Abstract
Expression of abnormally long polyglutamine (polyQ) tracks is the source of a range of dominant neurodegenerative diseases, such as Huntington disease. Currently, there is no treatment for this devastating disease, although some chemicals, e.g., metformin, have been proposed as therapeutic solutions. In this work, we show that metformin, together with salicylate, can synergistically reduce the number of aggregates produced after polyQ expression in Caenorhabditis elegans. Moreover, we demonstrate that incubation polyQ-stressed worms with low doses of both chemicals restores neuronal functionality. Both substances are pleitotropic and may activate a range of different targets. However, we demonstrate in this report that the beneficial effect induced by the combination of these drugs depends entirely on the catalytic action of AMPK, since loss of function mutants of aak-2/AMPKα2 do not respond to the treatment. To further investigate the mechanism of the synergetic activity of metformin/salicylate, we used CRISPR to generate mutant alleles of the scaffolding subunit of AMPK, aakb-1/AMPKβ1. In addition, we used an RNAi strategy to silence the expression of the second AMPKβ subunit in worms, namely aakb-2/AMPKβ2. In this work, we demonstrated that both regulatory subunits of AMPK are modulators of protein homeostasis. Interestingly, only aakb-2/AMPKβ2 is required for the synergistic action of metformin/salicylate to reduce polyQ aggregation. Finally, we showed that autophagy acts downstream of metformin/salicylate-related AMPK activation to promote healthy protein homeostasis in worms.
Keywords: Metformin, Salycilate, AMPK, polyQ toxicity, Caenorhabditis elegans, Synergy
1. Introduction
Several late-onset neurodegenerative diseases are caused by abnormal microsatellite expansions of CAG repeats, which encode tracts of polyglutamines (polyQs) (reviewed in Paulson [1]). These expansions lay within encoding regions of at least nine endogenous genes, which have unrelated functions (see for a review [2]). For example, HTT encodes huntingtin, a protein of unknown function that causes Huntington disease (HD) when more than 35 CAG triplets are present [3]. Other diseases caused by polyQ repeats include six of the spinocerebellar ataxias (SCA 1, 2, 3, 6, 7, and 17), dentatorubral-pallidoluysian atrophy (DRPLA), and spino-bulbar muscular atrophy (SBMA) [1]. The causative genes of the diseases encode proteins with different functions that vary from mRNA translation and RNA regulation to ubiquitin-proteasome function (ataxin-1 and ataxin-3 [4,5]). These molecules, which have important roles within cells, become gain-of-function toxic proteins when expansions of polyQs go beyond a pathological threshold [6]. These mutant proteins become unstable and show a tendency to misfold and, as a consequence, become very prone to aggregation. Therefore, the hallmark of all these diseases is the presence of aggregates, and eventually, inclusion bodies (IB), which include the mutant protein but also unrelated proteins sequestered within [7]. The fact that the same source of toxicity (i.e., polyQ expansions within endogenous proteins) produces different diseases may be related to the distinct expression patterns of these genes, which produce malfunction in different neuronal types [2].
Caenorhabditis elegans is a genetically tractable model, which has been widely used to study polyQ toxicity (see for example [8–10]). Worms expressing polyQs in different tissues helped to uncover many genes modulating the rate of aggregation and/or the toxicity induced by these molecules [8,11–14]. Using neuronal and muscular models of polyQ toxicity in C. elegans, we described AMP-activated protein kinase (AMPK) as a good candidate to manipulate aggregation and toxicity induced by these molecules [15,16]. Moreover, AMPK activation is neuroprotective in in vivo mouse models of HD [15–17].
AMPK is an obligate heterotrimer enzyme composed of a catalytic subunit (AMPKα) and two regulatory subunits (AMPKβ and AMPKγ). AMPK is a master regulator of energy and metabolism in cells [18]. In addition, this enzyme can react to different types of stress of a different nature, e.g., polyQ-induced toxicity [15–17]. The regulation of AMPK function is very complex. When ATP levels are low, the concentration of AMP rises, and this molecule binds to AMPKγ, a regulatory subunit of this enzyme, which in turn activates the catalytic subunit (AMPKα) of the complex, inducing the phosphorylation of several targets (reviewed by Kim et al. [19],). In addition to AMP, AMPK is activated by a plethora of metabolites, protein kinases, and synthetic compounds [19]. Among the synthetic activators, metformin is widely accepted to be a classic indirect activator of this enzyme [19]. This compound, which is widely used to treat type 2 diabetes, induces a mild inhibition of complex I of the electron transport chain in the mitochondria. Non-lethal mild inhibition of this complex increases AMP levels, which in turn activates AMPK.
Interestingly, metformin can reduce polyQ-induced aggregation in muscle cells and neuronal toxicity in C. elegans [15,16] and reduce behavioural phenotypes in mammalian models of HD [15–17,20,21]. Metformin has also beneficial effects when administered in HD patients. In this regard, we have analysed the so-called Enroll-HD database, which included at the time more than 8000 HD patients and healthy controls. Through this analysis, we demonstrated that HD patients who were also type 2 diabetics and were taking metformin to treat this condition, showed better marks in cognitive tests, compared to non-diabetic HD patients [22]. Another well-known modulator of AMPK activity is salicylate, which binds to AMPKβ (scaffolding subunit) to induce activation of the complex [19]. Some studies have shown that both compounds, metformin and salicylate, are able to activate AMPK in a synergistic way to improve insulin resistance [23]. Moreover, incubating cells with combinations of aspirin and metformin enhances apoptosis in in vitro models of breast cancer [24]. However, both substances act pleiotropically and therefore AMPK is not their only target. For example, it has been shown that metformin protects from cancer by indirect activation of AMPK, inhibition of Hexokinase II and suppression of gluconeogenesis, through inhibition of glycerol-3-phosphate dehydrogenase [25]. Moreover, metformin can also alter glucose transport and enhances HIF1A (Hipoxia Inducible Factor 1 Subunit Alpha) degradation [25]. In murine models of atherosclerosis it is protective by activation of AMPK, reducing the expression of AT1R (type 1 Angiotensin II Receptor) and increasing the expression of SOD1 (Superoxide dismutase 1) [26]. Salicylate shows also complex modulation of targets, different than AMPK. It is well-known that salicylate is able to promote the acetylation and inhibition of cyclooxygenases to reduce inflammation [27]. This substance is chemoprotective against colorectal cancer through acetylation of cellular cyclins (CDKs) [28], and it does suppress nuclear translocation of HsGAPDH preventing cell death [29]. Those are a few examples of the different nature of targets modulated by metformin and/or salicylate. In this work, we show that synergistic activation of AMPK, using low doses of metformin and salicylate, can reduce polyQ aggregation and neuronal impairment in C. elegans models of polyQ toxicity. Moreover, we show that metformin/salicylate synergy is dependent exclusively on AMPK. We also report that the benefitial effects produced by both drugs require a functional autophagy pathway.
2. Material and methods
2.1. Maintenance of C.elegans strains
All worm strains were maintained at 20 °C degrees as described elsewhere [30]. N2 standard wild type [30], AM141: rmIs133[unc-54p::40Q::YFP] X [8] and NL5901: pkIs2386[unc-54p::alphasynuclein::YFP + unc-119(+)] IV [31] strains were obtained from the Caenorhabditis Genetics Center (CGC, Minneapolis, MN, USA). The strains RVM131: vltEx131[mec-3p::112Q::TdTomato; myo-2p::GFP], RVM132: vltEx131[mec-3p::112Q::TdTomato; myo-2p::GFP]; aak-2(ok524) X, and RVM137: rmIs133[unc-54p::40Q::YFP] X; aak-2(ok524) X were described elsewhere [16]. In this work we have developed the following strains: RVM301: aakb-1(vlt18) X; RVM304: vltEx131[mec-3p::112Q::TdTomato; myo-2p::GFP]; aakb-1(vlt18) X; and RVM305: rmIs133[unc-54p::40Q::YFP] X; aakb-1(vlt18) X. All strains were out-crossed at least three times.
2.2. Generation of knock-out worms using CRISPR/Cas9 system
CRISPR/Cas9 was used to generate a knock-out in aakb-1 gene. We based our design and strategy on a modified protocol from the Ceron’s Lab (IDIBELL Institute, Barcelona, Spain) [32]. We used Alt-R™ S.p. Cas9 Nuclease 3NLS, and three RNAs to produce two gRNAs: Alt-R™ CRISPR-Cas9 tracrRNA and two AltR™ CRISPR-Cas9 crRNA from Integrated DNA Technologies (IDT DNA, Coralville, IA, USA). The gene dpy-10 was also disrupted as a selection marker, as described elsewhere [33]. The guide RNA sequences designed to target aakb-1 were: 5ʹ-ACG AGC TTG GAA TTC CAC CG-3ʹ and 5ʹ-AGA GGC TAA ATC CTT GTC GA-3ʹ. The RNA was resuspended in 20 μl of Nuclease-free Duplex Buffer from IDT DNA. The final concentrations of the CRISPR components were: Alt-R™ S.p. Cas9 Nuclease 3NLS: 4.5 μM; Alt-R™ CRISPR-Cas9 tracrRNA: 32 μM; target gene AltR™ CRISPR-Cas9 crRNA: 35 μM.
2.3. RNA interference
Worms were fed with E. coli strain HT115 expressing dsRNA of aakb-2, atg-18, bec-1 and lgg-1 genes into the pL4440 plasmid. A coding fragment of each gene was amplified using the Phusion high fidelity polymerase (Thermo Fisher Scientific, Waltham, MA, USA) and the following primers: FRW_aakb-2: 5ʹ- GGC AAC AAT CAG TCT GGA GG-3ʹ; REV_aakb-2: 5ʹ-TAC CAT CGG GAT CCG CGT CTG C-3ʹ; FRW_atg-18: 5´- CAC ACT GAC ATA TGA AGG CG-3´; REV_atg-18: 5´-AAG AGA TGA ACA GAT CCA GTG-3. FRW_bec-1: 5´-TGT GCT TCC ACA TTT GGG TTG ATG-3´; REV_bec-1: 5´-AGC CAT TGC ACG AGT CCA TCG-3´; FRW_lgg-1: 5ʹ-GAA TCA AAA TGA AGT GGG CTT AC-3ʹ; REV_lgg-1: 5ʹ-TCC TTC TTT TCG ACC TCT CCT CC-3ʹ.. PCR products were cloned into an EcoRV site of the pL4440 plasmid to generate RNAi vectors: pJB1_pL4440-lgg-1, pJB2_ pL4440-aakb-2, pJB3_pL4440-bec-1 and pJB4_pL4440-atg-18. All vectors, including the empty vector pL4440, were grown overnight at 37 °C in Luria-Bertani liquid medium containing 50 μg/mL carbenicillin to select positive colonies. We added 1 mM IPTG into grown feeding bacteria cultures and incubated for 2 h at 37 °C and shaking before seeding RNAi plates. This induction step allowed us to intensify RNAi effect in worms. Autophagy-related genes are essential for appropriate worm development so we induced the knock down expression after L3 stage, to avoid inducing developmental arrest of the worms. Feeding worms with the E. coli strain HT115 has been shown to change the expression of the promoter of the muscular unc-54/myosin gene [34]. This is the promoter that drives the expression of the polyQ construct in muscle cells. If the expression of the polyQs is reduced, then we would observe and artefactual reduction of the aggregation process, which is heavily influenced by the concentration of the polyQs. To avoid this, we also induced RNAi against non-lethal genes in L3 larvae. Therefore, synchronized populations were incubated in feeding RNAi plates to induce aakb-2, atg-18, bec-1 and lgg-1 silencing. We scored the number of polyQ aggregates after silencing for 24 h.
