Abstract
Background
It has proved that there is an association between cancer and volatile organic compounds (VOCs) of exhaled breath. This study targets on verifying the existence of specific VOCs in breathing in breast cancer patients, especially those with ductal carcinoma in situ (DCIS).
Methods
There were a total of 203 participants included in the final analysis, which included 71 (35.0%) patients with histologically confirmed breast cancer (including 13 with DCIS, 31 with lymph node metastasis‐negative status, and 27 with lymph node metastasis‐positive status), 78 (38.4%) healthy volunteers, and 54 (26.6%) patients with histologically confirmed gastric cancer. Gas chromatography‐mass spectrometry and solid‐phase microextraction were used to analyze the breath samples for the presence of VOCs.
Results
There were significant differences in the volatile organic metabolites between the DCIS, lymph node metastasis‐negative breast cancer, and lymph node metastasis‐positive breast cancer groups compared with the healthy controls as well as between the breast cancer and gastric cancer patients. An overlapping set of seven VOCs, including (S)‐1,2‐propanediol, cyclopentanone, ethylene carbonate, 3‐methoxy‐1,2‐propanediol, 3‐methylpyridine, phenol, and tetramethylsilane, was significantly different between the breast cancer patients and healthy individuals as well as between the breast cancer and gastric cancer patients. The combination of these seven compounds was considered as a biomarker for breast cancer. The sensitivity for predicting DCIS by this set of seven compounds was determined to be 80.77%, and the specificity was determined to be 100%.
Conclusions
This set of seven breast cancer‐specific VOCs can be regarded as one particular expiratory marker for DCIS and will help to establish new screening methods for early breast cancer.
Keywords: breast cancer, breath analysis, DCIS, early diagnosis of breast cancer, GC‐MS, volatile biomarker
Exhaled breath analysis using Gas Chromatography–Mass Spectrometry (GC/MS) can distinguish patients with breast cancer in situ from healthy people. And seven VOCs can be served as biomarkers, They are (S)‐1,2‐propanediol, Cyclopentanone, Ethylene carbonate, 3‐Methoxy‐1,2‐propanediol, 3‐Methylpyridine, phenol, and Tetramethylsilicane. The sensitivity for predicting DCIS by this seven compounds model is 80.77%.

1. BACKGROUND
Breast cancer is known as the most prevalent malignancy among women and the primary cause of female deaths worldwide. 1 In 2018, over two million new cases were diagnosed and 626 679 deaths due to breast cancer occurred worldwide. 2 In the United States, an estimated 249 260 new cases of breast cancer were diagnosed in 2018. 3
The prognoses in patients with breast cancer were depended mainly on the stage of the disease. 4 , 5 Ductal carcinoma in situ (DCIS) is the early stage of infiltrated breast cancer. It has been reported that the 10‐year overall survival for patients with DCIS could reach 98%‐99%. 6 In comparison, the 10‐year tumor‐free survival rate is 70%‐80% for patients with axillary lymph node metastasis‐negative breast cancer, while it is <30% for patients with axillary lymph node metastasis‐positive breast cancer. 4 Therefore, diagnosis and appropriate therapy at an early stage can effectively reduce the mortality of breast cancer. 4 , 7 , 8
There are several adjuvant screening methods (eg, mammography, ultrasonography, digital breast tomosynthesis [DBT], and magnetic resonance imaging [MRI]) for breast cancer. 9 , 10 , 11 , 12 However, the accuracy of these methods largely relies on the physicians’ experience and the tumor's histopathologic features; therefore, there may be a misinterpretation or a missed diagnosis. 9 , 11 , 12 , 13 According to the US Preventive Services Task Force (USPSTF), mammography is recommended for breast cancer screening of women aged 50‐74 years. 10 The USPSTF also announced that there is still a lack of sufficient evidence to define the balance of harms and benefits in mammography, ultrasonography, DBT, and MRI in all populations. 10 Furthermore, there is currently no reliable screening method for DCIS of the breast. 14 , 15 , 16 The chance of a cure improves considerably if the disease is diagnosed at an early stage when the tumor is still localized and asymptomatic. 17 A missed diagnosis of DCIS will make these people miss the best opportunity for treatment. However, up to 75% of DCIS patients will progress to invasive breast cancer. 18 , 19
With the rapid development of exhaled breath metabolomics in recent years, the association between volatile organic compounds (VOCs) of exhaled breath and cancer has attracted increasing attention. 20 , 21 , 22 , 23 , 24 , 25 Breath VOC analysis is appropriate for disease screening because it is noninvasive, portable, inexpensive, and easy for patients to accept. Besides, the technique has the potential for early diagnosis. 26 , 27 Preliminary studies have confirmed that the analysis of VOCs can distinguish breast cancer patients from healthy controls. 23 , 24 , 25 , 28 However, there is not enough evidence to define the specific biomarkers for the early stage of breast cancer.
Our previous analytical study reported that a set of three biomarkers (eg, 2,5,6‐trimethylolethane, 1,4‐dimethoxy‐2,3‐butanediol, and cyclohexanone) could be used to distinguish patients with breast cancer, cyclomastopathy, and mammary gland fibroma. 29 The present study expanded the sample size of patients based on our previous research. 29 The objective of this study was to explore the potential biomarkers of exhaled breath for the early stage of breast cancer.
2. METHODS
This was a prospective cohort study. The Ethics Committee of Harbin Medical University approved the study protocol (No. 201808), and each patient signed informed consent before study enrollment.
