Abstract
Background
Gastrin‐17 (G‐17) and Helicobacter pylori (H pylori) antibody are widely used in the screening of gastric diseases, especially in gastric cancer. In this study, we aimed to evaluate the value of G‐17 and H pylori antibody in gastric disease screening.
Methods
Healthy males and females (1368 and 1212, respectively) aged between 21‐80 years were recruited for the study. Serum G‐17 value was measured using ELISA, and H pylori antibodies were measured using Western blotting. Statistical analyses were performed using the chi‐square, Mann‐Whitney U, and Kruskal‐Wallis H tests.
Results
Serum G‐17 level was higher in the H pylori‐positive group than in the negative group. Serum G‐17 level was higher in the type 1 H pylori‐positive group than in the type 2 H pylori‐positive group. Further, serum G‐17 level was higher in females than in males and showed significant differences among different age‐groups, with changes in trend proportional to the age. The positive rate of H pylori infection in all the subjects was 58.29% and did not show a significant difference between males and females. However, it showed significant differences among different age‐groups, with the changing trend proportional to the age.
Conclusion
Analysis of serum G‐17 level and H pylori antibody typing is valuable in gastric disease screening. Every laboratory should establish its own reference interval for G‐17 level.
Keywords: age, gastric disease, gastrin, gender, Helicobacter pylori antibody
Serum G‐17 level was higher in females than in males, and the changing trend of serum G‐17 level was proportional to age.

1. INTRODUCTION
Helicobacter pylori (H pylori) is a gram‐negative, spiral‐shaped bacterium that is known to have infected more than half of the world's population and is the only pathogenic bacteria currently known to survive in the human stomach. Helicobacter pylori is listed as grade 1 carcinogen in the list of carcinogens published by the World Health Organization. It changes the structure and function of the gastric mucosa following infection, eventually leading to a variety of gastric diseases. 1 , 2 , 3
Gastrin‐17 (G‐17) is an important gastrointestinal hormone which is mainly secreted by the gastrointestinal G cells. It stimulates the secretion of gastric acid and pepsinogen, promotes proliferation of the gastric mucosal cells, enhances the motility of the gastrointestinal tract, and promotes the secretion of pancreatic juice and bile. It also reflects the functional status of the gastric mucosa and plays an important role in the occurrence and development of gastrointestinal tumors. 4 , 5 , 6
As an important constituent of "serological biopsy" of gastric mucosa, H pylori antibody and G‐17 are widely used in clinical diagnosis of gastric diseases. Serum G‐17 levels are thought to be closely related to H pylori infection, and several studies have attempted to explore and demonstrate their relationship. 7 , 8 , 9 Most of the studies were performed on patients with gastric diseases, and limited number of studies have used healthy human subjects. In this study, we analyzed the level of serum G‐17 and H pylori antibodies in healthy Chinese population to explore their diagnostic potential in the screening of gastric diseases.
2. MATERIALS AND METHODS
2.1. Subjects
In this study, we performed retrospective analysis of 1368 apparently healthy males and 1212 apparently healthy females (aged 21‐80 years) who performed physical examinations at the Health Examination Center of The People's Hospital of Guangxi Zhuang Autonomous Region between February 2018 and February 2020. Serum G‐17 and H pylori antibody were simultaneously tested as examination items in all the subjects. The exclusion criteria of the subjects in our study were as follows: 1. Individuals with a history of administration of special medication (including proton pump inhibitors and H2 receptor antagonists among others) within 2 weeks prior to the physical examination; 2. individuals with a history of gastric diseases such as gastritis, gastric ulcer, and gastric cancer; 3. individuals with a history of stomach surgery; 4. individuals with severe heart, liver, or kidney insufficiency; 5. individuals with a long‐term history of smoking or drinking; and 6. individuals with known infectious diseases. This study was approved by the ethics committee of The People's Hospital of Guangxi Zhuang Autonomous Region.
2.2. G‐17 Enzyme‐linked immunosorbent assay (ELISA)
All the individuals were required to maintain fasting for more than 10 hours prior to drawing of blood. Fasting blood samples were collected into BD Vacutainer blood collection tubes (Becton Dickinson, allowed to clot for at least 30 minutes at room temperature, and then centrifuged for 10 minutes at 1200 g to obtain the serum. Serum G‐17 value was measured using ELISA (Biohit). All procedures were carried out according to the manufacturer's instructions. Reference interval of G‐17 in normal population provided by the instructions was 1 ~ 7 pmol/L, but it was recommended that every laboratory establishes its own reference interval.
