Abstract
Objectives:
We aimed to evaluate the association between levosimendan treatment and acute kidney injury (AKI) as well as assess the clinical sequelae of AKI in cardiac surgery patients with depressed LV function (ejection fraction < 35%).
Methods:
Patients in the LEVO-CTS trial undergoing on-pump coronary artery bypass grafting (CABG), valve, or CABG/valve surgery were stratified by occurrence and severity of post-operative AKI using the AKIN classification. The association between levosimendan infusion and AKI was modeled using multivariable regression.
Results:
Among 854 LEVO-CTS patients, 231 (27.0%) experienced postoperative AKI, including 182 (21.3%) with stage 1, 35 (4.1%) with stage 2, and 14 (1.6%) with stage 3 AKI. The rate of AKI was similar between patients receiving levosimendan or placebo. The odds of 30-day mortality significantly increased by AKI stage compared to those without AKI (stage 1: adjusted odds ratio [aOR] 2.0, 95% CI 0.8–4.9; stage 2: aOR 9.1, 95% CI 3.2–25.7; stage 3: aOR 12.4, 95% CI 3.0–50.4). No association was observed between levosimendan, AKI stage, and odds of 30-day mortality (interaction p=0.69). Factors independently associated with AKI included increasing age, BMI, diabetes, and increasing baseline systolic blood pressure. Increasing baseline eGFR and aldosterone antagonist use were associated with a lower risk of AKI.
Conclusions:
Postoperative AKI is common among high-risk patients undergoing cardiac surgery and associated with significantly increased risk of 30-day death or dialysis. Levosimendan was not associated with the risk of AKI.
Keywords: Levosimendan, cardiac surgery, postoperative care, acute kidney injury
Introduction
Acute kidney injury (AKI) is common following cardiac surgery and is associated with a significant increase in short- and long-term morbidity, mortality, and treatment costs (1–4). While the definition of cardiac surgery-associated acute kidney injury (CS-AKI) varies, the reported incidence is as high as 40% (5, 6). The exact etiology of CS-AKI has yet to be fully elucidated but likely results from a complex array of physiologic insults including hemodynamic and inflammatory factors, ischemia-reperfusion injury, microembolization associated with cardiopulmonary bypass (CPB), circulating toxins, neurohormonal activation, and oxidative stress (5, 7, 8).
Levosimendan, a calcium-sensitizing inotrope and ATP-sensitive potassium channel opener, has been widely used in clinical practice for the treatment of acute heart failure and multiple meta-analyses have suggested improved survival in patients undergoing cardiac surgery (9, 10). Further, prior studies have also demonstrated a peri-operative renal protective effect associated with levosimendan, possibly related to its hemodynamic, anti-inflammatory, and antioxidant effects (10, 11). Despite these promising early results, findings from recent placebo-controlled randomized clinical trials have cast doubt on the beneficial role of levosimendan in cardiac surgery (12–14). The purpose of this study was to evaluate the association between perioperative levosimendan treatment and AKI as well as assess the clinical sequelae of AKI in cardiac surgery patients with depressed LV function using data collected in the Levosimendan in Patients with Left Ventricular Systolic Dysfunction Undergoing Cardiac Surgery on Cardiopulmonary Bypass (LEVO-CTS) trial.
Methods
This study is a post-hoc analysis of the LEVO-CTS trial, the design and results of which have been reported (12, 15). Briefly, LEVO-CTS enrolled 882 patients aged 18 years or older with an ejection fraction of 35% or less who were scheduled to undergo coronary artery bypass grafting (CABG), CABG plus aortic valve surgery, isolated mitral valve surgery, or a combination of these procedures with the use of CPB. Patients were randomized 1:1 to receive a 24-hour intravenous infusion of levosimendan or matching placebo beginning immediately prior to surgery. Perioperative laboratory data, including serum creatinine, were collected prior to surgery as well as daily on postoperative days 1–5.
In this analysis, the primary outcome was postoperative AKI as defined by the Acute Kidney Injury Network (AKIN) classification (16). The AKIN classification was used primarily instead of RIFLE (Risk, Injury, Failure, Loss of kidney function, and End-stage kidney disease) or KDIGO (Kidney Disease Improving Global Outcomes) classifications as the latter definitions were initially validated using 7 days of creatinine data, and only 5 days of data were collected in LEVO-CTS (17, 18). Unlike RIFLE and KGIDO, AKIN was validated using a 48-hour window; however, descriptive data pertaining to all three classification systems are presented. A modified AKIN classification was used, based solely on creatinine data and not urine output, as prior studies have questioned the validity of the urine output AKI criterion in patients undergoing cardiac surgery (19). For the classification of AKI, the highest 5-day postoperative value of creatinine was used in addition to the use of renal replacement therapy. Clinical endpoints included 30- and 90-day mortality and renal failure defined as the need for renal replacement therapy.