2.1. Participants
We recruited patients from The First Affiliated Hospital of Harbin Medical University from December 1, 2018, to February 1, 2020. The First Affiliated Hospital of Harbin Medical University is a comprehensive hospital with approximately 4398 beds. The eligibility criteria of the included participants were similar to those of a previous study. 29 The patients of the breast cancer cohort were recruited from the Breast Surgery Department. The inclusion criteria were as follows: (a) the patients were between 18 and 80 years old; (b) the physical status of the patients was defined according to the American Society of Anesthesiologists (ASA) physical status classification system as ASA I or ASA II 30 ; and (c) the patients were all scheduled for breast surgery within 2 days with histologically confirmed breast cancer. The control group of healthy female volunteers was recruited from the Medical Center. The inclusion criterion for healthy controls was as follows: (a) aged between 18 and 80 years old; (b) female; (c) confirmed as not having breast cancer by mammography or ultrasound examination; (d) no history of malignancies; (e) no current infectious diseases. Our team previously collected data from another control group of gastric cancer patients in 2015 for a previous study. The inclusion criteria for the gastric cancer cohort were as follows: (a) aged between 18 and 80 years old; (b) did not have breast cancer; (c) did not have other malignancies; (d) did not have a current infectious disease. All of the gastric cancer patients had signed a consent form agreeing with their data being reused in future research. The exclusion criteria for all included participants were as follows: (a) patients were currently breastfeeding, pregnant, or could become pregnant; (b) patients had congenital disease(s); (c) patients underwent chemotherapy or radiotherapy treatment before the testing or had another malignancy at the time of the testing; (d) patients had comorbidities such as obstructive lung disease, pulmonary tuberculosis, chronic asthma, or other pulmonary diseases; (e) patients had an inflammatory condition at the time of testing; (f) patients had symptoms of any acute illness during the previous 2 weeks.
2.2. Breath sample collection
Before breath sample collection, all participants were asked to strictly fast for 8 hours to minimize the influence of their diet and the environment on the composition of their exhaled breaths. Alveolar breath sampling was performed as described previously for other studies. 31 , 32 A gas‐tight syringe (50 mL; Agilent Inc) was used to draw and transfer the exhaled gas (20 mL) into an evacuated 20‐mL glass vial (Supelco Inc) for each participant. All vials were flushed and cleaned with nitrogen gas (Liming Gas Inc) thoroughly to remove any residual contaminants. 33 All of the samples were analyzed within 3 hours of sampling. The gastric cancer sample data in this experiment were all collected and interpreted under the same conditions.
2.3. Solid‐phase microextraction (SPME)
A 75‐mm‐thick SPME fiber (purchased from Supelco) was inserted into a vial, and the vial was exposed to the gaseous sample at 40°C for 20 minutes. In the hot gas chromatography injector at 200°C, the desorption of VOCs occurred for 2 minutes.
2.4. Gas chromatography‐mass spectrometry (GC/MS) analysis
All of the analyses were performed on a GC/MS (Shimadzu GC‐MS QP 2010, Shimadzu) equipped with a DB‐5MS (length: 30 m; ID: 0.250 µm; film thickness, 0.25 mm: Agilent Technologies) PLOT column. The injections were performed in the splitless mode, and the splitless time was 1 minutes. The injector temperature was set to 200°C, and the carrier gas was helium at a flow rate of 2 mL/min. The temperature in the column was held at 40°C for 2 minutes in order to condense the hydrocarbons. The temperature was then increased to 200°C at 70°C/min and held for 1 minutes. After that, the temperature ramped to 230°C at 20°C/min and stayed for 3 minutes. The MS analyses were performed in the full‐scan mode with an associated m/z range of 35‐200 amu. The ionization energy of 70 eV was used for each measurement, with the ion source maintained at 200°C.
2.5. Statistical analyses
Statistical analyses were performed using SIMCA‐p + 11 software. 34 Differences in VOCs between groups were tested with partial least‐squares discriminant analysis (PLS‐DA) and principal component analysis (PCA). The SIMCA‐p software was used to prevent overfitting by applying the default seven‐round cross‐validation. Also, permutation tests using 200 iterations were performed to validate the supervised model further. We selected the potential metabolic biomarkers based on the variable importance in the projection values calculated from the PLS‐DA model. For all data analyses, a P value <.05 indicated statistical significance. The area under the curve of the combined biomarkers and the sensitivity and specificity analyses were performed using R language software 3.2 (R Development Core Team 2011).
3. RESULTS
In this study, there were a total of 203 participants included in the final analysis, which included 71 (35.0%) patients with histologically confirmed breast cancer, 78 (38.4%) healthy volunteers, and 54 (26.6%) patients with histologically confirmed gastric cancer. For the breast cancer cohort, 13 patients had DCIS, 31 patients had lymph node metastasis‐negative breast cancer that was not DCIS, and 27 patients had lymph node metastasis‐positive breast cancer that was not DCIS. The mean age and histological features of each group are summarized in Table 1. The mean ages of each group/subgroup were not significantly different (P > .05).
TABLE 1.
Demographic characteristics of the study subjects
| Category | Characteristics | Number | Age (years old), (mean ± SD) |
|---|---|---|---|
| Healthy controls | No breast disease | 78 | 51.0 ± 10.0 |
|
Breast cancer (N = 71) |
Ductal carcinoma in situ (DCIS) | 13 | 55.0 ± 9.1 |
| Breast cancer (lymph node negative) | 31 | 53.6 ± 8.6 | |
| Breast cancer (lymph node positive) | 27 | 54.4 ± 8.8 | |
| Gastric cancer | Gastric adenocarcinoma | 54 | 57.1 ± 8.5 |
3.1. Patients with DCIS breast cancer vs healthy controls
A total of 411 metabolites were consistently detected in the samples from the DCIS patients and healthy controls. A total of 13 differential metabolites were identified between the two groups (Table 2). In addition, the two‐dimensional PCA score plot displayed a good separation trend (Figure 1A), and the PLS‐DA score plot demonstrated separation between the DCIS patients and the healthy controls using eight components (R2X = 0.868; R2Y = 0.806; Q2 = 0.531; Figure 1B). Moreover, in the validation plot, the R2 and Q2 values were found to be less than the original values. All of the above parameters confirmed the validity of the supervised model with the 13 VOCs (Figure 1C).