2.3. H pylori antibody testing using Western blot analysis
Samples were prepared as described above for G‐17. Serum H pylori antibodies were measured using Western blot analysis (Blot). The test principles were as follows: H pylori antigens were electrophoresed on sodium dodecyl sulfate (SDS)‐polyacrylamide gel, separated according to the molecular weights, and then transferred to nitrocellulose membrane. The H pylori antibodies present in the serum react with the antigens on the nitrocellulose membrane and were visualized with the addition of enzyme‐labeled antigens and color reagents. A positive zone appeared colored on the membrane. All procedures were carried out according to the manufacturer's instructions. Interpretations of the H pylori antibody typing were performed as follows: Negative result: only quality control zone appeared on the color rendering zone; type 1 H pylori antibody: CagA and VacA zone simultaneously appeared or either of the two appeared; and type 2 H pylori antibody: UreA and UreB zone simultaneously appeared or either of the two appeared, CagA and VacA zone did not appear.
2.4. Statistical analysis
All the experimental data were analyzed with SPSS statistics 22.0 software. Serum G‐17 levels were expressed by median (25th percentile, 75th percentile) (M; P25 ~ P75). Positive rates of H pylori between males and females, among the different age‐groups, were compared using chi‐square test; serum G‐17 levels between the males and females were compared using Mann‐Whitney U test; serum G‐17 levels among different age‐groups were compared using Kruskal‐Wallis H test; serum G‐17 levels among the three groups based on different H pylori infection status were compared using Kruskal‐Wallis H test at first, and Mann‐Whitney U test was then used to make pairwise comparison if a significant difference was detected by Kruskal‐Wallis H test. The level of statistical significance was set at P < .05.
3. RESULTS
3.1. Comparison of serum G‐17 levels among the three groups based on different H pylori infection status
The serum G‐17 level was 3.30 (1.51 ~ 6.61) in the 1023 subjects with type 1 H pylori infection, 1.50 (0.73 ~ 3.40) in the 481 subjects with type 2 H pylori infection, and 0.94 (0.57 ~ 1.77) in the 1076 subjects with no H pylori infection. The differences in the serum G‐17 levels among the three groups were significant (P = .000; Table 1).
Table 1.
Serum G‐17 levels in the three groups based on different H pylori infection status
| Hp infection status | Number | G‐17 (pmol/L) |
|---|---|---|
| Hp‐1 positive | 1023 | 3.30 (1.51 ~ 6.61) |
| Hp‐2 positive | 481 | 1.50 (0.73 ~ 3.40) |
| Hp negative | 1076 | 0.94 (0.57 ~ 1.77) |
3.2. Comparison of serum G‐17 level between males and females
The serum G‐17 level was 1.59 (0.76 ~ 4.04) in all the subjects, 1.50 (0.67 ~ 4.01) in males, and 1.68 (0.86 ~ 4.09) in females. Thus, the serum G‐17 level in females was higher than that in males (P = .005; Table 2; Figure 1).