2.4. Pharmacological assays
To treat worms in liquid culture, synchronized L1 animals were incubated in 50 mL conical tubes with a final volume of 5 mL, which contained E. coli strain OP50 as a source of food (OD600 = 0.5), 50 μg/mL streptomycin, 12.5 μg/mL nystatin, 5 μg/mL cholesterol, and the specific amount of drug or vehicle, using M9 1X buffer as solvent. L1 animals were treated with several doses of metformin (Sigma-Aldrich-Merck, St. Louis, MO, USA) and/or salicylate (Sigma-Aldrich-Merck) until they reached the young adult stage. Then, we scored the number of polyQ aggregates on the 40Q::YFP worms, and assayed the touch response on 112Q::TdTom animals. We also investigated the motor performance of worms expressing 40Q::YFP in muscle cells (see below). To evaluate whether the treatments (metformin, salicylate and both) could have an effect on already formed aggregates, we treated 40Q::YFP young adult animals and scored the number of polyQ aggregates in 2-day-old adults. Worms expressing the α-synuclein construct show a late aggregation pattern so we treated young adult animals, which do not show any signs of aggregation, and scored the number of α-syn aggregates and evaluated motility in 2-day-old adult animals. To inhibit autophagic flux, we added 10,000 μM chloroquine (Sigma-Aldrich-Merck) 24 h before scoring the number of inclusion bodies. Worms were incubated at 20 °C, under agitation, until they reached young adulthood or the 2-day-old adult stage.
2.5. In vivo scoring of polyQ aggregates in muscle cells
Expression of the 40Q::YFP transgene produces aggregates of polyQs in an age-dependent manner, which can be followed using a dissecting microscope equipped with fluorescence. At least twenty young adult were analysed for each genotype and each independent experiment for each condition using an M165FC Leica dissecting microscope (Leica, Wetzlar, Germany). To analyse the impact of metformin and salycilate treatment on already-formed aggregates, we assessed this possible effect in ten 2-day-old adults, repeating the analysis in three independent experiments.
2.6. Motility assay
To evaluate the fitness of animals with different genotypes and treatments, we evaluated motility in worms using thrashing assys, a measure of fitness which correlate with healthspan in C. elegans [35,36]. To asses the motility capacity of worms expressing the 40Q::YFP transgene, we counted the number of thrashes per treated animal for 30 s and we extrapolated the average data to represent thrashes per minute. Before scoring, each animal was aclimatted in M9 medium for 30 s. We analysed at least 30 untreated/treated animals (L4, young adult and 2-day-old adult depending on the experiment) per condition, and we performed each experiment three times to obtain reproducible values.
2.7. Evaluation of the touch response
The aggregation of polyQ-containing proteins in mechanosensory neurons in the 112Q::TdTomato worms, induces a touch response dysfunction. The touch response of at least 40 young adult animals was analysed by gently passing an eyelash mounted on a toothpick on the posterior part of the animals as described elsewhere [9]. Each animal was touched 10 times and we represented the percentage of times that the animal responded. Each assay was repeated at least three times. The touch response phenotype was scored using an MS5 dissecting microscope (Leica).
2.8. In vivo scoring of α-synuclein aggregates
In contrast to worms expressing polyQs in muscle cells, animals expressing the α-synuclein::YFP transgene, show aggregation in old worms (2-day-old adult animals or older). Therefore, we treated young adult animals, which did not show aggregation of this molecule yet, and we evaluated the number of α-syn aggregates in the area located between the two pharyngeal bulbs, in 2-day-old adult animals as described elsewhere [34]. We used a DM2500 (Leica) vertical fluorescence microscope for scoring. We analysed 30 untreated/treated 2-dayold adult animals in total per condition and we reproduced each experiment at least three times.
2.9. Fluorescent microscopy imaging
Images were collected using a Leica SP5 confocal microscope (Leica). Live animals were mounted onto 2% agar pads and anaesthetized with a drop of 0.05 M sodium azide.
2.10. Immunoblotting
For polyQ protein quantification, treated young adult animals were washed with M9 1X buffer before lysing the samples with RIPA buffer (Invitrogen, Carlsbad, CA, USA) and proteinase inhibitors cocktail (Complete, Roche, Basel, Switzerland). Samples were boiled at 100 °C for 10 min containing 4X SDS sample loading buffer. Protein extracts were separated by 8% sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene di-fluoride (PVDF) membranes (Bio-Rad Laboratories, Hercules, CA, USA) by semi-dry blotting (Trans-Blot Turbo, Bio-Rad). Blocking was done with 3% milk, according to the specification of the following primary antibodies: mouse anti-polyQ (1:1000, Sigma Ref. #P1874) and mouse anti-actin (1:500, Invitrogen ref. #MA5–11869) to normalize values. Primary antibodies were incubated overnight at 4 °C degrees with shaking. We used the secondary antibody anti-mouse conjugated to HRP (1:10000, Santa Cruz ref. #SC-2005, Santa Cruz Biotechnology, Dallas, TX, USA) to develop immunoblots. Images were obtained using Amersham Imager 600 and enhanced chemiluminescent (ECL) detection (GE Healthcare, Chicago, IL, USA). Quantification values were obtained using the Image J software.
2.11. Statistical analysis
Binomial logistic regression was used to study the synergistic effect of metformin treatment on the number of touch responses out of 10 trials. Also, due to the discrete nature of the variable, we carried out negative binomial logistic regression to study the influences of these drugs on the dynamics of aggregation. Thrashing measurements were analysed using linear regression models given its quantitative nature. R software (version 3.5.1) was used to perform the statistical analyses. The results are presented as Odds Ratio (OR) or Estimate, lower and upper confidence interval (CI95 %), and p-value to indicate the significance of the data. The experimenter was blinded to genotype and treatment in all experiments through the work.
3. Results and discussion
3.1. Metformin and salicylate synergise to reduce polyglutamine-induced toxicity
Aspirin and derivatives (such as salicylate) and metformin act pleiotropically to activate many different targets [37,38]. Moreover, these substances have side effects when used chronically, e.g., weight loss (metformin [39]) or gastrointestinal bleeding (aspirin [40]). However, both substances have been shown to synergise to improve insulin sensitivity [23]. Since synergic action requires lower doses of both substances, it may reduce the side effects of these drugs, probably through activation of a unique pathway. In this regard, metformin and salicylate have been shown to indirectly and directly activate AMPK, respectively [41,42]. However, both substances are also able to modulate the function of many other targets and cellular processes. For example, metformin inhibits mitochondrial complex I, which in turn raises AMP/ATP levels, thus activating AMPKγ, which activates AMPKα. However, this substance is also able to inhibit mTOR and increase insulin levels [43]. This pleiotropic behaviour applies to salicylate as well, which not only allosterically activates AMPK through binding to AMPKβ [41] but also modulates many other targets [44].
Therefore, the rationale of our study was to investigate whether metformin and salicylate can synergise to reduce toxicity induced by polyQs expression in vivo, by reducing their concentrations to a limit where other targets are unaffected. We also aimed to find out the mechanism behind AMPK-specific reduction of polyQ toxicity. To achieve this goal, we used a C. elegans model of neuronal toxicity, carrying a transgene that induced expression of 112Q fused to the dimeric fluorescent protein TdTomato (TdTom) in mechanosensory neurons, which in turn impaired their function (Fig. 1A) [16]. Therefore, we could assay mechanosensation in these worms by gently touching their tails with an eyelash mounted in a toothpick [16]. These animals normally respond only 25 % of the time, while wild type worms do it 7 out of 10 times (OR = 6.71, CI95 %[5.19, 8.73], p < 0.001) (Fig. 1B) [16]. We cultured worms in many different concentrations of both drugs separately and together to identify synergistic doses (data not shown). Firstly, we observed that treating the worms with 50 μM salicylate alone rescued neuronal function (OR = 2.18, CI95 %[1.83, 2.59], p < 0.001), to the same degree as 2000 μM metformin (Fig. 1B), as we had reported before [15,16].
Fig. 1.
