Abstract
Background
This study was to conduct a predictive model for the prognosis of aneurysmal subarachnoid hemorrhage (aSAH) and validate the clinical data.
Methods
A total of 235 aSAH patients were enrolled in this study, dividing into the favorable or poor prognosis groups based on Modified Rankin Scale (mRS) at 3 months postoperatively. Multivariate analysis was assessed using binary Logistic regression and Fisher discriminant analysis. The receiver operating characteristic (ROC) curve was used to determine the cut‐off value.
Results
Our findings showed that the high Glasgow Coma Scale (GCS) score 24‐hour after surgery reduced the risk of poor prognosis, and the surgical clipping and elevated neutrophil‐lymphocyte ratio (NLR) increased the risk of poor prognosis. The discriminant function was V = 0.881 × GCS score − 0.523 × NLR − 0.422 × therapeutic approach, and V = −0.689 served as a cut‐off value. When V ≥ −0.689, the good prognosis was considered among these patients with aSAH. The correctness for predicting the prognostic outcomes by self‐validation was 85.11%.
Conclusion
This predictive model established by a discriminant analysis is a useful tool for predicting the prognostic outcomes of aSAH patients, which may help clinicians identify patients at high risk for poor prognosis and optimize treatment after surgery.
Keywords: aneurysmal subarachnoid hemorrhage, discriminant analysis, prognosis, validation
The ROC curve was established according to V as the test value and mRS score results as the gold standard (AUC = 0.859, SE = .040, 95% CI: 0.781‐0.938) and the critical value of V (cut off) was −0.689. When V ≥ −0.689, the prognosis was considered as good, otherwise as poor
1. INTRODUCTION
Aneurysmal subarachnoid hemorrhage (aSAH) is an acute cerebrovascular disease that seriously damages the central nervous system and simultaneously exerts pathophysiological effects on multiple organs of the body, with high mortality and morbidity. 1 , 2 Approximately one‐third of the survivors suffer from severe disability and functional dependency. 3 Rebleeding is a major early complication of aSAH with the incidence of 6%‐23%, which most occurs within 72 hours of onset. 4 , 5 Previous studies reported that the mortality was 50%‐60% in the presence of rebleeding. 4 , 5 , 6 To date, craniotomy clipping and endovascular embolization serve as main treatments for aSAH to prevent and treat complications such as rebleeding, vasospasm and hydrocephalus, and to reduce the occurrences of deaths and disabilities. 7 , 8 , 9 , 10 , 11 Although improvements in intensive care and treatments in recent years, the prognosis is still a frequent concern for aSAH. To the best of our knowledge, the clinical data indicated that the prognosis of aSAH was poor after surgery. Therefore, prediction of prognostic outcomes is of great value for treatment options and assessment in patients with aSAH.
Several studies have mentioned that biochemical indicators could serve as predictive factors of the prognosis in patients with aSAH, such as lipoprotein‐associated phospholipase A2, high‐sensitivity C‐reactive protein. 12 , 13 However, the change of a single indicator may not provide strong and sufficient clinical evidences for clinicians to diagnose the diseases. The predictive models are a derivative tool in statistics which are useful to predict the prognostic outcomes on the basis of the pooled evaluations of physical, laboratory, and radiologic examinations. 14 High‐quality predictive models can be responsible for guiding clinical decisions and patient counseling, insuring rational allocation of resources to decline the healthcare costs, and improving the designs and analyses of clinical trials. 14 The optimal care for aSAH patients is applied in clinic, but high mortality cannot be avoided, and the long‐term quality of life among survivors is unsatisfactory postoperatively. The establishment of a productive model may be beneficial in the management of patients with aSAH.
In the current study, we conducted a predictive model for the prognosis of aSAH and validated the clinical data, which may be useful for clinicians to improve the prognostic outcomes of patients after surgery.
2. METHODS
2.1. Patients
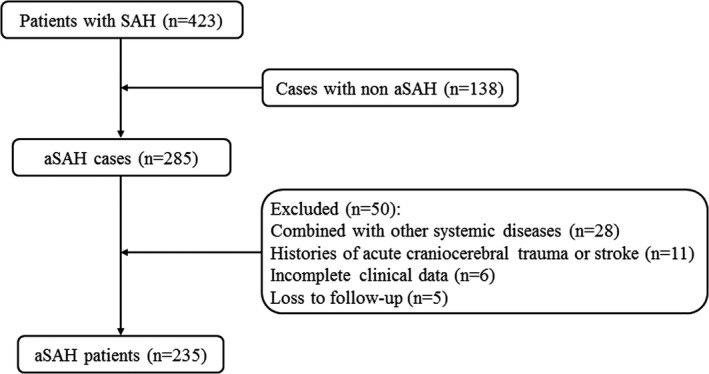
A total of 235 aSAH patients admitted to Meizhou People's Hospital were screened in this study between December 2016 and December 2018. All cases were consecutively recruited in the retrospective investigation that were divided into the favorable (n = 201) or poor (n = 34) prognosis groups based on Modified Rankin Scale (mRS) at 3 months postoperatively. 15 In this study, patients at score 4‐6 manifested poor prognosis, while patients at score 0‐3 showed favorable prognosis. The clinical indicators were noted including gender, age, body mass index (BMI), drinking, smoking, diabetes, hypertension, operation history, Hunt‐Hess grade, surgical techniques, surgical timing, initially ruptured aneurysm bleeding, aneurysm numbers and sizes, Glasgow Coma Scale (GCS) score, biochemical indexes, and prognostic outcomes. This study was approved by the Institutional Review Board (IRB) of Meizhou People's Hospital (approval number: No. 2016‐A‐39).