Baseline demographic and clinical characteristics were presented as median (25th – 75th percentiles) for continuous variables and count (%) for categorical variables, unless otherwise specified. Comparisons between AKI stage cohorts were performed using the Spearman rank sum test for continuous variables and the Mantel Haenszel Ordinal test for categorical variables. The association between baseline characteristics and postoperative AKI as well as the association between postoperative AKI and clinical endpoints were assessed using multivariable logistic regression, with AKI treated as a binary variable. Adjustment variables for the outcome of AKI were selected a priori and included levosimendan treatment group, age, body mass index (BMI), systolic blood pressure, baseline glomerular filtration rate (GFR), baseline left ventricular ejection fraction (LVEF), diabetes, preoperative use of an angiotensin-converting enzyme (ACE) inhibitor, aldosterone antagonist, as well as surgical procedure type and duration of CPB. Adjustment variables for the clinical endpoints of 30- and 90-day mortality were also selected a priori and included AKI stage, age, and baseline LVEF. Linearity of continuous variables with the logit of the outcome was assessed and piecewise linear splines were used when the linearity assumption was violated. Cox proportional hazards modeling was performed for 90-day mortality. The assumption of proportional hazards was verified. Descriptive analyses were performed using the LEVO-CTS intention to treat (ITT) population after excluding patients without both pre- and post-operative creatinine values recorded (n=854/882 included), while regression and descriptive analyses involving levosimendan were performed using the modified intention to treat (mITT) population (n=828 included). Multivariable modeling was performed as complete case analyses except for the covariate of BMI (14% missing), which was imputed randomly from the normal distribution.
Two-sided p-values ≤ 0.05 were considered statistically significant unless otherwise indicated. All statistical analyses were performed by statisticians at the Duke Clinical Research Institute (Duke University, Durham, NC) using SAS version 9.4 (SAS Institute, Inc., Cary, NC).
Results
Patient characteristics
In total, 854 patients were included, comprised of 623 (73%) patients that experienced no postoperative AKI and 231 (27%) that experienced AKI according to the AKIN classification. 182 (21%), 35 (4%), and 14 (2%) experienced AKIN stage 1, 2, and 3 kidney injury, respectively. Baseline demographic and clinical characteristics of the study population are summarized in Table 1. Patients who experienced AKI were older, had a higher baseline systolic blood pressure, were more likely to have a history of diabetes mellitus, and were more likely NYHA Class III or IV compared with patients who did not experience AKI. There were no significant differences in sex, race, history of peripheral vascular disease, or baseline cardiovascular medication usage between the two groups, except for aldosterone antagonist usage which was less frequent in the AKI group. In terms of procedural characteristics, the distribution of cardiac surgery procedure types was similar among patients who did and did not experience AKI. Patients who experienced AKI, however, had longer lengths of CPB and a longer duration of aortic cross clamp.
Table 1.
Demographic, clinical, and operative characteristics stratified by AKIN classification AKI stage
| Variable | All Patients | No AKI | AKI | p-value | ||
|---|---|---|---|---|---|---|
| (n=854) | (n=623) | Stage 1 (n=182) | Stage 2 (n=35) | Stage 3 (n=14) | ||
| Median age (years, IQR) | 65 (58–72) | 64 (58–71) | 67 (59–74) | 68 (59–74) | 69 (61–72) | 0.01 |
| Female sex | 170 (19.9%) | 132 (21.2%) | 26 (14.3%) | 9 (25.7%) | 3 (21.4%) | 0.46 |
| Race | 0.37 | |||||