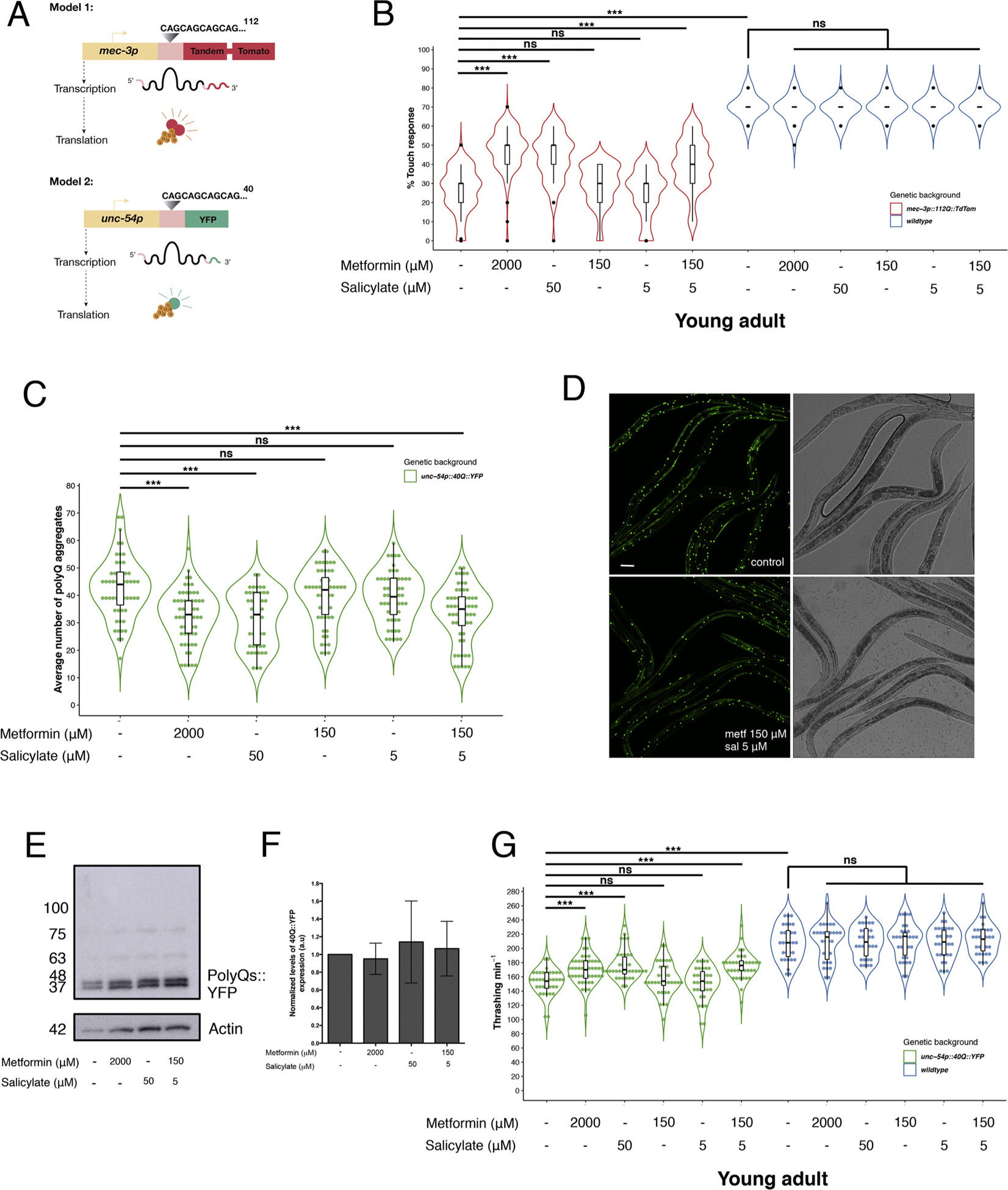
Metformin and salicylate act synergistically to reduce polyQ aggregation and restore neuronal function. A) Models of polyQ toxicity used for assessing the synergistic effect of metformin and salicylate. Model 1 (112Q:TdTom) is a model of neuronal toxicity, carrying a transgene that induces expression of 112Q fused to TdTomato fluorescent dimeric protein in mechanosensory neurons under the control of mec-3 promoter. Model 2 (40Q::YFP) is a model of polyQ muscle aggregation that carries a transgene that expresses 40Q fused to YFP (Yellow Fluorescent Protein), in muscle cells under the control of unc-54 promoter. B). The expression of 112Q:TdTom in mechanosensory neurons induces impairment of neuronal function compared to animals that do not have the transgene (OR = 6.71, CI95 %[5.19, 8.73], p < 0.001). Treatment with 2000 μM metformin and 50 μM salycilate separately rescue neuronal function in 112Q young adult animals compared to untreated animals (OR = 2.59, CI95 %[2.33, 2.88], p < 0.001; OR = 2.18, CI95 %[1.83, 2.60], p < 0.001; respectively). Lower doses of metformin/salycilate in combination (150 μM and 5 μM respectively) rescue neuronal function synergistically in 112Q young adult animals (OR = 1.86, CI95 %[1.46, 2.36], p < 0.001), while single treatments do not modify the touch response compared with 112Q control (OR = 1.16, CI95 %[0.91, 1.48], p = 0.214; OR = 0.92, CI95 %[0.76, 1.11], p = 0.374 respectively). All effective single and dual treatments do not change mechanosensorial function in wild type young adult animals (OR = 0.92, CI95 %[0.65, 1.31], p = 0.658; OR = 0.91, CI95 %[0.64, 1.29], p = 0.595; OR = 0.97, CI95 %[0.68, 1.37], p = 0.859 respectively). C) Single treatment with 2000 μM metformin and 50 μM salicylate reduce polyQ aggregation in 40Q young adult animals (OR = 0.79, CI95 %[0.69, 0.90], p < 0.001; OR = 0.80, CI95 %[0.70, 0.91], p = 0.001, respectively). Synergistic combination (150 μM metformin/ 5 μM salicylate) decreases polyQ aggregation at the same level of high dose of drugs (OR = 0.81, CI95 %[0.71, 0.92], p = 0.001). D) Representative photographs from untreated and treated (150 μM metformin/5 μM salycylate) 40Q::YFP young adult animals show a different polyQ aggregation pattern in body wall muscles. E) Inmunoblot of the total levels of polyQ::YFP proteins. Proteins weredetected using a mouse anti-polyQ primary antibody conjugated to HRP (1:10000, Santa Cruz ref. #SC-2005). Samples were obtained from untreated and treated 40Q young adult animals (2000 μM metformin, 50 μM salicylate and 150 μM metf/5 μM sal combination) as described in Materials and Methods. F) Levels of polyQ::YFP expression were quantified by Image J software and values were normalized to untreated 40Q::YFP worms. G) Metformin and salicylate induce rescue of motor function in 40Q young adult animals. 40Q young adult animals show a significant motor defect compared to wild type young adult animals (whithout the polyQ transgene) (Estimate = 54.73; CI95 %[44.42, 65.05], p < 0.001). Culturing worms in high doses of the drugs (2000 μM metformin and 50 μM salicylate) improve motility in 40Q young adults compared with untreated animals (Estimate = 17.66, CI95 %[8.72, 26.60], p < 0.001; Estimate = 23.70, CI95 %[14.13, 33.27], p < 0.001, respectively), while low doses separately (150 μM metformin or 5 μM salicylate) do not modify the motor phenotype (Estimate = 3.92, CI95 %[−5.78, 13.63], p = 0.427; Estimate = −2.02, CI95 %[−11.73, 7.69], p = 0.683 respectively). Dual treatment with low doses of metformin (150 μM) and salycilate (5 μM) rescues motility impairment in 40Q young adult animals significantly (Estimate = 22.20, CI95 %[12.63, 31.77], p < 0.001). Neither of single and mix treatments improve motility phenotype in wild type background. PolyQ aggregation analysis shows Odds Ratio (OR), lower and upper confidence interval 95 % (CI95 %) and p-value to indicate the significance of the data. For thrashing analysis, OR value is changed by Estimate value according to the type of the variable. At least thirty animals were tested and three independent experiments were performed in each case. Scale bar represents: 50 μm.
Secondly, we found that 150 μM metformin and 5 μM salicylate together was the minimal dose to synergistically rescue neuronal function in polyQ worms (Estimate = 0.55, CI95 %[1.20, 2.53], p = 0.004) (Supplemental Fig. S1), to the level of polyQ-stressed worms treated with either 2000 μM metformin or 50 μM salicylate alone (OR = 1.86, CI95 %[1.46, 2.36], p < 0.001) (Fig. 1B). In contrast to the dual combination (150 μM metformin/5 μM salicylate), both compounds were ineffective separately (OR = 1.16, CI95 %[0.91, 1.48], p = 0.214; OR = 0.92, CI95 %[0.76, 1.11], p = 0.374) respectively) (Fig. 1B). Pharmacological assays with 2000 μM metformin, 50 μM salicylate and combination of both (150 μM metformin/5 μM salicylate) improved neuronal mechanosensation in 112Q::TdTom worms while showed an innocuous effect in wild type animals (OR = 0.92, CI95 %[0.65, 1.31], p = 0.658; OR = 0.91, CI95 %[0.64, 1.29], p = 0.595; OR = 0.97, CI95 %[0.68, 1.37], p = 0.859, respectively), suggesting that this treatment specifically reduces polyQ neuronal stress in worms (Fig. 1B). As we have used ten-fold lower doses of each drug, this may reduce the chance of activation of undesired side targets, minimizing potential side effect of this treatment, which is relevant for future therapy in humans.
To obtain insight into the mechanisms of neuroprotection by metformin/salicylate, we sought to test whether these drugs altered the dynamics of polyQ aggregation. If that was the case, this would suggest that pathways of misfolded protein clearance were involved in neuroprotection by metformin/salicylate treatment. To test this hypothesis, we used worms that expressed 40 CAG repeats in frame with the yellow fluorescence protein (40Q::YFP) in muscle cells, which showed age-dependent aggregation of polyQs (Fig. 1A) [8]. In agreement with previous work [16] treatment with 2000 μM metformin (positive control) drastically reduced the number of polyQ aggregates compared to untreated young adult animals (OR = 0.80, CI95 % [0.69, 0.90], p < 0.001) (Fig. 1C). As expected, culturing 40Q worms in 50 μM salicylate also resulted in reduced number of aggregates (OR = 0.81, CI95 % [0.70, 0.91], p = 0.001) (Fig. 1C). Finally, culturing 40Q::YFP worms in 150 μM metformin plus 5 μM salicylate had a synergistic effect and significantly decreased the number of aggregates (OR = 0.80, CI95 % [0.71, 0.92], p = 0.001) (Fig. 1C and 1D), in contrast to worms treated with either 150 μM metformin (OR = 0.97, CI95 % [0.85, 1.09], p = 0.574) or 5 μM salicylate (OR = 0.98, CI95 % [0.86, 1.11], p = 0.749) alone (Fig. 1C). Pharmacological treatements did not affect the total expression of polyQ protein (Fig. 1E, F), suggesting that the activity of the unc-54 promoter, which drives the expression of the 40Q::YFP transgene, was unaltered by these compounds.
Reduction of polyQ aggregation may not be followed by better function of the muscle cells of the worms. To check whether treated animals showed better fitness we used thrashing assays. These assays consist of counting how many “thrashes”, or body bends, do the animals in liquid to avoid being pulled down by gravity [45]. It has been shown that animals performing better thrashing show also better lifespan and overall healthspan [36]. Moreover, worm models expressing polyQ-containing proteins in muscle cells have reduced thrashing in liquid [46,47]. As we expected, we observed that worms expressing polyQs in muscle cells showed reduced thrashing activity (Estimate = 54.73; CI95 %[44.42, 65.05], p < 0.001) (Fig. 1G). Interestingly, treated worms (with metformin 2000 μM, salicylate 50 μM and synergistic combination of both) had a better motility capacity than untreated animals (Estimate = 17.66, CI95 %[8.72, 26.60], p < 0.001; Estimate = 23.70, CI95 %[14.13, 33.27], p < 0.001; Estimate = 22.20, CI95 %[12.63, 31.77], p < 0.001, respectively) (Fig. 1G). In contrast, treated 40Q::YFP young adult animals with low dose of drugs (metformin or salicylate) alone did not show statistical differences in their motility capacity in comparison to untreated animals (Estimate = 3.92, CI95 %[−5.78, 13.63], p = 0.427; Estimate = −2.02, CI95 %[−11.73, 7.69], p = 0.683 respectively) (Fig. 1G). In agreement with the touch response assays, treated and untreated wild type young adult animals showed the same motility capacity values, suggesting that these drugs specifically reduce polyQ stress in muscle cells (Fig. 1G).
