Abstract
Polycyclic aromatic hydrocarbons (PAHs) are byproducts of incomplete combustion reactions and are ubiquitous in the environment, leading to widespread human exposure via inhalation and ingestion pathways. PAHs have been implicated as endocrine disrupting compounds in previous animal and in vitro studies, but human studies are currently lacking. Pregnant women and their developing fetuses are particularly susceptible populations to environmental contaminants, in part because alterations in hormone physiology during gestation can have adverse consequences on the health of the pregnancy. We utilized data on 659 pregnant women from the PROTECT longitudinal birth cohort in Puerto Rico to assess associations between repeated measures of 8 urinary hydroxylated PAH (OH-PAH) metabolites and 9 serum hormones during gestation. Urine samples were collected at 3 study visits (median gestational ages of 18, 22, and 26 weeks at each visit, respectively) and serum samples were collected at the first and third study visits. Linear mixed effects models were used to ascertain longitudinal associations between OH-PAHs and hormones, and sensitivity analyses were employed to assess potential nonlinearity and differences in associations on the basis of fetal sex and timing of biomarker measurement. Among the multiple positive associations we observed between OH-PAHs and CRH, estriol, progesterone, T3, and the ratio of T3 to T4, and inverse associations with testosterone, the most notable are a 24.3% increase (95% CI: 13.0, 36.7) in CRH with an interquartile range (IQR) increase in 1-hydroxyphenanthrene and a 17.2% decrease (95% CI: 8.13, 25.4) in testosterone with an IQR increase in 1-hydroxynapthalene. Many associations observed were dependent on fetal sex, and some relationships showed evidence of nonlinearity. These findings demonstrate the importance of studying PAH exposures during pregnancy and highlight the potential complexity of their impacts on the physiology of human pregnancy.
Keywords: Polycyclic aromatic hydrocarbon, endocrine disruption, progesterone, corticotropin releasing hormone, thyroid hormone, birth cohort
Graphical Abstract
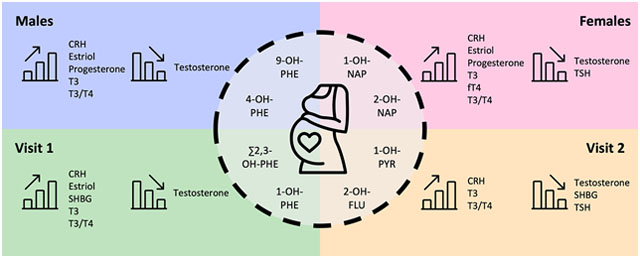
Introduction
Polycyclic aromatic hydrocarbons (PAHs) are natural byproducts of incomplete combustion reactions. Human exposure sources are diverse and include tobacco smoke, automobile exhaust, charred meats, asphalt particles, refinery and power plant emissions, generator use, forest fires, and volcanic eruptions (Srogi, 2007). Hydroxylated metabolites of PAHs (OH-PAHs) have been shown to be reliable biomarkers of PAH exposure (Strickland et al., 1996) and can be widely detected in humans around the globe (Cathey et al., 2018; Perera et al., 2005; Urbancova et al., 2016). Furthermore, consumption of chargrilled meats and airborne exposures have been shown to be predictive of urinary biomarkers of PAHs (Alghamdi et al., 2015).
Previous research has demonstrated that biomarkers of PAH exposure are associated with various health endpoints including asthma (Al-Daghri et al., 2013), breast cancer (Mordukhovich et al., 2016), cardiovascular disease (Brucker et al., 2014; Wang et al., 2016), and reduced sperm quality (Radwan et al., 2015; Sun et al., 2011). PAH exposure during pregnancy can also adversely affect the developing fetus. Maternal exposure of mice to PAHs damages the DNA and induces premature cell death of oocytes in the offspring (Jurisicova et al., 2007). In zebrafish, embryonic exposure to the PAH benzo(a)pyrene (BaP) impaired various reproductive endpoints (Gao et al., 2018). Humans exposed to PAHs in utero are also at risk for lower IQ in childhood (Vishnevetsky et al., 2015), low birth length (Yang et al., 2018), and attention deficit hyperactivity disorder (Perera et al., 2014). Increased concentrations of biomarkers of inflammation and oxidative stress have previously been observed among pregnant women in relation to PAH exposure (Ferguson et al., 2018, 2017, 2015), which may have adverse downstream effects on the pregnancy.
Significant associations between PAHs and endocrine disruption have been observed among non-pregnant populations including Iranian adolescents (Kelishadi et al., 2018), American men and women in NHANES (Jain, 2016; Wang et al., 2017), and Chinese men (Yang et al., 2017; Zhu et al., 2009). These studies provided evidence for changes in various hormone concentrations including thyroid stimulating hormone (TSH), free thyroxine (fT4), total triiodothyronine (T3), testosterone, estradiol, and sex hormone binding globulin (SHBG) with exposure to PAHs. However, each of these studies was limited by either a small sample size or a cross-sectional study design. While very little work has been done to investigate the endocrine disrupting potential of PAH exposure among pregnant women, previous in vitro studies have shown that PAHs alter secretion of progesterone and estradiol from placental and granulosa cell lines (Drwal et al., 2017; Zajda and Gregoraszczuk, 2020). Regulation of various hormone concentrations is essential for proper maintenance and progression of a healthy pregnancy, as well as fetal growth and development, and disruption of these hormones may contribute to adverse birth outcomes such as preterm birth, preeclampsia, gestational diabetes, low birth weight, altered reproductive tract or neurodevelopment, and potential for later developmental disorders.
The goal of this analysis was to investigate the endocrine disrupting potential of PAHs during pregnancy in a prospective human pregnancy cohort study. To achieve this, we utilized repeated biomarker measures over two time points during mid-gestation to assess associations between urinary low molecular weight PAH metabolites and serum hormones. Previous work has suggested that PAH exposure measured during each trimester may have differential effects on fetal outcomes (Choi et al., 2012), and that changes in hormone concentrations through pregnancy have varying effects on birth outcomes between male and female fetuses (Cathey et al, submitted). Based on these previous findings, we also aimed to investigate the effects of gestational age and fetal sex on the associations between OH-PAHs and hormone concentrations.
Methods
Study Population
Data for the present study were obtained from the Puerto Rico Testsite for Exploring Contamination Threats (PROTECT) prospective birth cohort. Pregnant women were recruited between 2012 and 2018 from seven hospitals and prenatal clinics in the northern karst region of Puerto Rico. Women were eligible to participate in the study if they participated in their first clinic visit before 20 weeks’ gestation, were free of obstetric or other preexisting medical conditions, had not used oral contraceptives within three months of getting pregnant, had not used in vitro fertilization to get pregnant, and were between the ages of 18 and 40. A total of 659 women provided urine samples for OH-PAH analysis at up to three time points during gestation (16–20 weeks, 20–24 weeks, and 24–28 weeks), and blood samples for hormone analysis at up to two of the time points (16–20 weeks and 24–28 weeks). Self-reported health information and demographics were provided at the first study visit. All women provided informed consent and all methods were approved by the research and ethics committees of the University of Michigan School of Public Health, University of Puerto Rico, Northeastern University, and participating hospitals and clinics.
PAH Exposure Assessment
Urine samples were collected for OH-PAH analysis at all three study visits. Liquid chromatography-mass spectrometry coupled with online solid phase extraction, which has been previously described (Cathey et al., 2018; Onyemauwa et al., 2009), was utilized to measure 8 hydroxylated PAH metabolites: 1-hydroxynapthalene (1-OH-NAP), 2-hydroxynapthalene (2-OH-NAP), 2-hydroxyfluorene (2-OH-FLU), 1-hydroxyphenanthrene (1-OH-PHE), the sum of 2-hydroxyphenanthrene and 3- hydroxyphenanthrene (Σ2,3-OH-PHE), 4-hydroxyphenanthrene (4-OH-PHE), 9-hydroxyphenanthrene (9-OH-PHE), and 1-hydroxypyrene (1-OH-PYR). Urine samples were analyzed at NSF International in Ann Arbor, Michigan. All OH-PAH concentrations detected below the limit of detection (LOD) were replaced by the LOD divided by the square root of two.