Table 2.
H pylori‐positive rates and serum G‐17 levels in different genders and age‐groups
| Age | Gender | Hp‐1 (%) | Hp‐2 (%) | Hp (%) | G‐17 (pmol/L) |
|---|---|---|---|---|---|
| 21‐30 | Male | 30.20 (74/245) | 14.29 (35/245) | 44.49 (109/245) | 1.06 (0.58 ~ 3.73) |
| Female | 28.81 (68/236) | 17.37 (41/236) | 46.19 (109/236) | 1.66 (0.88 ~ 3.78) | |
| Male + female | 29.52 (142/481) | 15.80 (76/481) | 45.32 (218/481) | 1.46 (0.67 ~ 3.77) | |
| 31‐40 | Male | 33.75 (107/317) | 17.03 (54/317) | 50.79 (161/317) | 1.28 (0.62 ~ 3.52) |
| Female | 39.41 (106/269) | 14.50 (39/269) | 53.90 (145/269) | 1.39 (0.70 ~ 4.52) | |
| Male + female | 36.35 (213/586) | 15.87 (93/586) | 52.22 (306/586) | 1.35 (0.66 ~ 4.01) | |
| 41‐50 | Male | 40.70 (116/285) | 19.30 (55/285) | 60.00 (171/285) | 1.47 (0.71 ~ 3.56) |
| Female | 41.59 (94/226) | 19.47 (44/226) | 61.06 (138/226) | 1.67 (0.90 ~ 4.04) | |
| Male + female | 41.10 (210/511) | 19.37 (99/511) | 60.47 (309/511) | 1.59 (0.79 ~ 3.80) | |
| 51‐60 | Male | 43.89 (115/262) | 21.76 (57/262) | 65.65 (172/262) | 1.82 (0.82 ~ 4.58) |
| Female | 47.60 (129/271) | 20.66 (56/271) | 68.27 (185/271) | 1.69 (0.93 ~ 3.98) | |
| Male + female | 45.78 (244/533) | 21.20 (113/533) | 66.98 (357/533) | 1.77 (0.85 ~ 4.26) | |
| >60 | Male | 48.65 (126/259) | 18.15 (47/259) | 66.80 (173/259) | 1.89 (0.78 ~ 4.27) |
| Female | 41.90 (88/210) | 25.24 (53/210) | 67.14 (141/210) | 1.89 (0.93 ~ 4.65) | |
| Male + female | 45.63 (214/469) | 21.32 (100/469) | 66.95 (314/469) | 1.89 (0.89 ~ 4.41) | |
| Total | Male | 39.33 (538/1368) | 18.13 (248/1368) | 57.46 (786/1368) | 1.50 (0.67 ~ 4.01) |
| Female | 40.02 (485/1212) | 19.22 (233/1212) | 59.24 (718/1212) | 1.68 (0.86 ~ 4.09) | |
| Male + female | 39.65 (1023/2580) | 18.64 (481/2580) | 58.29 (1504/2580) | 1.59 (0.76 ~ 4.04) |
FIGURE 1.
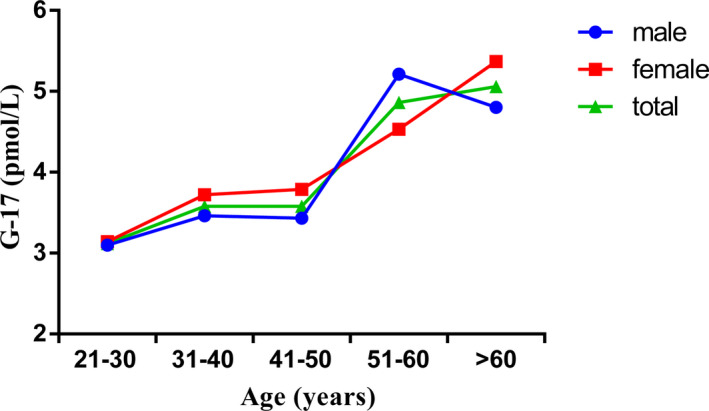
Serum G‐17 levels in different genders and age groups
3.3. Comparison of serum G‐17 level among different age‐groups
The serum G‐17 levels in the different age‐groups of 21‐30, 31‐40, 41‐50, 51‐60, and >60 years were 1.46 (0.67 ~ 3.77), 1.35 (0.66 ~ 4.01), 1.59 (0.79 ~ 3.80), 1.77 (0.85 ~ 4.26), and 1.89 (0.89 ~ 4.41), respectively. The serum G‐17 levels showed significant differences among the age‐groups (P = .000), with the changing trend proportional to the age (Table 2; Figure 1).
3.4. Comparison of H pylori positive rates between males and females
Helicobacter pylori ‐positive rate was 58.29% in all the subjects, 57.46% in males, and 59.24% in females. There was no significant difference in the positive rate between males and females (P = .379). The type 1 H pylori positive rates were 39.33% and 40.02% in males and females, respectively, and there was no significant difference between them (P = .747). The type 2 H pylori positive rates were 18.13% and 19.22% in males and females, respectively, and showed no significant difference (P = .479; Table 2; Figure 2).
FIGURE 2.
