR imaging assessment tool to define brain abnormalities in very preterm infants at term. AJNR Am J Neuroradiol 2013;34:2208–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klein A, et al. Evaluation of volume-based and surface-based brain image registration methods. Neuroimage 2010;51:214–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Van Essen DC, et al. An integrated software suite for surface-based analyses of cerebral cortex. J Am Med Inform Assoc 2001;8:443–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hill J, et al. A surface-based analysis of hemispheric asymmetries and folding of cerebral cortex in term-born human infants. J Neurosci 2010;30:2268–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith SM, et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage 2006;3:1487–505. [DOI] [PubMed] [Google Scholar]
- 19.Lean RE, Paul RA, Smyser CD, Rogers CE. Maternal intelligence quotient (IQ) predicts IQ and language in very preterm children at age 5 years. J Child Psychol Psychiatry 2018;59:150–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lean RE, Paul RA, Smyser TA, Smyser CD, Rogers CE. Social Adversity and Cognitive, Language, and Motor Development of Very Preterm Children from 2 to 5 Years of Age. J Pediatr 2018;203:177–84.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lean RE, et al. Maternal and family factors differentiate profiles of psychiatric impairments in very preterm children at age 5-years. J Child Psychol Psychiatry 2020;6:157–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matthews LG, et al. Brain growth in the NICU: critical periods of tissue-specific expansion. Pediatr Res 2018;83:976–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Makaryus R, et al. Brain maturation in neonatal rodents is impeded by sevoflurane anesthesia. Anesthesiology 2015;123:557–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Broad KD, et al. Isoflurane Exposure Induces Cell Death, Microglial Activation and Modifies the Expression of Genes Supporting Neurodevelopment and Cognitive Function in the Male Newborn Piglet Brain. PLoS One 2016;11:e0166784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jevtovic-Todorovic V, et al. Early exposure to common anesthetic agents causes widespread neurodegeneration in the developing rat brain and persistent learning deficits. J Neurosci 2003;23:876–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fredriksson A, Pontén E, Gordh T, Eriksson P. Neonatal exposure to a combination of N-methyl-D-aspartate and gamma-aminobutyric acid type A receptor anesthetic agents potentiates apoptotic neurodegeneration and persistent behavioral deficits. Anesthesiology 2007;107:427–36. [DOI] [PubMed] [Google Scholar]
- 27.Stratmann G, et al. Isoflurane differentially affects neurogenesis and long-term neurocognitive function in 60-day-old and 7-day-old rats. Anesthesiology 2009;110:834–48. [DOI] [PubMed] [Google Scholar]
- 28.Deng M, et al. Brain regional vulnerability to anaesthesia-induced neuroapoptosis shifts with age at exposure and extends into adulthood for some regions. Br J Anaesth 2014;113:443–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ing C, et al. Latent Class Analysis of Neurodevelopmental Deficit After Exposure to Anesthesia in Early Childhood. J Neurosurg Anesthesiol 2017;29:264–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dammann O, Leviton A. Maternal intrauterine infection, cytokines, and brain damage in the preterm newborn. Pediatr Res 1997;42:1–8. [DOI] [PubMed] [Google Scholar]
- 31.Lodygensky GA, et al. In vivo MRI analysis of an inflammatory injury in the developing brain. Brain Behav Immun 2010;24:759–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Volpe JJ. Brain injury in premature infants: a complex amalgam of destructive and developmental disturbances. Lancet Neurol 2009;8:110–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gano D, et al. Impaired cognitive performance in premature newborns with two or more surgeries prior to term-equivalent age. Pediatr Res 2015;78:323–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu G, et al. Late development of the GABAergic system in the human cerebral cortex and white matter. J Neuropathol Exp Neurol 2011;70:841–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moeskops P, et al. Development of Cortical Morphology Evaluated with Longitudinal MR Brain Images of Preterm Infants. PLoS One 2015;10:e0131552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zwicker JG, et al. Smaller Cerebellar Growth and Poorer Neurodevelopmental Outcomes in Very Preterm Infants Exposed to Neonatal Morphine. J Pediatr 2016;172:81–7.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McPherson C, et al. Brain Injury and Development in Preterm Infants Exposed to Fentanyl. Ann Pharmacother 2015;49:1291–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sinha SK, and Neogi S. Bedside Neonatal Intensive Care Unit Surgery-Myth or Reality! Journal of Neonatal Surgery 2013;2:20. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.