| White | 760 (89.8%) | 563 (90.8%) | 156 (87.2%) | 30 (85.7%) | 11 (91.7%) | |
| Black | 46 (5.4%) | 28 (4.5%) | 15 (8.4%) | 3 (8.6%) | - | |
| Other | 40 (4.7%) | 29 (4.7%) | 8 (4.5%) | 2 (5.7%) | 1 (8.3%) | |
| Median BMI (kg/m2, IQR) | 28.1 (25.0–31.7) | 27.8 (24.9–30.9) | 29.2 (25.4–33.2) | 30.9 (26.7–35.5) | 26.9 (24.9–31.8) | 0.002 |
| Median systolic BP (mmHg, IQR) | 123 (111–137) | 121 (110–135) | 126 (114–145) | 131 (114–143) | 126 (110–147) | <0.001 |
| Median LVEF (%, IQR) | 26 (23–32) | 26 (22–31) | 27 (25–33) | 25 (22–30) | 28 (25–30) | 0.36 |
| Medical history | ||||||
| Atrial fibrillation | 163 (19.1%) | 113 (18.1%) | 39 (21.4%) | 8 (22.9%) | 3 (21.4%) | 0.28 |
| Prior CABG or valve surgery | 66 (7.7%) | 46 (7.4%) | 15 (8.2%) | 4 (11.4%) | 1 (7.1%) | 0.51 |
| Prior MI | 446 (54.4%) | 336 (56.5%) | 83 (47.2%) | 20 (57.1%) | 7 (50.0%) | 0.17 |
| Prior stroke | 62 (7.3%) | 42 (6.8%) | 16 (8.9%) | 3 (8.6%) | 1 (7.1%) | - |
| Hypertension | 689 (81.3%) | 497 (80.7%) | 154 (84.6%) | 28 (80.0%) | 10 (71.4%) | 0.49 |
| Diabetes mellitus | 428 (50.2%) | 287 (46.1%) | 116 (63.7%) | 19 (54.3%) | 6 (42.9%) | <0.001 |
| Peripheral vascular disease | 120 (14.2%) | 85 (13.8%) | 27 (15.0%) | 6 (17.6%) | 2 (14.3%) | - |
| Heart failure or cardiogenic shock | 673 (81.0%) | 486 (80.1%) | 152 (85.9%) | 24 (68.6%) | 11 (91.7%) | 0.06 |
| Coronary artery disease | 768 (90.2%) | 563 (90.7%) | 161 (89.0%) | 30 (85.7%) | 14 (100.0%) | 0.42 |
| Current NYHA Class | - | |||||
| I | 38 (5.8%) | 30 (6.4%) | 7 (4.7%) | - | 1 (9.1%) | |
| II | 257 (39.5%) | 195 (41.7%) | 50 (33.6%) | 9 (39.1%) | 3 (27.3%) | |
| III | 285 (43.8%) | 192 (41.0%) | 73 (49.0%) | 13 (56.5%) | 7 (63.6%) | |
| IV | 71 (10.9%) | 51 (10.9%) | 19 (12.8%) | 1 (4.3%) | - | |
| Pre-operative medications | ||||||
| Aspirin | 575 (69.7%) | 412 (68.4%) | 123 (69.9%) | 27 (81.8%) | 13 (92.9%) | 0.10 |
| Beta-blockers | 662 (80.2%) | 487 (80.9%) | 140 (79.5%) | 26 (78.8%) | 9 (64.3%) | 0.47 |
| ACE inhibitors/ARB | 366 (44.4%) | 279 (46.3%) | 70 (39.8%) | 12 (36.4%) | 5 (35.7%) | 0.29 |
| Diuretics | 358 (43.4%) | 261 (43.4%) | 74 (42.0%) | 18 (54.5%) | 5 (35.7%) | 0.54 |
| Aldosterone antagonist | 114 (13.8%) | 100 (16.6%) | 10 (5.7%) | 3 (9.1%) | 1 (7.1%) | - |
| Operative characteristics | 0.07 | |||||
| CABG | 562 (66.6%) | 427 (69.1%) | 107 (59.4%) | 18 (54.5%) | 10 (76.9%) | |
| Valve | 90 (10.7%) | 66 (10.7%) | 21 (11.7%) | 3 (9.1%) | - | |
| CABG + valve | 192 (22.7%) | 125 (20.2%) | 52 (28.9%) | 12 (36.4%) | 3 (23.1%) | |
| Median cross clamp duration (minutes, IQR) | 79 (56–110) | 77 (55–105) | 85 (62–121) | 86 (59–143) | 53 (34–71) | <0.001 |
| Median CPB duration (minutes, IQR) | 112 (85–151) | 108 (81–147) | 123 (94–164) | 136 (84–190) | 83 (79–126) | <0.001 |
| Levosimendan infusiona | 418 (50.5%) | 304 (49.9%) | 97 (56.1%) | 10 (30.3%) | 7 (53.8%) | 0.80 |
Modified intention to treat population (n=828); AKI, acute kidney injury; IQR, interquartile range; BMI, body mass index; BP, blood pressure; LVEF, left ventricular ejection fraction; CABG, coronary artery bypass grafting; MI, myocardial infarction; NYHA, New York Heart Association; ACE, angiotensin converting enzyme; ARB, angiotensin receptor blocker; CPB, cardiopulmonary bypass
Perioperative renal function
Detailed data regarding perioperative renal function are presented in Table 2. Median preoperative creatinine and GFR were 91 umol/L (IQR 78–112) and 69 ml/min/1.73m2 (IQR 55–85), respectively. 67.1% (n=573) of patients experienced an increase in serum creatinine from baseline to the postoperative period, with a median change of +11 mmol/L (IQR −3-+29). 15.6% and 27.1% of patients were classified as having experienced AKI by RIFLE and KDIGO criteria, respectively. 2.7% (n=24) of patients required renal replacement therapy in the postoperative period, including 5 that continued to require it beyond 30 days.