An interesting question is whether protein aggregates can be removed, once they have been formed. To evaluate whether metformin and salicylate are protective in worms containing already aggregates, we treated 40Q::YFP young adult animals and we analysed the number of polyQ aggregates when they reached the 2-day-old adult stage. We did not observe differences between untreated/treated animals with 2000 μM metformin, 50 μM salicylate and synergistic combination of both (150 μM metf/5 sal μM) (OR = 0.98, CI95 %[0.91, 1.06], p = 0.661; OR = 0.98, CI95 %[0.91, 1.06], p = 0.616; OR = 0.97, CI95 %[0.90, 1.04], p = 0.355, respectively) (Supplemental Fig. S2). Therefore, the therapeutic effect of these drugs prevents or delays aggregation formation from early stages, but it is ineffective once the aggregates have been formed.
3.2. Synergistic reduction of polyQ aggregation requires AMPK catalytic function
Metformin and salicylate are well-known AMPK activators, though both drugs are pleiotropic so they can modulate the function of a wide range of targets (see for example [25–29]) show undesirable side effects [45,46]. Therefore, Next, we sought to test whether a reduction in the number of aggregates in 40Q::YFP worms treated with metformin and salicylate required AMPK function. To do so, we analysed worms defective of the only catalytic subunit of AMPK that affected the lifespan and health in C. elegans (aak-2/AMPKα2) [48]. Analysis of 40Q; aak-2(ok524) young adults showed a statistically significant increase in the number of polyQ aggregates in comparison to wild type animals (OR = 1.26, CI95 % [1.19, 1.33], p < 0.001) (Fig. 2C). Moreover, protection by metformin and salicylate was suppressed in 40Q; aak-2(ok524) worms (OR = 0.98, CI95 % [0.933, 1.037], p = 0.541) (Fig. 2C). In addition, aak-2 loss of function induced an aggravation of the motor defect in the young adult animals compared to 40Q controls (Estimate = −43.93, CI95 %[−48.13, −39.74], p < 0.001) (Fig. 2D) and the motility capacity of treated 40Q; aak-2(ok524) young adults was not rescued by the treatment with the combination of metformin and salicylate (Estimate = −3.80, CI95 %[−8.03, 0.43], p = 0.077) (Fig. 2D). Therefore, and in agreement with previous results [16,20], these data show that the catalytic subunit of AMPK is required for the protective effect of these drugs. The reduction of polyQ aggregates by metformin and salicylate, and the exclusive requirement of an active AMPK function is relevant from a therapeutic point of view, since by using low doses of these chemicals, it could prevent the activationof other targets, and therefore it could reduce undesired pharmacological effects associated with these drugs.
Fig. 2.
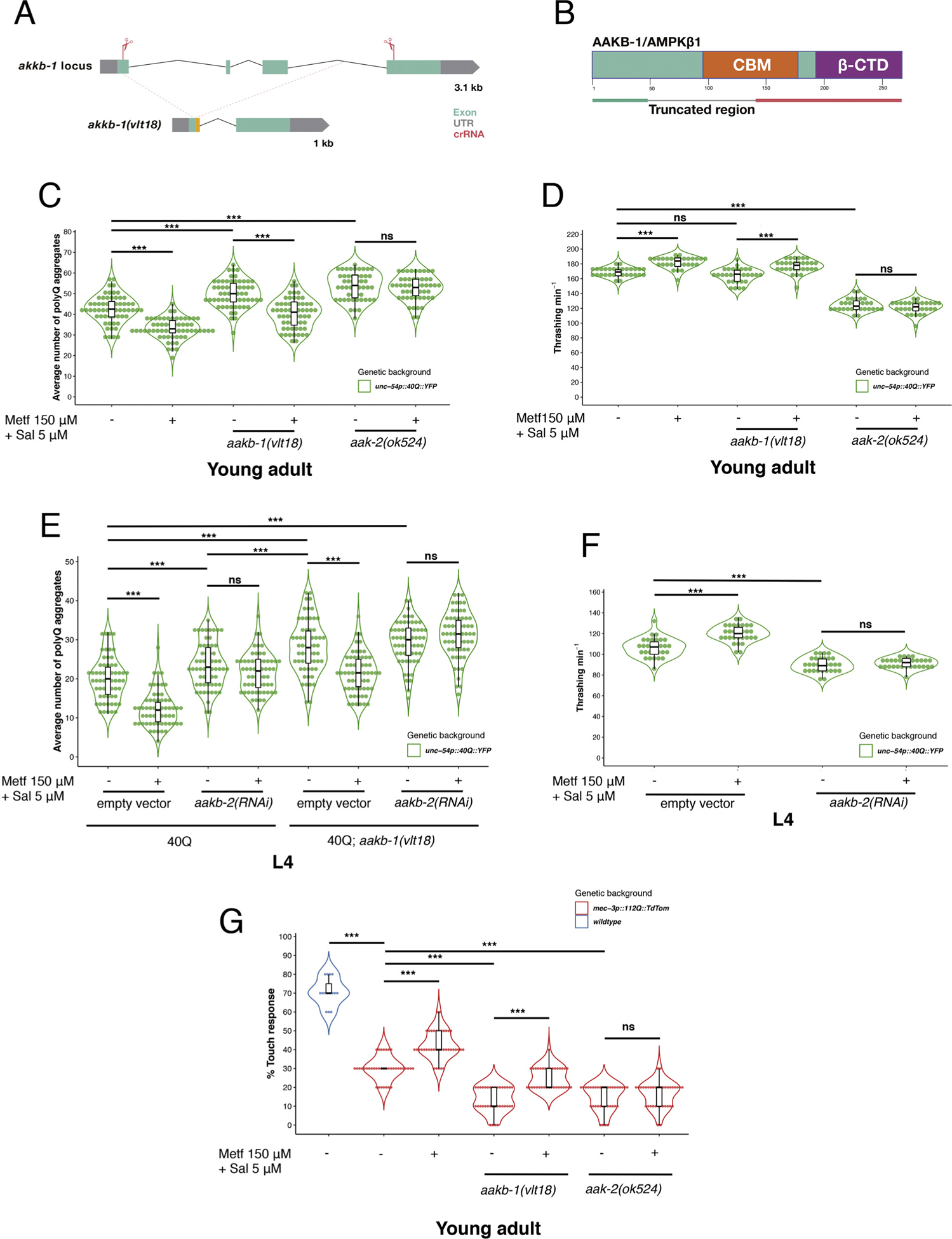
The beneficial synergistic effect of metformin and salicylate on polyQ aggregation requires AMPK activity. A) Diagram of the genomic locus encoding aakb-1 and the position of the guide RNAs (gRNA) used to produce a knock out of the gene by CRISPR/Cas9. Green boxes represent exons; thin black inclined lines indicate introns. The red scissors represent the Cas9 nuclease bound to the gRNA. The red lines indicate the place of probable cut within the gene. The result of deleting the region between the gRNAs is shown below the genomic locus of aakb-1 (1 kb length). This deletion includes the donor splicing site of the first exon (in yellow), so aakb-1(vlt18) is likely unable to splice correctly. B) Diagram representing the protein AAKB-1/AMPKβ1 and its functional domains. The truncated locus, after CRISPR treatment, may produce a truncated protein, lacking part of the CBM C) The graph represents the number of polyQ aggregates in several young adults of the following strains: 40Q, 40Q; aak-2(ok524) and 40Q; aakb-1(vlt18) treated with metformin 150 μM and salicylate 5 μM, or with vehicle (H2O). Reduction of polyQ aggregation, induced by synergistic metformin and salicylate treatment, depends on aak-2/AMPKα (OR = 0.98, CI95 % [0.933, 1.037], p = 0.541), while aakb-1/AMPKβ1 (OR = 0.81, CI95 % [0.76, 0.85], p < 0.001) is not implicated. D) Graph showing the results of the thrashing assays in the aakb-1(vlt18) and aak-2(ok524) mutants into a polyQ toxicity context. The ablation of aakb-1 does not modify motility of 40Q young adult animals (Estimate = −4.13, CI95 %[−8.30, 0.06], p = 0.053), in contrast to aak-2 ablated animals (Estimate = −43.93, CI95 %[−48.13, −39.74], p < 0.001). The protector role of the synergistic treatment is blocked in aak-2 ablated animals (Estimate = −3.80, CI95 %[−8.03, 0.43], p = 0.077), while aakb-1 mutants did not modify the effect of the drugs (Estimate = 12.13, CI95 %[7.31, 16.95], p < 0.001). E) Graph representing the average number of polyQ aggregates in treated (150 μM metformin/5 μM salycilate) and untreated 40Q, 40Q; aakb-1(vlt18), 40Q; aakb-2(RNAi) and double mutants L4 animals. Both subunits, AAKB-1 and AAKB-2, modulate polyQ aggregation, however only ablation of aakb-2 is essential for the response to the synergistic effect of metformin and salicylate in muscle aggregation. Double mutants aakb-1; aakb-2 failed to rescue polyQ aggregation phenotype upon metformin/salicylate treratment compared to untreated double mutants (OR = 1.06, CI95 %[0.99, 1.14], p = 0.10). F) Graph showing the data regarding the fitness of the worms. Ablation of aakb-2 by RNAi silencing disrupts motility capacity in L4 40Q animals (Estimate = −16.53, CI95 %[−20.88, −12.18], p < 0.001). Motility rescue by 150 μM metformin plus 5 μM salicylate is observed in 40Q L4 animals (Estimate = 13.73, CI95 %[9.16, 18.31], p < 0.001), while the beneficial effect is supressed in ablated aakb-2 L4 animals (Estimate = 1.73, CI95 %[−1.46, 4.91], p = 0.28). G) Mechanosensorial function is measured by percentage of touch response in 112Q::TdTom (denoted as 112Q), 112Q; aak-2(ok524) and 112Q; aakb-1(vlt18) after treatment or not with metformin 150 μM and salicylate 5 μM. Defective animals of aak-2 respond worse to metformin/salicylate treatment (OR = 1.14, CI95 % [0.76, 1.71], p = 0.53) while null aakb-1 animals show a significant response (OR = 2.17, CI95 % [1.48, 3.21], p < 0.001). PolyQ aggregation analysis show Odds Ratio (OR), lower and upper confidence interval 95 % (CI95 %) and p-value to indicate the significance of the data. For thrashing and touch analysis, OR value is changed by Estimate value according to the type of the variable At least thirty animals were tested and experiments were performed three independent times.
3.3. The mechanism of reduction of polyQ aggregation by metformin and salicylate involves the function of the regulatory AMPKβ2
To investigate the mechanistic aspects of metformin/salicylate synergism, we also tested whether the regulatory subunit AMPKβ, which could respond to direct binding of salicylate [41], was also required for the induced reduction of polyQ aggregates by these drugs. The genome of C. elegans encodes two isoforms of AMPKβ, aakb-1, and aakb-2. Since there was no available strain with a mutant allele of aakb-1 in the CGC worm collection, we generated the aakb-1(vlt18) mutant using CRISPR/Cas9 (Fig. 2A). To do so we designed two guide RNAs (gRNAs or crRNAs) against the first and last exon of aakb-1 (Fig. 2A), so the injection of ribonucleoproteins carrying both gRNAs would delete most of the coding region. Although we were not able to isolate a mutant that had a deletion between these exons, we isolated worms with vlt18, a deletion that covered most of the gene sequence (Fig. 2A). This deletion includes the donor splicing site of the first exon, so aakb-1 is likely unable to splice correctly (Fig. 2A). Even if the gene was correctly spliced and all coding sequences remained in-frame, it would produce a truncated protein (Fig. 2B) that would lack a large part of the N-terminal region, including half of the carbohydrate-binding-domain (CBM) (Fig. 2B), which is essential for salicylate activation of AMPK function [19].
