Abstract
Objectives:
Solid renal masses have unknown malignant potential with commonly utilized imaging. Biopsy can offer a diagnosis of cancer but has a high non-diagnostic rate and complications. Reported use of multiparametric magnetic resonance imaging (mpMRI) to diagnose aggressive histology (i.e., clear cell renal cell carcinoma (ccRCC)) via a clear cell likelihood score (ccLS) was based on retrospective review of cT1a tumors. We aim to retrospectively assess the diagnostic performance of ccLS prospectively assigned to renal masses of all stages evaluated with mpMRI prior to histopathologic evaluation.
Methods:
In this retrospective cohort study from June 2016 to November 2019, 434 patients with 454 renal masses from 2 institutions with heterogenous patient populations underwent mpMRI with prospective ccLS assignment and had pathologic diagnosis. ccLS performance was assessed by contingency table analysis. The association between ccLS and ccRCC was assessed with logistic regression.
Results:
Mean age and tumor size were 60±13 years and 5.4±3.8 cm. Characteristics were similar between institutions except for patient age and race (both p<0.001) and lesion laterality and histology (both p=0.04). The PPV of ccLS increased with each increment in ccLS (ccLS1: 5%[3/55], ccLS2: 6%[3/47]; ccLS3: 35%[20/57], ccLS4: 78%[85/109], ccLS5: 93%[173/186]). Pooled analysis for ccRCC diagnosis revealed sensitivity 91%(258/284), PPV 87%(258/295) for ccLS≥4; and specificity 56%(96/170), NPV 94%(96/102) for ccLS≤2. Diagnostic performance was similar between institutions.
Conclusions:
We confirm the optimal diagnostic performance of mpMRI to identify ccRCC in all clinical stages. High PPV and NPV of ccLS can help inform clinical management decision making.
Keywords: Multiparametric Magnetic Resonance Imaging; Carcinoma, Renal Cell; Kidney Neoplasms; Diagnostic Imaging
INTRODUCTION
With the increasing use of cross-sectional imaging [1], the rate of incidentally identified renal masses continues to increase [2]. Many of these masses will remain indolent with either no or very slow growth and require no intervention [3]. Accordingly, the American and European guidelines for the management of clinical stage 1 renal masses contemplates active surveillance (AS) as a valid option for patients with comorbidities and T1a (≤4cm) or T1b (4-7 cm) tumors [4,5]. The reported risk of metastasis even in larger tumors (i.e. cT1b/T2, >4 cm) on AS is very low [6] but varies substantially based on different histologic subtypes [7]. Data suggest that clear cell renal cell carcinoma (ccRCC), the most common subtype, carries a worse prognosis and higher metastatic risk than other RCC subtypes [8]. Discerning between these entities could expedite treatment of higher risk patients and in theory, avoid progression or metastasis.
While historically renal mass biopsy (RMB) was not endorsed due to diagnostic inaccuracy and concern for tract seeding, it is now an option to evaluate for malignancy [9]. However, its use remains controversial [10]. While some groups have advocated for routine use of RMB [11], this practice is not widely accepted. Diagnostic accuracy of RMB has improved with superior imaging/targeting but remains imperfect (14.1% non-diagnostic rate) [12] and not all masses are amenable to biopsy (e.g. hilar and anterior). RMB is also invasive, typically requires patient anesthetic, and has a small risk (1%) for hemorrhage requiring intervention [13].
Multiparametric magnetic resonance imaging (mpMRI) offers non-invasive, radiation-free characterization of renal masses with the goal of determining RCC subtype [14-16]. A Likert scale-based clear cell likelihood score (ccLS) to identify ccRCC has been proposed and demonstrated 79% accuracy, 78% sensitivity, 80% specificity, 80% positive (PPV) and 80% negative (NPV) predictive values for cT1a ccRCC in a retrospective review of 121 masses [17]. Inter-reader variability was found to be moderate to good (mean κ = 0.53). Based on these results, we incorporated the ccLS in clinical reports of MRI examinations and subsequently demonstrated good diagnostic performance in a small cohort of cT1a renal masses[18]. In this study, we performed a retrospective review to assess the diagnostic performance of ccLS prospectively assigned to renal masses of all sizes and stages evaluated with mpMRI prior to histopathologic evaluation in two diverse patient populations.
MATERIALS AND METHODS:
This bi-institutional (Institution 1 [University of Texas Southwestern Medical Center], Institution 2 [Parkland Health and Hospital System]) retrospective cohort study was approved by the Institutional Review Board. The requirement for informed consent was waived. The study was compliant with the Health Insurance Portability and Accountability Act. Diagnostic performance of ccLS in a small subgroup of 59 patients with cT1a masses (Institution 1) has been recently reported[18]. Institution 1 is an academic tertiary-care medical center whereas Institution 2 is the affiliated safety net hospital system for Dallas county.