TABLE 2.
Potential biomarkers
| Potential biomarker | RT | CAS Number | DCIS vs healthy controls |
Lymph node metastasis‐ negative breast cancer vs healthy controls |
Lymph node metastasis‐ positive breast cancer vs healthy controls |
Breast cancer vs gastric cancer |
|---|---|---|---|---|---|---|
| a (S)‐1,2‐Propanediol | 3.04 | 4254‐15‐3 | o | o | o | o |
| a Cyclopentanone | 3.93 | 120‐92‐3 | o | o | o | o |
| Methyl acrylic acid | 4.46 | 79‐41‐4 | o | o | o | |
| Cyclohexanone | 6.15 | 108‐94‐1 | o | o | o | |
| 2‐Butoxyethanol | 6.36 | 111‐76‐2 | o | o | ||
| a Ethylene carbonate | 7.58 | 96‐49‐1 | o | o | o | o |
| a 3‐Methoxy‐1,2‐propanediol | 7.66 | 623‐39‐2 | o | o | ||
| a 3‐Methylpyridine | 8.04 | 108‐99‐6 | o | o | ||
| a Phenol | 8.06 | 108‐95‐2 | o | o | o | o |
| 1,1,3,3‐Tetramethylurea | 8.34 | 632‐22‐4 | o | o | o | |
| 2‐Ethylhexanol | 9.27 | 104‐76‐7 | o | o | o | |
| 2,6‐Dimethyloctane | 10.05 | 2051‐30‐1 | o | o | ||
| 2‐Phenyl‐2‐propanol | 10.57 | 617‐94‐7 | o | o | o | |
| a Tetramethyl silicane | 11.64 | 75‐76‐3 | o | o | o | o |
| Cyclohexanol, 2‐(1‐methylethyl)‐ | 12.51 | 96‐07‐1 | o | o | ||
| Hexamethyldisilane | 15.1 | 1450‐14‐2 | o | o | ||
| Propane, 2‐methyl‐1,2‐bis (trimethylsiloxy)‐ | 18.23 | 99875‐05‐5 | o | o |
Abbreviations: CAS, chemical abstracts service; DCIS, ductal carcinoma in situ; RT, retention time.
Indicates the biomarkers that were differentially detected between cohorts or subcohorts. o indicates that the compound was identified as a potential marker.
FIGURE 1.
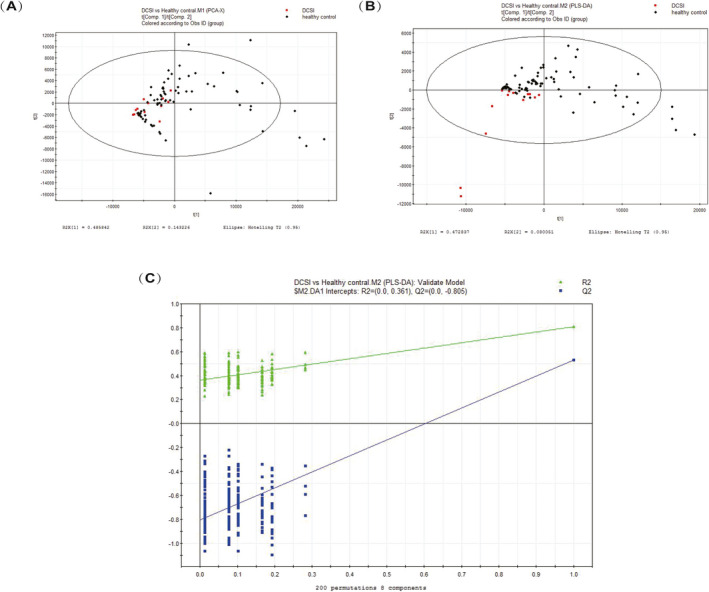
A, PCA score plot. B,: PLS‐DA score plot (eight components, R2X = 0.868; R2Y = 0.806; Q2 = 0.531). C, PLS‐DA validation plot intercepts: R2 = (0.0, 0.031); Q2 = (0.0, −0.805)
3.2. Patients with lymph node metastasis‐negative breast cancer vs healthy controls
A total of 411 metabolites were consistently detected in the samples from the lymph node metastasis‐negative breast cancer and the healthy controls. A total of 12 differential metabolites were identified between the two groups (Table 2). In addition, the two‐dimensional PCA score plot demonstrated a good separation trend (Figure 2A), and the PLS‐DA score plot demonstrated separation between the lymph node metastasis‐negative breast cancer patients and the healthy controls when using seven components (R2X = 0.84; R2Y = 0.771; Q2 = 0.425; Figure 2B). Moreover, in the validation plot, the R2 and Q2 values were found to be less than the original values. All of the above parameters confirmed the validity of the supervised model with the 12 VOCs (Figure 2C).
FIGURE 2.