Analysis of 40Q; aakb-1(vlt18) young adults showed a statistically significant increase in the number of polyQ aggregates (OR = 1.17, CI95 % [1.11,1.24], p < 0.001) (Fig. 2C). However, the effect of loss-of-function of the catalityc subunit (aak-2) was stronger than the one produced by the aakb-1 mutation, on polyQ aggregation (OR = 1.07, CI95 % [1.02, 1.13], p = 0.008) (Fig. 2C). This suggests that, although the function of the regulatory subunit (AMPKβ1) was important, complete removal of AMPKα catalytic subunit resulted in higher polyQ aggregation.
Aside from the impact of removing either of AMPK subunits on polyQ aggregation, metformin/salicylate treatment of these mutants showed remarkable differences. While removal of aakb-1 did not eliminate protection from polyQ toxicity by these drugs (OR = 0.81, CI95 % [0.76, 0.85], p < 0.001) (Fig. 2C), suppression of aak-2 completely blocked reduction of polyQ aggregation by metformin and salicylate (OR = 0.98, CI95 % [0.93, 1.04], p = 0.54) (Fig. 2C). In regard to the motor phenotype, ablation of aakb-1 showed a very small tendency to fall, compared with the drastic drop of aak-2 mutants, that was not statistically different from wild type worms (Estimate = −4.13, CI95 %[−8.33, 0.06], p = 0.053 (Fig. 2D). This apparent disconnection between the aggregation of polyQ-phenotype and motor behaviour may be due to the mild aggregation phenotype presented in the aakb-1 mutants. In any case, metformin and salicylate were able to rescue motility in aakb-1(vlt18) (Estimate = 12.13, CI95 %[7.31, 16.95], p < 0.001) (Fig. 2D) in these animals. Taken all together, these data suggest that, although aakb-1 is required to modulate polyQ aggregation, it does not participate of the synergistic effect of metformin and salicylate on protein homeostasis.
As aakb-1 is not involved in the synergistic activation of AMPK by metformin and salicylate, we sought to test whether the gene encoding the second isoform of AMPKβ present in C. elegans (aakb-2) could play a role in this process. To do so, we used initially the aakb-2(rr88) loss of function mutants. However, this strain had strong sterility (data not shown) which prevented our analysis. Therefore, we took advantage of the RNAi technology to suppress aakb-2 function, in both wild type and aakb-1 mutants expressing polyQs in muscle cells (40Q::YFP worms). Knocking down aakb-2 by RNAi in wild type 40Q::YFP animals slightly increased polyQ aggregation compared to the controls (OR = 1.174, CI95 % [1.07, 1.29], p = 0.001), though not to the level of the structural mutant aakb-1 (OR = 1.207, CI95 % [1.107, 1.316], p < 0.001) (Fig. 2E). This milder enhancement of polyQ aggregation may be due to a partial suppression of aakb-2 by RNAi, but it may also be due to a less relevant role of this isoform in regulating protein homeostasis. Although aakb-2(RNAi) worms showed lower number of polyQ aggregates than aakb-1(vlt18) mutants, aakb-2(RNAi) animals did not respond to metformin/salicylate treatment (OR = 0.92 CI95 % [0.84, 1.01], p = 0.07), which suggests that its function is important for the effect of these drugs (Fig. 2E). Double ablated animals (aakb-1(vlt18); aakb-2(RNAi)) showed the highest increase of polyQ aggregation, and they completely failed to respond to metformin/salicylate (OR = 1.063 CI95 % [0.99, 1.14], p = 0.10) (Fig. 2E). These results suggest that only aakb-2 is required for the action of metformin/salicylate to reduce polyQ aggregation, although both isoforms are essential for appropriate protein homeostasis in C. elegans.
We then analysed the motility capacity of aakb-2(RNAi) in untreated/treated worms. In this context, untreated aakb-2(RNAi) animals showed a lower number of thrashes compared to 40Q::YFP (Estimate = −16.53, CI95 %[−20.88, −12.18], p < 0.001) (Fig. 2F), suggesting that increased polyQ aggregation translates into worse motor performance. As we expected, reduction of aakb-2 expression blocked the rescue induced by metformin/salicylate combined treatment, observed in the wild type worms expressing polyQs (Estimate = 1.73, CI95 %[−1.45, 4.91], p = 0.28) (Fig. 2F). Hence, the aakb-2 isoform is required for the action of metformin and salicylate to restore healthspan disrupted by expression of polyQ-containing proteins in C. elegans.
3.4. Neuronal protection of metformin and salicylate is dependent on AMPK functional subunits
It has been shown before that AMPK function is important for appropriate mechanosensation in C. elegans neurons stressed by polyQs [9,12,13,15,16]. In agreement with these results, 112Q::TdTom worms showed reduced touch response when we introduced a mutant allele of AMPKα, aak-2(ok524), (OR = 0.39, CI95 % [0.27, 0.56], p < 0.001) (Fig. 2G). Analysis of wild type 112Q::TdTom worms treated with metformin/salicylate showed that the animals recovered neuronal function compared to untreated animals (OR = 1.73, CI95 % [1.29, 2.33], p < 0.001) (Fig. 2G). However, this recovery was absent in 112Q; aak-2(ok524) mutant worms (OR = 1.14, CI95 % [0.76, 1.71], p = 0.53) (Fig. 2G). Interestingly, the ablation of aakb-1/AMPKβ1 also drastically reduced the touch response of polyQ-stressed animals (OR = 0.36, CI95 % [0.25, 0.52], p < 0.001) (Fig. 2G). This result suggests that both AMPK subunits (aak-2 and aakb-1) are important for protein homeostasis in neurons, as it happens in muscle cells (Fig. 2C–F). aakb-1(vlt18) mutant worms responded well to metformin/salicylate treatment (OR = 2.17, CI95 % [1.48, 3.21], p < 0.001) (Fig. 2G), in contrast to the null response of animals defective of aak-2 (OR = 1.14, CI95 % [0.76, 1.71], p = 0.53) (Fig. 2G). These results suggest that aakb-1 plays a role in neuroprotection, although it is dispensable for the effects of metformin and salicylate to reduce polyQ-induced neuronal toxicity.
3.5. The beneficial effect of metformin/salicylate on polyQ aggregation is mediated through autophagy
We have demonstrated that metformin/salicylate synergistically reduce polyQ aggregation and toxicity in an AMPK-dependent manner. Since this enzyme is a direct activator of autophagy (reviewed by Li and Chen [49]), we sought to investigate whether autophagy was involved in the reduction of polyQ toxicity by these drugs. To investigate this possibility, we disrupted the autophagy pathway in metformin/salicylate treated animals, hypothesizing that the beneficial effect of the drugs would be lost. We used two approaches: 1) inhibition of the autophagic flux with chloroquine to block autophagosome-lysosome fusion and activity [50]; and 2) disruption of autophagy-related genes by knocking down LGG-1/LC3, ATG-18/ATG-18 and BEC-1/ATG-6, using RNAi technology [51,52]. In constrast to wild type animals, where metformin/salicylate reduced aggregation (OR = 0.67, CI95 % [0.58, 0.78], p < 0.001) (Fig. 3A), preventing autophagy with chloroquine completely blocked reduction of polyQ aggregation by metformin/salicylate, so the worms treated with all three substances (metformin/salicylate/chloroquine) had the same aggregation pattern as untreated worms (OR = 1.10, CI95 % [0.95, 1.27], p = 0.177) (Fig. 3A). All groups of animals treated with chloroquine (with and without metformin/salicylate) showed no statistically significant different number of polyQ aggregates (OR = 1.02, CI95 %[0.89, 1.17], p = 0.739) (Fig. 3A). From a functional point of view, we observed that chloroquine did not show a motility rescue in metformin/salicylate treated worms, compared to animals cultured with chloroquine alone (Estimate = 0.67, CI95 %[−7.88, 9.21], p = 0.874) or with untreated control L4 animals (Estimate = −1.47, CI95 %[−11.17, 8.24], p = 0.763) (Fig. 3B). These results confirmed our previous observation that inhibition of autophagic flux in polyQ-stressed worms reduced the benefit of metformin treatment [16].
Fig. 3.