Study Design:
The ccLS was incorporated into the clinical reports for mpMRI of both institutions in June 2016. Our study period extended from this date to November 2019. The inclusion criterion was patients who underwent a mpMRI to evaluate a solid renal mass during the study period with subsequent confirmatory histologic diagnosis. Exclusion criteria was: 1) unable to complete mpMRI or mpMRI performed without IV contrast; 2) associated histopathology or biopsy prior to mpMRI; 3) masses excluded by the ccLS algorithm (e.g. presence of macroscopic fat, masses with less than 25% solid component [19]). Patients with suboptimal mpMRI examinations (e.g. motion artifact) and tumors with a less than 75% cystic necrotic/hemorrhagic component were included in the analysis as they constitute an intent to treat cohort. Patient demographics and clinical details (including age, gender, tumor size and histology) were extracted by chart review.
Image Acquisition and Analysis:
All clinical mpMRIs were performed on a 1.5T or 3T whole-body scanner using an abdominal phased-array coil (Institution 1: Philips Ingenia 1.5T and 3T, Philips Achieva 1.5T and 3T, Siemens Avanto 1.5T, and Siemens Aera 1.5T; Institution 2: Siemens Aera 1.5T, Siemens Avanto Fit 1.5T, Siemens Skyra 3T). The mpMRI protocol has been previously described and represents a standard clinical MRI protocol without and with contrast at both institutions [17]. Briefly, it includes coronal and axial fat-saturated T2-weighted single-shot fast spin echo; axial chemical shift T1-weighted; DWI with b-values of 0, 50, 400, 800; and multiplanar fat suppressed, DCE T1-weighted imaging, including corticomedullary, late nephrographic, and excretory phases. ADC maps were generated at the scanner using all b- values and a monoexponential decay model.
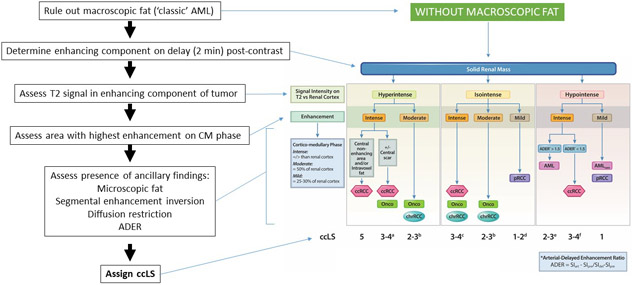
mpMRI interpretation was performed independently by one of 16 fellowship-trained abdominal radiologists covering the clinical service who had previously been trained in assigning ccLS. Each study was reviewed on a Picture Archiving and Communications System workstation (Institution 1: IntelliSpace, Philips Healthcare; Institution 2: Horizon Medical Imaging, McKesson). All mpMRIs were reported using a structured report that includes fields with all the relevant variables included in the algorithm and a final field to prospectively assign the ccLS [17,20,21] for each renal lesion identified. If multiple lesions were identified, a separate score was assigned to each. The ccLS is based on a previously reported diagnostic algorithm [20]. Although the diagnostic algorithm has not changed since its inception, specific rules for assigning ccLS have been implemented over time [22] (Figure 2). Quantitative analysis of signal intensity for assessment of various imaging features in the algorithm (e.g. corticomedullary enhancement [21], arterial-delayed enhancement ratio [23,24]) were calculated at the discretion of the radiologists interpreting the mpMRI. Generally, the designation of ‘intense enhancement’ requires an area in the renal mass that enhances to a degree similar to that of the renal cortex so qualitative assessment is sufficient. The distinction between mild and moderate enhancement (25-30% versus 50% relative to renal cortex) often requires quantitative assessment [22]. However, since interpretation was prospective and part of the clinical workload, the use of quantitative versus qualitative assessment was not tabulated.
Figure 2:
Clear Cell Likelihood Score (ccLS) diagnostic algorithm and image interpretation. The ccLS is a 5-tier classification that denotes the likelihood of a mass representing a clear cell carcinoma: 1, very unlikely; 2, unlikely; 3, equivocal; 4: likely; and 5, highly likely. First, reviewer must exclude the presence of macroscopic fat (i.e. ccLS is not applicable to masses with macroscopic fat), which would be diagnostic of angiomyolipoma. Second, assessment of delayed post-contrast images (2 minutes) helps define the enhancing portions of the mass. Renal mass signal characteristics and enhancement in subsequent steps is evaluated in the enhancing portion of the mass only (i.e. avoid non-enhancing portions, cystic degeneration, etc.). Third, the mass is classified as hyperintense, isointense, or hypointense relative to the renal cortex based on T2-weighted images (preferable on single-shot fast spin echo images without fat suppression). Fourth, renal mass is classified based on presence of intense (similar or higher), moderate (approximately 50%), or mild (approximately 25-30%) enhancement during the corticomedullary phase relative to the enhancement in the ipsilateral renal cortex. Assessment of enhancement is made with a region of interest (approximately 100 mm2) placed in the area of the tumor that demonstrates the most marked contrast enhancement during the corticomedullary phase on the basis of a visual assessment. Next, the mass is further characterized based on the presence of microscopic fat, segmental enhancement inversion, diffusion restriction (i.e. higher and lower signal intensity than renal parenchyma on b800 DWI and ADC, respectively), and the arterial-delayed enhancement ratio (ADER). Lastly, a ccLS is assigned. a – ccLS = 3 if segmental enhancement inversion (SEI) is present; b – ccLS = 2 if SEI is present; c – ccLS = 4 if microscopic fat is present; d – ccLS = 2 if enhancement is between 25% and 50%; e – ccLS = 2 if homogeneous or marked restriction on diffusion weighted images (DWI); f – ccLS = 3 if homogeneous or marked restriction on DWI, ccLS =4 if heterogeneous. AML = angiomyolipoma, CM = cortico-medullary, ccRCC = clear cell renal cell carcinoma, Onco = oncocytoma, chrRCC = chromophobe renal cell carcinoma, pRCC = papillary renal cell carcinoma, SIart = arterial phase signal intensity (SI), SIpre = pre-contrast SI, SIdel = delayed phase SI. (Modified with permission from [22])
Reference Standard
Histological analysis was performed by genitourinary pathologists according to the World Health Organization classification of renal neoplasms [25]. The subtype as determined on biopsy or extirpative surgery was the reference standard. In cases where biopsy was followed by extirpative surgery, the subtype as determined on extirpative surgery was the reference standard. Tumor stage was assigned per pathologic report for patients who underwent extirpative therapy and radiologic report for those undergoing RMB. Clinical stage was assigned based on review of imaging and notes in medical record.