Inclusion criteria: (a) 18‐80 years old; (b) clearly diagnosed as aSAH according to guidelines for the diagnosis and treatment of Chinese subarachnoid hemorrhage; (c) CT scan of the head confirmed aSAH; (d) patients with first rupture of aneurysm by CT angiography (CTA) and digital subtraction angiography (DSA); (e) admission within 24 hours of the initial symptom onset; (f) hospitalization ≥3 days in neurosurgery.
Excluded criteria: (a) aSAH patients caused by non‐aneurysmal causes such as arteriovenous malformation or arteriovenous fistula; (b) patients with histories of acute or chronic infections, autoimmune diseases, recent cardiovascular and cerebrovascular diseases, and other systemic diseases including malignant neoplasms, uremia, chronic heart disease, and chronic pulmonary disease; (c) previous use of anticoagulant/antiplatelet drugs; (d) patients with histories of acute craniocerebral trauma, brain tumor or stroke with neurological dysfunction; (e) incomplete clinical data; (f) patients with preoperative rebleeding of aneurysms and surgical treatment failure; (g) severe liver and kidney dysfunction; (h) refuse or lost to follow‐up; (i) patients who have taken other experimental drugs or ongoing clinical studies within 1 month.
2.2. Laboratory examination
The blood biochemical indexes were tested using the automatic biochemical analyzer. The clinical indicators consisted of high‐density lipoprotein (HDL), low‐density lipoprotein (LDL), heart rate (HR), neutrophil‐lymphocyte ratio (NLR), platelet‐lymphocyte ratio (PLR), white blood cell (WBC) count, platelet (PLT) count, neutrophil count (NEUT), lymphocyte count (LC), glutamic oxaloacetic transaminase (AST), albumin and fasting blood glucose (FBG).
2.3. GCS score
Glasgow Coma Scale score mainly includes Motor response (M), Verbal response (V), and eye‐opening (E). The degree of coma is evaluated based on the sum of M, V, and E scores. A score above 14 was considered as normal, a score below 7 was coma, and GCS score ≤3 was indicated brain death or poor prognosis.
2.4. Statistical analysis
Statistical analysis was performed using SPSS 23.0 (SPSS, Inc, Chicago, IL). Measuring data were presented as the mean ± standard and analyzed by ANOVA. Counting data were presented as n (%) with χ2 or Fisher tests. The single‐factor analysis of the prognosis at 3 months postoperatively was carried out by t, Kruskal‐Wallis or χ2 tests. Multivariate analysis was assessed using binary Logistic regression and Fisher discriminant analysis. The receiver operating characteristic (ROC) curve was used to determine the cut‐off value. P < .05 was considered statistically significant.
3. RESULTS
3.1. The baseline data of patients with aSAH
The process of patient selection was shown in Figure 1. Totally, 235 patients with aSAH were included in this study containing 93 males (39.60%) and 142 females (60.40%), with the mean age of (58.10 ± 9.97) years and the mean BMI of (23.08 ± 2.20). There were no statistical differences in gender (χ2 = 0.04, P = .836), age (t = −1.01, P = .311), BMI (t = 1.29, P = .199), drinking (χ2 = 0.016, P = .899), smoking (P = .378), diabetes (P = .068), hypertension (χ2 = 1.23, P = .268), operation history (P = .610), surgical timing (P = .345), initially ruptured aneurysm bleeding (P = .855), aneurysm numbers (χ2 = 1.956, P = .162), and sizes (P = .153) between favorable and poor prognosis groups. Significant differences in Hunt‐Hess grade (χ2 = 63.58, P < .001) and surgical techniques (χ2 = 14.31, P < .001) were showed between the two groups (Table 1).
Figure 1.
ROC curve of the prognosis in patients with aASH
Table 1.