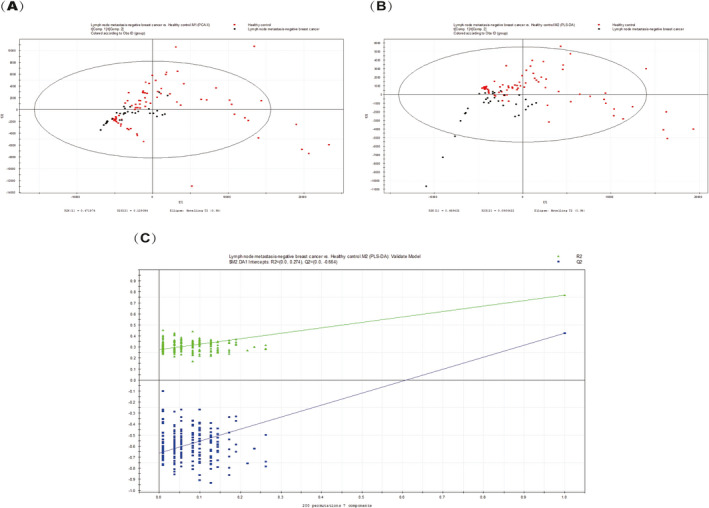
A, PCA score plot. B, PLS‐DA score plot (seven components, R2X = 0.84; R2Y = 0.771; Q2 = 0.425). C, PLS‐DA validation plot intercepts: R2 = (0.0, 0.274); Q2 = (0.0, −0.664)
3.3. Patients with lymph node metastasis‐positive breast cancer vs healthy controls
A total of 411 metabolites were consistently detected in the samples from the lymph node metastasis‐positive breast cancer patients and the healthy controls. A total of 17 differential metabolites were identified between the two groups (Table 2). In addition, the two‐dimensional PCA score plot demonstrated a trend for good separation (Figure 3A), and the PLS‐DA score plot showed separation between the lymph node metastasis‐positive breast cancer patients and the healthy controls using seven components (R2X = 0.841; R2Y = 0.761; Q2 = 0.555; Figure 3B). Moreover, in the validation plot, the R2 and Q2 values were found to be less than the original values. All of the above parameters confirmed the validity of the supervised model with the 17 VOCs (Figure 3C).
FIGURE 3.
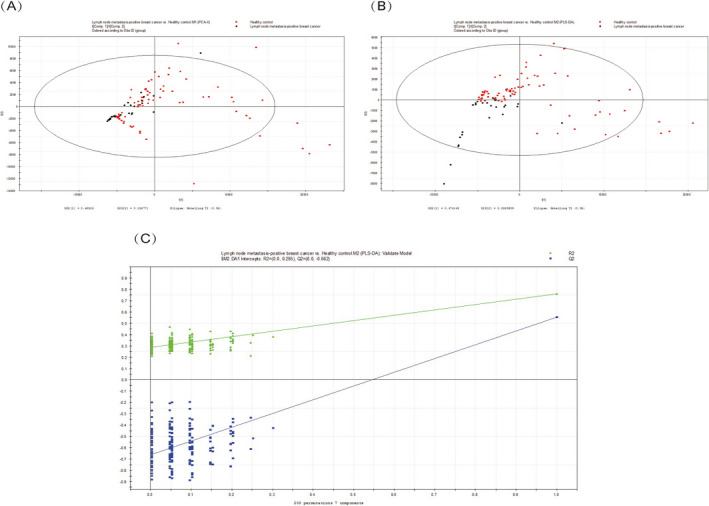
A, PCA score plot. B, PLS‐DA score plot (seven components, R2X = 0.841; R2Y = 0.761; Q2 = 0.555). C, PLS‐DA validation plot intercepts: R2 = (0.0, 0.275); Q2 = (0.0, −0.653)
3.4. Patients with breast cancer vs patients with gastric cancer
A total of 237 metabolites were consistently detected in the breast cancer and gastric cancer samples. A total of 17 differential metabolites were identified between the two groups, seven of which overlapped with the differential metabolites between the breast cancer patients and the healthy controls (Table 2). In addition, the two‐dimensional PCA score plot demonstrated a trend for good separation (Figure 4A), and the PLS‐DA score plot showed separation between the lymph node metastasis‐positive breast cancer patients and the gastric cancer patients using two components (R2X = 0.58; R2Y = 0.664; Q2 = 0.608; Figure 4B). Moreover, in the validation plot, the R2 and Q2 values were found to be less than the original values. All of the above parameters confirmed the validity of the supervised model with the 17 VOCs (Figure 4C).
FIGURE 4.
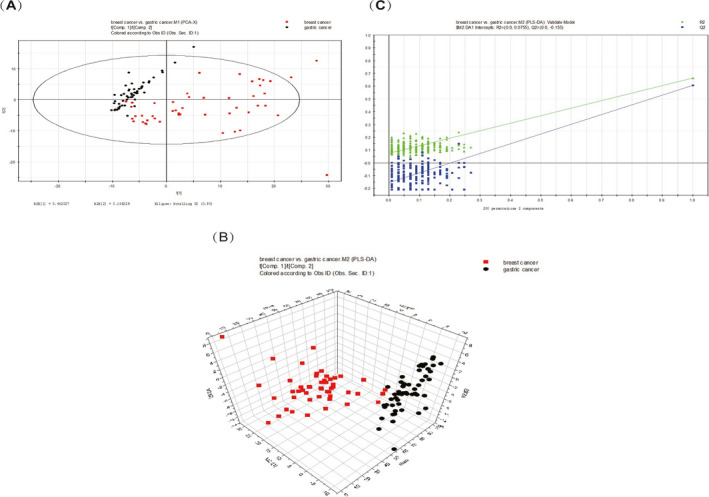
A, PCA score plot. B, PLS‐DA score 3D plot (two components, R2X = 0.58; R2Y = 0.664; Q2 = 0.608). C, PLS‐DA validation plot intercepts: R2 = (0.0, 0.0775); Q2 = (0.0, −0.155)
3.5. Combined biomarkers
We used the differential metabolites that overlapped between breast cancer vs gastric cancer and breast cancer vs healthy controls as potential breast cancer markers. An overlapping set of seven VOCs was significantly different between patients with breast cancer vs healthy people and patients with breast cancer vs patients with gastric cancer. The seven VOCs were (S)‐1,2‐propanediol, cyclopentanone, ethylene carbonate, 3‐methoxy‐1,2‐propanediol, 3‐methylpyridine, phenol, and tetramethylsilane. The combination of these seven potential biomarkers was used to analyze the sensitivity and specificity of their detection of the various breast cancer groups. The results are shown in Table 3 and Figure 5.
TABLE 3.