Statistical Analysis
For diagnostic performance assessment, renal masses were grouped according to ccLS into positive for ccRCC (ccLS≥4), negative for ccRCC (ccLS≤2), and equivocal (ccLS3). Contingency tables were constructed to assess sensitivity and specificity both between institutions and in a pooled fashion. Post-test probabilities (PPV and NPV) were calculated from the pooled data.
Patient and tumor-specific categorical variables were compared between institutions using Fisher’s exact test. Continuous variables were compared by Mann-Whitney U test. Some categories with few counts were excluded or combined before entering the tests. For race, we compared the distribution of White, African American, and Hispanic. For clinical stage, clinical T2, T3, and T4 patients were combined for analysis.
Logistic regression models were used to assess the association between ccLS and ccRCC for each institution. A conditional logistic regression was used to estimate the overall odds ratio (pooled). Odds ratios were estimated for ccLS univariately as well as adjusted for patient (age, sex, BMI, race) and tumor characteristics (laterality, tumor size, and clinical stage). Correlation between ccLS and clinical stage was assessed using Spearman rank correlation. Differences in the proportion of tumors with and without histopathologic confirmation across ccLS groups were analyzed with a Cochran-Armitage trend test.
All data analysis was performed using SAS, version 9.4 (SAS Institute Inc.). P values of <0.05 were considered statistically significant.
RESULTS:
Patient/Tumor Characteristics:
Between both centers, a total of 993 mpMRIs with ccLS were performed during the study period. Of these, 154 studies represented serial imaging of the same mass and were excluded. Only the most contemporaneous mpMRI exam in these patients prior to the histologic diagnosis was used for analysis. Further, 442 masses were excluded due to lack of pathologic confirmation, 25 due to RMB performed prior to mpMRI, and 15 for other exclusion criteria (Figure 1). In total, 434 patients (263 males, 171 females) with 454 renal masses and mean age of 60 years (range 18-91) were eligible for analysis. Table 1 details the patient and tumor characteristics as stratified by clinical stage. Overall mean tumor size was 5.4±3.8 cm. Extirpative surgery was performed for 387/454 masses (85%) while RMB was performed on the remaining 67/454 (15%). A final histopathologic diagnosis of ccRCC was found in 284/454 (63%) masses whereas 133/454 (29%) masses were non-ccRCC malignancies. Benign diseases (oncocytoma [n=25], angiomyolipoma [n=6], fibrosis/sclerosis/inflammation [n=2], metanephric adenoma [n=2], malakoplakia [n=1], hyperplastic lymph node [n=1]) were identified in 37/454 (8%) masses.
Figure 1.