Hormone Measurements
Serum samples were collected at the first and third study visits for hormone analysis. All serum samples collected were analyzed at the Central Ligand Assay Satellite Services (CLASS) laboratory in the Department of Epidemiology at the University of Michigan School of Public Health. Progesterone, SHBG, testosterone, T3, total thyroxine (T4), fT4 and TSH were measured using a chemiluminescence immunoassay. Estriol (E3) and corticotropin releasing hormone (CRH) were measured using an enzyme immunoassay. Some hormone concentrations were not available for all participants due to volume limitations. The ratios of progesterone to estriol (Prog/E3) and T3 to T4 (T3/T4) were assessed in addition to measured hormones because previous research indicated their relevance to adverse birth outcomes (Dietrich et al., 2012; Romero et al., 1988; Ruiz et al., 2008). All hormone concentrations detected below the LOD were replaced by the LOD divided by the square root of two.
Statistical Analyses
Demographics and other relevant heath characteristics of the study population including age, education level, marital status, employment status, annual household income, alcohol consumption, tobacco use and exposure to environmental tobacco smoke, number of previous live births, pre-pregnancy BMI, and fetal sex were tabulated. Distributions of pregnancy average and visit-specific PAH metabolite and hormone concentrations were calculated. Visit-specific concentrations were assessed for temporal variability by calculating intraclass correlation coefficients (ICCs), which assess between- and within-individual variability of OH-PAH concentrations across study visits. Spearman correlations were calculated between pregnancy average OH-PAH concentrations.
All demographic and health characteristics were considered for inclusion in final statistical models. Criteria for covariate selection included associations with exposure and outcome measures and impacting the main effect estimate by at least 10% when included in a linear model (in addition to specific gravity to account for differences in urinary dilution). Final models were adjusted for continuous maternal age, categorical maternal education level, categorical maternal exposure to environmental tobacco smoke (ETS), and specific gravity, resulting in a final sample size of 659 women (sample size is slightly less for some hormones due to sample volume limitations).
Linear mixed effects (LME) models with random intercepts, which are useful for correlated and unbalanced outcome data, were used to test for associations between repeated measures of urinary PAH metabolites (visits 1 and 3 only) and serum hormone concentrations. All PAH metabolites (excluding 4-OH-PHE and 9-OH-PHE due to low detection rates) were initially assessed as continuous exposure variables for associations with hormone concentrations. As a sensitivity analysis, indicator variables for fetal sex and for study visit were then used (not simultaneously) in interaction terms with exposure variables to test for differences in exposure-outcome associations by fetal sex or study visit. OH-PAH concentrations were subsequently modeled as quartiles to explore possibilities of nonlinear associations with hormone concentrations. Because fewer than 70% of samples had detectable concentrations of 4-OH-PHE and 9-OH-PHE, these metabolites were modeled as categorical variables with 3 levels, the lowest level corresponding to concentrations measured below the LOD and the higher two levels split evenly among the detected samples. Statistical significance was set to alpha=0.05, and all analyses were run using R version 3.5.1.
Results
Demographic and other relevant health information of the study population is shown in Table 1. The majority of women were under 30 years old, had attained at least some college education, were employed, lived in a home earning less than $30k per year, were married, had never smoked or been exposed to ETS, did not drink alcohol during their pregnancy, had given birth to less than 2 previous children, and had a BMI under 26 before becoming pregnant. Distributions of exposure and outcome biomarkers averaged across visits are shown in Table 2 (visit-specific distributions are shown in Supplementary Table 1). With adjustment for specific gravity, OH-PAH ICCs were the lowest for 4-OH-PHE (0.187) and the highest for 2-OH-NAP (0.524). Concentrations of E3 and progesterone demonstrated significant within-individual compared to between-individual variability over the study period (ICCs −0.233 and 0.088, respectively), and both hormones were much higher later in pregnancy when compared to the first visit. In contrast, other hormone concentrations had lower within-person variability compared to between-person variability across study visits (ICCs range: 0.538–0.766). Only some PAH metabolites were highly correlated with one another (R > 0.50), and metabolites from the same parent compound did not necessarily correlate (Supplementary Table 2). For example 1-OH-PHE and Σ2,3-OH-PHE were highly correlated (R=0.822), but 1-OH-NAP and 2-OH-NAP were not correlated (R=0.049). Phenanthrene metabolites were also highly correlated with 1-OH-PYR and 2-OH-FLU (R=0.539–0.780).
Table 1:
Demographics and other health information for 659 women in PROTECT.
| N (%) | |
|---|---|
| Maternal Age | |
| 18–24 | 240 (36.4%) |
| 25–29 | 204 (31%) |
| 30–34 | 133 (20.2%) |
| 35–41 | 82 (12.4%) |
| Maternal Education | |
| GED or less | 129 (19.6%) |
| Some College | 233 (35.4%) |
| Bachelors or Higher | 297 (45.1%) |
| Currently Employed | |
| No | 236 (36.1%) |
| Yes | 418 (63.9%) |
| Annual Household Income | |
| <10k | 173 (29.8%) |
| 10k–<30k | 187 (32.2%) |
| 30k–<50k | 140 (24.1%) |
| >=50k | 81 (13.9%) |
| Marital Status | |
| Single | 129 (19.6%) |
| Married | 354 (53.9%) |
| Cohabitating | 174 (26.5%) |
| Smoking Status | |
| Never | 562 (85.4%) |
| Ever | 86 (13.1%) |
| Current | 10 (1.52%) |
| ETS | |
| Never | 598 (90.7%) |
| 1 Hour or less | 25 (3.79%) |
| >1 Hour | 36 (5.46%) |
| Alcohol | |
| Never | 318 (48.5%) |
| Yes, before Pregnancy | 300 (45.7%) |
| Yes, currently | 38 (5.79%) |
| Number of Children | |
| 0 | 246 (43.4%) |
| 1 | 257 (45.3%) |
| 2 to 5 | 64 (11.3%) |
| Pre-Pregnancy BMI | |
| [0,25] | 344 (55%) |
| (25, 30] | 174 (27.8%) |
| Above 30 | 107 (17.1%) |
| Fetal Sex | |
| Female | 316 (48.5%) |
| Male | 336 (51.5%) |
Table 2:
Distributions of gestational average urinary exposure biomarkers and serum hormone concentrations. All PAH concentrations have been corrected for specific gravity.