Table 2.
Perioperative renal function
| Variable | Intention to treat population (n=854) |
|---|---|
| Median baseline creatinine (umol/L, IQR) | 91.1 (77.8–112.3) |
| Median baseline GFR (mL/min/1.73m2) | 69.4 (54.7–85.0) |
| Median postoperative peak creatinine (umol/L, IQR) | 102.0 (82.2–132.6) |
| Median postoperative trough GFR (mL/min/1.73m2) | 63.5 (45.4–82.4) |
| Median change in creatinine (umol/L, IQR)a | +10.6 (−2.7−+29.2) |
| Perioperative increase in creatinine | 573 (67.1%) |
| Median change in GFR (mL/min/1.73m2)a | −5.0 (−16.8−+6.5) |
| Perioperative decrease in GFR | 510 (59.9%) |
| RIFLE classification | |
| No AKI | 721 (84.4%) |
| Risk | 84 (9.8%) |
| Injury | 35 (4.1%) |
| Failure | 14 (1.6%) |
| KDIGO classification | |
| No AKI | 623 (72.9%) |
| I | 181 (21.2%) |
| II | 35 (4.1%) |
| III | 16 (1.9%) |
| Patients requiring renal replacement therapy (RRT) in 30 days after surgery | 24 (2.7%) |
| Median time from surgery to RRT initiation (days, IQR) | 3 (2–6) |
| Median duration of RRT (days, IQR) | 3.5 (1.5–16) |
| Requiring RRT beyond 30 days | 5 (0.6%) |
Postoperative - preoperative; GFR, glomerular filtration rate
Adjusted analysis of factors associated with AKI
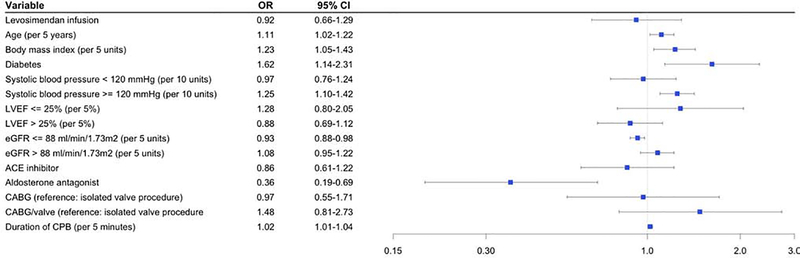
Multivariable logistic regression identified patient baseline and operative characteristics independently associated with developing postoperative AKI (Figure 1). There was no significant association between treatment with levosimendan and AKI (OR 0.92, 95% CI 0.66–1.29). Factors associated with an increased risk of developing AKI included increasing systolic blood pressure over 120 mmHg (OR 1.25 per 10 units, 95% CI 1.10–1.42), diabetes (OR 1.62, 95% CI 1.14–2.31), increasing duration of CPB (OR 1.02 per 5 minutes, 95% CI 1.01–1.04), increasing BMI (OR 1.23 per 5 units, 95% CI 1.05–1.43), and increasing age (OR 1.11 per 5 years, 95% CI 1.02–1.22). Factors associated with a decreased likelihood of developing AKI included baseline aldosterone antagonist usage (OR 0.36, 95% CI 0.19–0.69) and increasing GFR up to 88 ml/min/1.73m2 (OR 0.93 per 5 units, 95% CI 0.88–0.98). Surgical subtype was not associated with the risk of AKI (CABG vs valve OR 0.97, 95% CI 0.55–1.71; CABG/valve vs valve OR 1.48, 95% CI 0.81–2.73).
Figure 1.
Baseline patient factors associated with acute kidney injury
Multivariable logistic regression model for postoperative acute kidney injury (AKI). AKI defined as stage 1 or greater using AKIN classification.
Analysis of 30-day outcomes
Unadjusted descriptive outcomes stratified by AKIN AKI stage are presented in Table 3. Patients who experienced AKI were more likely to require secondary inotropes (≥24 hours after study drug initiation), postoperative inotropes/vasopressors overall, and intraaortic balloon pump (IABP) support. Left ventricular assist device (LVAD) and extracorporeal membrane oxygenation (ECMO) usage was uncommon but more frequent among patients with AKI. In addition, patients with AKI had significantly longer intensive care unit (ICU) and hospital lengths of stay as well as significantly higher rates of 30-day mortality and renal failure.