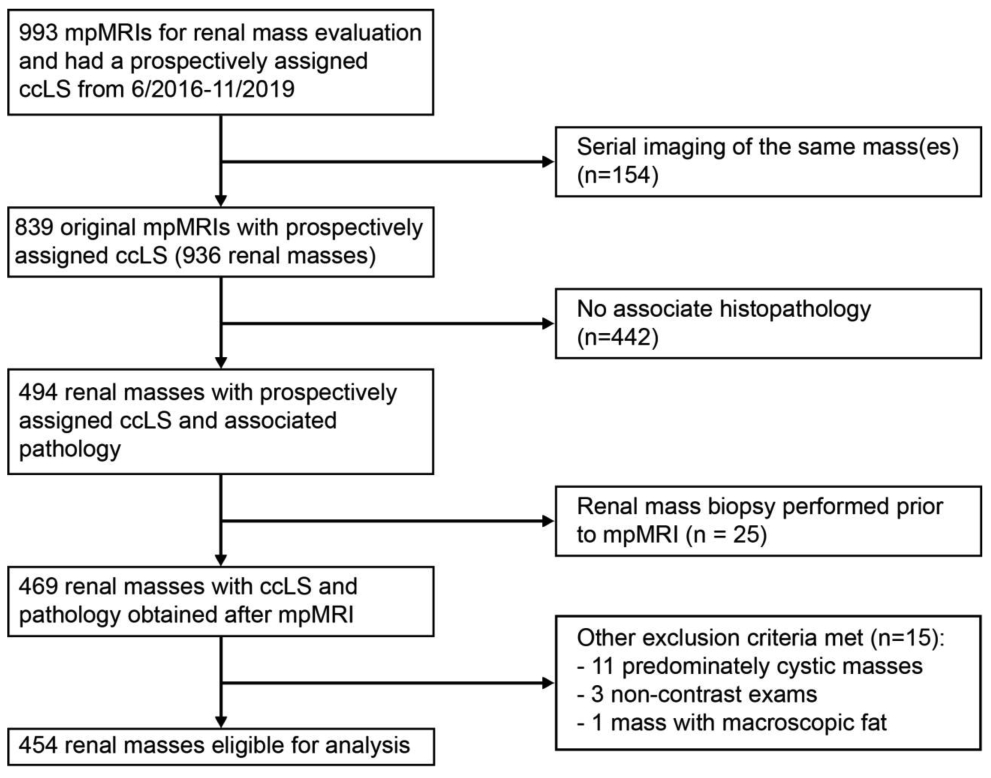
Flowsheet of multiparametric magnetic resonance images (mpMRIs) reviewed and assigned a clear cell likelihood score (ccLS) for study inclusion with exclusion criteria specified.
Table 1.
Baseline patient demographics and tumor characteristics as stratified by clinical stage
| cT1a | cT1b | cT1 | cT2-4 | All Stages | |
|---|---|---|---|---|---|
| No Patients (%) | 204 (47%) | 94 (22%) | 298 (69%) | 136 (31%) | 434 (100%) |
| No. Lesions (%) | 213 (47%) | 99 (22%) | 312 (69%) | 142 (31%) | 454 (100%) |
| Mean Age (years) ± SD | 59 ± 13 | 63 ± 12 | 60 ± 13 | 61 ± 13 | 60 ± 13 |
| Gender | |||||
| Male | 110 (54%) | 61 (65%) | 171 (57%) | 92 (68%) | 263 (61%) |
| Female | 94 (46%) | 33 (35%) | 127 (43%) | 44 (32%) | 171 (39%) |
| Race | |||||
| White | 101 (50%) | 56 (60%) | 157 (53%) | 85 (62%) | 242 (56%) |
| African American | 48 (24%) | 16 (17%) | 64 (21%) | 15 (11%) | 79 (18%) |
| Hispanic | 34 (17%) | 16 (17%) | 50 (17%) | 31 (23%) | 81 (19%) |
| Other | 9 (4%) | 2 (2%) | 11 (4%) | 2 (1%) | 13 (3%) |
| Unknown | 12 (6%) | 4 (4%) | 16 (5%) | 3 (2%) | 19 (4%) |
| Mean BMI ± SD | 30.2 ± 6.5 | 30.4 ± 6.3 | 30.2 ± 6.4 | 28.6 ± 6.2 | 29.7 ± 6.4 |
| Mean Tumor Size (cm) ± SD | 2.7 ± 0.8 | 5.1 ± 0.8 | 3.5 ± 1.3 | 9.7 ± 4.0 | 5.4 ± 3.8 |
| Tumor Laterality | |||||
| Left | 106 (50%) | 48 (48%) | 154 (49%) | 74 (52%) | 228 (50%) |
| Right | 107 (50%) | 51 (52%) | 158 (51%) | 68 (48%) | 226 (50%) |
| Pathology Source | |||||
| Extirpative | 170 (80%) | 86 (87%) | 256 (82%) | 131 (92%) | 387 (86%) |
| Renal Mass Biopsy | 43 (20%) | 13 (13%) | 56 (18%) | 11 (8%) | 67 (15%) |
| Histology | |||||
| Clear Cell RCC | 120 (56%) | 63 (64%) | 183 (59%) | 101 (71%) | 284 (63%) |
| Papillary RCC | 44 (21%) | 14 (14%) | 58 (18%) | 16 (11%) | 74 (16%) |
| Chromophobe RCC | 8 (4%) | 6 (6%) | 14 (4%) | 8 (6%) | 22 (5%) |
| Oncocytoma | 14 (7%) | 8 (8%) | 22 (7%) | 3 (2%) | 25 (6%) |
| Oncocytic Neoplasm | 2 (1%) | 2 (2%) | 4 (1%) | 1 (1%) | 5 (1%) |
| RCC NOS | 15 (7%) | 4 (4%) | 19 (6%) | 6 (4%) | 25 (6%) |
| TCC | 0 (0%) | 0 (0%) | 0 (0%) | 1 (1%) | 1 (0%) |
| Primary Renal Sarcoma | 0 (0%) | 0 (0%) | 0 (0%) | 1 (1%) | 1 (0%) |
| Renal Medullary | |||||
| Carcinoma | 0 (0%) | 0 (0%) | 0 (0%) | 1 (1%) | 1 (0%) |