The characteristics of various prognostic outcomes in patients with aSAH
| Variables | Favorable prognosis | Poor prognosis | Total | χ2/t | P |
|---|---|---|---|---|---|
| Gender, n (%) | |||||
| Male | 79 (84.90) | 14 (15.10) | 93 (39.60) | 0.04 | .836 |
| Female | 122 (85.90) | 20 (14.10) | 142 (60.40) | ||
| Age, years, | 57.83 ± 9.87 | 59.71 ± 10.53 | 58.10 ± 9.97 | −1.01 | .311 |
| BMI, Kg/m2, | 23.16 ± 2.19 | 22.64 ± 2.23 | 23.08 ± 2.20 | 1.29 | .199 |
| Drinking, n (%) | |||||
| No | 126 (84.60) | 23 (15.40) | 149 (78.40) | 0.016 | .899 |
| Yes | 35 (85.40) | 6 (14.60) | 41 (21.60) | ||
| Smoking a , n (%) | |||||
| No | 131 (83.40) | 26 (16.60) | 157 (85.80) | .378 | |
| Yes | 24 (92.30) | 2 (7.70) | 26 (14.20) | ||
| Diabetes a , n (%) | |||||
| No | 143 (85.60) | 24 (14.40) | 167 (91.80) | .068 | |
| Yes | 10 (66.70) | 5 (33.30) | 15 (8.20) | ||
| Hypertension, n (%) | |||||
| No | 73 (82.00) | 16 (18.00) | 89 (44.10) | 1.23 | .268 |
| Yes | 99 (87.60) | 14 (12.4) | 113 (55.90) | ||
| Operation history, n (%) | |||||
| No | 141 (83.90) | 27 (16.10) | 168 (95.50) | .610 | |
| Yes | 8 (100.00) | 0 (0.00) | 8 (4.50) | ||
| Hunt‐Hess grade, n (%) | |||||
| I‐III | 178 (94.70) | 10 (5.30) | 188 (80.00) | 63.58 | <.001 |
| IV‐V | 23 (48.90) | 24 (51.10) | 47 (20.00) | ||
| Surgical techniques, n (%) | |||||
| Endovascular therapy | 114 (94.20) | 7 (5.80) | 121 (51.50) | 14.31 | <.001 |
| Surgical clipping | 87 (77.00) | 26 (23.00) | 113 (48.10) | ||
| Surgical timing a , n (%) | |||||
| Early stage | 183 (86.30) | 29 (13.70) | 212 (90.20) | .345 | |
| Delay | 18 (78.30) | 5 (21.70) | 23 (9.80) | ||
| Initially ruptured aneurysm bleeding a , n (%) | |||||
| No | 1 (100.00) | 0 (0.00) | 1 (0.40) | .855 | |
| Yes | 199 (85.40) | 34 (14.60) | 233 (99.60) | ||
| Aneurysm, n (%) | |||||
| Single | 179 (86.50) | 28 (13.50) | 207 (88.20) | 1.956 | .162 |
| Multiple | 19 (76.00) | 6 (24.00) | 25 (10.89) | ||
| Aneurysm size a , n (%) | |||||
| <13 mm | 198 (86.10) | 32 (13.90) | 230 (97.90) | .153 | |
| ≥13 mm | 3 (60.00) | 2 (40.00) | 5 (2.10) | ||
Using Fisher test.
3.2. Single‐factor regression analysis for the prognosis at 3 months postoperatively
The GCS score (H = 54.46, P < .001) and albumin (t = 2.70, P = .008) in the favorable prognosis group was higher than that in the poor prognosis group, with statistical differences. The levels of NLR (t′ = −3.68, P = .001), PLR (t′ = −2.23, P < .001), HR (t′ = −2.70, P = .010), WBC count (t′ = −3.61, P < .001), NEUT (t = 3.79, P < .001), AST (t′ = −2.45, P = .015), and FBG (t′ = −2.32, P = .028) in the poor prognosis group were higher than the favorable group. We also found that there were no statistical differences in rebleeding or death 24 hours after onset (P = .054), HDL (z = 1.39, P = .326), LDL (t = 1.35, P = .177), PLT count (t = 0.20, P = .838), and LC (z = 1.53, P = .295) between the two groups (Table 2).
Table 2.
