Abstract
Objective:
To understand osteoporosis screening practices, particularly in men, by a diverse cohort of physicians, including primary care physicians, endocrinologists and geriatricians.
Methods:
We surveyed randomly selected members of the American Academy of Family Practice (AAFP), Endocrine Society and American Geriatrics Society. Respondents were asked to rate how often they would screen for osteoporosis in four different clinical scenarios by ordering a bone density scan. Multivariable logistic regression analyses were conducted to determine factors associated with offering osteoporosis screening in men in each clinical scenario. Physicians were also asked to note factors that would lead to osteoporosis screening in men.
Results:
Response rate was 63% (359/566). While 90% respondents reported that they would always or frequently screen for osteoporosis in a 65-year old postmenopausal woman, only 22% reported they would screen a 74-year-old man with no significant past medical history. Endocrinologists were more likely to screen a 74-year-old man compared to primary care physicians [odds ratio (OR) 2.32, 95% confidence interval (CI) 1.10–4.88]. In addition to chronic steroid use (94%), history of non-traumatic fractures (88%) and androgen-deprivation therapy for prostate cancer (82%), more than half the physicians reported suppressive doses of thyroid hormone (64%) and history of falls (52%) as factors leading to screening for osteoporosis in men.
Conclusion:
Our survey results highlight heterogeneity in osteoporosis screening in men, with underscreening in some scenarios compared to women, and identifies factors that lead to screening in men. These findings can help design interventions to improve osteoporosis screening in men.
Keywords: osteoporosis, screening, men, survey
INTRODUCTION
Osteoporosis is a significant public health issue and is estimated to affect more than 10 million adults over the age of 50 in the United States with approximately 2 million being men (1, 2). Moreover, healthcare utilization and costs associated with management of osteoporosis-related complications are immense, resulting in half a million hospitalizations and about 2.6 million physician office visits per year with an estimated healthcare expenditure of 19 billion dollars annually (3). Overall, one in two women and one in four men above the age of 50 are at risk for fragility fractures (4, 5). While women are at a greater risk of developing osteoporosis, prior studies have shown that men have higher mortality rates and worse functional outcomes than women following a fragility fracture (6–10). Specifically, men are twice as likely as women to die after a hip fracture (10).
Despite increased awareness regarding osteoporosis, its consequences, and availability of multiple widely disseminated practice guidelines recommending osteoporosis screening in older adults, osteoporosis remains underdiagnosed and undertreated in men, leaving them vulnerable to early death and disability (11–17). Even though multiple factors likely contribute to the suboptimal evaluation of men at risk for osteoporosis, little is known about current clinical practices regarding osteoporosis screening across different specialties.
Our study was designed to further understand osteoporosis screening practices in men among a diverse cohort of physicians. In addition, we sought to identify factors that lead physicians to screen for osteoporosis in men. We conducted a nationwide survey of endocrinologists, primary care physicians and geriatricians, reflective of a real-world setting, in order to provide additional, clinically relevant data on where potential interventions are needed to improve osteoporosis screening in men.
METHODS
Study Population and Data Collection
We conducted a nationwide survey of randomly selected active members of the Endocrine Society, American Academy of Family Practice and American Geriatrics Society. We employed a modified Dillman method of survey administration in order to enhance our response rate (18). An initial mailing to the physicians included an introductory letter, survey instrument, postage-paid return envelope and a small monetary gift. Following three weeks, a postcard reminder was sent to all selected physicians. A second survey instrument with a postage-paid return envelope was sent to non-responders three weeks later. A total of 600 physicians were surveyed. Physicians completed the surveys in 2018.
As shown in Figure 1, a total of 34 physicians were ineligible because they were deceased, retired, not treating patients with osteoporosis or had an incorrect mailing address. Of the remaining 566 response-eligible physicians, 359 completed the survey with a 63% (359/566) response rate. Data from the survey were de-identified and logged using a double entry method to ensure <1% error. The study was granted exemption by the University of Michigan Institutional Review Board.
Figure 1.
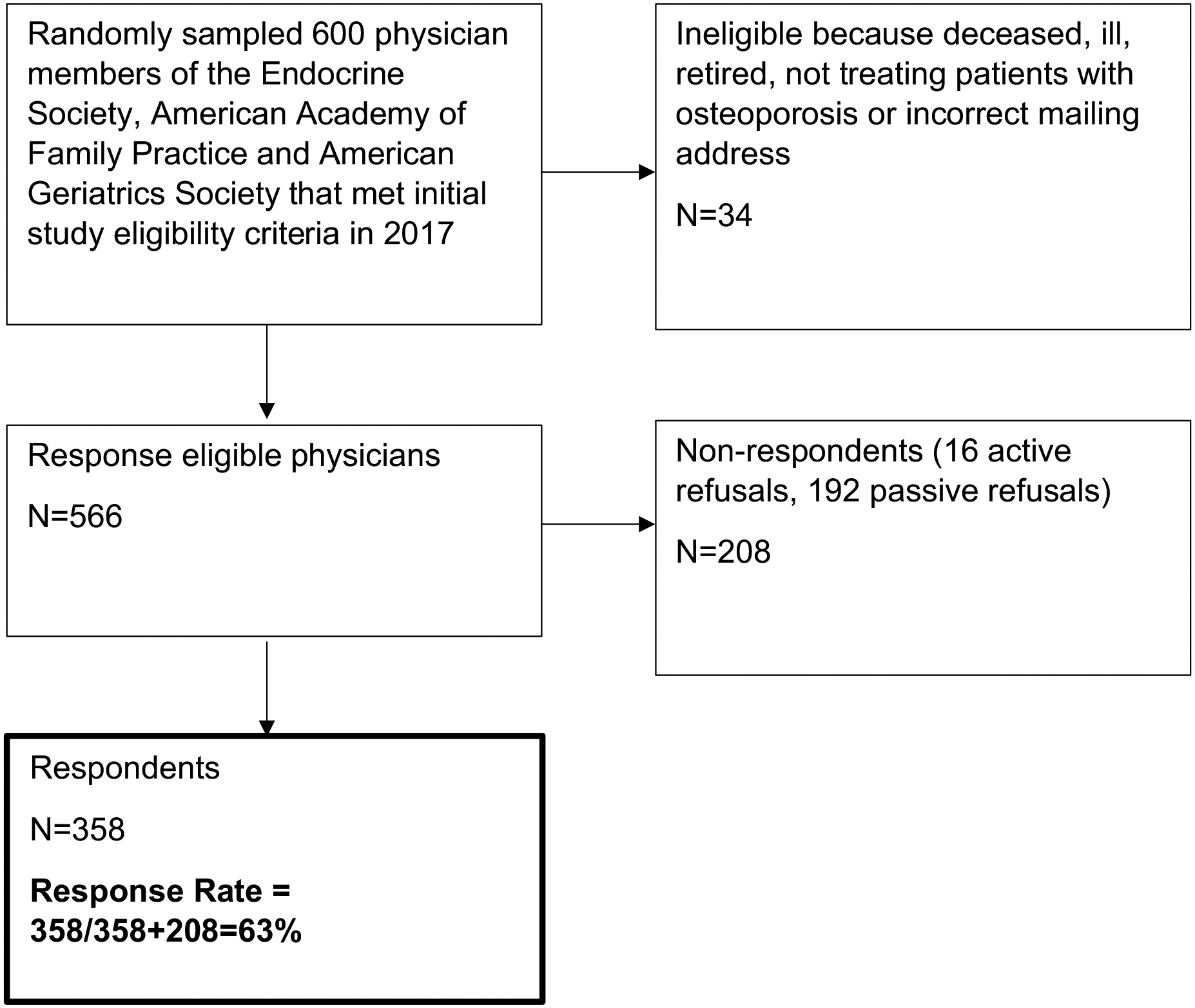
Flow diagram of survey respondents.