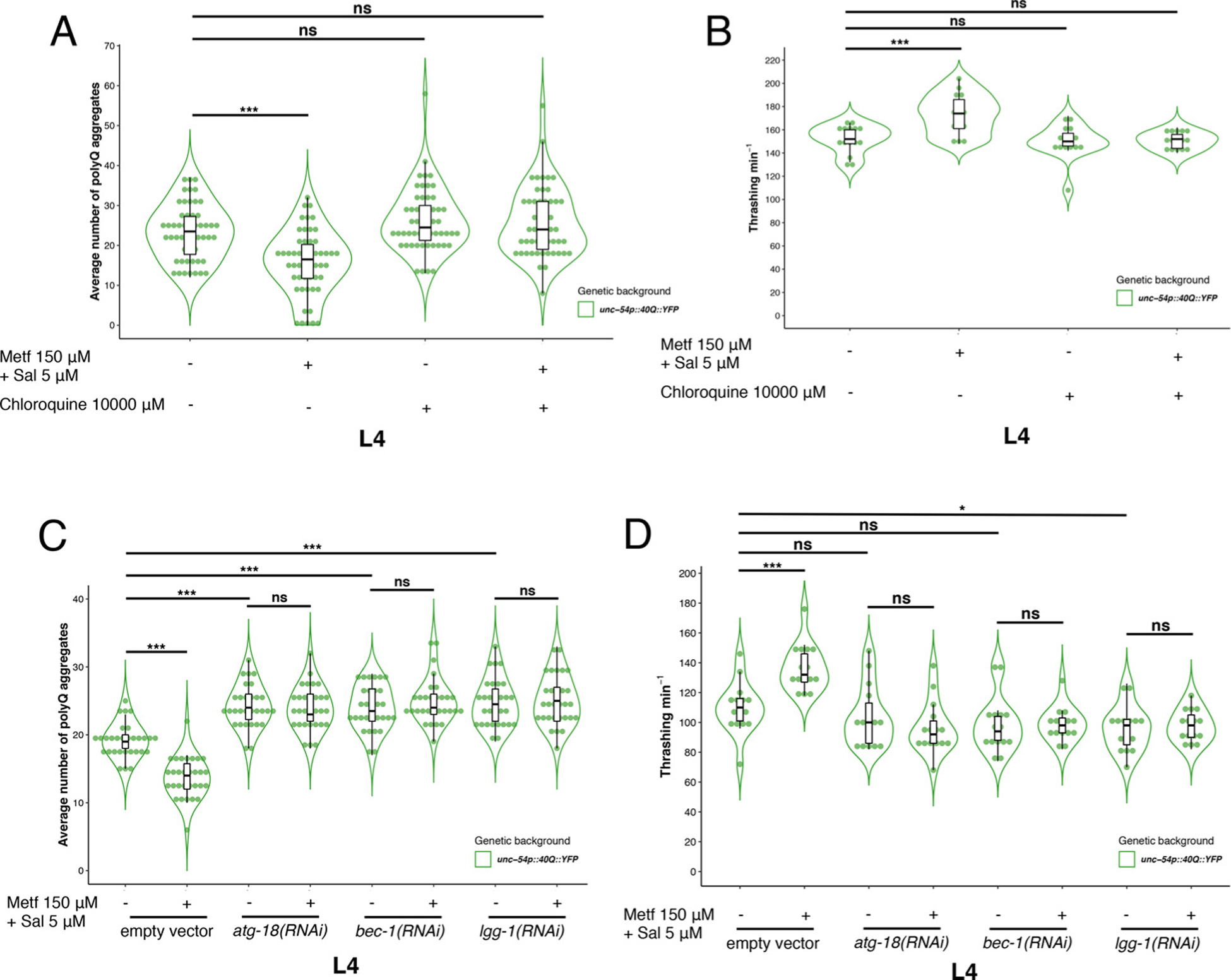
Autophagy is required to induce the beneficial synergistic effect of metformin and salicylate on polyQ aggregation. A) The blocking effect of chloroquine on lysosomal flow suppresses protection by metformin/salicylate in L4 treated larvae with 10 mM chloroquine (OR = 1.10, CI95 % [0.95, 1.27], p = 0.177). B) Metformin and salycilate together improve motility of 40Q L4 animals (Estimate = 21.73, CI95 %[12.03, 31.44], p < 0.001). After chloroquine treatment, 40Q L4 animals treated with the synergistic combination of drugs failed to rescue motility (Estimate = −1.47, CI95 %[−11.17, 8.24], p = 0.763). C) The reduction of the function of atg-18, bec-1 and lgg-1 increase polyQ aggregation in muscle cells (OR = 1.26, CI95 %[1.13, 1.41], p < 0.001; OR = 1.25, CI95 %[1.12, 1.39], p < 0.001; OR = 1.28, CI95 %[1.15, 1.43], p < 0.001, respectively). RNAi treatment of lgg-1, atg-18 and bec-1 supresses the protective effect of the dual treatment (150 μM metformin/5 μM salycilate) compared to control 40Q L4 animals (OR = 0.99, CI95 %[0.89, 1.09], p = 0.792; OR = 1.05, CI95 %[0.94, 1.16], p = 0.389; OR = 1.02, CI95 %[0.92, 1.12], p = 0.775, respectively). D) Knock down of atg-18 and bec-1 does not modify motility in 40Q L4 animals, although ablation of lgg-1 slightly modifies the motility phenotype (Estimate = −14.13, CI95 %[−27,16, −1.11], p = 0.034). Metformin/salicylate treated animals showed higher motility than control untreated animals (Estimate = 26.53, CI95 %[14.46, 38.61], p < 0.001). Reduction of function of atg-18, bec-1 and lgg-1 avoid the protective effect of the synergic drugs over the motility phenotype (Estimate = −7.73, CI95 %[−21.90, 6.44], p = 0.273; Estimate = 0.40, CI95 %[−10.93, 11.73], p = 0.943; Estimate = 2.13, CI95 %[−7.50, 11.76], p = 0.653, respectively). PolyQ aggregation analysis show Odds Ratio (OR), lower and upper confidence interval 95 % (CI95 %) and p-value to indicate the significance of the data. For thrashing, OR value is changed by Estimate value according to the type of the variable At least thirty animals were tested in each case and we performed three independent experiments.
It is known that inactivation of autophagy by silencing several essential genes, such as atg-18 and bec-1, modifies aggregation pattern in 40Q animals [53]. In addition, it has been shown that aspirin, a synthetic derivative of salicylate, causes activation of autophagy through lgg-1 in C. elegans [54]. Therefore, we tested metformin/salicylate effects in 40Q::YFP animals depleted of LGG-1, ATG-18 and BEC-1 (lgg-1(RNAi), atg-18(RNAi) and bec-1(RNAi) worms). First, we observed that after 24 h of feeding 40Q L4 animals with RNAi against the atg-18, bec-1 and lgg-1 genes, silenced 40Q L4 animals showed an increment of polyQ aggregation pattern, compared with wild type 40Q animals (OR = 1.26, CI95 %[1.13, 1.41], p < 0.001; OR = 1.25, CI95 %[1.12, 1.39], p < 0.001; OR = 1.28, CI95 %[1.15, 1.43], p < 0.001, respectively) (Fig. 3C). We also observed that animals silenced by RNAi (atg-18, bec-1 and lgg-1) were unresponsive to the beneficial effect of metformin and salicylate, since the number of polyQ aggregates were statistically similar to untreated silenced animals (OR = 0.99, CI95 %[0.89, 1.09], p = 0.792; OR = 1.05, CI95 %[0.94, 1.16], p = 0.389; OR = 1.02, CI95 %[0.92, 1.12], p = 0.775, respectively) (Fig. 3C). In agreement with our previous results, treated silenced worms did not show a different motility pattern compared with untreated silenced animals for atg-18, bec-1 and lgg-1 (Estimate = −7.73, CI95 %[−21.90, 6.44], p = 0.273; Estimate = 0.40, CI95 %[−10.93, 11.73], p = 0.943; Estimate = 2.13, CI95 %[−7.50, 11.76], p = 0.653, respectively) (Fig. 3D). These results are consistent with previous work from other authors, who showed that metformin could extend lifespan in C. elegans by activation of the lysosomal pathway, in an lgg-1-dependent manner [55]. Altogether, these results show that the autophagy flow is required for the synergistic effect of metformin/salicylate on polyQ aggregation and motility. Although is tempting to speculate that AMPK activation, by metformin and salicylate, is inducing autophagy, which in turns reduces polyQ aggregation and enhances motility, we cannot rule out the possibility that AMPK activation and autophagy may run in parallel.
3.6. α-synuclein aggregation pattern is rescued by AMPK activators
We sought to test whether AMPK activators could influence the toxicity induced by α-synuclein, to broaden the range of diseases in which these compounds could be useful. To do so, we used a well-known C. elegans model of Parkinson’s disease, the strain NL5901, which expresses α-synuclein::YFP in muscle cells (α-syn from now on) [31]. The expression of this construct produces aggregation of this peptide in old adult worms (from 2-day-old adult animals onwards), which we could analyse using a fluorescence microscope. Hence, we treated young adult α-syn::YFP animals, which do not show yet aggregates, with 2000 μM metformin, and then analysed 2-day-old adult stage. This analysis showed that these worms had a reduction of α-syn aggregates compared to untreated animals (OR = 0.55, CI95 %[0.45, 0.69], p < 0.001) (Fig. 4A). Treating these animals with 50 μM salicylate showed a similar result (OR = 0.61, CI95 %[0.49, 0.75], p < 0.001) (Fig. 4A). As we expected, we could not observe differences between animals treated just with low doses of metformin or salicylate (150 μM metformin or 5 μM salicylate), and untreated worms (OR = 0.94, CI95 %[0.76, 1.16], p = 0.545; OR = 1.05, CI95 %[0.85, 1.3], p = 0.679, respectively) (Fig. 4A). However, synergistic combination of both reduced significantly α-syn aggregates in muscle cells compared to control animals (OR = 0.63, CI95 %[0.50, 0.78], p < 0.001) (Fig. 4A and B).
Fig. 4.
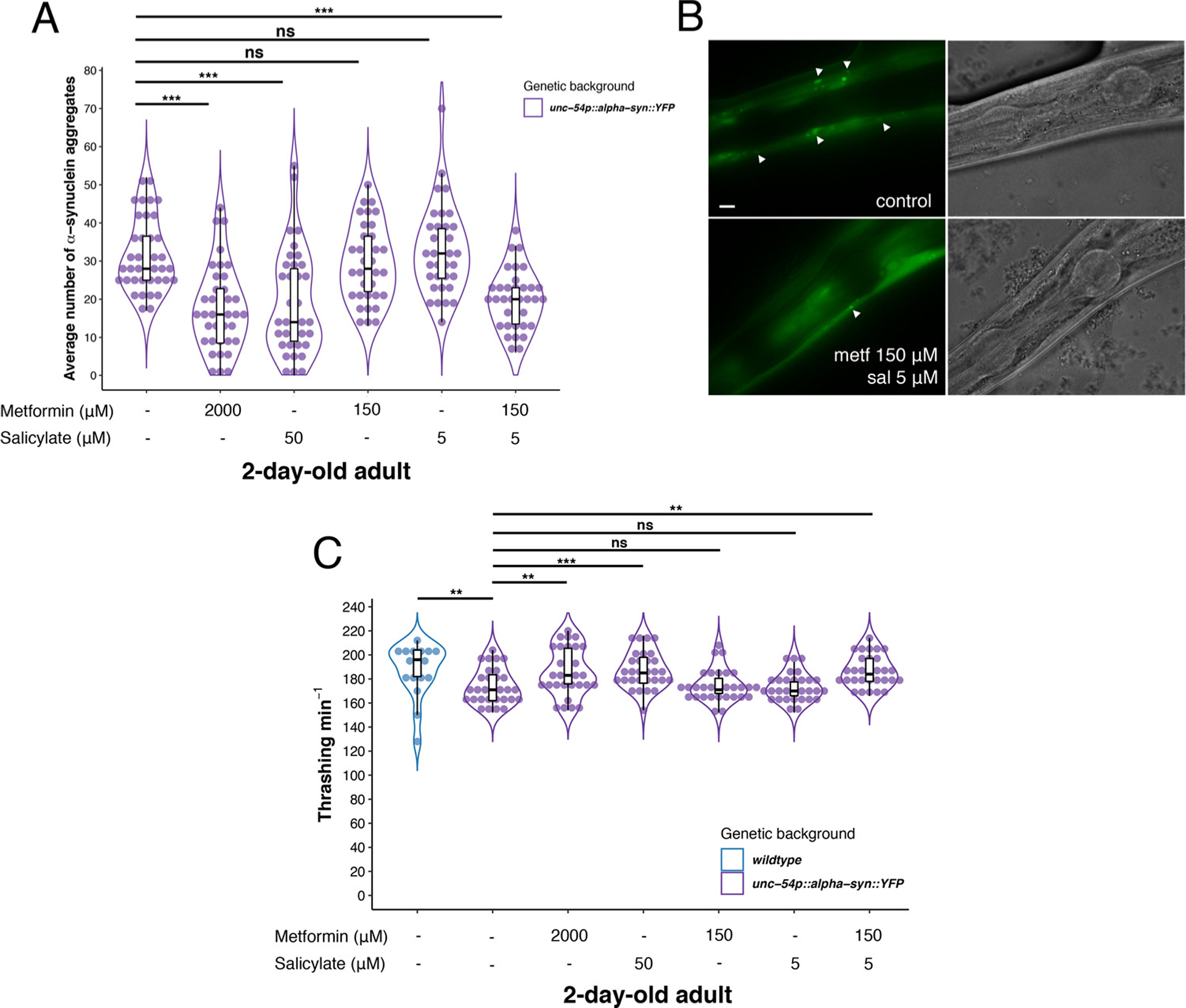
Synergistic treatment with metformin and salicylate reduce α-synuclein aggregation and restore motor capacity. A) Treated animals with 2000 μM metformin or 50 μM salicylate show a reduction of α-synuclein (α-syn) aggregates compared to untreated α-syn::YFP 2-day-old adult animals (OR = 0.55, CI95 %[0.45, 0.69], p < 0.001; OR = 0.61, CI95 %[0.49, 0.75], p < 0.001, respectively). Synergistic dose of metformin (150 μM) and salicylate (5 μM) in combination reduces α-syn aggregation compared to untreated animals, to the same level of the respective high doses separately (OR = 0.63, CI95 %[0.50, 0.78], p < 0.001). B) Photographs from untreated/treated (150 μM metf/ 5 μM sal) α-syn::YFP 2-day-old adult animals. Images show α-syn aggregates (white arrows) in the area located between two pharyngeal bulbs. C) α-syn aggregates induce an impairment of motility compared with wild type animals (OR = −14.02, CI95 %[−23.26, −4.77], p = 0.003). Treated worms with high doses of metformin (2000 μM) or salicylate (50 μM) and synergistic doses of both (metformin 150 μM/salicylate 5 μM) rescue motility capacity of α-syn::YFP 2-day-old adult animals to the level of wild type animals (not expressing transgenes) (Estimate = 12.60, CI95 %[4.74, 20.47], p = 0.002; Estimate = 13.47, CI95 %[5.60, 21.33], p = 0.001; Estimate = 12.60, CI95 %[4.74, 20.47], p = 0.002, respectively). PolyQ aggregation analysis show Odds Ratio (OR), lower and upper confidence interval 95 % (CI95 %) and p-value to indicate the significance of the data. For thrashing analysis, OR value is changed by Estimate value according to the type of the variable. At least twenty animals were tested and experiments were performed three independent times. Scale bar represents: 10 μm.