The characteristics of aSAH patients between the two groups at 3 mo postoperatively
| Variables | Favorable prognosis | Poor prognosis | Total | P | |
|---|---|---|---|---|---|
| GCS score, n (%) | |||||
| 3‐8 | 22 (10.95) | 21 (61.80) | 43 (18.40) | H = 54.46 | <.001 |
| 9‐12 | 10 (4.98) | 4 (11.80) | 14 (6.00) | ||
| 13‐15 | 168 (83.58) | 9 (26.50) | 177 (75.60) | ||
| Rebleeding or death 24 h after onset a , n (%) | |||||
| None | 196 (99.49) | 32 (94.12) | 228 (98.70) | .054 | |
| Rebleeding or death | 1 (0.51) | 2 (5.88) | 3 (1.30) | ||
| HDL b , mmol/L, M(P25, P75) | 1.35 (1.15, 1.59) | 1.30 (1.10, 1.77) | 1.34 (1.14, 1.62) | z = 1.39 | .326 |
| LDL, mmol/L, /M(P25, P75) | 3.14 ± 0.89 | 2.90 ± 0.99 | 3.11 (2.55,3.60) | t = 1.35 | .177 |
| NLR c , | 7.04 (5.10, 11.62) | 11.20 (7.17, 17.44) | 7.37 (5.54, 12.31) | t′ = −3.68 | .001 |
| PLR c , M(P25, P75) | 157.39 (111.90, 203.56) | 180.80 (122.00, 252.88) | 160.79 (112.30, 206.81) | t′ = −2.23 | .033 |
| HR c , beats/min, | 81.51 ± 9.48 | 89.50 ± 16.79 | 82.67 ± 11.17 | t′ = −2.70 | .010 |
| WBC count, | 13.89 ± 4.64 | 17.04 ± 4.74 | 14.34 ± 4.77 | t = −3.61 | <.001 |
| PLT count, | 229.73 ± 56.97 | 227.48 ± 67.09 | 229.41 ± 58.35 | t = 0.20 | .838 |
| NEUT, | 11.49 ± 4.48 | 14.65 ± 4.27 | 11.93 ± 4.58 | t = 3.79 | <.001 |
| LC b , M(P25, P75) | 1.50 (1.10, 2.00) | 1.10 (0.90, 1.90) | 1.40 (1.10, 2.00) | z = 1.53 | .295 |
| AST, U/L, | 26.61 ± 12.84 | 32.79 ± 15.09 | 27.50 ± 13.33 | t = −2.45 | .015 |
| Albumin, g/L, | 42.85 ± 4.35 | 40.50 ± 6.06 | 42.51 ± 4.70 | t = 2.70 | .008 |
| FBG c , mmol/L, | 7.02 ± 2.53 | 8.69 ± 3.39 | 7.22 ± 2.69 | t′ = −2.32 | .028 |
Abbreviations: AST, glutamic oxaloacetic transaminase; FBG, fasting blood‐glucose; GCS, Glasgow Coma Scale; HDL, high‐density lipoprotein; HR, heart rate; LC, lymphocyte count; LDL, low‐density lipoprotein; NEUT, neutrophil count; NLR, neutrophil‐lymphocyte ratio; PLR, platelet‐lymphocyte ratio; PLT, platelet; WBC, white blood cell.
Using Fisher test;
Using Mann‐Whitney U test;
Using t test.
3.3. Multivariate Logistic regression analysis for the prognosis at 3 months postoperatively
In Table 3, the stepwise regression analysis was used to analyze the influence factors of the prognosis at 3 months postoperatively in patients with aSAH. The findings showed that the higher the GCS score 24‐hour after surgery, the lower the risk of poor prognosis (OR = 0.34, 95% CI: 0.18‐0.63, P = .001). The risk of poor prognosis in the surgical clipping was higher than that in the endovascular therapy (OR = 3.34, 95% CI: 1.02‐10.99, P = .033). In addition, the higher the NLR, the higher the risk of poor prognosis (OR = 1.13, 95% CI: 1.03‐1.24, P = .008).
Table 3.
Multivariate Logistic regression analysis for the prognosis at 3 mo postoperatively
| Variables | OR (95% CI) | B | P |
|---|---|---|---|
| GCS scoring at 24 h after surgery | 0.34 (0.18‐0.63) | −1.09 | .001 |
| Surgical techniques | |||
| Endovascular therapy | — | ||
| Surgical clipping | 3.34 (1.02‐10.99) | 1.21 | .033 |
| NLR | 1.13 (1.03‐1.24) | 0.12 | .008 |
Abbreviations: CI, confidence interval; GCS, Glasgow Coma Scale; NLR, neutrophil‐lymphocyte ratio; OR, odds ratio.
3.4. The predictive model for the prognosis at 3 months postoperatively
The GCS score 24‐hour after surgery, surgical techniques, and NLR was as independent variables, the prognosis at 3 months postoperatively was as the grouping variable, and Fisher discriminant analysis was carried out. Then, the discriminative equation was as follows: V = 0.881 × GCS score − 0.523 × NLR − 0.422 × surgical techniques. The value of Wilks'Lambda was 0.712 (χ2 = 77.36, P < .001), with significant differences.
The GCS score (score 3‐8 as 1, score 9‐12 as 2 and 13‐15 as 3) and surgical techniques (endovascular therapy as 1 and surgical clipping as 2) were assigned in this study. The V‐value was calculated based on the discriminative equation. The ROC curve (Figure 2) was established according to V as the test value and mRS score results as the gold standard (AUC = 0.859, SE = .040, 95% CI: 0.781‐0.938) and the critical value of V (cut off) was −0.689. When V ≥ −0.689, the prognosis was considered as good, otherwise as poor.
Figure 2.