Survey Design and Measures
The survey instrument was developed based on the research questions and systematic review of the literature. Standard techniques were used to assess content validity including review by endocrinologists, primary care physicians, geriatricians and survey methodologists, and pilot testing in a selected multidisciplinary group of physicians involved in osteoporosis screening at the University of Michigan.
Information collected on physician respondents
We collected information on physician specialty, practice setting, years in practice since completion of residency, percentage of male patients treated in clinic, number of days spent providing care in an average week, and guidelines read on osteoporosis. Physician specialty was categorized as endocrinology, primary care (including those reporting internal medicine and family medicine), and geriatrics. Practice setting was categorized as academic tertiary care center, private practice and community-based academic affiliate. In regards to guidelines, physicians were asked to state whether they had read any of the following guidelines: 2008 Screening for Osteoporosis in Men: A Clinical Practice Guideline from the American College of Physicians (19); 2008 National Osteoporosis Foundation Clinical Practice Guidelines (20); 2012 Osteoporosis in Men: An Endocrine Society Clinical Practice Guideline (21); 2014 National Osteoporosis Foundation: Clinician’s Guide to Prevention and Treatment of Osteoporosis (22); 2017 Treatment of Low Bone Density or Osteoporosis to Prevent Fractures in Men and Women: A Clinical Practice Guideline Update from the American College of Physicians (23).
Physician reported frequency of screening for osteoporosis
Physicians were asked how often they would screen for osteoporosis by ordering a bone density scan in the following four different clinical scenarios: a) a 74-year-old man with no significant past medical history, b) a 65-year-old postmenopausal woman, c) a 55-year-old man with no significant past medical history and d) a 55-year-old man with a history of thyroid cancer on long term suppressive doses of thyroid hormone. Response categories were based on a 5-point Likert scale as follows: always, frequently, occasionally, rarely and never.
Factors reported by physicians to lead to osteoporosis screening in men
Physicians were also asked to select all factors that led them to screen for osteoporosis in their male patients. Factors included chronic use of steroids, previous non-traumatic fracture, androgen-deprivation therapy for prostate cancer, primary hyperparathyroidism, hypogonadism, suppressive doses of thyroid hormone, history of falls, rheumatoid arthritis, alcohol abuse, smoking, family history of osteoporosis, history of parental hip fracture under the age of 80, previous traumatic fracture, severe osteoarthritis, vegetarian diet and other.
Statistical Analyses
Descriptive data were generated with frequencies and percentages. Multivariable logistic regression analyses were conducted to determine factors associated with offering osteoporosis in men screening in each clinical scenario (Likert scale was dichotomized as never/rarely/occasionally versus frequently/always). Using the Pearson chi-square test, we performed a univariate analysis to determine the association between use of suppressive doses of thyroid hormone leading to osteoporosis screening in men and physician specialty. A univariate analysis on the association between history of falls as a factor leading to osteoporosis screening in men and physician specialty was also conducted. Missing data were <5% per survey item and were not included in the analyses. All statistical analyses were performed using R version 3.5.2. A 95% CI not including the null value was considered statistically significant. A p-value of <0.05 was considered statistically significant.
RESULTS
Of the 566 response-eligible physicians, 359 (63%) completed the survey. The respondent characteristics are shown in Table 1. Of the 359 respondents, 128 (36%) were primary care physicians, 114 (32%) were endocrinologists and 113 (32%) were geriatricians. Majority of the physicians were in private practice (51%). More than half the physicians reported being in practice for over 20 years (52%). Overall, 78.9% of endocrinologists, 53.1% of geriatricians and 33.6% of primary care physicians reported having read at least one guideline on osteoporosis screening (see Appendix for details).
Table 1.
Survey participant characteristics (N=359)*
| Physician Characteristics | N (%) |
|---|---|
| Specialty | |
| Primary Care | 128 (36.1) |
| Endocrinology | 114 (32.1) |
| Geriatrics | 113 (31.8) |
| Practice Setting | |
| Private Practice | 173 (50.6) |
| Community-Based Academic Affiliate | 106 (31.0) |
| Academic Tertiary Care Center | 63 (18.4) |
| Years in Practice | |
| 0–10 | 67 (18.9) |
| 11–20 | 102 (28.7) |
| >20 | 186 (52.4) |
| Days per Week Providing Patient Care | |
| 0.5–2 days | 62 (18.0) |
| 3–5 days | 290 (82.0) |
| Percent Patients who are Male | |
| 0–25% | 44 (12.4) |
| 26–50% | 259 (73.2) |
| 51–100% | 51 (14.4) |
| Read Guidelines on Osteoporosis | |
| No | 165 (46.0) |
| Yes | 194 (54.0) |
Figures 2a–d demonstrate the frequency of screening for osteoporosis based on physician responses to the four different clinical scenarios described above. Herein, only 22% of physicians reported they would frequently to always screen a 74-year-old man with no significant medical history (Figure 2a) compared to 90% of physicians who reported they would frequently to always screen a 65-year-old woman for osteoporosis (Figure 2b). Of those physicians that stated that they would frequently to always screen a 74-year-old man with no significant medical history, 46% were endocrinologists, 32% were geriatricians, and 22% were primary care physicians. While fewer than 2% of the physicians reported that they would frequently to always screen a 55-year-old man with no significant medical history (Figure 2c), 58% stated they would screen a 55-year-old man with history of thyroid cancer on suppressive doses of thyroid hormone (Figure 2d). Results from multivariable analyses indicated that endocrinologists were more likely to screen a 74-year-old man without a significant past medical history compared to primary care physicians (OR 2.32, 95% CI 1.10–4.88). Other results did not reach statistical significance.