| N | 25th | 50th | 75th | 90th | 95th | Max | Geo.Mean | Geo.SD | ICC (95% CI) | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1-OH-NAP | 659 | 97.5 | 191 | 375 | 820 | 1436 | 25299 | 504 | 3.08 | 0.39 (0.26, 0.50) |
| 2-OH-NAP | 659 | 2640 | 4253 | 7548 | 14021 | 20179 | 87689 | 6768 | 2.29 | 0.56 (0.46, 0.64) |
| 1-OH-PYR | 659 | 62.1 | 98.9 | 154 | 248 | 316 | 2231 | 132 | 2.01 | 0.49 (0.37, 0.58) |
| 2-OH-FLU | 659 | 54.1 | 76.9 | 115 | 168 | 244 | 1312 | 105 | 1.91 | 0.43 (0.30, 0.53) |
| 1-OH-PHE | 659 | 57.6 | 89.0 | 132 | 202 | 255 | 1667 | 113 | 1.97 | 0.52 (0.41, 0.60) |
| Σ2,3-OH-PHE | 659 | 55.1 | 80.2 | 121 | 175 | 231 | 1465 | 104 | 1.86 | 0.40 (0.27, 0.51) |
| 4-OH-PHE | 659 | 10.0 | 14.9 | 22.4 | 34.9 | 50.8 | 349.9 | 21.1 | 1.92 | 0.19 (0.03, 0.33) |
| 9-OH-PHE | 659 | 8.38 | 11.7 | 19.4 | 38.0 | 57.6 | 967 | 20.9 | 2.07 | 0.21 (0.05, 0.34) |
| CRH (pg/mL) | 658 | 21.6 | 57.8 | 96.7 | 131 | 161 | 243 | 65.5 | 2.51 | 0.63 (0.54, 0.70) |
| Estriol (mg/mL) | 655 | 16.1 | 23.8 | 34.8 | 46.7 | 60.8 | 265 | 28.3 | 1.82 | −0.17 (−0.38, 0.01) |
| Progesterone (ng/mL) | 658 | 40.4 | 53.8 | 74.5 | 104 | 136 | 1037 | 65 | 1.67 | 0.09 (−0.09, 0.24) |
| Testosterone (pg/mL) | 658 | 48.0 | 77.9 | 406 | 733 | 898 | 1496 | 246 | 3.33 | 0.75 (0.69, 0.80) |
| SHBG (pg/mL) | 659 | 441 | 561 | 690 | 842 | 937 | 1404 | 582 | 1.44 | 0.77 (0.71, 0.81) |
| TSH (uIU/mL) | 655 | 0.77 | 1.20 | 1.79 | 2.51 | 3.10 | 8.92 | 1.41 | 1.97 | 0.66 (0.58, 0.72) |
| T3 (mg/mL) | 658 | 1.34 | 1.78 | 2.09 | 2.36 | 2.50 | 3.19 | 1.71 | 1.55 | 0.68 (0.61, 0.74) |
| fT4 (ng/dL) | 658 | 0.93 | 1.03 | 1.13 | 1.21 | 1.28 | 1.72 | 1.03 | 1.18 | 0.55 (0.45, 0.64) |
| T4 (ug/dL) | 655 | 10.5 | 11.8 | 13.2 | 14.4 | 15.2 | 19.0 | 11.9 | 1.19 | 0.76 (0.69, 0.80) |
CRH and Reproductive Hormones
When PAH metabolite biomarkers were modeled as continuous variables, all metabolites except for 2-OH-NAP showed significant associations with multiple reproductive hormones (Table 3). Significant increases in CRH concentrations were observed with IQR increases in 1-OH-NAP (%Δ: 14.0, 95% CI: 4.06, 24.9), 1-OH-PHE (%Δ: 24.3, 95% CI: 12.9, 36.7), Σ2,3-OH-PHE (%Δ: 18.1, 95% CI: 7.00, 30.4), and 2-OH-FLU (%Δ: 15.3, 95% CI: 4.54, 27.1). IQR increases in 1-OH-PHE and 1-OH-PYR were associated with 11.3% (95% CI: 3.94, 19.3) and 13.2% (95% CI: 4.73, 22.3) increases in E3, respectively, and 9.53% (95% CI: 3.47, 15.9) and 8.11% (95% CI: 1.37, 15.3) increases in progesterone, respectively. An IQR increase in Σ2,3-OH-PHE was also associated with a 10.1% increase (95% CI: 2.47, 18.3) in E3, a 5.60% decrease (95% CI: 0.15, 10.8) in prog/E3, and a 4.00% increase (95% CI: 0.22, 7.94) in SHBG. Conversely, decreased testosterone concentrations were observed with IQR increases in 1-OH-NAP (%Δ: −17.2, 95% CI: −25.4, −8.13), 1-OH-PHE (%Δ: −18.0, 95% CI: −26.6, −8.28), 1-OH-PYR (%Δ: −12.4, 95% CI: −22.6, −0.88), and 2-OH-FLU (%Δ: −14.0, 95% CI: −23.1, −3.82).
Table 3:
Results from linear mixed models depicting the percent changes in CRH and reproductive hormone concentrations associated with an interquartile range increase in PAH metabolite concentrations over the study period.
| CRH | Estriol | Progesterone | ||||||
|---|---|---|---|---|---|---|---|---|
| %Δ (95% CI) | P-Value | %Δ (95% CI) | P-Value | %Δ (95% CI) | P-Value | |||
| 1-OH-NAP | 14.0 (4.06, 24.9) | 0.005 | −0.33 (−6.93, 6.75) | 0.925 | 1.67 (−3.93, 7.60) | 0.568 | ||
| 2-OH-NAP | −6.36 (−15.3, 3.54) | 0.202 | −3.30 (−9.98, 3.87) | 0.359 | −2.23 (−7.85, 3.73) | 0.456 | ||
| 1-OH-PYR | 9.21 (−1.93, 21.6) | 0.110 | 13.2 (4.73, 22.3) | 0.002 | 8.11 (1.37, 15.3) | 0.019 | ||
| 2-OH-FLU | 15.3 (4.54, 27.1) | 0.005 | 2.92 (−4.13, 10.5) | 0.428 | 2.91 (−2.98, 9.16) | 0.342 | ||
| 1-OH-PHE | 24.3 (13.0, 36.7) | 0.000 | 11.3 (3.94, 19.23 | 0.003 | 9.53 (3.47, 15.9) | 0.002 | ||
| Σ2,3-OH-PHE | 18.1 (7.00, 30.4) | 0.001 | 10.1 (2.47, 18.3) | 0.009 | 4.52 (−1.55, 11.0) | 0.149 | ||
| Progesterone/Estriol | Testosterone | SHBG | ||||||
| %Δ (95% CI) | P-Value | %Δ (95% CI) | P-Value | %Δ (95% CI) | P-Value | |||
| 1-OH-NAP | 2.53 (−2.76, 8.11) | 0.357 | −17.2 (−25.4, −8.13) | 0.000 | −0.18 (−3.28, 3.02) | 0.910 | ||
| 2-OH-NAP | 0.76 (−4.74, 6.57) | 0.792 | 7.30 (−4.66, 20.8) | 0.244 | −3.39 (−6.79, 0.13) | 0.061 | ||
| 1-OH-PYR | −5.64 (−11.2, 0.27) | 0.063 | −12.4 (−22.6, −0.88) | 0.037 | 4.00 (0.22, 7.94) | 0.039 | ||
| 2-OH-FLU | −0.65 (−6.00, 5.01) | 0.819 | −14.0 (−23.1, −3.82) | 0.009 | 0.11 (−3.22, 3.56) | 0.947 | ||
| 1-OH-PHE | −2.67 (−7.81, 2.75) | 0.329 | −18.0 (−26.6, −8.28) | 0.001 | 3.38 (−0.06, 6.93) | 0.055 | ||
| Σ2,3-OH-PHE | −5.60 (−10.8, −0.15) | 0.046 | −4.76 (−15.0, 6.67) | 0.400 | 1.10 (−2.31, 4.62) | 0.533 | ||
All models adjusted for continuous maternal age, categorical maternal education, categorical maternal exposure to environmental tobacco smoke, and specific gravity.
Most associations appeared linear when OH-PAH concentrations were modeled as quartiles (Figure 1). There was additional evidence of a positive linear association between 9-OH-PHE and E3 and progesterone. Modeling Σ2,3-OH-PHE continuously did not demonstrate any associations with progesterone, however quartile modeling revealed an increase in progesterone with high concentrations of Σ2,3-OH-PHE compared to the lowest quartile (Q4 %Δ: 17.1, 95% CI: 2.27, 34.0). Quartile modeling of 1-OH-PYR also provided evidence of a possible non-monotonic association with E3 and progesterone, with the effect estimate at the third quartile of exposure being lower than that of the second and fourth quartiles of exposure. Quartile modeling attenuated the association between SHBG and Σ2,3-OH-PHE, but also provided evidence of nonlinear associations with 1-OH-PHE, 1-OH-PYR, and 9-OH-PHE. There was also suggestive evidence of nonlinear associations between testosterone and 2-OH-NAP and 9-OH-PHE.