| Unknown Carcinoma | 0 (0%) | 0 (0%) | 0 (0%) | 1 (1%) | 1 (0%) |
| Metastatic Lesion | 0 (0%) | 1 (1%) | 1 (0%) | 2 (1%) | 3 (1%) |
| Benign | 10 (5%) | 1 (1%) | 11 (4%) | 1 (1%) | 12 (3%) |
| Assigned ccLS | |||||
| 1 | 32 (15%) | 13 (13%) | 45 (14%) | 10 (7%) | 55 (12%) |
| 2 | 24 (11%) | 8 (8%) | 32 (10%) | 15 (11%) | 47 (10%) |
| 3 | 34 (16%) | 11 (11%) | 45 (14%) | 12 (8%) | 57 (13%) |
| 4 | 59 (28%) | 21 (21%) | 80 (26%) | 29 (20%) | 109 (24%) |
| 5 | 64 (30%) | 46 (46%) | 110 (35%) | 76 (54%) | 186 (41%) |
No. = Number; SD = standard deviation; BMI = body mass index; RCC = renal cell carcinoma; NOS= not otherwise specified; TCC = urothelial cell carcinoma; ccLS = clear cell likelihood score
Thirty-seven patients underwent RMB prior to extirpative therapy, of which 13 were ccLS≤2, 8 was ccLS3, and 16 were ccLS≥4. On pathology, the majority of ccLS≤2 masses were found to be pRCC (n=7), with the remainder chrRCC (n=2), ccRCC (n=2), unclassified RCC (n=1), and oncocytoma (n=1)., Three of the ccLS3 lesions were ccRCC with the remainder pRCC (n=2), chrRCC (n=2), and unclassified RCC (n=1). Of those masses with a ccLS≥4, 14 were ccRCC, 1 was a clear cell papillary variant, and 1 was hereditary leiomyomatosis and RCC-associated RCC on final pathology and genetic testing. There were 3 instances of histologic discordance between biopsy and extirpative pathology: a ccLS3 lesion noted to be ccRCC on biopsy was later re-classified as unclassified RCC on extirpative pathology; one ccLS2 and one ccLS3 lesions were labelled as oncocytic neoplasms on biopsy but later further subtyped as an oncocytoma and chrRCC on extirpative pathology, respectively.
One hundred and twenty-four patients had a mass <3 cm in diameter, of which 30 were ccLS≤2, 20 were ccLS3, and 74 were ccLS≤4. Two lesions (2/30, 7%) were found to harbor ccRCC in the ccLS≤2 group, while 10 (10/20, 50%) in the ccLS3 and 57 (57/74, 77%) in the ccLS≥4 groups were also ccRCC.
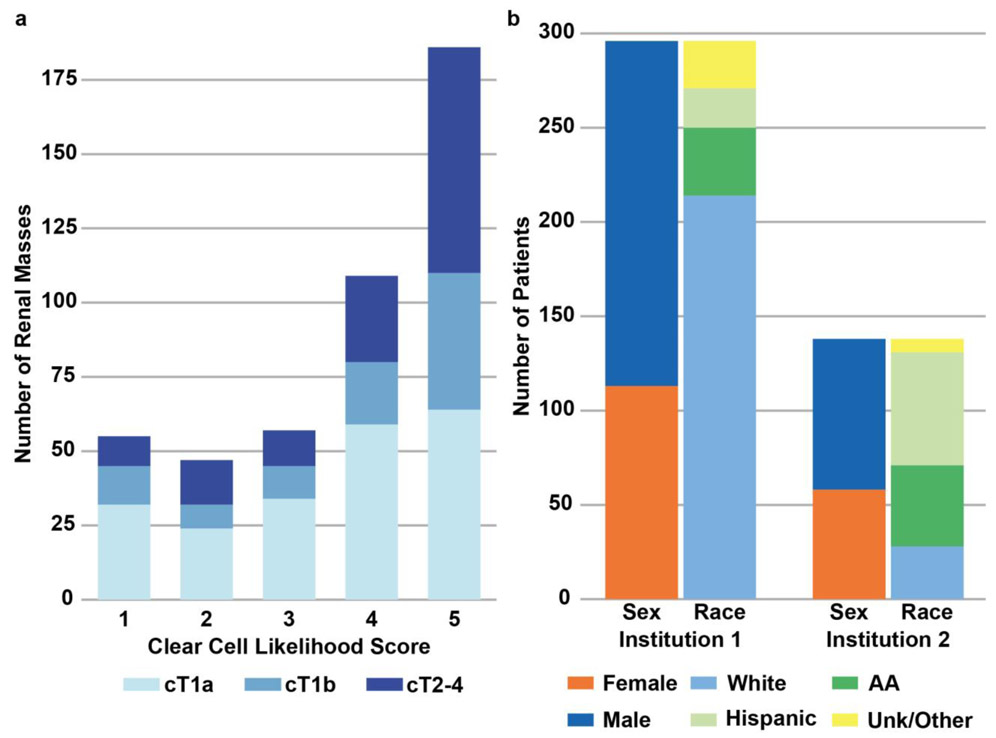
Higher tumor stage was associated with higher ccLS (p<0.001, Figure 3a). Figure 3b shows patient demographics by institution. Patient and tumor characteristics were similar between both institutions, though statistical differences were noted in patient age and race, as well as tumor laterality and histology (Table 2).