Figure 2a.
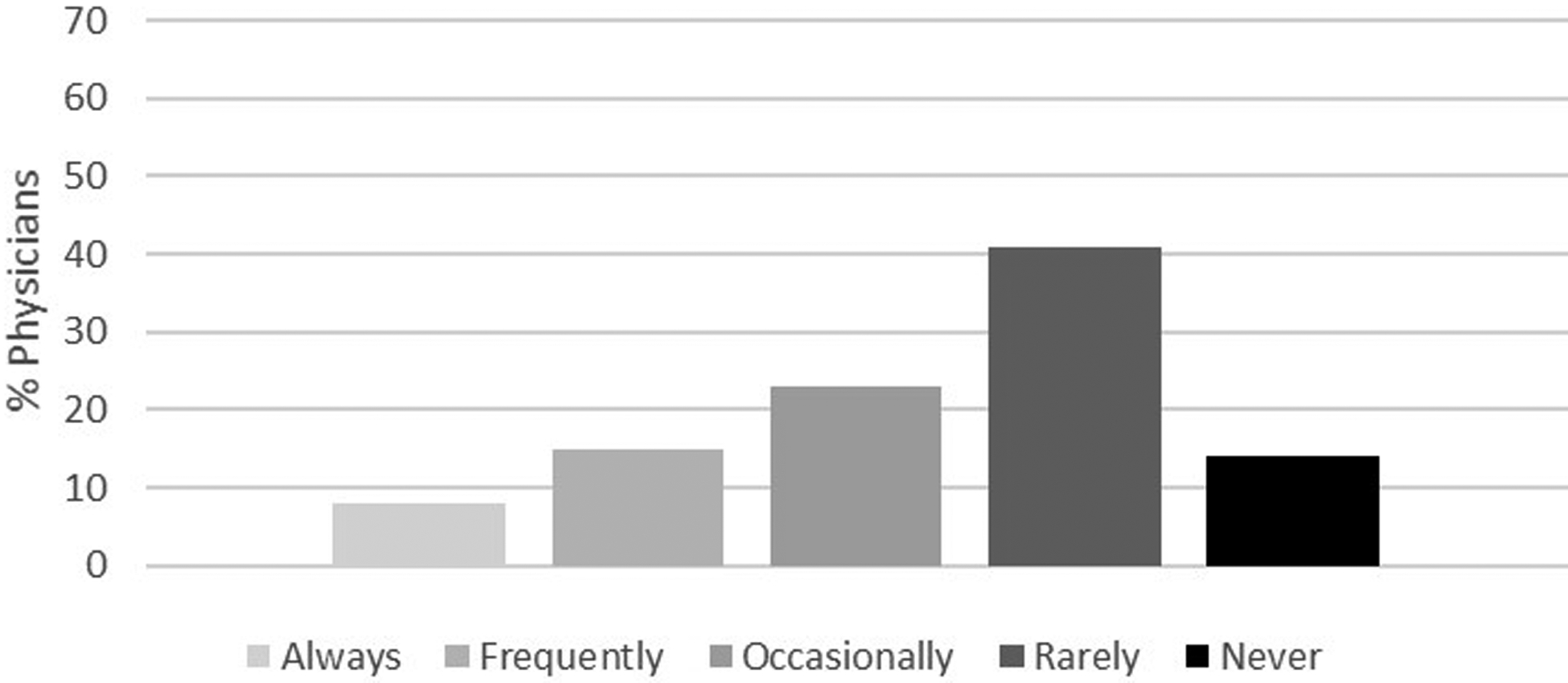
Likelihood of offering osteoporosis screening in a 74-year-old man with no significant past medical history. Response categories were based on a 5-point Likert scale as follows: always, frequently, occasionally, rarely and never.
Figure 2d.