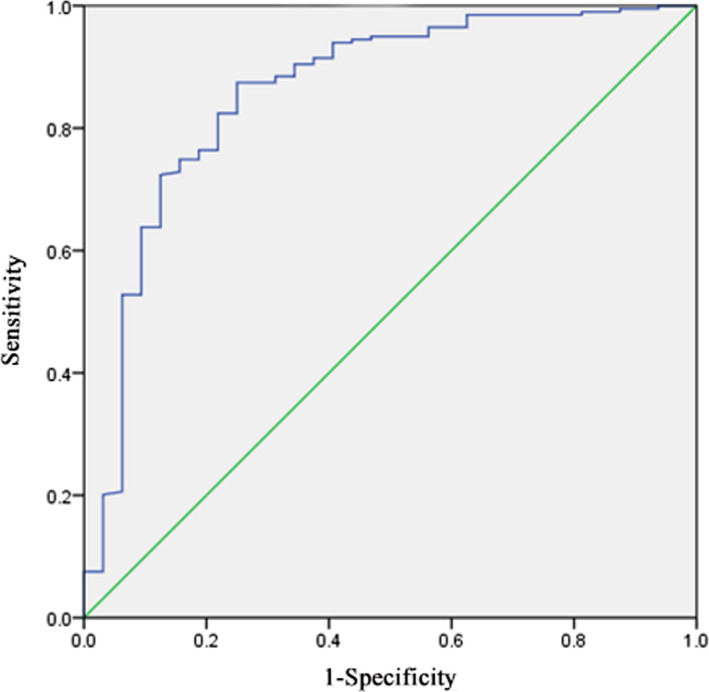
ROC curve of the prognosis in patients with aASH
The self‐validation method was used to assess the efficacy of the predictive model in Table 4. The V‐value of 182 patients with aSAH was >−0.689, indicating favorable prognosis, while 53 cases were poor prognosis (V<−0.689). The results showed that 86.57% of patients were accurately recognized with a good prognosis via the predictive model in the favorable prognosis group. 76.47% of subjects were correctly judged as having a bad prognosis in the poor prognosis group. The accuracy of this predictive model obtained by the discriminant analysis was 85.11%. It is indicated that the predictive effect of the model was relatively reasonable.
Table 4.
The self‐validation of the predictive model
| Predictive outcomes | Favorable prognosis (score 0‐3) | Poor prognosis (score 4‐6) |
|---|---|---|
| Favorable prognosis | 86.57% (174/201) | 23.53% (8/34) |
| Poor prognosis | 13.43% (27/201) | 76.47% (26/34) |
4. DISCUSSION
Aneurysmal subarachnoid hemorrhage is a serious disease that threatens human health. Previous studies reported that >30% of mortality was related to the existence of aSAH, and merely about 30% of aSAH patients could return to independent living. 16 Approximately 10%‐25% of acute aSAH patients die after bleeding or before arrival at the hospital. 17 Thus, it is of great value to pay attention to the prognostic outcomes of aSAH patients to improve their quality of life and survival. In this study, we established a predictive model to assess the prognosis of aSAH using a discriminant analysis, and to conduct an internal validation to identify the effectiveness of this model. Totally, 235 aSAH patients were screened and observed the recovery 3 months after surgery. Patients at score 4‐6 manifested poor prognosis, while patients at score 0‐3 showed favorable prognosis. The prognostic parameters including the GCS score 24‐hour after surgery, surgical clipping, and NLR were used to establish a discriminant function, with an accuracy of 85.11%. Our findings showed that high GCS score 24‐hour after surgery was a protective factor for the risk of poor prognosis, and the surgical clipping and NLR were risk factors for the occurrence of poor prognosis in patients with aSAH.
Studies have shown that the discriminant analysis has been clinically used to select significant indicators. 18 , 19 , 20 In this work, we first applied this analysis to establish a predictive model to screen the major factors of prognosis in patients with aSAH, which may be availably applied in clinic. Our findings from multivariate Logistic analysis showed that the high GCS score 24‐hour after surgery could reduce the risk of poor prognosis, and the surgical clipping and elevated NLR could increase the risk of poor prognosis. The discriminant function was conducted with these significant variables, V = 0.881 × GCS score − 0.523 × NLR − 0.422 × surgical techniques, and V = −0.689 served as a cut‐off value. When V ≥ −0.689, the good prognosis was considered among these patients with aSAH. We further demonstrated that the accuracy of the discriminant analysis for predicting the prognostic outcomes was 85.11%, of which 96.57% in the feature of favorable prognosis and 76.47% of poor prognosis. It was indicated that the predictive effectiveness of this model was relatively reasonable, which may be generalized to the clinic for the prediction of the prognostic outcomes among aSAH cases, improving the living quality and survival condition.
Previous studies have demonstrated that NLR was associated with the outcomes and could predict the courses of different medical conditions, such as ischemic stroke, 21 , 22 cerebral hemorrhage, 23 , 24 and major cardiac events. 25 , 26 In our study, we found that NLR was associated with the prognostic outcomes of aSAH patients. The high level of NLR could increase the risk of poor prognosis at 3 months postoperatively. One mechanism by which neutrophils can contribute to unfavorable outcomes following aSAH is the synthesis and secretion of matrix metalloproteinase. These are enzymes able to degrade any component of the extracellular matrix and play role in brain‐barrier damage and development of secondary brain injury. 27 In addition, the mechanism of NLR‐induced bleeding may be contributed by multifactorial pathophysiology, such as inflammatory reaction, immune dysfunction, and the production of reactive oxygen species (ROS). 5 , 28 , 29 , 30 , 31 , 32 aSAH can induce the leukocytosis and increased neutrophils by stimulating the systemic cellular responses that can cause the brain injury and delayed cerebral ischemia. 33 , 34 Early studies showed that the growth and rupture of cerebral aneurysms were promoted via the leukocyte infiltration and inflammatory reaction in the wall of aneurysms. 35 , 36 , 37 , 38 The results of histopathology found that the thinned wall of aneurysms was related to leukocytosis. 39 Furthermore, Sheheryar et al mentioned that elevated NLR at admission could predict higher inpatient mortality among aSAH patients. 40 Giede‐Jeppe et al 29 reported NLR as an independent factor for unfavorable functional outcome in aSAH. These were consistent with our findings, indicating the high level of NLR may increase the risk of poor prognosis in patients with aSAH.