Figure 3.
A) Overall distribution of clear cell likelihood score (ccLS) and tumor stage in study cohort. B) Patients demographics for each institution. AA = African-American; Unk = Unknown
Table 2.
Baseline patient demographics and tumor characteristics as stratified by institution
| Institution 1 | Institution 2 | Pooled | P-value | |
|---|---|---|---|---|
| No Patients (%) | 296 (68%) | 138 (32%) | 434 (100%) | |
| No. Lesions (%) | 315 (69%) | 139 (31%) | 454 (100%) | |
| Mean Age (years) ± SD | 62 ± 13 | 56 ± 11 | 60 ± 13 | <.001 |
| Gender | 0.5 | |||
| Male | 183 (62%) | 80 (58%) | 263 (61%) | |
| Female | 113 (38%) | 58 (41%) | 171 (39%) | |
| Race | <.001 | |||
| White | 217 (72%) | 30 (21%) | 247 (56%) | |
| African American | 37 (12%) | 43 (30%) | 80 (18%) | |
| Hispanic | 21 (7%) | 63 (44%) | 84 (19%) | |
| Other | 9 (3%) | 4 (3%) | 13 (3%) | |
| Unknown | 16 (5%) | 3 (2%) | 19 (4%) | |
| Mean BMI ± SD | 29.6 ± 6.2 | 30.1 ± 6.9 | 29.7 ± 6.4 | 0.3 |
| Mean Tumor Size (cm) ± SD | 5.3 ± 3.7 | 5.8 ± 4.0 | 5.4 ± 3.8 | 0.07 |
| Tumor Laterality | 0.04 | |||
| Left | 148 (47%) | 80 (58%) | 228 (50%) | |
| Right | 167 (53%) | 59 (42%) | 226 (50%) | |
| Pathology Source | 0.7 | |||
| Extirpative | 270 (86%) | 117 (84%) | 387 (85%) | |
| Renal Mass Biopsy | 45 (14%) | 22 (16%) | 67 (15%) | |
| Histology | 0.04 | |||
| Clear Cell RCC | 201 (64%) | 83 (60%) | 284 (63%) | |
| Papillary RCC | 43 (14%) | 31 (22%) | 74 (16%) | |
| Chromophobe RCC | 14 (4%) | 8 (6%) | 22 (5%) | |
| Oncocytoma | 21 (7%) | 4 (3%) | 25 (6%) | |
| Oncocytic Neoplasm | 4 (1%) | 1 (1%) | 5 (1%) | |
| RCC NOS | 14 (4%) | 11 (8%) | 25 (6%) | |
| TCC | 1 (0%) | 0 (0%) | 1 (0%) | |
| Primary Renal Sarcoma | 1 (0%) | 0 (0%) | 1 (0%) | |
| Renal Medullary Carcinoma | 0 (0%) | 1 (1%) | 1 (0%) | |
| Unknown Carcinoma | 1 (0%) | 0 (0%) | 1 (0%) | |
| Metastatic Lesion | 3 (1%) | 0 (0%) | 3 (1%) | |
| Benign | 12 (4%) | 0 (0%) | 12 (3%) | |
| Assigned ccLS | 0.4 | |||
| 1 | 32 (10%) | 23 (17%) | 56 (12%) | |
| 2 | 34 (11%) | 13 (9%) | 47 (10%) | |
| 3 | 39 (12%) | 18 (13%) | 57 (13%) | |
| 4 | 80 (25%) | 29 (21%) | 109 (24%) | |
| 5 | 130 (41%) | 56 (40%) | 186 (41%) |
No. = number; SD = standard deviation; BMI = body mass index; RCC = renal cell carcinoma; NOS= not otherwise specified; TCC = urothelial cell carcinoma; ccLS = clear cell likelihood score
ccLS score and tumor size distribution in tumors both with and without histologic confirmation are shown in Supplemental Figure 1. Histopathologic confirmation was lower in smaller tumors (<4cm) than larger tumors (>4 cm) or locally advanced stage (T3-T4). The proportion of tumors with histopathologic confirmation increased in higher ccLS groups (p<0.001).
Association between ccLS and ccRCC:
The prevalence of ccRCC among solid renal masses between institutions were comparable (Institution 1: 64%; Institution 2: 60%; p=0.46) and the distribution of ccLS between institutions were similar. Univariate logistic regression identified an approximately 4- to 5-fold increase in the odds for ccRCC with each increasing ccLS level at both institutions, as well as in pooled analysis (Table 3). Multivariable regression, controlling for the previously mentioned covariates, confirmed these findings.
Table 3.