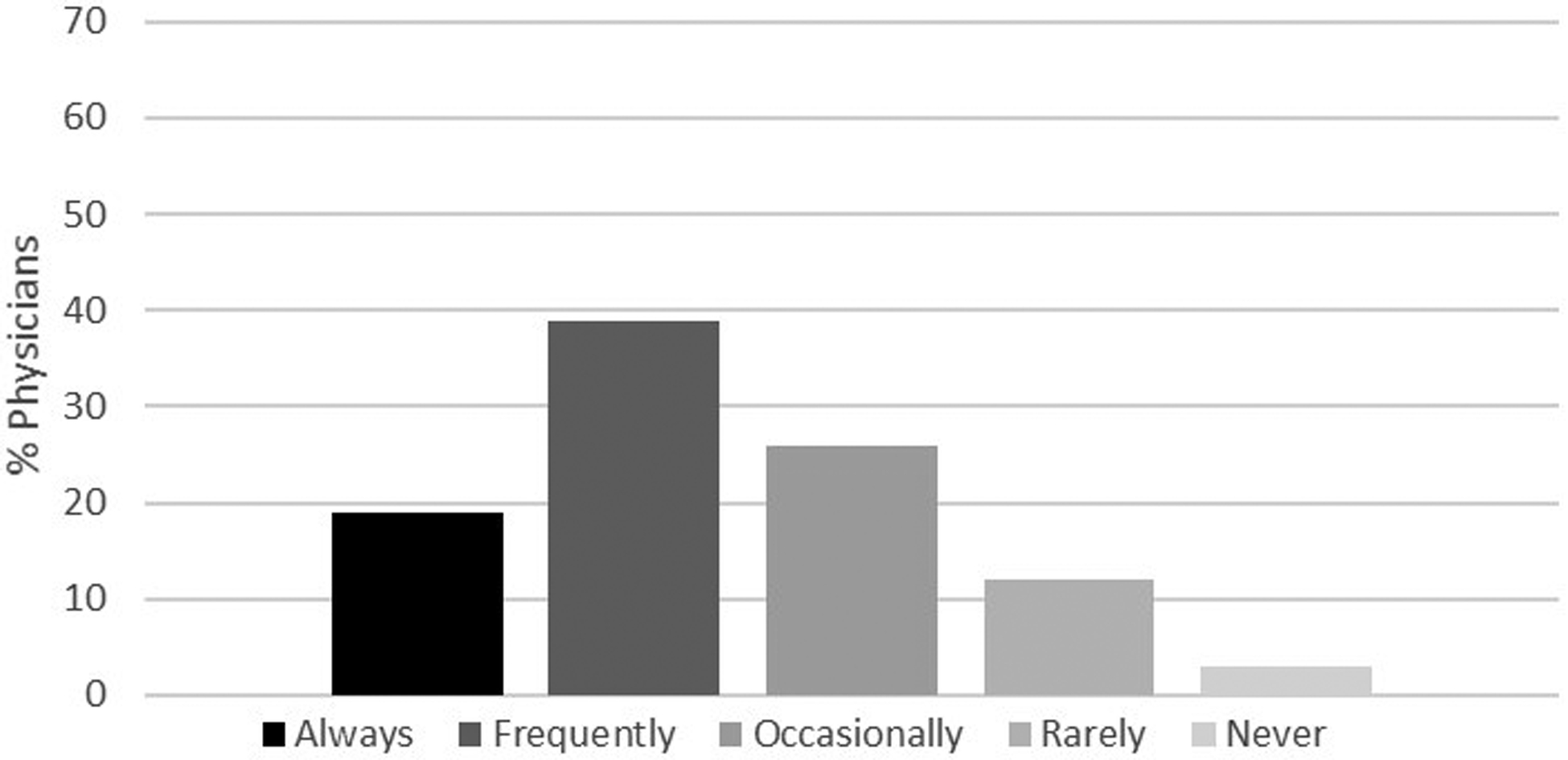
Likelihood of offering osteoporosis screening in a 55-year-old man with a history of thyroid cancer on long term suppressive doses of thyroid hormone. Response categories were based on a 5-point Likert scale as follows: always, frequently, occasionally, rarely and never.
Figure 2b.
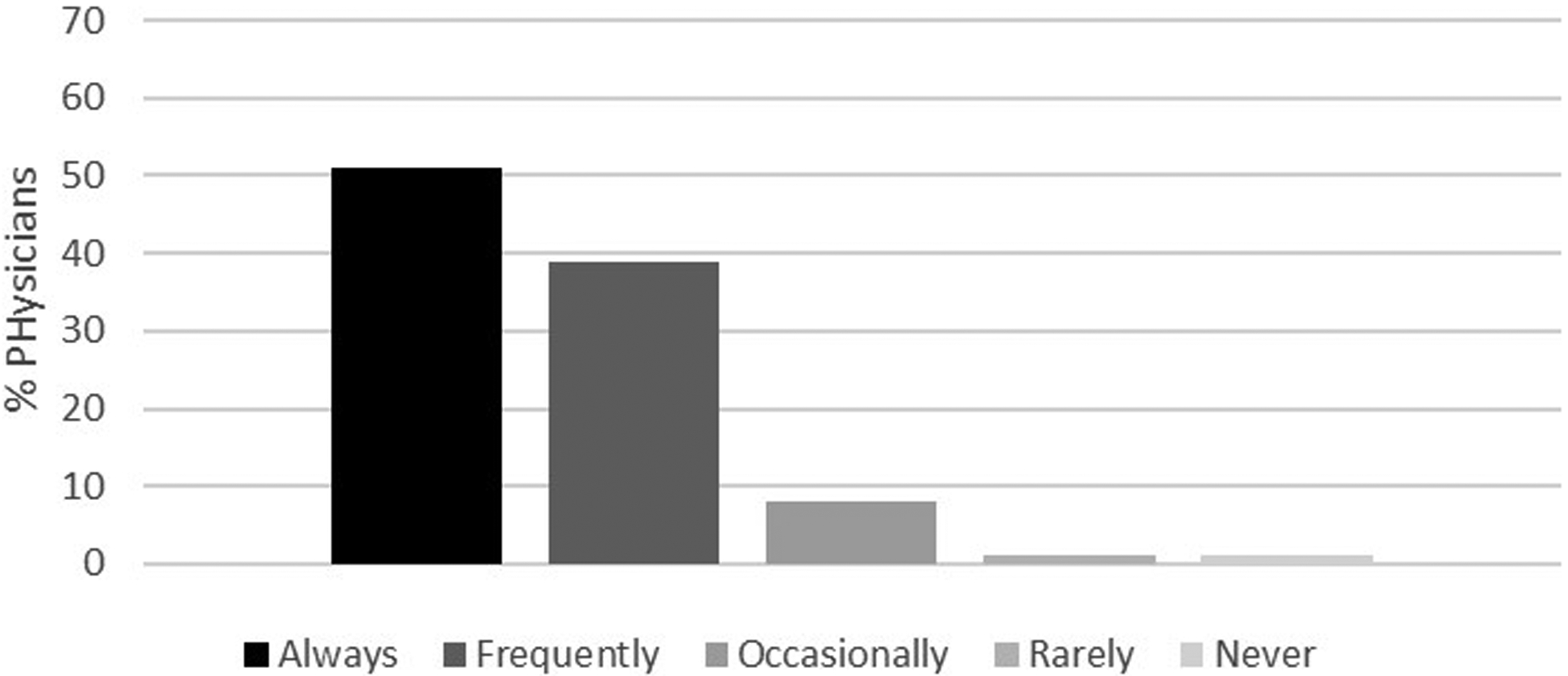
Likelihood of offering osteoporosis screening in a 65-year-old postmenopausal woman. Response categories were based on a 5-point Likert scale as follows: always, frequently, occasionally, rarely and never.
Figure 2c.