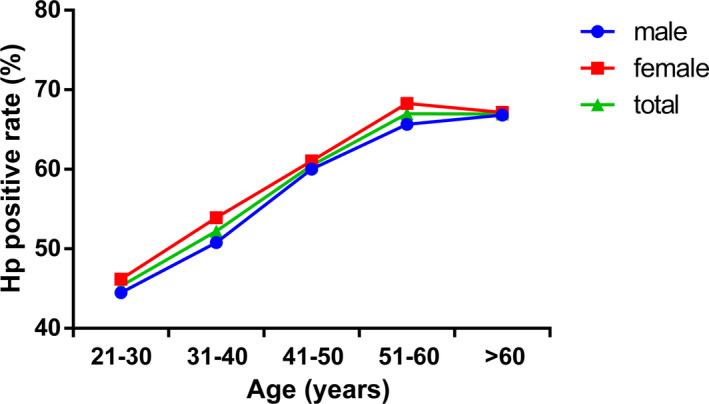
H pylori positive rates in different genders and age groups
3.5. Comparison of H pylori positive rates among different age‐groups
Helicobacter pylori positive rates in the different age‐groups of 21‐30, 31‐40, 41‐50, 51‐60, and >60 years were 45.32%, 52.22%, 60.47%, 66.98%, and 66.95%, respectively, and showed significant differences (P = .000) among the age‐groups. The changes in the trend were proportional to the age (Table 2; Figure 2).
4. DISCUSSION
Gastrin is mainly synthesized and secreted by the G cells in the gastric antrum. G‐17 is the most important form of gastrin in blood circulation. 6 , 10 , 11 In our study, serum G‐17 levels were higher in females than in males. However, in age‐group of 51‐60 years, the serum G‐17 levels were higher in males than in females. This difference may be related to the changes in hormones that occur in women during menopause. Some previous studies have reported differences in serum G‐17 levels between males and females, while some others have reported no difference. 12 , 13 , 14 G‐17 is an unstable indicator which is affected by many factors. It shows variations in different regions and populations, such that the difference between males and females also varied. Serum G‐17 levels also showed significant differences in different age‐groups, the change in the trend was proportional to the age.
Helicobacter pylori is a curved gram‐negative bacillus which is found in the gastric mucosa and is typically acquired during childhood. Helicobacter pylori infection rates change with public sanitary conditions, socioeconomic factors, and hygiene habits and tend to increase with age. Helicobacter pylori infection is globally distributed, and more than half of the world's population are carriers of the microorganism, with a higher prevalence in the developing countries compared to the developed countries. 15 , 16 , 17 Previous studies have reported an H pylori infection rate of 50 ~ 80% in China. Consistent with this report, the H pylori infection rate in our study was 58.29%. 18 , 19 Measurement of H pylori antibody in our study was conducted using a typing test. The type 1 H pylori produces vacuolating cytotoxin (VacA) and cytotoxin‐associated protein (CagA) and is more infectious and pathogenic than the type 2 H pylori which produces urease subunit A/B (UreA/B) and no cytotoxins. 20 , 21 , 22
In the H pylori‐infected group, 68.02% were infected with type 1 H pylori, and 31.98% were infected with type 2 H pylori. These data showed that most of the H pylori infection in our region were caused by type 1 H pylori and demonstrated that the type 1 H pylori was more infectious than type 2 H pylori. The positive rate of H pylori infection showed no significant difference between males and females. Some previous studies reported differences in the positive rate of H pylori infection between males and females, while some others reported no difference. 23 , 24 H pylori infection status also changed depending on different regions and populations, such that the difference between males and females also varied. The positive rate of H pylori infection among different age‐groups showed significant differences, and the changes in the trends were proportional to the age.
Studies have suggested that H pylori infection stimulates gastrin secretion, leading to an increase in the serum G‐17 level. 25 , 26 In our study, the serum G‐17 level in H pylori‐positive group was higher than in the H pylori‐negative group. Further, the serum G‐17 level in type 1 H pylori‐positive group was also higher than in the type 2 H pylori‐positive group. It is reported that H pylori infection increases urea and ammonia in the stomach, and decreases gastric acid, resulting in an increase in G‐17 secretion. 27 , 28 Compared to type 2 H pylori, the type 1 H pylori produces VacA and CagA, which was more pathogenic to the gastric mucosa. The serum G‐17 levels in subjects with type 1 H pylori infection were higher than in subjects with type 2 H pylori infection, which also demonstrates that type 1 H pylori has more pathogenic to the gastric mucosa than type 2 H pylori.
The reference interval of serum G‐17 level in normal population according to the manufacturer's instructions was 1 ~ 7 pmol/L. However, it was recommended that each laboratory needs to establish its own reference interval. Both, increase or decrease of serum G‐17 levels indicates a risk of gastric disease. Gastrin is mainly synthesized and secreted by the G cells in the gastric antrum. Gastrin levels often decrease when a lesion develops in the gastric antrum and conversely increase when a lesion develops in the gastric body. 29 Serum G‐17 levels in our study were slightly lower than that in previous reports. 30 , 31 , 32 As an unstable indicator, G‐17 is influenced by many factors, including people from different regions with different diets and lifestyle habits. Given that several factors may affect serum G‐17 levels, every laboratory needs to establish its own reference interval for G‐17, and the statistical data pertaining to the serum G‐17 levels in our study would serve as a reference for our laboratory.