In addition, we also discovered that the risk of poor prognosis in the surgical clipping was higher in comparison with the endovascular therapy. The surgical clipping is a gold standard treatment in recent decades, and Yasargil applied the microsurgical techniques to neurosurgery to improve the surgical approach to intracranial aneurysms. 41 Endovascular therapy was initially based on the use of inflatable balloons in the aneurysmal cavity in 1970s. 42 Endovascular coiling has been served as a succedaneous method for treating the ruptured or unruptured intracranial aneurysms after approval of Food and Drug Administration (FDA) in 1995. Previous studies reported the better effectiveness of endovascular therapy in quality of life and survival in comparison with surgical clipping. 43 , 44 Compared with those treated with surgical clipping, the mortality and disability of ruptured intracranial patients receiving 1‐year endovascular therapy were lower, with a reduced absolute risk of 7.4%. 44 These were similar with our results, suggesting the surgical clipping for aSAH may increase the risk of poor prognosis.
A strength of this study is that we established a predictive model for the prognosis of aSAH, and validated with the internal data. The high GCS score 24‐hour after surgery was a protective factor, and the surgical clipping and NLR were risk factors for the occurrence of poor prognosis in aSAH patients. The establishment of our discriminant function had a good performance for predicting the prognostic outcomes in aSAH patients. There were some limitations in our study. First, the sample size may reduce the statistical power. Second, only a small number of enrolled patients developed poor prognosis, which may affect the statistical outcomes. Third, due to the possible influence of treatments, the longitudinal assessment of laboratory indices over time was not carried out. Additionally, the predictive value of this model was evaluated base on an internal data, lacking of the external validation. These should be cautious in interpreting the results. Hence, future studies should further validate the results of the present study.
5. CONCLUSION
We established a predictive model to assess the prognosis of aSAH using a discriminant analysis, and to conduct an internal validation to identify the effectiveness of this model. Our results revealed that the correctness for predicting the favorable prognosis was 85.67%, as well as for predicting the poor prognosis was 76.47%. The accuracy obtained by discriminant analysis was 85.11%, indicating that the effectiveness of this predictive model was relatively reasonable. These findings obtained from our study may help clinicians identify patients at high risk for poor prognosis and optimize treatment after surgery.
Lai X, Zhang W, Ye M, Liu X, Luo X. Development and validation of a predictive model for the prognosis in aneurysmal subarachnoid hemorrhage. J Clin Lab Anal. 2020;34:e23542 10.1002/jcla.23542
Funding information
Research and Education Project of Meizhou People's Hospital (No. PY‐2019003).
REFERENCES
- 1. Virani SS, Alonso A, Benjamin EJ, et al. Heart disease and stroke statistics‐2020 update: a report from the American Heart Association. Circulation. 2020;141(9):e139‐e596. [DOI] [PubMed] [Google Scholar]
- 2. Connolly ES Jr, Rabinstein AA, Carhuapoma JR, et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a guideline for healthcare professionals from the American Heart Association/american Stroke Association. Stroke. 2012;43(6):1711‐1737. [DOI] [PubMed] [Google Scholar]
- 3. Rinkel GJ, Algra A. Long‐term outcomes of patients with aneurysmal subarachnoid haemorrhage. Lancet Neurol. 2011;10(4):349‐356. [DOI] [PubMed] [Google Scholar]
- 4. Lu VM, Graffeo CS, Perry A, et al. Rebleeding drives poor outcome in aneurysmal subarachnoid hemorrhage independent of delayed cerebral ischemia: a propensity‐score matched cohort study. J Neurosurg. 2019;1‐9. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 5. van Donkelaar CE, Bakker NA, Veeger NJ, et al. Predictive factors for rebleeding after aneurysmal subarachnoid hemorrhage: rebleeding aneurysmal subarachnoid hemorrhage study. Stroke. 2015;46(8):2100‐2106. [DOI] [PubMed] [Google Scholar]
- 6. Stienen MN, Germans M, Burkhardt JK, et al. Predictors of in‐hospital death after aneurysmal subarachnoid hemorrhage: analysis of a nationwide database (Swiss SOS [Swiss Study on Aneurysmal Subarachnoid Hemorrhage]). Stroke. 2018;49(2):333‐340. [DOI] [PubMed] [Google Scholar]