Table 3.
Unadjusted outcomes stratified by AKIN classification AKI stage
| Variable | All Patients | No AKI | AKI | p-value | ||
|---|---|---|---|---|---|---|
| (n=854) | (n=623) | Stage 1 (n=182) | Stage 2 (n=35) | Stage 3 (n=14) | ||
| Inotrope use ≥24 hours after study drug initiation | 486 (56.9%) | 332 (53.3%) | 121 (66.5%) | 25 (71.4%) | 8 (57.1%) | 0.004 |
| Postoperative inotrope/vasopressor use | ||||||
| Dopamine | 33 (3.9%) | 18 (2.9%) | 13 (7.1%) | 2 (5.7%) | - | 0.06 |
| Norepinephrine | 396 (46.4%) | 277 (44.5%) | 88 (48.4%) | 23 (65.7%) | 8 (57.1%) | 0.07 |
| Epinephrine | 185 (21.7%) | 122 (19.6%) | 40 (22.0%) | 18 (51.4%) | 5 (35.7%) | <0.001 |
| Vasopressin | 189 (22.1%) | 112 (18.0%) | 57 (31.3%) | 13 (37.1%) | 7 (50.0%) | <0.001 |
| Phenylephrine | 87 (10.2%) | 58 (9.3%) | 22 (12.1%) | 5 (14.3%) | 2 (14.3%) | 0.388 |
| Dobutamine | 136 (15.9%) | 87 (14.0%) | 40 (22.0%) | 7 (20.0%) | 2 (14.3%) | 0.07 |
| Milrinone | 140 (16.4%) | 88 (14.1%) | 37 (20.3%) | 11 (31.4%) | 4 (28.6%) | 0.009 |
| Postoperative MCS use | ||||||
| IABP | 73 (8.5%) | 44 (7.1%) | 19 (10.4%) | 8 (22.9%) | 2 (14.3%) | 0.008 |
| ECMO | 7 (0.8%) | 3 (0.5%) | 1 (0.5%) | 2 (5.7%) | 1 (7.1%) | 0.008 |
| LVAD | 8 (0.9%) | 3 (0.5%) | 1 (0.5%) | 3 (8.6%) | 1 (7.1%) | 0.001 |
| Median ICU length of stay (days, IQR) | 4 (3–6) | 3 (3–5) | 5 (3–8) | 8 (5–16) | 6.5 (3–10) | <0.001 |
| Median hospital length of stay (days, IQR) | 8 (7–11) | 8 (6–11) | 9 (7–15) | 14 (8–23) | 10 (7–14) | <0.001 |
| 30-day mortalitya | 31 (3.7%) | 14 (2.3%) | 8 (4.6%) | 6 (18.2%) | 3 (23.1%) | <0.001 |
| 30-day renal failurea | 34 (4.1%) | 2 (0.3%) | 8 (4.6%) | 12 (36.4%) | 12 (92.3%) | <0.001 |
Modified intention to treat (n=828); AKI, acute kidney injury; MCS, mechanical circulatory support; IABP, intraaortic balloon pump; ECMO, extracorporeal membrane oxygenation; LVAD, left ventricular assist device; ICU, intensive care unit; IQR, interquartile range
In the unadjusted regression as well as after adjustment for age and baseline ejection fraction (Table 4), patients who experienced AKI were significantly more likely to experience 30- or 90-day mortality. The risk of both endpoints increased according to AKI stage. Levosimendan infusion did not affect the association between AKI stage and the odds of 30-day mortality (interaction p=0.69).
Table 4.
Unadjusted and adjusted association between AKI and mortality
| Outcome | Unadjusted | Adjusteda | ||
|---|---|---|---|---|
| 30 day mortality | OR (95% CI) | p-value | OR (95% CI) | p-value |
| No AKI | Ref | Ref | Ref | Ref |
| Stage I | 2.06 (0.85–4.99) | 0.11 | 1.99 (0.82–4.85) | 0.13 |
| Stage II | 9.43 (3.36–26.44) | <0.001 | 9.13 (3.24–25.73) | <0.001 |
| Stage III | 12.73 (3.16–51.35) | <0.001 | 12.38 (3.04–50.40) | <0.001 |
| 90 day mortality | HR (95% CI) | p-value | HR (95% CI) | p-value |
| No AKI | Ref | Ref | Ref | Ref |
| Stage I | 2.32 (1.15–4.65) | 0.018 | 2.18 (1.08–4.40) | 0.029 |
| Stage II | 11.81 (5.66–24.66) | <0.001 | 10.87 (5.20–22.75) | <0.001 |
| Stage III | 8.38 (2.49–28.19) | <0.001 | 8.43 (2.50–28.43) | <0.001 |
Adjusted for age and baseline left ventricular ejection fraction; AKI, acute kidney injury
Discussion
In this post-hoc analysis of the LEVO-CTS trial, we demonstrate no significant association between perioperative levosimendan infusion and the occurrence of postoperative AKI. In addition, perioperative levosimendan infusion did not affect the association between the degree of kidney injury and the risk of mortality. We did, however, demonstrate that AKI is common among patients with reduced EF undergoing elective cardiac surgery and is associated with a significantly increased likelihood of 30-day renal replacement therapy and both 30- and 90-day mortality.