Although none of the subjects in our study had any history of gastric diseases, screening of serum G‐17 and H pylori antibody can help in the identification of high‐risk population and allow for intervention in the early stage of the disease.
ACKNOWLEDGMENT
This study is supported by the self‐funded research project of The Health Committee of Guangxi Zhuang Autonomous Regions (No. Z2016629).
Liu W, Sun Y, Yuan Y. Analysis of serum gastrin‐17 and Helicobacter pylori antibody in healthy Chinese population. J Clin Lab Anal. 2020;34:e23518 10.1002/jcla.23518
REFERENCES
- 1. Eusebi LH, Zagari RM, Bazzoli F. Epidemiology of Helicobacter pylori infection. Helicobacter. 2014;19(1):1‐5. [DOI] [PubMed] [Google Scholar]
- 2. Sipponen P, Kosunen TU, Valle J, Riihela M, Seppala K. Helicobacter pylori infection and chronic gastritis in gastric cancer. J Clin Pathol. 1992;45(4):319‐323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Malfertheiner P, Megraud F, Bazzoli F, et al. Current concepts in the management of Helicobacter pylori infection: the Maastricht III Consensus Report. Gut. 2007;56:772‐781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Farias CB, Lima RC, Lima LO, et al. Stimulation of proliferation of U138‐MG glioblastoma cells by gastrin‐releasing peptide in combination with agents that enhance cAMP signaling. Oncology. 2008;75:27‐31. [DOI] [PubMed] [Google Scholar]
- 5. Schuburt ML, Peura DA. Control of gastric acid secretion in health and disease. Gastroenterology. 2008;134:1842‐1860. [DOI] [PubMed] [Google Scholar]
- 6. Dockray GJ, Varro A, Dimaline R, Wang T. The gastrins: their production and biological activities. Annu Rev Physiol. 2001;63:119‐139. [DOI] [PubMed] [Google Scholar]
- 7. Shimoyama T, Chinda D, Matsuzaka M, et al. Decrease of serum level of gastrin in healthy Japanese adults by the change of Helicobacter pylori infection. J Gastroenterol Hepatol. 2014;29(4):25‐28. [DOI] [PubMed] [Google Scholar]
- 8. Testino G, Cornaggia M, De Laco F. Helicobacter pylori influence on gastric acid secretion in duodenal ulcer patients diagnosed for the first time. Panminerva Med. 2002;44(1):19‐22. [PubMed] [Google Scholar]
- 9. Beales I, Blaser MJ, Srinivasan S, et al. Effect of Helicobacter pylori products and recombinant cytokines on gastrin release from cultured canine G cells. Gastroenterology. 1997;113(2):465‐471. [DOI] [PubMed] [Google Scholar]
- 10. Huang YN, Tang SX. The research progress of gastrin and gastric cancer. J Military Surg Southwest China. 2012;14(5):757‐760. [Google Scholar]
- 11. Vananen H, Vauhkonen M, Helske T, et al. Non‐endoscopic diagnosis of atrophic gastritis with a blood test. Correlation between gastric histology and serum levels of gastrin‐17 and pepsinogen Ⅰ: a multicentre study. Eur J Gastroenterol Hepatol. 2003;15(8):885‐891. [DOI] [PubMed] [Google Scholar]
- 12. Zhang Z, Sun LP, Gong YH, et al. Factors affecting the serum gastrin 17 level: an evidence‐based analysis of 3906 serum samples among Chinese. J Dig Dis. 2007;8(2):72‐76. [DOI] [PubMed] [Google Scholar]
- 13. Chen MY, Xu Q, Sun LP, et al. Correlation between gastric cancer, precancerous diseases and serum gastrin 17 levels. Clin J Gastroenterol Hepatol. 2015;24(2):161‐165. [Google Scholar]
- 14. Sousa JB, Etchebehere RM, Queiroz DMM, et al. Increased serum gastrin in patients with different clinical forms of Chagas disease coinfected with Helicobacter pylori . Rev Inst Med Trop Sao Paulo. 2019;61:e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pellicano R, Ribaldone DG, Fagoonee S, et al. A 2016 panorama of Helicobacter pylori infection: key messages for clinicians. Panminerva Med. 2016;58(4):304‐317. [PubMed] [Google Scholar]
- 16. Kato S, Ozawa K, Koike T, et al. Effect of Helicobacter pylori infection on gastric acid secretion and meal‐stimulated serum gastrin in children. Helicobacter. 2004;9:100‐105. [DOI] [PubMed] [Google Scholar]