Area under the ROC curve values, sensitivities, and specificities in four different comparisons
| DCIS vs healthy controls | Breast cancer (Lymph node negative) vs healthy controls | Breast cancer (Lymph node positive) vs healthy controls | Breast cancer vs healthy controls | |
|---|---|---|---|---|
| AUC | 0.9380 | 0.9430 | 0.8640 | 0.9190 |
| Sensitivity | 0.8077 | 0.8205 | 0.9615 | 0.9359 |
| Specificity | 1.0000 | 0.9032 | 0.6296 | 0.7162 |
Abbreviation: AUC, Area under the ROC curve.
FIGURE 5.
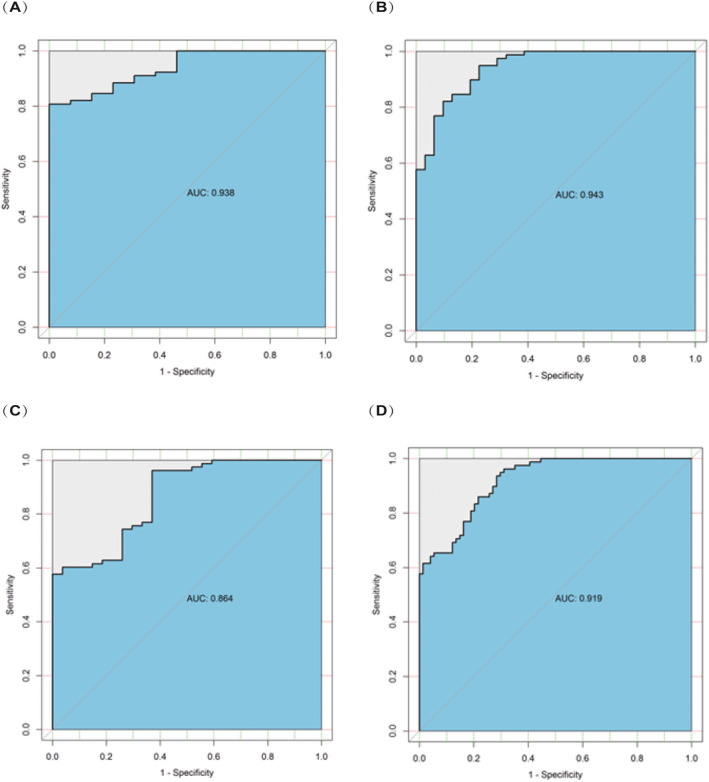
A, The ROC curve of healthy controls vs DCIS patients obtained by using the combination of seven biomarkers. B, The ROC curve of healthy controls vs lymph node‐negative breast cancer patients obtained by using the combination of seven biomarkers. C, The ROC curve of healthy controls vs lymph node‐positive breast cancer patients obtained by using the combination of seven biomarkers. D, The ROC curve of healthy controls vs breast cancer patients obtained by using the combination of seven biomarkers
4. DISCUSSION
Breast cancer is a global problem, and breast cancer screening is believed to reduce breast cancer mortality by 19% (range: 12%‐26%). 35 The currently available techniques (eg, mammography and MRI) for breast cancer screening cannot always reliably distinguish between cancer patients and healthy subjects, especially those with early breast cancer.
The analysis of VOCs is a new frontier for cancer diagnosis because it is noninvasive and uses potentially inexpensive methods; thus, it has attracted increasing attention by researchers. In particular, several studies have confirmed that some specific VOCs are present in abnormal concentrations in the exhalations of patients with breast cancer, and the origin of these compounds has been analyzed. 21 , 24 , 25 , 28 , 29 , 36 , 37 , 38 The principle behind the analysis of VOCs is based on cell biology. Gene and/or protein changes due to tumor growth may lead to peroxidation of the cell membrane species, which emit VOCs. 39 These VOCs can be detected either through exhaled breath 40 , 41 , 42 or directly from the headspace of cancer cells, 43 as cancer‐related changes in the blood chemistry lead to measurable changes in breathing by exchange through the lung. 42
To date, there is no uniform standard for candidate tumor markers in exhaled VOCs from breast cancer patients. Phillips et al have reported that methylated alkane derivatives and alkanes could be utilized as specific VOCs for breast cancer. 44 , 45 In 2010, Peng et al demonstrated that significantly different levels of five volatile compounds, including 3,3‐dimethyl pentane, 2‐amino‐5‐isopropyl‐8‐methyl‐1‐azulenecarbonitrile, 2,3,4‐trimethyl decane, 5‐(2‐methylpropyl)nonane, and 6‐ethyl‐3‐octyl ester 2‐trifluoromethyl benzoic acid in the exhaled breaths between breast cancer patients and healthy controls In addition, as reported by Kneepkens et al 46 , pentane concentrations in exhaled breath samples from breast cancer patients were found significantly increased. The changes in lipid and amino acid metabolism in cells are most likely the main reasons for the modifications of VOCs. 47 However, these previous studies did not supply any evidence of VOC changes according to different stages of breast cancer. In our research, we categorized the patients with breast cancer into three groups: DCIS cohort, lymph node metastasis‐negative cohort, and lymph node metastasis‐positive cohort. Each of these three cohorts was compared with healthy controls, respectively, and potential biomarkers were successfully isolated. A total of 17 differential metabolites were identified in the comparison of healthy controls and DCIS. Most of the VOCs identified in this study were alkanes, ketones, aldehydes, alcohols, or olefins. Furthermore, with cancer progression, the number of differential volatile markers gradually increased. Among them, methyl acrylic acid is a physiological substrate of the valine pathway and is metabolized to carbon dioxide by two substrates of the citric acid cycle: methylmalonate and succinyl‐CoA. Moreover, amino acids are one type of common metabolic marker in breast cancer metabolism analysis. 48 , 49 The metabolism of amino acids also is affected by oxidative stress, which may lead to a change of VOCs. 47 Consistent with our results, Lavra et al 50 have reported that 2‐ethyl hexanol can be considered as a biomarker for breast cancer growth and malignancy. However, this marker was not statistically different between the three‐breast cancer sub‐cohorts analyzed in this study.