Figure 1:
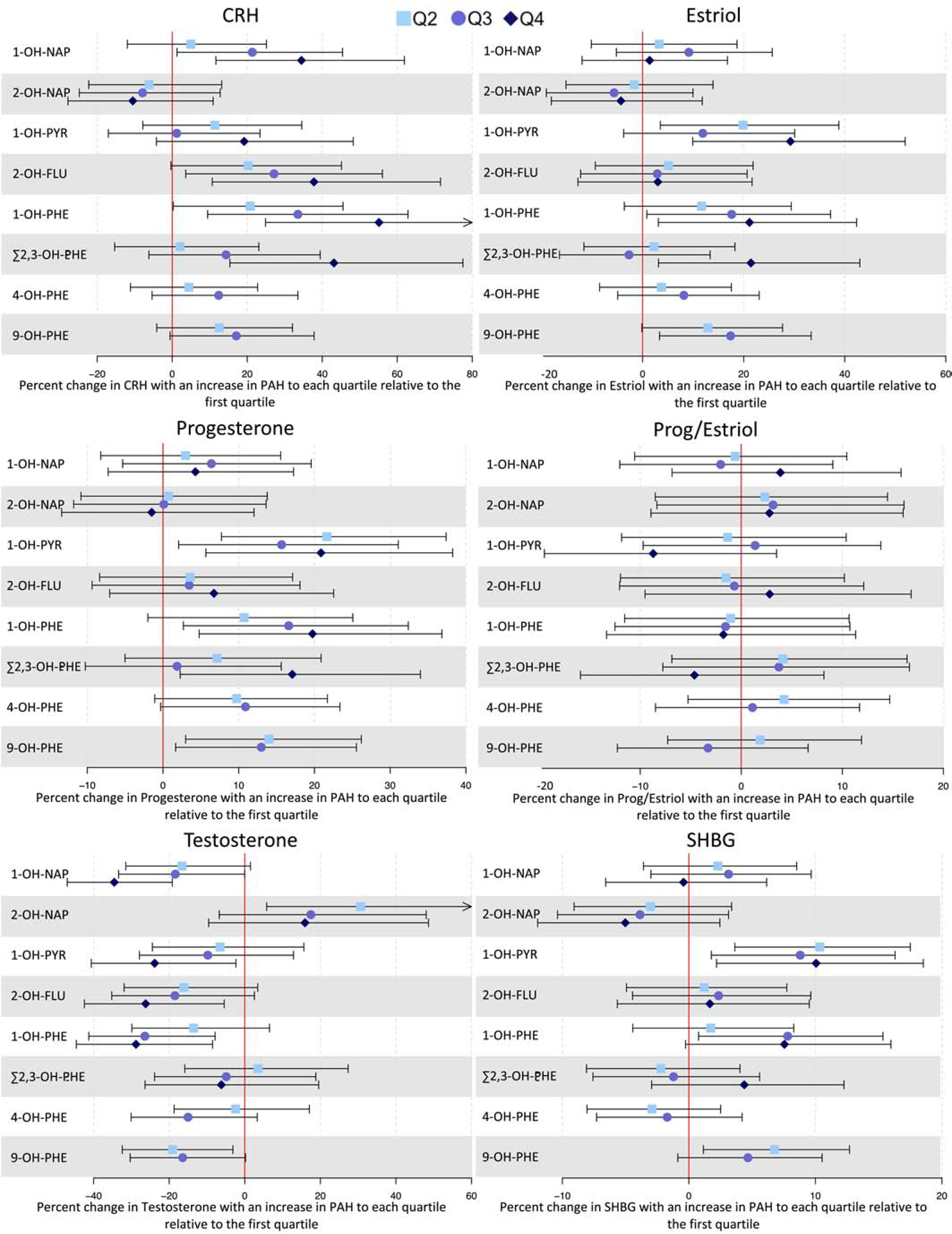
Forest plot depicting percent changes (colored points) and 95% confidence intervals (bars) of CRH and reproductive hormones with increases from the first quartile of PAH metabolite concentration to higher quartiles. Arrows indicate that the confidence interval extends beyond the bounds of the plot.
*Reference category corresponds to PAH metabolite concentrations measured below the LOD. Among PAH measurements above the LOD, the boxes are estimates corresponding to PAH concentrations at or below the median, and the circles are estimates corresponding to concentrations above the median.
There was a significant difference in the association between 2-OH-NAP and CRH among male versus female fetal sexes (p=0.028 for interaction, Supplementary Table 3). An IQR increase in 2-OH-NAP was associated with a decrease in CRH among male fetuses (%Δ: −15.8, 95% CI: −26.9, −3.13) but not among female fetuses, and categorical modeling of 2-OH-NAP showed evidence of a linear relationship among male fetuses. There was also evidence of a linear relationship between CRH, E3, progesterone, and testosterone and 9-OH-PHE among female, but not male, fetuses. Progesterone also showed a linear association with 4-OH-PHE among female fetuses (data not shown).
Significant associations between PAH metabolites and CRH were greater in magnitude at the first study visit relative to the third, but this difference was only significant for Σ2,3-OH-PHE (p=0.028, Supplementary Table 4). Categorical modeling suggested a similar result for 9-OH-PHE (data not shown). Associations between E3 and 1-OH-PHE, 1-OH-PYR, and Σ2,3-OH-PHE were significant only at the first study visit, but differences between study visits were not significant. Quartile modeling suggested that these early associations between E3 and 1-OH-PHE and 1-OH-PYR may not be linear. Only relatively low concentrations of 2-OH-NAP were associated with progesterone, and this relationship was only significant at the third study visit (Q2 %Δ: −14.1, 95% CI: −25.4, −1.07) (data not shown). An IQR increase in 1-OH-PYR was associated with increased SHBG at the first study visit only (p=0.028 between visits). Finally, there was a marginally significant difference between study visits on the association between 1-OH-NAP and testosterone (p=0.052), with effect estimates being greater in magnitude at the first study visit.
Thyroid Hormones
Associations between OH-PAHs and thyroid hormones are shown in Table 4. Significant increases in T3 concentrations were observed with IQR increases in 1-OH-NAP (%Δ: 5.16, 95% CI: 2.12, 8.20), 1-OH-PHE (%Δ: 7.99, 95% CI: 4.79, 11.2), 1-OH-PYR (%Δ: 6.90, 95% CI: 3.34, 10.5), and 2-OH-FLU (%Δ: 3.98, 95% CI: 0.72, 7.25). A suggestive increase was observed with an IQR increase in Σ2,3-OH-PHE (%Δ: 3.29, 95% CI: −0.02, 6.60). Similar results were found for the ratio of T3/T4. TSH concentrations decreased by 8.35% (95% CI: −14.7, −1.58) with an IQR increase in 2-OH-NAP.
Table 4.
Results from linear mixed models depicting the percent changes in thyroid hormone concentrations associated with an interquartile range increase in PAH metabolite concentrations over the study period.
| TSH | T3 | fT4 | ||||
|---|---|---|---|---|---|---|
| %Δ (95% CI) | p-Value | %Δ (95% CI) | p-Value | %Δ (95% Cl) | p-Value | |
| 1-OH-NAI’ | 4.78 (−1.79, 11.8) | 0.159 | 5.16 (2.12, 8.20) | 0.001 | 1.40 (− 0.09, 2.89) | 0.068 |
| 2-OH-NAP | −8.35 (−14.7, −1.58) | 0.018 | 0.77 (−2.64, 4.18) | 0.659 | −1.00 (−2.64, 0.63) | 0.230 |
| 1-OH-PYR | 1.77 (−5.66, 9.80) | 0.650 | 6.90 (3.34, 10.5) | 0.000 | 0.93 (−0.81, 2.67) | 0.296 |
| 2-OH-FLU | −1.92 (−8.45, 5.08) | 0.582 | 3.98 (0.72, 7.25) | 0.018 | 1.25 (−033, 2.84} | 0.123 |
| 1-OH-PHE | 1.14 (−5.53, 8.29) | 0.744 | 7.99 (4.79, 11.2) | 0.000 | 1.51 (−0.04, 3.07) | 0.058 |
| Σ2,3-OH-PHE | 0(−6.74, 7.23) | 1.000 | 3.29 (−0.02, 6.60) | 0.053 | 0.15 (−1.46, 1.77) | 0.853 |
| T4 | T3/T4 | |||||
| %Δ (95% CI) | p-Value | %Δ (95% CI) | p-Value | |||
| 1-OH-NAP | −0.44 (−1.93, 1.06) | 0.569 | 5.31 (2.15, 8.48) | 0.001 | ||
| 2-OH-NAP | −0.71 (−2.37, 0.95) | 0.402 | 1.51 (−2.02, 5.03) | 0.404 | ||
| 1-OH-PYR | 1.30 (−0.47, 3.07) | 0.151 | 5.64 (1.92, 9.35) | 0.003 | ||
| 2-OH-FLU | −0.90 (−2.50, 0.70) | 0.273 | 5.42 (2.05, 8.8) | 0.002 | ||
| 1-OH-PHE | 0.21 (−1.39, 1.81) | 0.796 | 7.98 (4.66, 11.31) | 0.000 | ||
| Σ2,3-OH-PHE | −0.63 (−2.25, 0.99) | 0.449 | 4.58 (1.16, 8.01) | 0.009 | ||
All models adjusted for continuous maternal age, categorical maternal education, categorical maternal exposure to environmental tobacco smoke, and specific gravity. Boldface indicates p-values of less than 0.055
Modeling OH-PAH concentrations as quartiles revealed that the associations between T3 and 1-OH-NAP and 2-OH-FLU may not be linear. Furthermore, categorical modeling of 4-OH-PHE and 9-OH-PHE suggested that T3 concentrations increased linearly with increasing 4-OHPHE and nonlinearly with increasing 9-OH-PHE (Figure 2). Increased concentrations of fT4 were observed only among the highest concentrations of 1-OH-NAP, relative to the lowest quartile (%Δ: 3.67, 95% CI: 0.63, 6.71), and among the second quartile of 2-OH-FLU, relative to the lowest (%Δ: 3.38, 95% CI: 0.27, 6.49). There was evidence of nonlinear associations between the ratio of T3/T4 and 1-OH-NAP, 1-OH-PHE, Σ2,3-OH-PHE, and 9-OH-PHE. Like T3, T3/T4 appeared to increase linearly with increasing concentrations of 4-OH-PHE.