Diagnostic odds ratio of clear cell likelihood score as predictive of clear cell renal cell carcinoma histopathology
| Institution 1 | Institution 2 | Pooled | |
|---|---|---|---|
| Univariate Odds Ratio (95% CI) | 5.21 (3.68-7.37) | 4.36 (2.77-6.86) | 4.85 (3.68-6.38) |
| Adjusted Odds Ratio (95% CI) | 4.95 (3.44-7.12) | 4.83 (2.54-9.19) | 4.66 (3.44-6.30) |
CI = Confidence interval
Diagnostic Performance:
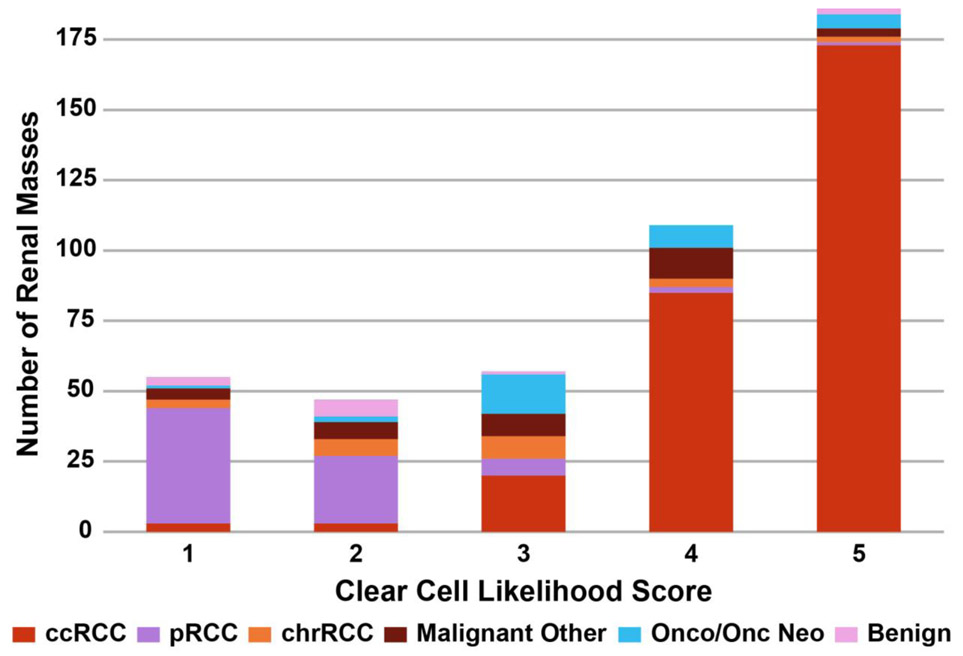
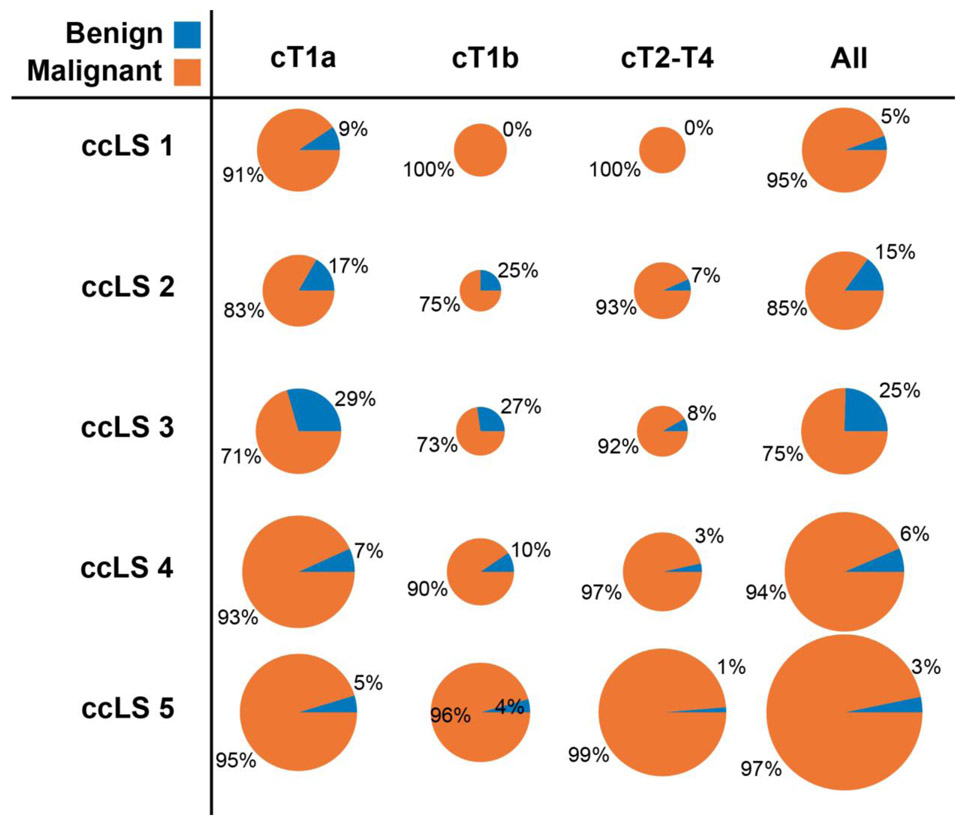
Of 284 histologically proven ccRCCs, 258 were assigned ccLS≥4 (sensitivity=91%). Of 170 histologically proven non-ccRCCs, 96 were assigned ccLS≤2 (specificity=56%). Table 4 lists the diagnostic accuracy stratified by clinical stage and per institution. Similarity of diagnostic performance between institutions was supported by the overlaying confidence intervals. Figure 4 demonstrates the distribution of pathologies across the ccLS strata. Figure 5 further demonstrates the percentages of all malignant pathologies by both ccLS and clinical stage with 94% of ccLS4 and 97% of ccLS5 representing malignant disease. Importantly, the number of tumors in these two categories constitute majority of all tumors (24% ccLS4; 41% ccLS5). In two cases, a mass assigned cT3-4 was benign on pathology: one case of malakoplakia with extension beyond Gerota’s fascia and one case of oncocytoma with extension into the perirenal fat [26].