The overexpression of human epidermal growth factor receptor 2 (HER2, c‐erbB2) is found in up to 30% of breast cancers; in addition, compared to patients with HER2‐negative breast cancer, patients with HER2‐positive breast cancer were associated with significantly worse outcomes. 51 Many studies have demonstrated that HER2 is also present in other cancers, particularly in gastric cancer. 52 , 53 , 54 , 55 Trastuzumab is a critical drug for the treatment of HER2‐positive breast cancer that also has been shown to be useful for the treatment of HER2‐positive gastric cancer 56 ; it is approved by the European Union, the United States, and China for the treatment of gastric cancer. Currently, there is no report comparing the exhaled metabolomics of these two types of cancer. We compared breast cancer and gastric cancer in the present study and found that the PCA scores were higher in the gastric cancer patients than in the breast cancer patients and healthy controls. A total of 14 differential metabolites were isolated, of which seven differential metabolites overlapped between the control groups, thus narrowing our search for breast cancer biomarkers. The combination of these seven markers can predict breast cancer, with a sensitivity of 93.59% and a specificity of 71.62%; meanwhile, the sensitivity and specificity for DCIS are 80.77% and 100%, respectively. Currently, mammography is the most popular method used for breast cancer detection, which may cause radiation‐induced mutations to participants due to the use of X‐rays. The sensitivity range of mammography is 71%‐96% for detecting breast cancer. Furthermore, patients with dense breast tissue have even lower mammographic sensitivities, from 48% to 70%. 8 The sensitivity of ultrasonography is higher than that of mammography at detecting lesions in women with dense breast tissue. 57 , 58 However, ultrasonography cannot detect most microcalcifications, which are the typical findings in DCIS. It has been reported that about 25% of cancers missed by ultrasonography were invasive carcinomas, while 75% were DCIS. 59 Besides, the results of ultrasonography can vary widely due to the diverse expertise of the technicians. 13 , 60
GC‐MS applied in our research is currently considered the gold standard technique in breath analysis, and widely used for separation and identification of unknown substances present in gaseous samples. GC‐MS can analyze multiple compounds simultaneously and its detection limit is low. But there are still some limitations, the instrument is expensive, bulky, and time‐consuming; therefore, it is not suitable for on‐line monitoring and can lead to sample contamination and loss. However, up to now, there is still a lack of compound‐specific devices for breath analysis, high‐performance equipment, such as GC‐MS, will remain indispensable to expand our basic knowledge and to search biomarkers.
5. CONCLUSION
Applying HS‐GCMS‐SPME detection, the combination of (S)‐1,2‐propanediol, cyclopentanone, ethylene carbonate, 3‐methoxy‐1,2‐propanediol, 3‐methylpyridine, phenol, and tetramethylsilane can be regarded as a specific expiratory marker for DCIS, which will help to establish new screening methods for early breast cancer. However, the number of patients in this study is relatively small, further studies in larger populations are required in order to confirm these findings.
Zhang Y, Guo L, Qiu Z, Lv Y, Chen G, Li E. Early diagnosis of breast cancer from exhaled breath by gas chromatography‐mass spectrometry (GC/MS) analysis: A prospective cohort study. J Clin Lab Anal. 2020;34:e23526 10.1002/jcla.23526
REFERENCES
- 1. Ferlay J, Soerjomataram I, Ervik M, et al. Cancer incidence and mortality worldwide: IARC CancerBase No. 11 Lyon. France: International Agency for Research on Cancer; 2013. [Google Scholar]
- 2. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394‐424. [DOI] [PubMed] [Google Scholar]
- 3. Segal R, Miller K, Jemal A. Cancer statistics, 2018. Ca Cancer J Clin. 2018;68(1):7‐30. [DOI] [PubMed] [Google Scholar]
- 4. Tabar L, Vitak B, Chen HH, et al. The Swedish Two‐County trial twenty years later. Updated mortality results and new insights from long‐term follow‐up. Radiol Clin North Am. 2000;38(4):625‐651. [DOI] [PubMed] [Google Scholar]
- 5. Engel J, Eckel R, Aydemir U, et al. Determinants and prognoses of locoregional and distant progression in breast cancer. Int J Radiat Oncol Biol Phys. 2003;55(5):1186‐1195. [DOI] [PubMed] [Google Scholar]
- 6. Schmidt C. Can some DCIS patients avoid adjuvant therapy? Still unknown. J Natl Cancer Inst. 2011;103(7):530‐531. [DOI] [PubMed] [Google Scholar]
- 7. Nystrom L, Rutqvist LE, Wall S, et al. Breast cancer screening with mammography: overview of Swedish randomised trials. Lancet. 1993;341(8851):973‐978. [DOI] [PubMed] [Google Scholar]
- 8. Humphrey LL, Helfand M, Chan BK, Woolf SH. Breast cancer screening: a summary of the evidence for the U.S. Preventive services task force. Ann Intern Med. 2002;137(5_Part_1):347‐360. [DOI] [PubMed] [Google Scholar]
- 9. Johnson MM. Full‐field digital mammography and digital breast tomosynthesis. Radiol Technol. 2017;88(3):299m‐319m. [PubMed] [Google Scholar]