Figure 2:
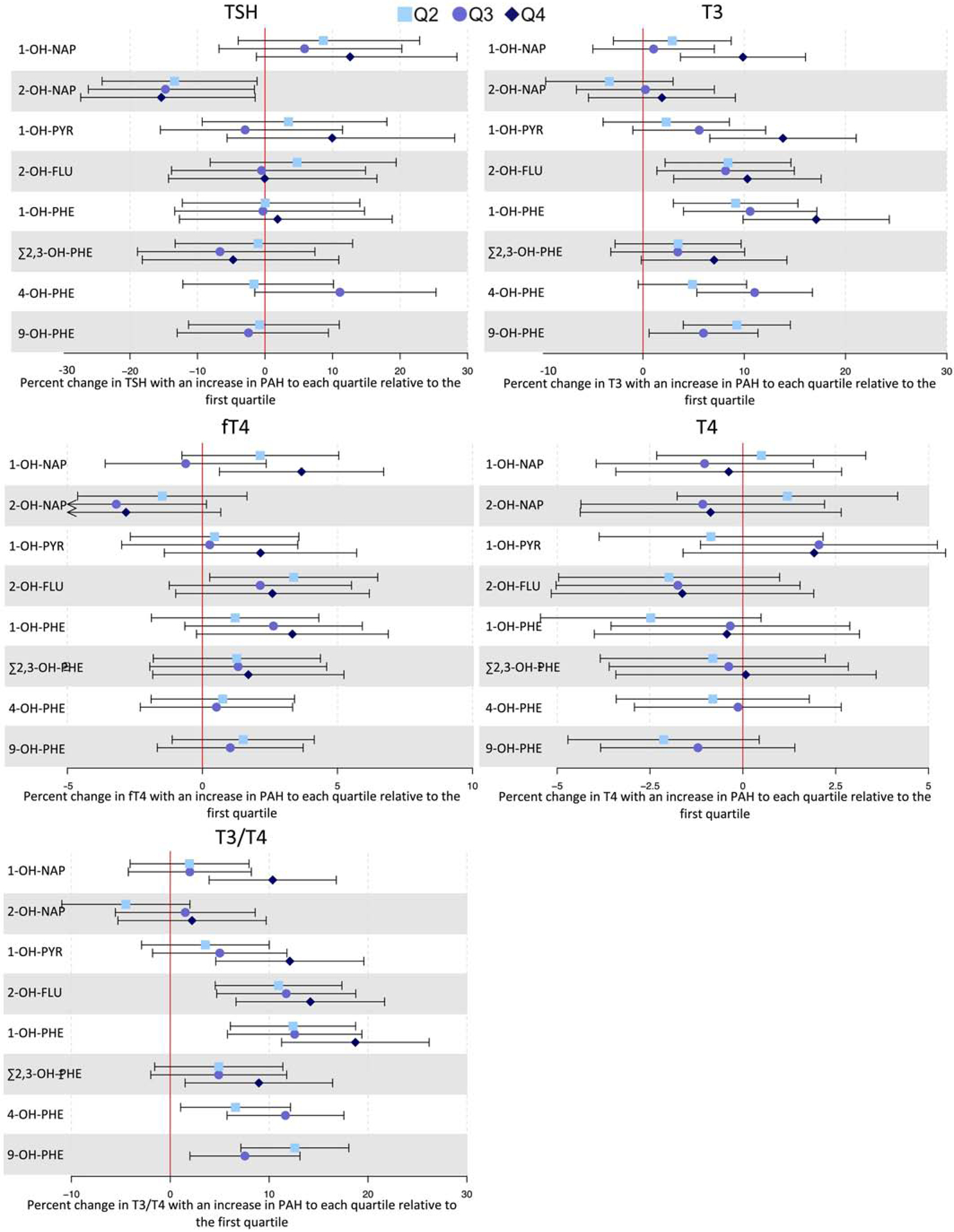
Forest plot depicting percent changes (colored points) and 95% confidence intervals (bars) of thyroid hormones with increases from the first quartile of PAH metabolite concentration to higher quartiles. Arrows indicate that the confidence interval extends beyond the bounds of the plot.
*Reference category corresponds to PAH metabolite concentrations measured below the LOD. Among PAH measurements above the LOD, the boxes are estimates corresponding to PAH concentrations at or below the median, and the circles are estimates corresponding to concentrations above the median.
There was a significant difference in the association between 2-OH-FLU and fT4 among male versus female fetal sexes (p=0.039 for difference) (Supplementary Table 5), with an IQR increase in 2-OH-FLU resulting in an increase in fT4 among only pregnancies with female fetuses (%Δ: 2.49, 95% CI: 0.51, 4.48). Quartile modeling of 2-OH-FLU suggested that the nonlinear relationship with fT4 described above was specific to women carrying female fetuses. There was also evidence of a nonlinear association between 4-OH-PHE and fT4 among only female pregnancies (data not shown).
The associations between T3 and 1-OH-NAP and Σ2,3-OH-PHE were significantly different between study visits (p=0.04 and p=0.039 for difference, respectively) (Supplementary Table 6), and associations were positive at the first study visit only. T3 concentrations were also observed to change nonlinearly with increasing concentrations of 9-OH-PHE at the third study visit only (data not shown). Similarly, the association between Σ2,3-OH-PHE and the ratio of T3/T4 was significantly different between study visits (p=0.037 for difference), with a positive association being observed at the first study visit only. Quartile modeling of OH-PAHs revealed that the association between Σ2,3-OH-PHE and T3/T4 was nonlinear at the first study visit, and that 2-OH-NAP resulted in decreased T3/T4 at the third study visit (data not shown).
Discussion
Here we show significant alteration of numerous hormone concentrations associated with urinary biomarkers of PAH exposure during pregnancy in a human cohort study of 707 women. Because a balance of critical hormones is necessary for endocrine regulation of pregnancy and fetal development, these changes could have implications for birth outcomes (Smith et al., 2009; Snegovskikh and Park, 2006). CRH concentrations were positively associated with most OH-PAHs, and most of these associations were greater among women carrying male fetuses. Moreover, we observed a decreased ratio of progesterone to estriol with increasing PAH exposure. Of potential importance, both elevated CRH and decreased progesterone to estriol ratio may have implications for the timing of labor. Testosterone concentrations decreased with greater PAH exposure, which interestingly did not depend on fetal sex. Finally, OH-PAHs were widely associated with increased T3 and T3/T4, but not TSH or T4 concentrations, suggesting a lack of negative feedback on TSH by T3 and overall increased thyroid activity.
We observed strongly positive associations between CRH serum concentrations and four of the six PAH metabolites we assayed in urine. A previous study conducted on benthic marine fish that were treated with naphthalene and phenanthrene showed that exposed fish secreted more cortisol than control fish, demonstrating an increased stress response with PAH exposure (Reddam et al., 2017). Because CRH is an upstream activator of cortisol secretion in humans (Taylor and Fishman, 1988), the stress response displayed in these marine fish may be analogous to increased concentrations of CRH with PAH exposure among the women in our study. To our knowledge, no previous studies have investigated effects of PAH exposure on CRH in women. CRH concentrations slightly rise during the first two trimesters of pregnancy and then exponentially increase as labor approaches, acting as a “placental clock” for the timing of labor (McLean et al., 1995). A prior study showed that percent daily change in CRH concentrations at 26 weeks were greater among women who eventually delivered preterm compared to full term (Smith et al., 2009), and we previously showed that women carrying a male fetus were at increased risk of delivering preterm when they had greater concentrations of CRH measured before the third trimester (Cathey et al, submitted). Because an association between PAH exposure during pregnancy and preterm birth has been described previously (Agarwal et al., 2018; Padula et al., 2014), our finding of an association between CRH and PAH metabolite concentrations could shed light on the mechanism by which PAHs impact preterm birth. The greater magnitude of the associations between OH-PAHs and CRH among male fetuses in the present study may reflect their greater sensitivity to changing CRH concentrations, and thus confer greater risk of preterm birth later in the pregnancy.