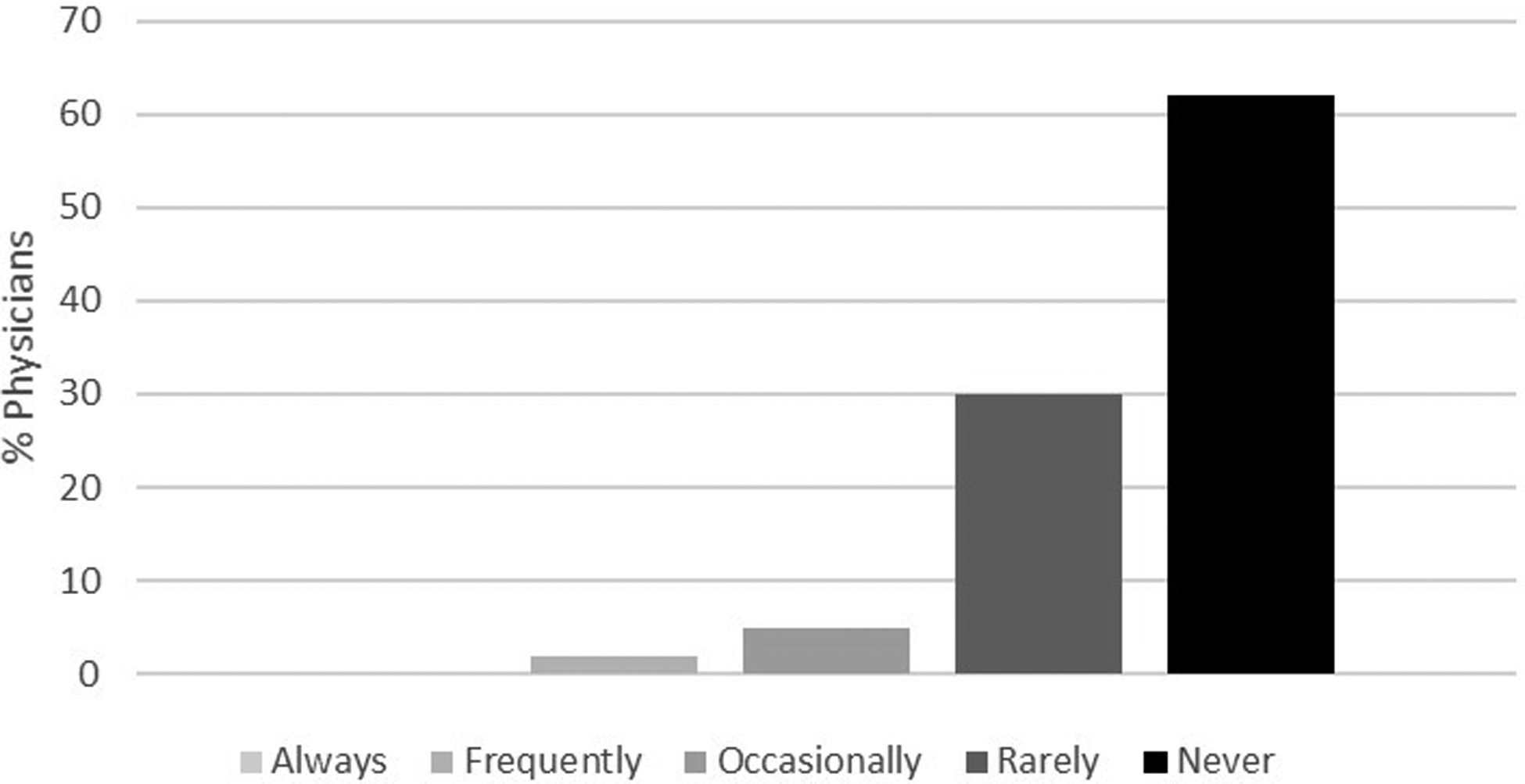
Likelihood of offering osteoporosis screening in a 55-year-old man with no significant past medical history. Response categories were based on a 5-point Likert scale as follows: always, frequently, occasionally, rarely and never.
Figure 3 shows the factors reported by physicians that would lead them to screen for osteoporosis in their male patients. Chronic use of steroids (94%), previous non-traumatic fracture (88%) and androgen-deprivation therapy for prostate cancer (82%) were the most common physician-reported factors that would lead to osteoporosis screening in men. Suppressive doses of thyroid hormone (64%) and history of falls (52%) were also reported as factors that would lead to osteoporosis screening in men. In univariate analyses, endocrinologists were more likely to order a bone density scan for osteoporosis screening in men taking suppressive doses of thyroid hormone (p<0.001) (data not shown). Physician specialty was not associated with screening for osteoporosis in men with a history of falls (p=0.508).
Figure 3.