- 10. Siu AL. Screening for breast cancer: U.S. preventive services task force recommendation statement. Ann Intern Med. 2016;164(4):279‐296. [DOI] [PubMed] [Google Scholar]
- 11. Mootz AR, Madhuranthakam AJ, Dogan B. Changing paradigms in breast cancer screening: abbreviated breast MRI. Eur J Breast Health. 2019;15(1):1‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Brem RF, Lenihan MJ, Lieberman J, Torrente J. Screening breast ultrasound: past, present, and future. AJR Am J Roentgenol. 2015;204(2):234‐240. [DOI] [PubMed] [Google Scholar]
- 13. Blair S, McElroy M, Middleton MS, et al. The efficacy of breast MRI in predicting breast conservation therapy. J Surg Oncol. 2006;94(3):220‐225. [DOI] [PubMed] [Google Scholar]
- 14. Kim MY, Kim HS, Choi N, Yang JH, Yoo YB, Park KS. Screening mammography‐detected ductal carcinoma in situ: mammographic features based on breast cancer subtypes. Clin Imaging. 2015;39(6):983‐986. [DOI] [PubMed] [Google Scholar]
- 15. Rauch GM, Kuerer HM, Scoggins ME, et al. Clinicopathologic, mammographic, and sonographic features in 1187 patients with pure ductal carcinoma in situ of the breast by estrogen receptor status. Breast Cancer Res Treat. 2013;139(3):639‐647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pang JM, Gorringe KL, Fox SB. Ductal carcinoma in situ ‐ update on risk assessment and management. Histopathology. 2016;68(1):96‐109. [DOI] [PubMed] [Google Scholar]
- 17. Berry DA, Cronin K, Plevritis S, et al. Effect of screening and adjuvant therapy on mortality from breast cancer. N Engl J Med. 2005;353(17):1784‐1792. [DOI] [PubMed] [Google Scholar]
- 18. Sanders ME, Schuyler PA, Dupont WD, Page DL. The natural history of low‐grade ductal carcinoma in situ of the breast in women treated by biopsy only revealed over 30 years of long‐term follow‐up. Cancer. 2005;103(12):2481‐2484. [DOI] [PubMed] [Google Scholar]
- 19. Leonard GD, Swain SM. Ductal carcinoma in situ, complexities and challenges. J Natl Cancer Inst. 2004;96(12):906‐920. [DOI] [PubMed] [Google Scholar]
- 20. Song G, Qin T, Liu H, et al. Quantitative breath analysis of volatile organic compounds of lung cancer patients. Lung Cancer. 2010;67(2):227‐231. [DOI] [PubMed] [Google Scholar]
- 21. Peng G, Hakim M, Broza YY, et al. Detection of lung, breast, colorectal, and prostate cancers from exhaled breath using a single array of nanosensors. Br J Cancer. 2010;103(4):542‐551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Poli D, Goldoni M, Corradi M, et al. Determination of aldehydes in exhaled breath of patients with lung cancer by means of on‐fiber‐derivatisation SPME‐GC/MS. J Chromatogr B Analyt Technol Biomed Life Sci. 2010;878(27):2643‐2651. [DOI] [PubMed] [Google Scholar]
- 23. Phillips M, Cataneo RN, Saunders C, Hope P, Schmitt P, Wai J. Volatile biomarkers in the breath of women with breast cancer. J Breath Res. 2010;4(2):026003. [DOI] [PubMed] [Google Scholar]
- 24. Patterson SG, Bayer CW, Hendry RJ, et al. Breath analysis by mass spectrometry: a new tool for breast cancer detection? Am Surg. 2011;77(6):747‐751. [PubMed] [Google Scholar]
- 25. Shuster G, Gallimidi Z, Reiss AH, et al. Classification of breast cancer precursors through exhaled breath. Breast Cancer Res Treat. 2011;126(3):791‐796. [DOI] [PubMed] [Google Scholar]
- 26. Queralto N, Berliner AN, Goldsmith B, Martino R, Rhodes P, Lim SH. Detecting cancer by breath volatile organic compound analysis: a review of array‐based sensors. J Breath Res. 2014;8(2):027112. [DOI] [PubMed] [Google Scholar]
- 27. Sun X, Shao K, Wang T. Detection of volatile organic compounds (VOCs) from exhaled breath as noninvasive methods for cancer diagnosis. Anal Bioanal Chem. 2016;408(11):2759‐2780. [DOI] [PubMed] [Google Scholar]
- 28. Mangler M, Freitag C, Lanowska M, Staeck O, Schneider A, Speiser D. Volatile organic compounds (VOCs) in exhaled breath of patients with breast cancer in a clinical setting. Ginekol Pol. 2012;83(10):730‐736. [PubMed] [Google Scholar]
- 29. Wang C, Sun B, Guo L, et al. Volatile organic metabolites identify patients with breast cancer, cyclomastopathy, and mammary gland fibroma. Sci Rep. 2014;4:5383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Doyle DJ, Garmon EH. American Society of Anesthesiologists classification (ASA class). StatPearls [Internet]. edn. Treasure Island: StatPearls Publishing; 2019. [PubMed] [Google Scholar]
- 31. Birken T, Schubert J, Miekisch W, Noldge‐Schomburg G. A novel visually CO2 controlled alveolar breath sampling technique. Technol Health Care. 2006;14(6):499‐506. [PubMed] [Google Scholar]
- 32. Miekisch W, Kischkel S, Sawacki A, Liebau T, Mieth M, Schubert JK. Impact of sampling procedures on the results of breath analysis. J Breath Res. 2008;2(2):026007. [DOI] [PubMed] [Google Scholar]
- 33. Bajtarevic A, Ager C, Pienz M, et al. Noninvasive detection of lung cancer by analysis of exhaled breath. BMC Cancer. 2009;9:348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. AB U. SIMCA‐P and SIMCA‐P+ 11 user guide and tutorial Umetrics AB: Umeå. 2005. [Google Scholar]
- 35. Glasziou P, Houssami N. The evidence base for breast cancer screening. Prev Med. 2011;53(3):100‐102. [DOI] [PubMed] [Google Scholar]