- 7. Reith W. Endovascular therapy options for aneurysmal subarachnoid hemorrhage. Radiologe. 2011;51(2):113‐119. [DOI] [PubMed] [Google Scholar]
- 8. Yamada S, Ishikawa M, Yamamoto K, Ino T, Kimura T, Kobayashi S. Aneurysm location and clipping versus coiling for development of secondary normal‐pressure hydrocephalus after aneurysmal subarachnoid hemorrhage: Japanese Stroke DataBank. J Neurosurg. 2015;123(6):1555‐1561. [DOI] [PubMed] [Google Scholar]
- 9. Hoh BL, Topcuoglu MA, Singhal AB, et al. Effect of clipping, craniotomy, or intravascular coiling on cerebral vasospasm and patient outcome after aneurysmal subarachnoid hemorrhage. Neurosurgery. 2004;55(4):779‐789, discussion 786–779. [DOI] [PubMed] [Google Scholar]
- 10. Teleb MS, Pandya DJ, Castonguay AC, et al. Safety and predictors of aneurysm retreatment for remnant intracranial aneurysm after initial endovascular embolization. J Neurointerv Surg. 2014;6(7):490‐494. [DOI] [PubMed] [Google Scholar]
- 11. Lanzino G, Fraser K, Kanaan Y, Wagenbach A. Treatment of ruptured intracranial aneurysms since the International Subarachnoid Aneurysm Trial: practice utilizing clip ligation and coil embolization as individual or complementary therapies. J Neurosurg. 2006;104(3):344‐349. [DOI] [PubMed] [Google Scholar]
- 12. Ding CY, Cai HP, Ge HL, Yu LH, Lin YX, Kang DZ. Assessment of lipoprotein‐associated phospholipase A2 level and its changes in the early stages as predictors of delayed cerebral ischemia in patients with aneurysmal subarachnoid hemorrhage. J Neurosurg. 2020;132(1):62‐68. [DOI] [PubMed] [Google Scholar]
- 13. Yang BH, He Q, Ding CY, Kang DZ, Tang QX. High‐sensitivity C‐reactive protein as a predictive factor of acute kidney injury following aneurysmal subarachnoid hemorrhage: a prospective observational study. Acta Neurochir (Wien). 2019;161(9):1783‐1791. [DOI] [PubMed] [Google Scholar]
- 14. Steyerberg EW. Clinical prediction models: a practical approach to development, validation, and updating. J R Statal Soc. 2010;66(2):661‐662. [Google Scholar]
- 15. Patel N, Rao VA, Heilman‐Espinoza ER, Lai R, Quesada RA, Flint AC. Simple and reliable determination of the modified rankin scale score in neurosurgical and neurological patients: the mRS‐9Q. Neurosurgery. 2012;71(5):971‐975; discussion 975. [DOI] [PubMed] [Google Scholar]
- 16. Kundra S, Mahendru V, Gupta V, Choudhary AK. Principles of neuroanesthesia in aneurysmal subarachnoid hemorrhage. J Anaesthesiol Clin Pharmacol. 2014;30(3):328‐337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Van Gijn J, Kerr RS, Rinkel GJ. Subarachnoid haemorrhage. Lancet. 2007;369(9558):306‐318. [DOI] [PubMed] [Google Scholar]
- 18. Kohri T, Sugano M, Kawashima O, et al. Prognostic model of stage II non‐small cell lung cancer by a discriminant analysis of the immunohistochemical protein expression. Surg Today. 2006;36(12):1039‐1046. [DOI] [PubMed] [Google Scholar]
- 19. Debik J, Euceda LR, Lundgren S, et al. Assessing treatment response and prognosis by serum and tissue metabolomics in breast cancer patients. J Proteome Res. 2019;18(10):3649‐3660. [DOI] [PubMed] [Google Scholar]
- 20. Mitchell RA, Partin JS, Arcinue EL, Partin JC, Ram ML, Sarnaik AP. Prognosis and diagnosis of Reye syndrome by discriminant analysis. Exp Mol Pathol. 1985;43(2):268‐273. [DOI] [PubMed] [Google Scholar]
- 21. Celikbilek A, Ismailogullari S, Zararsiz G. Neutrophil to lymphocyte ratio predicts poor prognosis in ischemic cerebrovascular disease. J Clin Lab Anal. 2014;28(1):27‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Liu YL, Lu JK, Yin HP, et al. High neutrophil‐to‐lymphocyte ratio predicts hemorrhagic transformation in acute ischemic stroke patients treated with intravenous thrombolysis. Int J Hypertens. 2020;2020:e5980261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lattanzi S, Brigo F, Trinka E, Cagnetti C, Di Napoli M, Silvestrini M. Neutrophil‐to‐lymphocyte ratio in acute cerebral hemorrhage: a system review. Transl Stroke Res. 2019;10(2):137‐145. [DOI] [PubMed] [Google Scholar]
- 24. Lattanzi S, Cagnetti C, Rinaldi C, Angelocola S, Provinciali L, Silvestrini M. Neutrophil‐to‐lymphocyte ratio improves outcome prediction of acute intracerebral hemorrhage. J Neurol Sci. 2018;387:98‐102. [DOI] [PubMed] [Google Scholar]