In the present study we observed that several PAH metabolites, including 1-OH-PHE and Σ2,3-OH-PHE, were associated with increased circulating concentrations of estriol and progesterone. In line with our results, previous in vitro studies demonstrated estrogenic activity of phenanthrene via interaction with the estrogen receptor alpha (Hayakawa et al., 2007; Kamelia et al., 2018; Vrabie et al., 2011). Hayakawa and colleagues specifically showed strong estrogenic activity of some PAH metabolites, but those metabolites were not consistent with those included in the present study. Conversely, previous studies have also shown decreased estradiol concentrations with phenanthrene exposure in zebrafish (Peng et al., 2019) and in pregnant women living near an e-waste site (Huang et al., 2020), and decreased progesterone secretion upon exposure to phenanthrene during human pregnancy (Drwal et al., 2017). The ratio of progesterone to estriol has been suggested as a more important measure for studying birth outcomes than either hormone on its own (Romero et al., 1988; Ruiz et al., 2008). Concentrations of both estriol and progesterone rise steadily throughout pregnancy, but the ratio begins to favor estriol as term approaches. In women, a functional withdrawal of progesterone, rather than decreasing concentrations, helps drive the initiation of labor (Mesiano and Welsh, 2007; Snegovskikh and Park, 2006). In this study, we observed that greater concentrations of Σ2,3-OH-PHE were associated with a decreased ratio of progesterone to estriol, suggesting that phenanthrene exposure may push the progesterone/estriol ratio towards estriol and potentially disrupt the endocrine regulation of labor. Although the effect of phenanthrene exposure on estriol and progesterone is inconsistent across studies, it remains plausible that any alteration of the ratio of progesterone to estriol may have significant consequences on human pregnancies.
Few studies have investigated the effects of PAH exposure on testosterone concentrations, but one in vitro study did observe that high concentrations of pyrene and phenanthrene inhibit the androgen receptor (Vinggaard et al., 2000). We observed that many PAH metabolites were inversely associated with testosterone concentration. The strongest reduction in testosterone concentrations occurred with the highest quartiles of exposure to pyrene and most phenanthrene metabolites. Although we did not observe significant differences by fetal sex on the association between OH-PAHs and testosterone, we previously showed that women who experienced preterm or spontaneous preterm birth and were carrying a male fetus had lower concentrations of testosterone throughout pregnancy compared to women who experienced term labor (Cathey et al., submitted). Thus, PAH exposure may contribute to reduced testosterone concentrations during gestation which differentially influences risk of preterm birth based on fetal sex.
We observed significant positive associations between PAH exposure and T3 and the ratio of T3 to T4, but not TSH or T4 concentrations. A previous in vitro study using human cell-based reported gene assays showed that diesel exhaust particles containing PAHs activated the thyroid receptor alpha and increased the receptor activation by endogenous T3 (Pencikova et al., 2019). Increased T3 and T3/T4 concentrations alongside unchanged TSH and T4 concentrations may suggest that PAH exposure interferes with the normal negative feedback loop from T3 to TSH. Other studies, however, have reported reduced thyroid function with PAH exposure. Common carp that were exposed to pyrene demonstrated significantly reduced concentrations of both T3 and T4 (Shirdel et al., 2016), and rockfish embryos exposed to pyrene also showed reduced concentrations of T3 and reduced expression of thyroid receptor genes (He et al., 2012). There may be inherent differences in how PAH exposure affects the thyroid axis in humans versus aquatic species, or in pregnant adults versus developing embryos, but more work must be done to better understand these relationships.
The present study was subject to several limitations. First, two OH-PAH measurements during pregnancy may not be adequate to ascertain the true exposure profiles of these women. However, because ICC values were moderate it is likely that PAH exposure sources for each woman do not change appreciably throughout her pregnancy, and thus the resulting changes in hormone concentrations are likely sustained over time. Because our detection rates of 4-OH-PHE and 9-OH-PHE were low, we could not assess these metabolites as continuous variables and consequently lost statistical power by analyzing them only as categorical variables. We also only measured metabolites from four different parent PAH compounds and do not have exposure information for other high molecular weight PAHs among the women in our cohort. However, a large portion of the published literature on health effects of PAHs focuses on high molecular weight compounds, so the present study contributes to a gap in current knowledge. Finally, our ability to interpret OH-PAH associations with thyroid hormones may be reduced because we did not collect thyroid autoantibody status in our participants, which may affect patterns of thyroid hormone concentrations through pregnancy.
Despite these limitations, this study was strong in several ways. To our knowledge, this is the first longitudinal study to assess associations between OH-PAHs and hormones during pregnancy, significantly adding to the current state of understanding the impacts of PAH exposures on pregnancy. This study also improves on previous PAH studies because of our use of biomarkers instead of air monitoring or food frequency questionnaires as the exposure assessment tool, which allowed for more specific individual exposure assessment and for the capture of all exposure routes rather than only inhalation or ingestion. We included a broad scope of hormones in the study, allowing us to evaluate PAH effects on the thyroid, adrenal, and gonadal hormonal axes. There are also no previous studies assessing PAH impacts on CRH, testosterone, or the ratio of progesterone to estriol in humans, which is a significant gap in the literature given their important roles during pregnancy. Finally, we were able to distinguish endocrine disruption of PAHs between fetal sexes and between exposures at different times during pregnancy.
Conclusions
In conclusion, we have provided evidence that PAH exposure during pregnancy is associated with altered hormone levels which may be indicative of endocrine disruption. Specifically, greater OH-PAH concentrations throughout pregnancy were associated with increased CRH, estriol, and T3, as well as decreased testosterone concentrations. These hormone changes may have downstream consequences for pregnancy and labor with potential implications for fetal and child health. Evidence of differing associations between OH-PAHs and hormones based on fetal sex suggests that endocrine systems may be differentially vulnerable to environmental exposures during gestation. Future research will aim to assess relationships between PAH exposures and adverse birth outcomes within the PROTECT cohort, and to understand how PAH exposures may be related to and interact with other environmental exposures occurring in Puerto Rico.
Supplementary Material
Highlights.