- 36. Stolarek RA, Potargowicz E, Seklewska E, et al. Increased H2O2 level in exhaled breath condensate in primary breast cancer patients. J Cancer Res Clin Oncol. 2010;136(6):923‐930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Xu Y, Lee H, Hu Y, Huang J, Kim S, Yun M. Detection and identification of breast cancer volatile organic compounds biomarkers using highly‐sensitive single nanowire array on a chip. J Biomed Nanotechnol. 2013;9(7):1164‐1172. [DOI] [PubMed] [Google Scholar]
- 38. Phillips M, Beatty JD, Cataneo RN, et al. Rapid point‐of‐care breath test for biomarkers of breast cancer and abnormal mammograms. PLoS One. 2014;9(3):e90226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Singer SJ, Nicolson GL. The fluid mosaic model of the structure of cell membranes. Science. 1972;175(4023):720‐731. [DOI] [PubMed] [Google Scholar]
- 40. Amann A, Spanel P, Smith D. Breath analysis: the approach towards clinical applications. Mini Rev Med Chem. 2007;7(2):115‐129. [DOI] [PubMed] [Google Scholar]
- 41. Ligor T, Ligor M, Amann A, et al. The analysis of healthy volunteers' exhaled breath by the use of solid‐phase microextraction and GC‐MS. J Breath Res. 2008;2(4):046006. [DOI] [PubMed] [Google Scholar]
- 42. Horvath I, Lazar Z, Gyulai N, Kollai M, Losonczy G. Exhaled biomarkers in lung cancer. Eur Respir J. 2009;34(1):261‐275. [DOI] [PubMed] [Google Scholar]
- 43. Filipiak W, Sponring A, Filipiak A, et al. TD‐GC‐MS analysis of volatile metabolites of human lung cancer and normal cells in vitro. Cancer Epidemiol Biomarkers Prev. 2010;19(1):182‐195. [DOI] [PubMed] [Google Scholar]
- 44. Phillips M, Cataneo RN, Ditkoff BA, et al. Volatile markers of breast cancer in the breath. Breast J. 2003;9(3):184‐191. [DOI] [PubMed] [Google Scholar]
- 45. Phillips M, Cataneo RN, Ditkoff BA, et al. Prediction of breast cancer using volatile biomarkers in the breath. Breast Cancer Res Treat. 2006;99(1):19‐21. [DOI] [PubMed] [Google Scholar]
- 46. Kneepkens C, Ferreira C, Lepage G, Roy C. The hydrocarbon breath test in the study of lipid peroxidation: principles and practice. Clin Invest Med. 1992;15(2):163‐186. [PubMed] [Google Scholar]
- 47. Liu Y, Li W, Duan Y. Effect of H2O2 induced oxidative stress (OS) on volatile organic compounds (VOCs) and intracellular metabolism in MCF‐7 breast cancer cells. J Breath Res. 2019;13(3):036005. [DOI] [PubMed] [Google Scholar]
- 48. Willmann L, Schlimpert M, Halbach S, Erbes T, Stickeler E, Kammerer B. Metabolic profiling of breast cancer: Differences in central metabolism between subtypes of breast cancer cell lines. J Chromatogr B. 2015;1000:95‐104. [DOI] [PubMed] [Google Scholar]
- 49. Shen J, Yan L, Liu S, Ambrosone CB, Zhao H. Plasma metabolomic profiles in breast cancer patients and healthy controls: by race and tumor receptor subtypes. Transl Oncol. 2013;6(6):757‐765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lavra L, Catini A, Ulivieri A, et al. Investigation of VOCs associated with different characteristics of breast cancer cells. Sci Rep. 2015;5:13246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER‐2/neu oncogene. Science. 1987;235(4785):177‐182. [DOI] [PubMed] [Google Scholar]
- 52. Sbitti Y, Essaidi I, Debbagh A, et al. Is there any advantage to combined trastuzumab and chemotherapy in perioperative setting her 2neu positive localized gastric adenocarcinoma? World J Surg Oncol. 2011;9:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wang J, Saukel GW, Garberoglio CA, Srikureja W, Hsueh CT. Pathological complete response after neoadjuvant chemotherapy with trastuzumab‐containing regimen in gastric cancer: a case report. J Hematol Oncol. 2010;3:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Keller R, Krause K. Explorative phase II study of perioperative treatment in patients with adenocarcinoma of the gastroesophageal junction or stomach (HerFLOT)(NCT01472029). In. 2012.
- 55. Abali H, Yalcin S, Onal HC, et al. A study of the combination of oxaliplatin, capecitabine, and herceptin (trastuzumab) and chemoradiotherapy in the adjuvant setting in operated patients with HER2+ gastric or gastro‐esophageal junction cancer (TOXAG study). Am Soc Clin Oncol. 2016;34(4_suppl):TPS182. [DOI] [PubMed] [Google Scholar]
- 56. Bang Y‐J, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2‐positive advanced gastric or gastro‐oesophageal junction cancer (ToGA): a phase 3, open‐label, randomised controlled trial. Lancet. 2010;376(9742):687‐697. [DOI] [PubMed] [Google Scholar]
- 57. Zonderland HM, Coerkamp EG, Hermans J, van de Vijver MJ, van Voorthuisen AE. Diagnosis of breast cancer: contribution of US as an adjunct to mammography. Radiology. 1999;213(2):413‐422. [DOI] [PubMed] [Google Scholar]
- 58. Skaane P. The additional value of US to mammography in the diagnosis of breast cancer. A prospective study. Acta Radiol. 1999;40(5):486‐490. [DOI] [PubMed] [Google Scholar]
- 59. Nemec CF, Listinsky J, Rim A. How should we screen for breast cancer? Mammography, ultrasonography, MRI. Cleve Clin J Med. 2007;74(12):897‐904. [DOI] [PubMed] [Google Scholar]
- 60. Akazawa K, Tamaki Y, Taguchi T, et al. Preoperative evaluation of residual tumor extent by three‐dimensional magnetic resonance imaging in breast cancer patients treated with neoadjuvant chemotherapy. Breast J. 2006;12(2):130‐137. [DOI] [PubMed] [Google Scholar]