The association between perioperative levosimendan infusion and the occurrence of postoperative AKI is controversial. In a 2016 meta-analysis of randomized controlled trials including 1,345 adult patients undergoing cardiac surgery, Zhou and colleagues found that perioperative levosimendan infusion reduced the incidence of postoperative AKI (OR 0.51, 95% CI 0.34–0.76), although not all of the studies examined were placebo controlled, the included populations were heterogeneous with regard to baseline cardiac function, the timing and duration of levosimendan infusion varied greatly, and the definition of renal outcomes were inconsistent (10). Similarly, meta-analyses by Qiang and colleagues in 2018, which included 3,246 adult cardiac surgery patients, and Sanfilippo and colleagues in 2017, which included 1,224 high risk cardiac surgery patients (LVEF <35% or low cardiac output syndrome, LCOS), both found improved renal outcomes associated with levosimendan usage (20, 21). In contrast, a 2019 meta-analysis by Zhu and colleagues which examined five trials of LCOS patients undergoing cardiac surgery, all placebo controlled with preoperative levosimendan infusion, found no significant association between levosimendan infusion and the incidence of AKI (22). Unlike the Zhu study, the positive renal protective effect demonstrated in many of the prior meta-analyses was strongly driven by the 2008 and 2009 Levin trials, which were not placebo controlled (23, 24). Indeed, like LEVO-CTS, the recent LICORN and CHEETAH trials both demonstrated neutral findings regarding the association between perioperative levosimendan infusion and renal outcomes (13, 14). LEVO-CTS, however, specified initiation of study drug before skin incision and enrolled patients with low LVEF at high risk for LCOS.
While levosimendan treatment was not associated with postoperative AKI in LEVO-CTS, we identified several baseline characteristics that were—baseline hypertension, diabetes, renal insufficiency, increasing BMI and age, as well as increasing duration of CPB. Aldosterone antagonist usage was protective against AKI, although we are only capable of identifying association and not causation. The relationship between many of these factors and AKI are well supported in the literature; however, the potential protective effect of aldosterone antagonist usage is more controversial (2, 25–27). Several pre-clinical studies have demonstrated improved resilience to, and recovery from, ischemic kidney injury with the use of mineralocorticoid receptor blockade (28, 29). Results from a 2017 placebo controlled randomized clinical trial by Barba-Navarro and colleagues examining the effect of spironolactone on CSA-AKI among 233 patients undergoing on-pump cardiac surgery were neutral, however (aOR 1.48, 95% CI 0.82–2.66) (30). Given the significant differences in clinical characteristics of the study populations between LEVO-CTS and the Barba-Navarro trial, including baseline cardiac function and chronicity of aldosterone antagonist usage, further investigation is certainly warranted.
There are several important limitations to this analysis. First, as a post-hoc observational analysis of clinical trial data, there is significant potential for residual confounding as LEVO-CTS was not designed specifically to examine the outcome of postoperative AKI. Further, as with any subgroup analysis of clinical trial data, multiple comparison testing may lead to an increased likelihood of error. In addition, the multicentric design of LEVO-CTS may have blunted the potential observed effect of levosimendan as there is significant heterogeneity of anesthetic and ICU management among centers resulting in varying rates of AKI (31). Lastly, our use of a modified KDIGO criteria to classify AKI, compared with the relatively more conservative RIFLE criteria, may have impacted our findings. The primary outcome of the study would likely not have changed significantly with the use of RIFLE, however, as the proportion of patients with AKI in the levosimendan arm would have been similar (52% vs 48%).