In parallel, we tested whether drug treatment improved health condition of α-syn::YFP animals by conducting the thrashing assay. In this case, treated 2-day-old adult worms with 2000 μM metformin or 50 μM salicylate separately showed an improvement of motility capacity compared to untreated animals (Estimate = 12.60, CI95 %[4.74, 20.47], p = 0.002; Estimate = 13.47, CI95 %[5.60, 21.33], p = 0.001, respectively) (Fig. 4C). In contrast, single low dose of drugs (metformin 150 μM and salicylate 5 μM) showed to be ineffective (Estimate = 0.733, CI95 %[−7.13, 8.60], p = 0.854; Estimate = −0.93, CI95 %[−8.80, 6.93], p = 0.815, respectively), while combination of both, increased significalty motility of animals compared to untreated α-syn::YFP 2-day-old adult animals (Estimate = 12.60, CI95 %[4.74, 20.47], p = 0.002) (Fig. 4C). These results suggest that metformin, salicylate and synergistic combination of them are able to reduce protein aggregation from other toxic species, and restore healthspan.
4. Conclusions
Our study shows for the first time that synergistic activation of AMPK, using low doses of metformin and salicylate, alleviates the neurotoxic effects of expanded polyQs and α-synuclein in vivo. Activation of this enzyme, using metformin, has been shown to be a magnificent strategy to treat behavioural and molecular symtopms in diferent murine models of HD. However metformin is a pleitotropic drug, as well as is salicylate, and therefore it is expected that using them to treat HD may produce undesired secondary effects in HD patients. Therefore, using low doses of both drugs may prevent from activation of unwanted targets, while activation of AMPK will be maintained to induce cell protection. Moreover, we show that protection induced by synergetic treatment with both chemicals requires autophagic flow, a cellular process which is disrupted in animal models and patients of HD.
Both AMPK and components of the autophagy process are strongly conserved between C. elegans and mammals, and hence it is not unfair to hypothesise that synergistic activation of AMPK may also be protective in humans. Therefore, pre-clinical trials in murine models of HD using combinations of both drugs are justified.
Supplementary Material
Acknowledgements
We thank the CGC, funded by the NIH Office of Research Infrastructure Programs (P40 OD010440), for worm strains. We also thank Andrew Fire for kindly providing the L4440 plasmid vector and the HT115 strain. We would also like to thank Ma Pilar Marín (Microscopy Unit of IIS-La Fe) and Carlos Mora Martínez (Institute of Biotechnology, University of Helsinki, Finland) for her kind help. RPVM is a Miguel Servet type II researcher (CPII16/00004) funded by Instituto de Salud Carlos III (ISCIII, Madrid, Spain). Grants from the ISCIII were used to perform this work (PI14/00949 and PI17/00011). All grants from ISCIII are co-financed by the European Development Regional Fund ”A way to achieve Europe” (ERDF). JBY holds a grant from the Generalitat Valenciana and the European Social Fund (ACIF/2019/249). Some equipment used in this work has been funded in partnership between the Generalitat Valenciana (Conselleria de Sanitat I Salut Pública, Valencian Community, Spain) and European Funds (ERDF/FSE), through the call “Improvement of research infrastructures for rare diseases” CV FEDER 2014-2020. This work has been partially supported by a grant from the Fundació Telemarató de la TV3 (Reference 559), which covered the work of MDS. The funds from the ISCIII are partially supported by the European Regional Development Fund. RPVM is also a Marie Curie fellow (CIG322034, EU). This work has been partially supported by a grant from the CIBERER (ACCI2016), a grant from the Fundación Ramón Areces (CIVP19S8119) and an Ayuda Miguel Gil grant to RPVM (VII Convocatoria Ayudas a la Investigación MHER, 2019).
Footnotes
Declaration of Competing Interest
The authors state that there is no conflict of interest.
Appendix A. Supplementary data
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.phrs.2020.105105.
References
- [1].Paulson H, Repeat expansion diseases, Handbook of Clinical Neurology vol. 147, Elsevier, 2018, pp. 105–123 ISBN 978-0-444-63233-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Lieberman AP, Shakkottai VG, Albin RL, Polyglutamine repeats in neurodegenerative diseases, Annu. Rev. Pathol. Mech. Dis 14 (2019) 1–27, 10.1146/annurev-pathmechdis-012418-012857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Macdonald M, A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington’s disease chromosomes, Cell 72 (1993) 971–983, 10.1016/0092-8674(93)90585-E. [DOI] [PubMed] [Google Scholar]
- [4].Evers MM, Toonen LJA, van Roon-Mom WMC, Ataxin-3 protein and RNA toxicity in spinocerebellar Ataxia type 3: current insights and emerging therapeutic strategies, Mol. Neurobiol (2013), 10.1007/s12035-013-8596-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Irwin S, Vandelft M, Pinchev D, Howell JL, Graczyk J, Orr HT, Truant R, RNA association and nucleocytoplasmic shuttling by ataxin-1, J. Cell. Sci 118 (2005) 233–242, 10.1242/jcs.01611. [DOI] [PubMed] [Google Scholar]
- [6].Ortega Z, Lucas JJ, Ubiquitin-proteasome system involvement in Huntington’s disease, Front. Mol. Neurosci 7 (2014), 10.3389/fnmol.2014.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Kopito RR, Aggresomes, inclusion bodies and protein aggregation, Trends Cell Biol 10 (2000) 524–530, 10.1016/S0962-8924(00)01852-3. [DOI] [PubMed] [Google Scholar]
- [8].Morley JF, Brignull HR, Weyers JJ, Morimoto RI, The threshold for polyglutamine-expansion protein aggregation and cellular toxicity is dynamic and influenced by aging in Caenorhabditis elegans, Proc. Natl. Acad. Sci 99 (2002) 10417–10422, 10.1073/pnas.152161099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Parker JA, Connolly JB, Wellington C, Hayden M, Dausset J, Neri C, Expanded polyglutamines in Caenorhabditis elegans cause axonal abnormalities and severe dysfunction of PLM mechanosensory neurons without cell death, Proc. Natl. Acad. Sci 98 (2001) 13318–13323, 10.1073/pnas.231476398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Satyal SH, Schmidt E, Kitagawa K, Sondheimer N, Lindquist S, Kramer JM, Morimoto RI, Polyglutamine aggregates alter protein folding homeostasis in Caenorhabditis elegans, Proc. Natl. Acad. Sci 97 (2000) 5750–5755, 10.1073/pnas.100107297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Nollen EAA, Garcia SM, van Haaften G, Kim S, Chavez A, Morimoto RI, Plasterk RHA, From the Cover: genome-wide RNA interference screen identifies previously undescribed regulators of polyglutamine aggregation, Proc. Natl. Acad. Sci 101 (2004) 6403–6408, 10.1073/pnas.0307697101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Parker JA, Vazquez-Manrique RP, Tourette C, Farina F, Offner N, Mukhopadhyay A, Orfila A-M, Darbois A, Menet S, Tissenbaum HA, et al. , Integration of β-Catenin, sirtuin, and FOXO signaling protects from mutant huntingtin toxicity, J. Neurosci 32 (2012) 12630–12640, 10.1523/JNEUROSCI.0277-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Parker JA, Arango M, Abderrahmane S, Lambert E, Tourette C, Catoire H, Néri C, Resveratrol rescues mutant polyglutamine cytotoxicity in nematode and mammalian neurons, Nat. Genet 37 (2005) 349–350, 10.1038/ng1534. [DOI] [PubMed] [Google Scholar]
- [14].Tourette C, Farina F, Vazquez-Manrique RP, Orfila A-M, Voisin J, Hernandez S, Offner N, Parker JA, Menet S, Kim J, et al. , The wnt receptor Ryk reduces neuronal and cell survival capacity by repressing FOXO activity during the early phases of mutant huntingtin pathogenicity, PLoS Biol 12 (2014) e1001895, 10.1371/journal.pbio.1001895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Vazquez-Manrique RP, Farina F, Cambon K, Dolores Sequedo M, Parker AJ, Millan JM, Weiss A, Deglon N, Neri C, AMPK activation protects from neuronal dysfunction and vulnerability across nematode, cellular and mouse models of Huntington’s disease, Hum. Mol. Genet 25 (2016) 1043–1058, 10.1093/hmg/ddv513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Sanchis A, García-Gimeno MA, Cañada-Martínez AJ, Sequedo MD, Millán JM, Sanz P, Vázquez-Manrique RP, Metformin treatment reduces motor and neuropsychiatric phenotypes in the zQ175 mouse model of Huntington disease, Exp. Mol. Med 51 (2019) 1–16, 10.1038/s12276-019-0264-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Walter C, Clemens LE, Müller AJ, Fallier-Becker P, Proikas-Cezanne T, Riess O, Metzger S, Nguyen HP, Activation of AMPK-induced autophagy ameliorates Huntington disease pathology in vitro, Neuropharmacology 108 (2016) 24–38, 10.1016/j.neuropharm.2016.04.041. [DOI] [PubMed] [Google Scholar]
- [18].Barnes K, Ingram JC, Porras OH, Barros LF, Hudson ER, Fryer LGD, Foufelle F, Carling D, Hardie DG, Baldwin SA, Activation of GLUT1 by metabolic and osmotic stress: potential involvement of AMP-activated protein kinase (AMPK), J. Cell. Sci 115 (2002) 2433–2442. [DOI] [PubMed] [Google Scholar]