- 17. Sugano K, Tack J, Kuipers E, et al. Kyoto global consensus report on Helicobacter pylori gastritis. Gut. 2015;64(9):1353‐1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schottker B, Adamu MA, Weck MN, et al. Helicobacter pylori infection, chronic atrophic gastritis and major cardiovascular events: a population‐based cohort study. Atherosclerosis. 2012;220:569‐574. [DOI] [PubMed] [Google Scholar]
- 19. Lee M, Baek H, Park JS, et al. Current Helicobacter pylori infection is significantly associated with subclinical coronary atherosclerosis in healthy subjects: a cross‐sectional study. PLoS ONE. 2018;13(3):1‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhang H, Liao Y, Zhang H, Wu J, Zheng D, Chen Z. Helicobacter pylori Cytotoxin‐associated gene A increases carcinogenicity of in colorectal adenoma. Int J Biol Markers. 2020;35(1):19‐25. [DOI] [PubMed] [Google Scholar]
- 21. Santolaria S, Benito R, Piazuelo E, Lanas A, Sainz R. CagA and VacA cytotoxin antibodies and risk for peptic ulcer disease in patients with Helicobacter pylori infection. Med Clin (Barc). 2001;16(17):641‐644. [PubMed] [Google Scholar]
- 22. Ai F, Hua X, Liu Y, Lin J, Feng Z. Preliminary study of pancreatic cancer associated with Helicobacter pylori infection. Cell Biochem Biophys. 2015;71(1):1‐4. [DOI] [PubMed] [Google Scholar]
- 23. Guo C, Liu F, Zhu L, et al. Analysis of culturable microbiota present in the stomach of children with gastric symptoms. Braz J Microbiol. 2019;50(1):107‐115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shan JH, Bai XJ, Han LL, Yuan Y, Sun XF. Helicobacter pylori Changes with aging in gastric biomarkers levels and in biochemical factors associated with infection in asymptomatic Chinese population. World J Gastroenterol. 2017;23(32):5945‐5953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Takamura A, Ito M, Boda T, et al. High expression of gastrin receptor protein in injured mucosa of Helicobacter pylori‐positive gastritis. Dig Dis Sci. 2013;58(3):634‐640. [DOI] [PubMed] [Google Scholar]
- 26. Tu H, Sun L, Dong X, et al. Serum anti‐Helicobacter pylori immunoglobulin G titer correlates with grade of histological gastritis, mucosal bacterial density, and levels of serum biomarkers. Scand J Gastroenterol. 2014;49(3):259‐266. [DOI] [PubMed] [Google Scholar]
- 27. Germana B, Di Mario F, Cavallaro LG, et al. Clinical usefulness of serum pepsinogens I and II, gastrin‐17 and anti‐Helicobacter pylori antibodies in the management of dyspeptic patients in primary care. Dig Liver Dis. 2005;37(7):501‐508. [DOI] [PubMed] [Google Scholar]
- 28. Zhang QY, Lv Z, Sun LP, et al. H. pyloriClinical significance of serum markers reflecting gastric function and infection in colorectal cancer. J Cancer. 2019;10(10):2229‐2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wang X, Ling L, Li S, et al. The diagnostic value of gastrin‐17 detection in atrophic gastritis: a meta‐analysis. Medicine. 2016;95(18):1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sun L, Tu H, Liu J, et al. A comprehensive evaluation of fasting serum gastrin‐17 as a predictor of diseased stomach in Chinese population. Scand J Gastroenterol. 2014;49(10):1164‐1172. [DOI] [PubMed] [Google Scholar]
- 31. Shan JH, Bai XJ, Han LL, et al. Changes with aging in gastric biomarkers levels and in biochemical factors associated with Helicobacter pylori infection in asymptomatic Chinese population. World J Gastroenterol. 2017;23(32):5945‐5953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gong Y, Wei W, Yuan Y. Association between abnormal gastric function risk and Helicobacter pylori infection assessed by ELISA and 14C‐urea breath test. Diagn Microbiol Infect Dis. 2014;80(4):316‐320. [DOI] [PubMed] [Google Scholar]