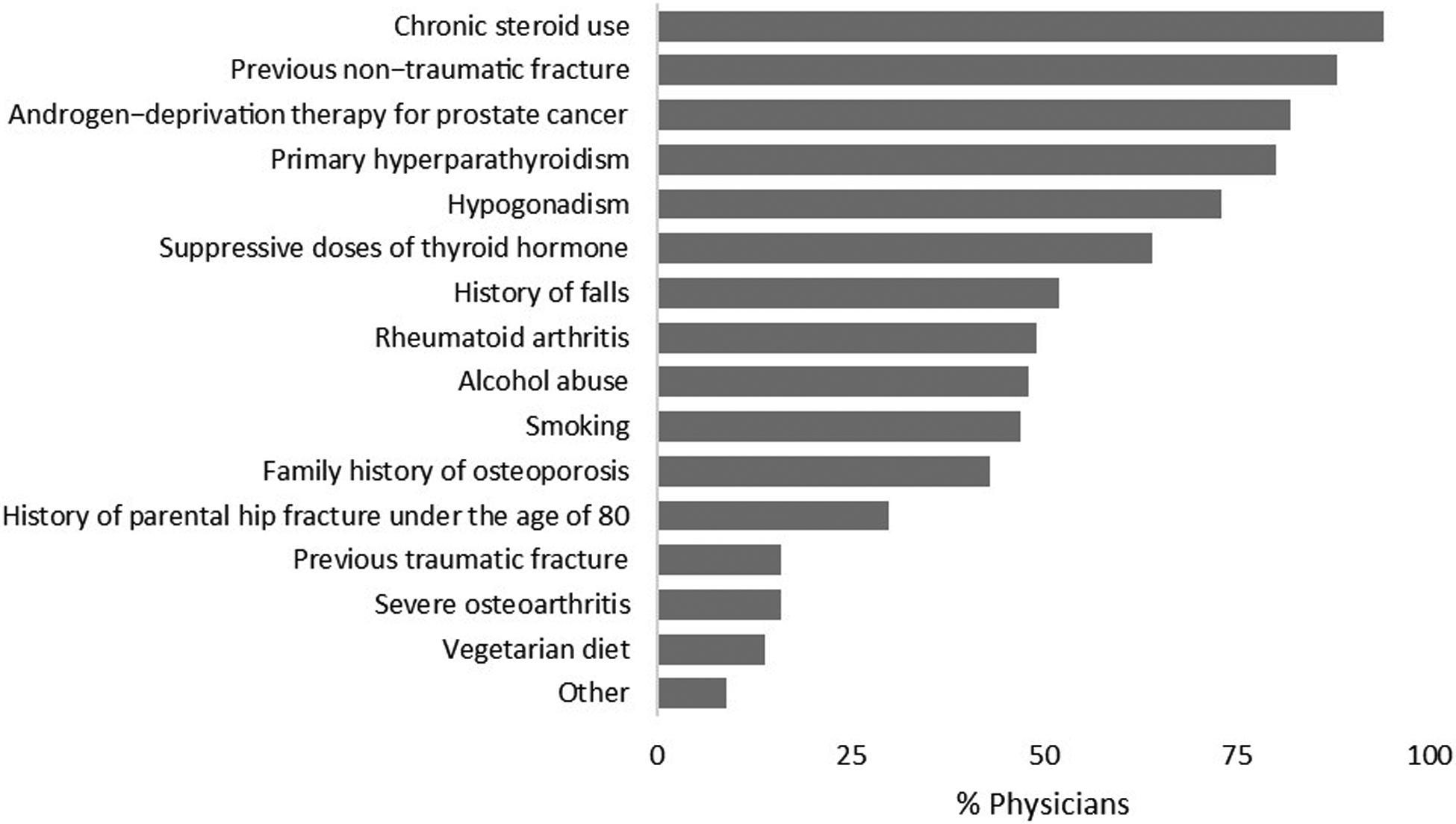
Factors reported by physicians to lead to osteoporosis screening in men.
DISCUSSION
Our nationwide survey of primary care physicians, endocrinologists and geriatricians showed that while the majority of physicians reported screening a 65-year-old postmenopausal woman with no significant risk factors for osteoporosis, fewer than one-fourth of physicians would screen a 74-year-old man with no past medical history, with endocrinologists being more likely to do so. Chronic use of steroids, previous non-traumatic fracture and androgen-deprivation therapy for prostate cancer were the most commonly reported factors that led to osteoporosis screening in men. Less expected was the finding that more than half of physicians reported suppressive doses of thyroid hormone and history of falls as factors leading to osteoporosis screening in men.
Complementing findings from prior studies, the results of our study demonstrate that there is heterogeneity in osteoporosis screening practices in men, compared to women, likely reflective of clinical uncertainty and possibly leading to underscreening in men (24–26). This partially stems from the lack of consensus among published guidelines and recommendations on advised screening for osteoporosis in men (19–23, 27). For example, the AAFP supports the United States Preventive Services Task Force (USPSTF) recommendations to screen women over the age of 65 and those younger than age 65 with additional risk factors. There are no clear recommendations regarding men citing insufficient evidence (27). In contrast, the National Osteoporosis Foundation and Endocrine Society recommend screening in men aged 70 years and older and those aged 50 years or older with risk factors for fractures (21, 22).
Prior retrospective single institution studies have similarly demonstrated low rates of osteoporosis screening in men despite aforementioned guideline recommendations (11–13). In a single institution retrospective study of 342 men aged 70–75 years old seen by a primary care physician at a university-based outpatient clinic, only 63 (18.4%) underwent a bone density scan (11). In another retrospective single-institution study of men aged 70 years and older evaluated in a primary care setting (N=310), only 11.3% were screened (12). In addition, in this other study, none of the men screened for osteoporosis were 90 years or older (12). Despite these studies, nationwide, population-based data are scarce and considerations that osteoporosis screening is undertaken by a multitude of physicians, both primary care physicians and specialists, is lacking. Our study fills these knowledge gaps.
We found that commonly reported factors that led to screening for osteoporosis in men, such as chronic use of steroids, non-traumatic fractures and androgen-deprivation therapy in men with prostate cancer, are reflective of well-recognized causes of secondary bone loss (28, 29). We also found that almost half of the physicians reported that they screen for osteoporosis in men who are at risk for falls. Falls are common in older adults with one-third of community-dwelling adults over the age of 65 and nearly one-half of those over the age of 80 sustaining a fall each year (30). Even though we know from clinical experience that the vast majority of fractures result from falls, epidemiologic data show that only 10–15% falls in older adults actually result in fractures (31). Despite falls not being incorporated in some algorithms assessing fracture risk such as FRAX (32–34), recent studies have shown that falls may be an independent predictive risk factor for future fractures in both women and men (35–37). Our finding that physicians consider history of falls as a factor leading them to screen for osteoporosis in men indicates that clinical judgement in addition to guidelines is important in decision-making. Whether men and women have different fall risk profiles and whether fall risk may be more strongly predictive of falls leading to fractures in men remain unknown.
We found that 64% of respondents cited use of suppressive doses of thyroid hormone as a risk factor that would lead to osteoporosis screening in men, with endocrinologists being more likely to do so. Additionally, when presented with a clinical scenario asking whether physicians would screen a 55-year-old man with history of thyroid cancer on suppressive doses of thyroid hormone for osteoporosis by ordering a bone density scan, more than half of physicians stated that they would. While untreated long-standing hyperthyroidism is a known risk factor for osteoporosis and is also used in calculating the probability of a fracture using the Fracture Risk Assessment Tool (FRAX), robust data on the deleterious effects of exogenous hyperthyroidism resulting from suppressive doses of thyroid hormone on bone in men are limited (38). In a case-control study by our team using a largely male cohort of 10,370 veterans with thyroid cancer and 10,370 age-, sex-, weight- and steroid use- matched controls, osteoporosis was more frequent in patients with thyroid cancer compared to controls (7.3% versus 5.3%, OR 1.33 95% CI 1.18–1.49). Even though low thyroid stimulating hormone (TSH) was associated with higher incidence of osteoporosis in the thyroid cancer patients, it was not associated with increased fractures eliciting the issue of screening bias in this population (39). Our survey results indicate that some physicians make an effort to address possible adverse effects of iatrogenic hyperthyroidism on bone in men, even in view of limited existing data. It remains unclear whether this is appropriate, as male patients on suppressive doses of thyroid hormone may be subjected to increased screening with bone density scans irrespective of their overall risk for osteoporosis and fractures.