- 25. Park JS, Seo KW, Choi BJ, et al. Importance of prognostic value of neutrophil to lymphocyte ratio in patients with ST‐elevation myocardial infarction. Medicine. 2018;97(48):e13471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tamhane UU, Aneja S, Montgomery D, Rogers EK, Eagle KA, Gurm HS. Association between admission neutrophil to lymphocyte ratio and outcomes in patients with acute coronary syndrome. Am J Cardi. 2008;102(6):653‐657. [DOI] [PubMed] [Google Scholar]
- 27. Lattanzi S, Di Napoli M, Ricci S, Divani AA. Matrix metalloproteinases in acute intracerebral hemorrhage. Neurotherapeutics. 2020;17(2):484‐496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wu Y, He Q, Wei Y, et al. The association of neutrophil‐to‐lymphocyte ratio and delayed cerebral ischemia in patients with aneurysmal subarachnoid hemorrhage: possible involvement of cerebral blood perfusion. Neuropsychiatr Dis Treat. 2019;15:1001‐1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Giede‐Jeppe A, Reichl J, Sprügel MI, et al. Neutrophil‐to‐lymphocyte ratio as an independent predictor for unfavorable functional outcome in aneurysmal subarachnoid hemorrhage. J Neurosurg. 2019;132(2):400‐407. [DOI] [PubMed] [Google Scholar]
- 30. Tao C, Wang J, Hu X, Ma J, Li H, You C. Clinical value of neutrophil to lymphocyte and platelet to lymphocyte ratio after aneurysmal subarachnoid hemorrhage. Neurocrit Care. 2017;26(3):393‐401. [DOI] [PubMed] [Google Scholar]
- 31. Al‐Mufti F, Amuluru K, Damodara N, et al. Admission neutrophil‐lymphocyte ratio predicts delayed cerebral ischemia following aneurysmal subarachnoid hemorrhage. J Neurointerv Surg. 2019;11(11):1135‐1140. [DOI] [PubMed] [Google Scholar]
- 32. Long B, Koyfman A, Runyon MS. Subarachnoid hemorrhage: updates in diagnosis and management. Emerg Med Clin North Am. 2017;35(4):803‐824. [DOI] [PubMed] [Google Scholar]
- 33. Al‐Mufti F, Misiolek KA, Roh D, et al. White blood cell count improves prediction of delayed cerebral ischemia following aneurysmal subarachnoid hemorrhage. Neurosurgery. 2019;84(2):397‐403. [DOI] [PubMed] [Google Scholar]
- 34. Yao PS, Chen GR, Xie XL, et al. Higher leukocyte count predicts 3‐month poor outcome of ruptured cerebral aneurysms. Sci Rep. 2018;8(1):5799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chalouhi N, Ali MS, Jabbour PM, et al. Biology of intracranial aneurysms: role of inflammation. J Cereb Blood Flow Metab. 2012;32(9):1659‐1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fennell VS, Kalani MY, Atwal G, Martirosyan NL, Spetzler RF. Biology of saccular cerebral aneurysms: a review of current understanding and future directions. Front Surg. 2016;3:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sawyer DM, Amenta PS, Medel R, Dumont AS. Inflammatory mediators in vascular disease: identifying promising targets for intracranial aneurysm research. Mediators Inflamm. 2015;2015:e896283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chalouhi N, Ali MS, Starke RM, et al. Cigarette smoke and inflammation: role in cerebral aneurysm formation and rupture. Mediators Inflamm. 2012;2012:e271582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kataoka K, Taneda M, Asai T, Kinoshita A, Ito M, Kuroda R. Structural fragility and inflammatory response of ruptured cerebral aneurysms. A comparative study between ruptured and unruptured cerebral aneurysms. Stroke. 1999;30(7):1396‐1401. [DOI] [PubMed] [Google Scholar]
- 40. Jamali SA, Turnbull MT, Kanekiyo T, et al. Elevated neutrophil‐lymphocyte ratio is predictive of poor outcomes following aneurysmal subarachnoid hemorrhage. J Stroke Cerebrovasc Dis. 2020;29(4):104631. [DOI] [PubMed] [Google Scholar]
- 41. Dandy WE. Intracranial aneurysm of the internal carotid artery: cured by operation. Ann Surg. 1938;107(5):654‐659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Serbinenko FA. Balloon occlusion of saccular aneurysms of the cerebral arteries. Voprosy neirokhirurgii. 1974;4:8‐15. [PubMed] [Google Scholar]
- 43. Molyneux A, Kerr R, Stratton I, et al. International Subarachnoid Aneurysm Trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised trial. Lancet. 2002;360(9342):1267‐1274. [DOI] [PubMed] [Google Scholar]
- 44. Molyneux AJ, Kerr RS, Yu LM, et al. International subarachnoid aneurysm trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised comparison of effects on survival, dependency, seizures, rebleeding, subgroups, and aneurysm occlusion. Lancet. 2005;366(9488):809‐817. [DOI] [PubMed] [Google Scholar]