Assessed associations between repeated gestational PAH and hormone measurements
PAH exposure resulted in increased concentrations of CRH, estriol and T3
PAH exposure resulted in decreased concentrations of testosterone and prog/estriol
Changes in hormone concentrations were dependent on fetal sex and gestational age
Gestational PAH exposure alters hormones that may result in disruption of labor
Acknowledgements
This study was supported by the Superfund Research Program of the National Institute of Environmental Health Sciences (NIEHS), National Institutes of Health (NIH; grant number P42ES017198) and the National Institute on Minority Health and Health Disparities (award number U54 MD007600). Additional support was provided from NIEHS grant number P30ES017885 and the Environmental influences on Child Health Outcomes (ECHO) program grant number UH3OD023251. ECHO is a nationwide research program supported by the NIH, Office of the Director to enhance child health. We would like to extend our gratitude to all PROTECT study participants and their families. The authors also thank the nurses and research staff who participated in cohort recruitment and follow up, as well as the Federally Qualified Health Centers (FQHC) in Puerto Rico that facilitated participant recruitment, including Morovis Community Health Center, Prymed in Ciales, Camuy Health Services, Inc. and the Delta OBGyn Group in Manati, as well as the Manati Medical Center and the Metro Pavia Hospital in Arecibo.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Agarwal P, Singh L, Anand M, Taneja A, 2018. Association Between Placental Polycyclic Aromatic Hydrocarbons (PAHS), Oxidative Stress, and Preterm Delivery: A Case-Control Study. Arch. Environ. Contam. Toxicol 74, 218–227. 10.1007/s00244-017-0455-0 [DOI] [PubMed] [Google Scholar]
- Al-Daghri NM, Alokail MS, Abd-Alrahman SH, Draz HM, Yakout SM, Clerici M, 2013. Polycyclic aromatic hydrocarbon exposure and pediatric asthma in children: a case-control study. Environ. Health 12, 1 10.1186/1476-069X-12-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alghamdi MA, Alam MS, Stark C, Mohammed N, Harrison RM, Shamy M, Khoder MI, Shabbaj II, Goen T, 2015. Urinary metabolites of polycyclic aromatic hydrocarbons in Saudi Arabian schoolchildren in relation to sources of exposure. Environ. Res 140, 495–501. 10.1016/j.envres.2015.04.023 [DOI] [PubMed] [Google Scholar]
- Brucker N, Charao MF, Moro AM, Ferrari P, Bubols G, Sauer E, Fracasso R, Durgante J, Thiesen FV, Duarte MM, Gioda A, Castro I, Saldiva PH, Garcia SC, 2014. Atherosclerotic process in taxi drivers occupationally exposed to air pollution and comorbidities. Environ. Res 131, 31–38. 10.1016/j.envres.2014.02.012 [DOI] [PubMed] [Google Scholar]
- Cathey A, Ferguson KK, Mcelrath TF, Cantonwine DE, Pace G, Alshawabkeh A, Cordero JF, Meeker JD, 2018. Distribution and predictors of urinary polycyclic aromatic hydrocarbon metabolites in two pregnancy cohort studies. Environ. Pollut 232, 556–562. 10.1016/j.envpol.2017.09.087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi H, Wang L, Lin X, Spengler JD, Perera FP, 2012. Fetal window of vulnerability to airborne polycyclic aromatic hydrocarbons on proportional intrauterine growth restriction. PLoS One 7, e35464 10.1371/journal.pone.0035464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich JW, Landgrafe G, Fotiadou EH, 2012. TSH and thyrotropic agonists: Key actors in thyroid homeostasis. J. Thyroid Res 2012 10.1155/2012/351864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drwal E, Rak A, Grochowalski A, Milewicz T, Gregoraszczuk EL, 2017. Cell-specific and dose-dependent effects of PAHs on proliferation, cell cycle, and apoptosis protein expression and hormone secretion by placental cell lines. Toxicol. Lett 280, 10–19. 10.1016/j.toxlet.2017.08.002 [DOI] [PubMed] [Google Scholar]
- Ferguson KK, Kamai EM, Cantonwine DE, Mukherjee B, Meeker JD, McElrath TF, 2018. Associations between repeated ultrasound measures of fetal growth and biomarkers of maternal oxidative stress and inflammation in pregnancy. Am. J. Reprod. Immunol 80, e13017 10.1111/aji.13017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson KK, McElrath TF, Chen Y-H, Loch-Caruso R, Mukherjee B, Meeker JD, 2015. Repeated measures of urinary oxidative stress biomarkers during pregnancy and preterm birth. Am. J. Obstet. Gynecol 212, 208.e1–8. 10.1016/j.ajog.2014.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson KK, McElrath TF, Pace GG, Weller D, Zeng L, Pennathur S, Cantonwine DE, Meeker JD, 2017. Urinary Polycyclic Aromatic Hydrocarbon Metabolite Associations with Biomarkers of Inflammation, Angiogenesis, and Oxidative Stress in Pregnant Women. Environ. Sci. Technol 51, 4652–4660. 10.1021/acs.est.7b01252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao D, Lin J, Ou K, Chen Y, Li H, Dai Q, Yu Z, Zuo Z, Wang C, 2018. Embryonic exposure to benzo(a)pyrene inhibits reproductive capability in adult female zebrafish and correlation with DNA methylation. Environ. Pollut 240, 403–411. 10.1016/j.envpol.2018.04.139 [DOI] [PubMed] [Google Scholar]
- Hayakawa K, Onoda Y, Tachikawa C, Hosoi S, Yoshita M, Chung SW, Kizu R, Toriba A, Kameda T, Tang N, 2007. Estrogenic/Antiestrogenic Activities of Polycyclic Aromatic Hydrocarbons and Their Monohydroxylated Derivatives by Yeast Two-Hybrid Assay. J. Heal. Sci 53, 562–570. 10.1248/jhs.53.562 [DOI] [Google Scholar]
- He C, Zuo Z, Shi X, Sun L, Wang C, 2012. Pyrene exposure influences the thyroid development of Sebastiscus marmoratus embryos. Aquat. Toxicol 124–125, 28–33. 10.1016/j.aquatox.2012.07.007 [DOI] [PubMed] [Google Scholar]
- Huang X, Xu X, Dai Y, Cheng Z, Zheng X, Huo X, 2020. Association of prenatal exposure to PAHs with anti-Mullerian hormone (AMH) levels and birth outcomes of newborns. Sci. Total Environ 723, 138009 10.1016/j.scitotenv.2020.138009 [DOI] [PubMed] [Google Scholar]
- Jain RB, 2016. Association between polycyclic aromatic hydrocarbons and thyroid function among males and females: data from NHANES 2007–2008. Int. J. Environ. Health Res 26, 405–419. 10.1080/09603123.2015.1135311 [DOI] [PubMed] [Google Scholar]
- Jurisicova A, Taniuchi A, Li H, Shang Y, Antenos M, Detmar J, Xu J, Matikainen T, Benito Hernandez A, Nunez G, Casper RF, 2007. Maternal exposure to polycyclic aromatic hydrocarbons diminishes murine ovarian reserve via induction of Harakiri. J. Clin. Invest 117, 3971–3978. 10.1172/JCI28493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamelia L, Louisse J, de Haan L, Maslowska-Gornicz A, Ketelslegers HB, Brouwer A, Rietjens IMCM, Boogaard PJ, 2018. The Role of Endocrine and Dioxin-Like Activity of Extracts of Petroleum Substances in Developmental Toxicity as Detected in a Panel of CALUX Reporter Gene Assays. Toxicol. Sci 164, 576–591. 10.1093/toxsci/kfy114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelishadi R, Sobhani P, Poursafa P, Amin MM, Ebrahimpour K, Hovsepian S, Mansourian M, Najafi R, Hashemipour M, 2018. Is there any association between urinary metabolites of polycyclic aromatic hydrocarbons and thyroid hormone levels in children and adolescents? Environ. Sci. Pollut. Res. Int 25, 1962–1968. 10.1007/s11356-017-0577-y [DOI] [PubMed] [Google Scholar]
- McLean M, Bisits A, Davies J, Woods R, Lowry P, Smith R, 1995. A placental clock controlling the length of human pregnancy. Nat. Med 1, 460–463. 10.1038/nm0595-460 [DOI] [PubMed] [Google Scholar]
- Mesiano S, Welsh TN, 2007. Steroid hormone control of myometrial contractility and parturition. Semin. Cell Dev. Biol 18, 321–331. 10.1016/j.semcdb.2007.05.003 [DOI] [PubMed] [Google Scholar]