The results from this survey study provide additional data regarding heterogeneity in osteoporosis screening practices in men and on factors that lead to osteoporosis screening in men using real-life clinically relevant scenarios. Strengths of our study are the inclusion of a diverse cohort of physicians including primary care physicians and specialists, sampling at a national level and a high response rate. However, there are potential limitations that should be considered. With the use of a survey instrument there is a possibility of non-response bias. However, the high response rate of 63% mitigates this risk. Additionally, even though our survey included a comprehensive list of known risk factors for osteoporosis that may lead to screening in men, there may be factors that were not included in the survey. Lastly, responses provided in the survey may not reflect actual practice.
In summary, our study has important implications for both patients and physicians. Our findings highlight the heterogeneity in osteoporosis screening practices in men, with underscreening prevalent in some scenarios, most likely related to lack of consensus across guidelines. While no clear guidelines are available for osteoporosis screening in men, fractures in men lead to substantial morbidity and mortality compared to women. There is a need for more research on men with osteoporosis which will help lead to targeted interventions to personalize screening in men at risk for osteoporosis and fractures.
Supplementary Material
Acknowledgment:
This work is supported by K08 AG049684 from the National Institute on Aging to Dr. Papaleontiou.
Footnotes
Disclosures: Authors have nothing to disclose.
Contributor Information
Palak Choksi, Division of Metabolism, Endocrinology and Diabetes, University of Michigan, Domino’s Farm, Lobby C, 24 Frank Lloyd Wright Drive, Ann Arbor, MI 48106; Department of Internal Medicine, University of Michigan, Domino’s Farm, Lobby C, 24 Frank Lloyd Wright Drive, Ann Arbor, MI 48106.
Brittany L. Gay, Division of Metabolism, Endocrinology and Diabetes, North Campus Research Complex, 2800 Plymouth Rd. Bldg. 16, 100S-23, Ann Arbor, MI 48109; Department of Internal Medicine, University of Michigan, North Campus Research Complex, 2800 Plymouth Rd. Bldg. 16, 100S-23, Ann Arbor, MI 48109.
David Reyes-Gastelum, Division of Metabolism, Endocrinology and Diabetes, North Campus Research Complex, 2800 Plymouth Rd. Bldg. 16, 400S-20, Ann Arbor, MI 48109; Department of Internal Medicine, University of Michigan, North Campus Research Complex, 2800 Plymouth Rd. Bldg. 16, 400S-20, Ann Arbor, MI 48109.
Megan R. Haymart, Division of Metabolism, Endocrinology and Diabetes, North Campus Research Complex, 2800 Plymouth Road, Bldg 16, Rm 408E, Ann Arbor, MI 48109; Department of Internal Medicine, University of Michigan, North Campus Research Complex, 2800 Plymouth Road, Bldg 16, Rm 408E, Ann Arbor, MI 48109.
Maria Papaleontiou, Division of Metabolism, Endocrinology and Diabetes, North Campus Research Complex, 2800 Plymouth Road, Bldg 16, Rm 453S, Ann Arbor, MI 48109; Department of Internal Medicine, University of Michigan, North Campus Research Complex, 2800 Plymouth Road, Bldg 16, Rm 453S, Ann Arbor, MI 48109.
REFERENCES
- 1.Wade SW, Strader C, Fitzpatrick LA, Anthony MS, O’Malley CD. Estimating prevalence of osteoporosis: examples from industrialized countries. Arch Osteoporos. 2014;9:182. [DOI] [PubMed] [Google Scholar]
- 2.Wright NC, Looker AC, Saag KG, et al. The recent prevalence of osteoporosis and low bone mass in the United States based on bone mineral density at the femoral neck or lumbar spine. J Bone Miner Res. 2014;29:2520–2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005–2025. J Bone Miner Res. 2007;22:465–475. [DOI] [PubMed] [Google Scholar]
- 4.U.S. Department of Health and Human Services. Bone Health and Osteoporosis: A Report of the Surgeon General. Rockville, MD: U.S. Department of Health and Human Services, Office of the Surgeon General, 2004. Available at: https://www.ncbi.nlm.nih.gov/books/NBK45513/ Accessed December 8, 2019. [Google Scholar]
- 5.Hopkins RB, Pullenayegum E, Goeree R, et al. Estimation of the lifetime risk of hip fracture for women and men in Canada. Osteoporos Int. 2012;23:921–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Center JR, Nguyen TV, Schneider D, Sambrook PN, Eisman JA. Mortality after all major types of osteoporotic fracture in men and women: an observational study. Lancet. 1999;353:878–882. [DOI] [PubMed] [Google Scholar]
- 7.Feldstein A, Elmer PJ, Orwoll E, Herson M, Hillier T. Bone mineral density measurement and treatment for osteoporosis in older individuals with fractures: a gap in evidence-based practice guideline implementation. Arch Intern Med. 2003;163:2165–2172. [DOI] [PubMed] [Google Scholar]
- 8.Forsen L, Sogaard AJ, Meyer HE, Edna T, Kopjar B. Survival after hip fracture: short- and long-term excess mortality according to age and gender. Osteoporos Int. 1999;10:73–78. [DOI] [PubMed] [Google Scholar]
- 9.Cooper C, Cole ZA, Holroyd CR, et al. Secular trends in the incidence of hip and other osteoporotic fractures. Osteoporos Int. 2011;22:1277–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kannegaard PN, van der Mark S, Eiken P, Abrahamsen B. Excess mortality in men compared with women following a hip fracture. National analysis of comedications, comorbidity and survival. Age Ageing. 2010;39:203–209. [DOI] [PubMed] [Google Scholar]
- 11.Alswat K, Adler SM. Gender differences in osteoporosis screening: retrospective analysis. Arch Osteoporos. 2012;7:311–313. [DOI] [PubMed] [Google Scholar]