Conclusions
In patients with reduced baseline ejection fraction undergoing cardiac surgery in the LEVO-CTS trial, perioperative infusion of levosimendan was not associated with the occurrence of AKI and did not influence the association between AKI and 30-day mortality. In this high-risk population of patients, however, AKI was common and was associated with a significantly increased risk of 30-day renal replacement therapy and mortality. Further research is needed into renal protection strategies in the multidisciplinary perioperative management of high-risk patients undergoing cardiothoracic surgery.
Acknowledgements
Funding for this study was provided by the Duke Clinical Research Institute (DCRI). Dr. Jawitz received funding from National Institutes of Health grant 5T32HL069749.
Funding: Funding was provided by NIH grant 5T32HL069749 and the Duke Clinical Research Institute
Footnotes
Disclosures: The authors have no relevant conflicts of interest to disclose. Dr. Lopes receives grants and personal fees from Bristol-Myers Squibb and Pfizer, personal fees from Boehringer Ingelheim and Bayer AG and grants from Amgen Inc, GlaxoSmithKline, Medtronic PLC, and Sanofi Aventis outside the submitted work.
This abstract was presented at the 2019 AHA Scientific Sessions in Philadelphia, PA
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Corredor C, Thomson R, Al-Subaie N: Long-Term Consequences of Acute Kidney Injury After Cardiac Surgery: A Systematic Review and Meta-Analysis. J Cardiothorac Vasc Anesth 2016; 30(1):69–75 [DOI] [PubMed] [Google Scholar]
- 2.O’Neal JB, Shaw AD, Billings FTt: Acute kidney injury following cardiac surgery: current understanding and future directions. Crit Care 2016; 20(1):187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Karkouti K, Wijeysundera DN, Yau TM, Callum JL, et al. : Acute kidney injury after cardiac surgery: focus on modifiable risk factors. Circulation 2009; 119(4):495–502 [DOI] [PubMed] [Google Scholar]
- 4.Loef BG, Epema AH, Smilde TD, Henning RH, et al. : Immediate postoperative renal function deterioration in cardiac surgical patients predicts in-hospital mortality and long-term survival. J Am Soc Nephrol 2005; 16(1):195–200 [DOI] [PubMed] [Google Scholar]
- 5.Vives M, Hernandez A, Parramon F, Estanyol N, et al. : Acute kidney injury after cardiac surgery: prevalence, impact and management challenges. Int J Nephrol Renovasc Dis 2019; 12:153–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown JR, Kramer RS, Coca SG, Parikh CR: Duration of acute kidney injury impacts long-term survival after cardiac surgery. Ann Thorac Surg 2010; 90(4):1142–1148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krian A: Incidence, prevention, and treatment of acute renal failure following cardiopulmonary bypass. Int Anesthesiol Clin 1976; 14(3):87–101 [DOI] [PubMed] [Google Scholar]
- 8.Abuelo JG: Normotensive ischemic acute renal failure. N Engl J Med 2007; 357(8):797–805 [DOI] [PubMed] [Google Scholar]
- 9.Tena MA, Urso S, Gonzalez JM, Santana L, et al. : Levosimendan versus placebo in cardiac surgery: a systematic review and meta-analysis. Interact Cardiovasc Thorac Surg 2018; 27(5):677–685 [DOI] [PubMed] [Google Scholar]
- 10.Zhou C, Gong J, Chen D, Wang W, et al. : Levosimendan for Prevention of Acute Kidney Injury After Cardiac Surgery: A Meta-analysis of Randomized Controlled Trials. Am J Kidney Dis 2016; 67(3):408–416 [DOI] [PubMed] [Google Scholar]
- 11.Zangrillo A, Alvaro G, Belletti A, Pisano A, et al. : Effect of Levosimendan on Renal Outcome in Cardiac Surgery Patients With Chronic Kidney Disease and Perioperative Cardiovascular Dysfunction: A Substudy of a Multicenter Randomized Trial. J Cardiothorac Vasc Anesth 2018; 32(5):2152–2159 [DOI] [PubMed] [Google Scholar]
- 12.Mehta RH, Leimberger JD, van Diepen S, Meza J, et al. : Levosimendan in Patients with Left Ventricular Dysfunction Undergoing Cardiac Surgery. N Engl J Med 2017; 376(21):2032–2042 [DOI] [PubMed] [Google Scholar]
- 13.Landoni G, Lomivorotov VV, Alvaro G, Lobreglio R, et al. : Levosimendan for Hemodynamic Support after Cardiac Surgery. N Engl J Med 2017; 376(21):2021–2031 [DOI] [PubMed] [Google Scholar]