- Mordukhovich I, Beyea J, Herring AH, Hatch M, Stellman SD, Teitelbaum SL, Richardson DB, Millikan RC, Engel LS, Shantakumar S, Steck SE, Neugut AI, Rossner PJ, Santella RM, Gammon MD, 2016. Vehicular Traffic-Related Polycyclic Aromatic Hydrocarbon Exposure and Breast Cancer Incidence: The Long Island Breast Cancer Study Project (LIBCSP). Environ. Health Perspect 124, 30–38. 10.1289/ehp.1307736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onyemauwa F, Rappaport SM, Sobus JR, Gajdosova D, Wu R, Waidyanatha S, 2009. Using liquid chromatography-tandem mass spectrometry to quantify monohydroxylated metabolites of polycyclic aromatic hydrocarbons in urine. J. Chromatogr. B, Anal. Technol. Biomed. life Sci 877, 1117–1125. 10.1016/j.jchromb.2009.02.067 [DOI] [PubMed] [Google Scholar]
- Padula AM, Noth EM, Hammond SK, Lurmann FW, Yang W, Tager IB, Shaw GM, 2014. Exposure to airborne polycyclic aromatic hydrocarbons during pregnancy and risk of preterm birth. Environ. Res 135, 221–226. 10.1016/j.envres.2014.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pencikova K, Ciganek M, Neca J, Illes P, Dvorak Z, Vondracek J, Machala M, 2019. Modulation of endocrine nuclear receptor activities by polyaromatic compounds present in fractionated extracts of diesel exhaust particles. Sci. Total Environ 677, 626–636. 10.1016/j.scitotenv.2019.04.390 [DOI] [PubMed] [Google Scholar]
- Peng X, Sun X, Yu M, Fu W, Chen H, Chen J, 2019. Chronic exposure to environmental concentrations of phenanthrene impairs zebrafish reproduction. Ecotoxicol. Environ. Saf 182, 109376 10.1016/j.ecoenv.2019.109376 [DOI] [PubMed] [Google Scholar]
- Perera F, Tang D, Whyatt R, Lederman SA, Jedrychowski W, 2005. DNA damage from polycyclic aromatic hydrocarbons measured by benzo[a]pyrene-DNA adducts in mothers and newborns from Northern Manhattan, the World Trade Center Area, Poland, and China. Cancer Epidemiol. Biomarkers Prev 14, 709–714. 10.1158/1055-9965.EPI-04-0457 [DOI] [PubMed] [Google Scholar]
- Perera FP, Chang H, Tang D, Roen EL, Herbstman J, Margolis A, Huang T-J, Miller RL, Wang S, Rauh V, 2014. Early-life exposure to polycyclic aromatic hydrocarbons and ADHD behavior problems. PLoS One 9, e111670 10.1371/journal.pone.0111670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radwan M, Jurewicz J, Sobala W, Brze Nicki S, Radwan P, Jakubowski L, Hawula W, Ulanska A, Hanke W, 2015. Human sperm aneuploidy after exposure to polycyclic aromatic hydrocarbons. Reprod. Fertil. Dev 10.1071/RD14063 [DOI] [PubMed] [Google Scholar]
- Reddam A, Mager EM, Grosell M, McDonald MD, 2017. The impact of acute PAH exposure on the toadfish glucocorticoid stress response. Aquat. Toxicol 192, 89–96. 10.1016/j.aquatox.2017.08.014 [DOI] [PubMed] [Google Scholar]
- Romero R, Scoccia B, Mazor M, Wu YK, Benveniste R, 1988. Evidence for a local change in the progesterone/ estrogen ratio in human parturition at term. Am. J. Obstet. Gynecol 159, 657–660. [DOI] [PubMed] [Google Scholar]
- Ruiz RJ, Saade GR, Brown CEL, Nelson-Becker C, Tan A, Bishop S, Bukowski R, 2008. The effect of acculturation on progesterone/estriol ratios and preterm birth in hispanics. Obstet. Gynecol 111, 309–316. 10.1097/01.AOG.0000297896.00491.2c [DOI] [PubMed] [Google Scholar]
- Shirdel I, Kalbassi MR, Shokri M, Olyaei R, Sharifpour I, 2016. The response of thyroid hormones, biochemical and enzymological biomarkers to pyrene exposure in common carp (Cyprinus carpio). Ecotoxicol. Environ. Saf 130, 207–213. 10.1016/j.ecoenv.2016.03.023 [DOI] [PubMed] [Google Scholar]
- Smith R, Smith JI, Shen X, Engel PJ, Bowman ME, McGrath SA, Bisits AM, McElduff P, Giles WB, Smith DW, 2009. Patterns of plasma corticotropin-releasing hormone, progesterone, estradiol, and estriol change and the onset of human labor. J. Clin. Endocrinol. Metab 94, 2066–2074. 10.1210/jc.2008-2257 [DOI] [PubMed] [Google Scholar]
- Snegovskikh V, Park JS, 2006. Endocrinology of Parturition. Endocrinol. Metab. Clin. North Am 35, 173–191. 10.1016/j.ecl.2005.09.012 [DOI] [PubMed] [Google Scholar]
- Srogi K, 2007. Monitoring of environmental exposure to polycyclic aromatic hydrocarbons: a review. Environ. Chem. Lett 5, 169–195. 10.1007/s10311-007-0095-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strickland P, Kang D, Sithisarankul P, 1996. Polycyclic aromatic hydrocarbon metabolites in urine as biomarkers of exposure and effect. Environ. Health Perspect 104 Suppl, 927–932. 10.1289/ehp.96104s5927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L, Zuo Z, Luo H, Chen M, Zhong Y, Chen Y, Wang C, 2011. Chronic exposure to phenanthrene influences the spermatogenesis of male Sebastiscus marmoratus: U-shaped effects and the reason for them. Environ. Sci. Technol 45, 10212–10218. 10.1021/es202684w [DOI] [PubMed] [Google Scholar]
- Taylor AL, Fishman LM, 1988. Corticotropin-releasing hormone. N. Engl. J. Med 319, 213–222. 10.1056/NEJM198807283190405 [DOI] [PubMed] [Google Scholar]
- Urbancova K, Lankova D, Rossner P, Rossnerova A, Svecova V, Tomaniova M, Veleminsky MJ, Sram RJ, Hajslova J, Pulkrabova J, 2016. Evaluation of 11 polycyclic aromatic hydrocarbon metabolites in urine of Czech mothers and newborns. Sci. Total Environ 10.1016/j.scitotenv.2016.10.165 [DOI] [PubMed] [Google Scholar]
- Vinggaard AM, Hnida C, Larsen JC, 2000. Environmental polycyclic aromatic hydrocarbons affect androgen receptor activation in vitro. Toxicology 145, 173–183. 10.1016/s0300-483x(00)00143-8 [DOI] [PubMed] [Google Scholar]
- Vishnevetsky J, Tang D, Chang H-W, Roen EL, Wang Y, Rauh V, Wang S, Miller RL, Herbstman J, Perera FP, 2015. Combined effects of prenatal polycyclic aromatic hydrocarbons and material hardship on child IQ. Neurotoxicol. Teratol 49, 74–80. 10.1016/j.ntt.2015.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrabie CM, Candido A, van den Berg H, Murk AJ, van Duursen MBM, Jonker MTO, 2011. Specific in vitro toxicity of crude and refined petroleum products: 3. Estrogenic responses in mammalian assays. Environ. Toxicol. Chem 30, 973–980. 10.1002/etc.463 [DOI] [PubMed] [Google Scholar]
- Wang L, Hu W, Xia Y, Wang X, 2017. Associations between urinary polycyclic aromatic hydrocarbon metabolites and serum testosterone in U.S. adult males: National Health and nutrition examination survey 2011–2012. Environ. Sci. Pollut. Res. Int 24, 7607–7616. 10.1007/s11356-017-8407-9 [DOI] [PubMed] [Google Scholar]
- Wang S, Bai Y, Deng Q, Chen Z, Dai J, Li X, Zhang W, Zhang X, He M, Wu T, Guo H, 2016. Polycyclic aromatic hydrocarbons exposure and lung function decline among coke-oven workers: A four-year follow-up study. Environ. Res 150, 14–22. 10.1016/j.envres.2016.05.025 [DOI] [PubMed] [Google Scholar]
- Yang P, Gong Y-J, Cao W-C, Wang R-X, Wang Y-X, Liu C, Chen Y-J, Huang L-L, Ai S-H, Lu W-Q, Zeng Q, 2018. Prenatal urinary polycyclic aromatic hydrocarbon metabolites, global DNA methylation in cord blood, and birth outcomes: A cohort study in China. Environ. Pollut 234, 396–405. 10.1016/j.envpol.2017.11.082 [DOI] [PubMed] [Google Scholar]
- Yang P, Sun H, Gong YJ, Wang YX, Liu C, Chen YJ, Sun L, Huang LL, Ai SH, Lu WQ, Zeng Q, 2017. Repeated measures of urinary polycyclic aromatic hydrocarbon metabolites in relation to altered reproductive hormones: A cross-sectional study in China. Int. J. Hyg. Environ. Health 220, 1340–1346. 10.1016/j.ijheh.2017.09.004 [DOI] [PubMed] [Google Scholar]
- Zajda K, Gregoraszczuk EL, 2020. Environmental polycyclic aromatic hydrocarbons mixture, in human blood levels, decreased oestradiol secretion by granulosa cells via ESR1 and GPER1 but not ESR2 receptor. Hum. Exp. Toxicol 39, 276–289. 10.1177/0960327119886027 [DOI] [PubMed] [Google Scholar]
- Zhu P, Bian Z, Xia Y, Han Y, Qiao S, Zhao R, Jin N, Wang S, Peng Y, Wang X, 2009. Relationship between urinary metabolites of polycyclic aromatic hydrocarbons and thyroid hormone levels in Chinese non-occupational exposure adult males. Chemosphere 77, 883–888. 10.1016/j.chemosphere.2009.08.054 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