- 12.Lim SY, Lim JH, Nguyen D, et al. Screening for osteoporosis in men aged 70 years and older in a primary care setting in the United States. Am J Mens Health. 2013;7:350–354. [DOI] [PubMed] [Google Scholar]
- 13.Antonelli M, Einstadter D, Magrey M. Screening and treatment of osteoporosis after hip fracture: comparison of sex and race. J Clin Densitom. 2014;17:479–483. [DOI] [PubMed] [Google Scholar]
- 14.Bor A, Matuz M, Gyimesi N, Biczok Z, Soos G, Doro P. Gender inequalities in the treatment of osteoporosis. Maturitas. 2015;80:162–169. [DOI] [PubMed] [Google Scholar]
- 15.Solomon DH, Johnston SS, Boytsov NN, McMorrow D, Lane JM, Krohn KD. Osteoporosis medication use after hip fracture in U.S. patients between 2002 and 2011. J Bone Miner Res. 2014;29:1929–1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feldstein AC, Nichols G, Orwoll E, et al. The near absence of osteoporosis treatment in older men with fractures. Osteoporos Int. 2005;16:953–962. [DOI] [PubMed] [Google Scholar]
- 17.Kiebzak GM, Beinart GA, Perser K, Ambrose CG, Siff SJ, Heggeness MH. Undertreatment of osteoporosis in men with hip fracture. Arch Intern Med. 2002;162:2217–2222. [DOI] [PubMed] [Google Scholar]
- 18.Dillman DA. Mail and Internet Surveys: The Tailored Design Method (ed Second). New York, New York: Wiley, 2007. [Google Scholar]
- 19.Qaseem A, Snow V, Shekelle P, et al. Screening for osteoporosis in men: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2008;148:680–684. [DOI] [PubMed] [Google Scholar]
- 20.Dawson-Hughes B, National Osteoporosis Foundation Guide C. A revised clinician’s guide to the prevention and treatment of osteoporosis. J Clin Endocrinol Metab. 2008;93:2463–2465. [DOI] [PubMed] [Google Scholar]
- 21.Watts NB, Adler RA, Bilezikian JP, et al. Osteoporosis in men: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2012;97:1802–1822. [DOI] [PubMed] [Google Scholar]
- 22.Cosman F, de Beur SJ, LeBoff MS, et al. Clinician’s Guide to Prevention and Treatment of Osteoporosis. Osteoporos Int. 2014;25:2359–2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qaseem A, Forciea MA, McLean RM, Denberg TD, Clinical Guidelines Committee of the American College of P. Treatment of Low Bone Density or Osteoporosis to Prevent Fractures in Men and Women: A Clinical Practice Guideline Update From the American College of Physicians. Ann Intern Med. 2017;166:818–839. [DOI] [PubMed] [Google Scholar]
- 24.Amarnath AL, Franks P, Robbins JA, Xing G, Fenton JJ. Underuse and Overuse of Osteoporosis Screening in a Regional Health System: a Retrospective Cohort Study. J Gen Intern Med. 2015;30:1733–1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gillespie CW, Morin PE. Trends and Disparities in Osteoporosis Screening Among Women in the United States, 2008–2014. Am J Med. 2017;130:306–316. [DOI] [PubMed] [Google Scholar]
- 26.Jain S, Bilori B, Gupta A, Spanos P, Singh M. Are Men at High Risk for Osteoporosis Underscreened? A Quality Improvement Project. Perm J. 2016;20:60–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Force USPST Curry SJ, Krist AH, et al. Screening for Osteoporosis to Prevent Fractures: US Preventive Services Task Force Recommendation Statement. JAMA. 2018;319:2521–2531. [DOI] [PubMed] [Google Scholar]
- 28.Khosla S, Amin S, Orwoll E. Osteoporosis in men. Endocr Rev. 2008;29:441–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Riggs BL, Melton LJ 3rd Involutional osteoporosis. N Engl J Med. 1986;314:1676–1686. [DOI] [PubMed] [Google Scholar]
- 30.Tinetti ME, Speechley M, Ginter SF. Risk factors for falls among elderly persons living in the community. N Engl J Med. 1988;319:1701–1707. [DOI] [PubMed] [Google Scholar]
- 31.Tinetti ME, Doucette J, Claus E, Marottoli R. Risk factors for serious injury during falls by older persons in the community. J Am Geriatr Soc. 1995;43:1214–1221. [DOI] [PubMed] [Google Scholar]
- 32.Kanis JA obotWHOSG. Assessment of osteoporosis at the primary healthcare level Technical Report. WHO Collaborating Centre. 2007 Sheffield, UK: University of Sheffield, 2007. [Google Scholar]
- 33.Kanis JA, Oden A, Johnell O, et al. The use of clinical risk factors enhances the performance of BMD in the prediction of hip and osteoporotic fractures in men and women. Osteoporos Int. 2007;18:1033–1046. [DOI] [PubMed] [Google Scholar]
- 34.Kanis JA, McCloskey EV, Johansson H, et al. Case finding for the management of osteoporosis with FRAX--assessment and intervention thresholds for the UK. Osteoporos Int. 2008;19:1395–1408. [DOI] [PubMed] [Google Scholar]
- 35.Edwards MH, Jameson K, Denison H, et al. Clinical risk factors, bone density and fall history in the prediction of incident fracture among men and women. Bone. 2013;52:541–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Harvey NC, Oden A, Orwoll E, et al. Falls Predict Fractures Independently of FRAX Probability: A Meta-Analysis of the Osteoporotic Fractures in Men (MrOS) Study. J Bone Miner Res. 2018;33:510–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nilsson M, Eriksson J, Larsson B, Oden A, Johansson H, Lorentzon M. Fall Risk Assessment Predicts Fall-Related Injury, Hip Fracture, and Head Injury in Older Adults. J Am Geriatr Soc. 2016;64:2242–2250. [DOI] [PubMed] [Google Scholar]
- 38.Papaleontiou M, Hawley ST, Haymart MR. Effect of Thyrotropin Suppression Therapy on Bone in Thyroid Cancer Patients. Oncologist. 2016;21:165–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Papaleontiou M, Banerjee M, Reyes-Gastelum D, Hawley ST, Haymart MR. Risk of Osteoporosis and Fractures in Patients with Thyroid Cancer: A Case-Control Study in U.S. Veterans. Oncologist. 2019;24:1166–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