- 14.Cholley B, Caruba T, Grosjean S, Amour J, et al. : Effect of Levosimendan on Low Cardiac Output Syndrome in Patients With Low Ejection Fraction Undergoing Coronary Artery Bypass Grafting With Cardiopulmonary Bypass: The LICORN Randomized Clinical Trial. JAMA 2017; 318(6):548–556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mehta RH, Van Diepen S, Meza J, Bokesch P, et al. : Levosimendan in patients with left ventricular systolic dysfunction undergoing cardiac surgery on cardiopulmonary bypass: Rationale and study design of the Levosimendan in Patients with Left Ventricular Systolic Dysfunction Undergoing Cardiac Surgery Requiring Cardiopulmonary Bypass (LEVO-CTS) trial. Am Heart J 2016; 182:62–71 [DOI] [PubMed] [Google Scholar]
- 16.Mehta RL, Kellum JA, Shah SV, Molitoris BA, et al. : Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care 2007; 11(2):R31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bellomo R, Ronco C, Kellum JA, Mehta RL, et al. : Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care 2004; 8(4):R204–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khwaja A: KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract 2012; 120(4):c179–184 [DOI] [PubMed] [Google Scholar]
- 19.Lagny MG, Jouret F, Koch JN, Blaffart F, et al. : Incidence and outcomes of acute kidney injury after cardiac surgery using either criteria of the RIFLE classification. BMC Nephrol 2015; 16:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qiang H, Luo X, Huo JH, Wang ZQ: Perioperative Use of Levosimendan Improves Clinical Outcomes in Patients After Cardiac Surgery: A Systematic Review and Meta-Analysis. J Cardiovasc Pharmacol 2018; 72(1):11–18 [DOI] [PubMed] [Google Scholar]
- 21.Sanfilippo F, Knight JB, Scolletta S, Santonocito C, et al. : Levosimendan for patients with severely reduced left ventricular systolic function and/or low cardiac output syndrome undergoing cardiac surgery: a systematic review and meta-analysis. Crit Care 2017; 21(1):252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhu J, Zhang Y, Chen L, He Y, et al. : Levosimendan in patients with low cardiac output syndrome undergoing cardiac surgery: A systematic review and meta-analysis. Anaesth Crit Care Pain Med 2019; 38(3):243–249 [DOI] [PubMed] [Google Scholar]
- 23.Levin RL, Degrange MA, Porcile R, Salvagio F, et al. : [The calcium sensitizer levosimendan gives superior results to dobutamine in postoperative low cardiac output syndrome]. Rev Esp Cardiol 2008; 61(5):471–479 [PubMed] [Google Scholar]
- 24.Levin R, Porcile R, Salvagio F, Del Mazo C, et al. : Levosimendan Reduces Mortality in Postoperative Low Cardiac Output Syndrome After Coronary Surgery. Circulation 2009; 120(18):S987–S988 [Google Scholar]
- 25.Ortega-Loubon C, Fernandez-Molina M, Carrascal-Hinojal Y, Fulquet-Carreras E: Cardiac surgery-associated acute kidney injury. Ann Card Anaesth 2016; 19(4):687–698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rosner MH, Okusa MD: Acute kidney injury associated with cardiac surgery. Clin J Am Soc Nephrol 2006; 1(1):19–32 [DOI] [PubMed] [Google Scholar]
- 27.Wang Y, Bellomo R: Cardiac surgery-associated acute kidney injury: risk factors, pathophysiology and treatment. Nat Rev Nephrol 2017; 13(11):697–711 [DOI] [PubMed] [Google Scholar]
- 28.Mejia-Vilet JM, Ramirez V, Cruz C, Uribe N, et al. : Renal ischemia-reperfusion injury is prevented by the mineralocorticoid receptor blocker spironolactone. Am J Physiol Renal Physiol 2007; 293(1):F78–86 [DOI] [PubMed] [Google Scholar]
- 29.Sanchez-Pozos K, Barrera-Chimal J, Garzon-Muvdi J, Perez-Villalva R, et al. : Recovery from ischemic acute kidney injury by spironolactone administration. Nephrol Dial Transplant 2012; 27(8):3160–3169 [DOI] [PubMed] [Google Scholar]
- 30.Barba-Navarro R, Tapia-Silva M, Garza-Garcia C, Lopez-Giacoman S, et al. : The Effect of Spironolactone on Acute Kidney Injury After Cardiac Surgery: A Randomized, Placebo-Controlled Trial. Am J Kidney Dis 2017; 69(2):192–199 [DOI] [PubMed] [Google Scholar]
- 31.Heringlake M, Knappe M, Vargas Hein O, Lufft H, et al. : Renal dysfunction according to the ADQI-RIFLE system and clinical practice patterns after cardiac surgery in Germany. Minerva Anestesiol 2006; 72(7–8):645–654 [PubMed] [Google Scholar]