- [19].Kim J, Yang G, Kim Y, Kim J, Ha J, AMPK activators: mechanisms of action and physiological activities, Exp. Mol. Med 48 (2016), 10.1038/emm.2016.16 e224–e224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Arnoux I, Willam M, Griesche N, Krummeich J, Watari H, Offermann N, Weber S, Narayan Dey P, Chen C, Monteiro O, et al. , Metformin reverses early cortical network dysfunction and behavior changes in Huntington’s disease, eLife 7 (2018) e38744, 10.7554/eLife.38744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Ma TC, Buescher JL, Oatis B, Funk JA, Nash AJ, Carrier RL, Hoyt KR, Metformin therapy in a transgenic mouse model of Huntington’s disease, Neurosci. Lett 411 (2007) 98–103, 10.1016/j.neulet.2006.10.039. [DOI] [PubMed] [Google Scholar]
- [22].Hervás D, Fornés-Ferrer V, Gómez-Escribano AP, Sequedo MD, Peiró C, Millán JM, Vázquez-Manrique RP, Metformin intake associates with better cognitive function in patients with Huntington’s disease , PLoS One 12 (2017) e0179283, 10.1371/journal.pone.0179283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Ford RJ, Fullerton MD, Pinkosky SL, Day EA, Scott JW, Oakhill JS, Bujak AL, Smith BK, Crane JD, Blümer RM, et al. , Metformin and salicylate synergistically activate liver AMPK, inhibit lipogenesis and improve insulin sensitivity, Biochem. J 468 (2015) 125–132, 10.1042/BJ20150125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Talarico G, Orecchioni S, Dallaglio K, Reggiani F, Mancuso P, Calleri A, Gregato G, Labanca V, Rossi T, Noonan DM, et al. , Aspirin and atenolol enhance metformin activity against breast cancer by targeting both neoplastic and micro-environment cells, Sci. Rep 6 (2016) 18673, 10.1038/srep18673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Schulten H-J, Pleiotropic effects of metformin on Cancer, IJMS 19 (2018) 2850, 10.3390/ijms19102850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Forouzandeh F, Salazar G, Patrushev N, Xiong S, Hilenski L, Fei B, Alexander RW, Metformin beyond diabetes: pleiotropic benefits of metformin in attenuation of atherosclerosis, JAHA 3 (2014), 10.1161/JAHA.114.001202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Alfonso Lf., Srivenugopal KS, Bath GJD, Oes aspirin acetylate multiple cellular proteins? (Review), Mol. Med. Rep 2 (2009), 10.3892/mmr_00000132. [DOI] [PubMed] [Google Scholar]
- [28].Dachineni R, Kumar DR, Callegari E, Kesharwani SS, Sankaranarayanan R, Seefeldt T, Tummala H, Bhat GJ, Salicylic acid metabolites and derivatives inhibit CDK activity: novel insights into aspirin’s chemopreventive effects against colorectal cancer, Int. J. Oncol 51 (2017) 1661–1673, 10.3892/ijo.2017.4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Choi HW, Tian M, Manohar M, Harraz MM, Park S-W, Schroeder FC, Snyder SH, Klessig DF, Human GAPDH is a target of aspirin’s primary metabolite salicylic acid and its derivatives, PLoS One 10 (2015) e0143447, 10.1371/journal.pone.0143447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Brenner S, The genetics of Caenorhabditis elegans, Genetics 77 (1974) 71–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].van Ham TJ, Thijssen KL, Breitling R, Hofstra RMW, Plasterk RHA, Nollen EAAC, Elegans model identifies genetic modifiers of α-Synuclein inclusion formation during aging, PLoS Genet. 4 (2008) e1000027, 10.1371/journal.pgen.1000027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Vicencio J, Martínez-Fernández C, Serrat X, Cerón J, Efficient generation of endogenous fluorescent reporters by nested CRISPR in Caenorhabditis elegans, Genetics 211 (2019) 1143–1154, 10.1534/genetics.119.301965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Prior H, Jawad AK, MacConnachie L, Beg AA, Highly efficient, rapid and Co-CRISPR-Independent genome editing in Caenorhabditis elegans, G3 7 (2017) 3693–3698, 10.1534/g3.117.300216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Muñoz-Lobato F, Rodríguez-Palero MJ, Naranjo-Galindo FJ, Shephard F, Gaffney CJ, Szewczyk NJ, Hamamichi S, Caldwell KA, Caldwell GA, Link CD, et al. , Protective role of DNJ-27/ERdj5 in Caenorhabditis elegans models of human neurodegenerative diseases, Antioxid. Redox Signal 20 (2014) 217–235, 10.1089/ars.2012.5051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Hahm J-H, Kim S, DiLoreto R, Shi C, Lee S-JV, Murphy CT, Nam HGC, Elegans maximum velocity correlates with healthspan and is maintained in worms with an insulin receptor mutation, Nat. Commun 6 (2015) 8919, 10.1038/ncomms9919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Bansal A, Zhu LJ, Yen K, Tissenbaum HA, Uncoupling lifespan and healthspan in Caenorhabditis elegans longevity mutants, Proc. Natl. Acad. Sci. U.S.A 112 (2015) E277–E286, 10.1073/pnas.1412192112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Rena G, Sakamoto K, Salicylic acid: old and new implications for the treatment of type 2 diabetes? Diabetol. Int 5 (2014) 212–218, 10.1007/s13340-014-0177-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Rena G, Hardie DG, Pearson ER, The mechanisms of action of metformin, Diabetologia 60 (2017) 1577–1585, 10.1007/s00125-017-4342-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].The Diabetes Prevention Program Research Group Long-Term Safety, Tolerability, and weight loss associated with metformin in the diabetes prevention program outcomes study, Diabetes Care 35 (2012) 731–737, 10.2337/dc11-1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Huang ES, Strate LL, Ho WW, Lee SS, Chan AT, Long-term use of aspirin and the risk of gastrointestinal bleeding, Am. J. Med 124 (2011) 426–433, 10.1016/j.amjmed.2010.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Hawley SA, Fullerton MD, Ross FA, Schertzer JD, Chevtzoff C, Walker KJ, Peggie MW, Zibrova D, Green KA, Mustard KJ, et al. , The ancient drug salicylate directly activates AMP-Activated protein kinase, Science 336 (2012) 918–922, 10.1126/science.1215327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Owen MR, Doran E, Halestrap AP, Evidence that metformin exerts its anti-diabetic effects through inhibition of complex 1 of the mitochondrial respiratory chain, Biochem. J 348 (Pt 3) (2000) 607–614. [PMC free article] [PubMed] [Google Scholar]
- [43].Barzilai N, Crandall JP, Kritchevsky SB, Espeland MA, Metformin as a tool to target aging, Cell Metab 23 (2016) 1060–1065, 10.1016/j.cmet.2016.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Wu K, Salicylates and their Spectrum of activity, AIAAMC 6 (2007) 278–292, 10.2174/187152307783220031. [DOI] [Google Scholar]
- [45].Hart A, Behavior. WormBook, (2006), 10.1895/wormbook.1.87.1. [DOI] [Google Scholar]
- [46].Lee AL, Ung HM, Sands LP, Kikis EA, A new Caenorhabditis elegans model of human huntingtin 513 aggregation and toxicity in body wall muscles, PLoS One 12 (2017) e0173644, 10.1371/journal.pone.0173644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Guerrero-Gómez D, Mora-Lorca JA, Sáenz-Narciso B, Naranjo-Galindo FJ, Muñoz-Lobato F, Parrado-Fernández C, Goikolea J, Cedazo-Minguez Á, Link CD, Neri C, et al. , Loss of glutathione redox homeostasis impairs proteostasis by inhibiting autophagy-dependent protein degradation, Cell Death Differ 26 (2019) 1545–1565, 10.1038/s41418-018-0270-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Apfeld J, The AMP-activated protein kinase AAK-2 links energy levels and insulin-like signals to lifespan in C. Elegans, Genes Dev 18 (2004) 3004–3009, 10.1101/gad.1255404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Li Y, Chen Y, AMPK and autophagy. In autophagy: biology and diseases, in: Qin Z-H (Ed.), Advances in Experimental Medicine and Biology, vol. 1206, Springer Singapore, Singapore, 2019, pp. 85–108 ISBN 9789811506017. [DOI] [PubMed] [Google Scholar]
- [50].Mauthe M, Orhon I, Rocchi C, Zhou X, Luhr M, Hijlkema K-J, Coppes RP, Engedal N, Mari M, Reggiori F, Chloroquine inhibits autophagic flux by decreasing autophagosome-lysosome fusion, Autophagy 14 (2018) 1435–1455, 10.1080/15548627.2018.1474314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Kabeya Y, LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing, EMBO J 19 (2000) 5720–5728, 10.1093/emboj/19.21.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Melendez A, Autophagy genes are essential for dauer development and life-span extension in C. Elegans, Science 301 (2003) 1387–1391, 10.1126/science.1087782. [DOI] [PubMed] [Google Scholar]
- [53].Jia K, Hart AC, Levine B, Autophagy genes protect against disease caused by polyglutamine expansion proteins in Caenorhabditis elegans, Autophagy 3 (2007) 21–25, 10.4161/auto.3528. [DOI] [PubMed] [Google Scholar]
- [54].Pietrocola F, Castoldi F, Markaki M, Lachkar S, Chen G, Enot DP, Durand S, Bossut N, Tong M, Malik SA, et al. , Aspirin recapitulates features of caloric restriction, Cell Rep 22 (2018) 2395–2407, 10.1016/j.celrep.2018.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Chen J, Ou Y, Li Y, Hu S, Shao L-W, Liu Y, Metformin extends C Elegans lifespan through lysosomal pathway, eLife 6 (2017) e31268, 10.7554/eLife.31268. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