Table 4.
Diagnostic accuracy of the clear cell likelihood score stratified by clinical stage and institution.
| By Clinical Stage | By Institution | All Lesions |
|||||
|---|---|---|---|---|---|---|---|
| cT1a | cT1b | cT1 | cT2-4 | Inst. 1 | Inst. 2 | ||
| Number of Lesions | 213 | 99 | 312 | 142 | 315 | 139 | 454 |
| 47% | 22% | 69% | 31% | 69% | 31% | 100% | |
| Accuracy | 157/213 | 79/99 | 236/312 | 118/142 | 248/315 | 106/139 | 354/454 |
| (95% CI) | 74%(67-79%) | 80%(71-87%) | 76%(71-80%) | 83%(76-88%) | 79%(74-83%) | 76%(69-83%) | 78%(74-82%) |
| Sensitivity | 103/120 | 59/63 | 162/183 | 96/101 | 186/201 | 72/83 | 258/284 |
| (95% CI) | 86%(78-91%) | 94%(85-98%) | 89%(83-92%) | 95%(89-98%) | 93%(88-95%) | 87%(79-92%) | 91%(87-94%) |
| Specificity | 54/93 | 20/36 | 74/129 | 22/41 | 62/114 | 34/56 | 96/170 |
| (95% CI) | 58%(48-68%) | 56%(40-70%) | 57%(49-66%) | 54%(39-68%) | 54%(45-63%) | 61%(48-72%) | 56%(49-64%) |
| PPV | 103/123 | 59/67 | 162/190 | 96/105 | 186/210 | 72/85 | 258/295 |
| (95% CI) | 84%(76-89%) | 88%(78-94%) | 85%(80-90%) | 91%(85-95%) | 89%(84-92%) | 85%(76-91%) | 87%(83-91%) |
| NPV | 54/56 | 20/21 | 74/77 | 22/25 | 62/66 | 34/36 | 96/102 |
| (95% CI) | 96%(88-99%) | 95%(77-99%) | 96%(89-99%) | 88%(70-96%) | 94%(85-98%) | 94%(82-98%) | 94%(88-97%) |
Inst. = Institution; PPV = Positive predictive value; NPV = Negative predictive value; CI = Confidence interval
Figure 4.
Distribution of histopathologic findings as stratified by clear cell likelihood score (ccLS). ccRCC = clear cell renal cell carcinoma; pRCC = papillary renal cell carcinoma; chrRCC = chromophobe renal cell carcinoma; Onco = oncocytoma; Onc Neo = oncocytic neoplasm
Figure 5.
Incidence of malignant histology when stratified by clear cell likelihood score (ccLS) and clinical stage. The area of each disk corresponds directly to the number of masses falling in the corresponding category.
DISCUSSION:
Classically, renal masses without macroscopic fat on imaging were considered renal cell carcinomas requiring definitive extirpative therapy regardless of size. RMB was advised against due to concerns for tract seeding and diagnostic inaccuracy. Over the past 20 years, there have been significant paradigm shifts in managing renal masses, including AS, ablative therapy, and improved RMB accuracy. More recently, neoadjuvant therapy is being tested in patients with advanced localized disease [27]. As medical imaging capabilities further advance, evaluating the diagnostic abilities of imaging may reduce the need to utilize currently available, yet invasive, techniques (e.g. RMB).
We acknowledge that imaging does not completely replace formal histopathologic evaluation and that ccLS focuses only on determination of the pre-test probability of ccRCC (or non-ccRCC) to guide management decision making. Indeed, the ccLS is not intended to classify tumors as malignant versus benign. Furthermore, ccLS does not differentiate low- versus high-grade ccRCC nor does it differentiate other less common aggressive non-ccRCC malignancies (e.g. type 2 pRCC). However, the use of ccLS as a tool to manage patients with renal masses has merits.
First, we find it useful in patients with small renal masses (<4cm, cT1a) who are potential candidates for both AS and surgical treatment. It can help the patient and treating urologist decide between definitive treatment (ccLS≥4), biopsy (ccLS3), or AS (ccLS≤2), depending on other patient factors and shared decision making [22]. Applying this approach to our cohort would have theoretically resulted in definitive treatment of a truly benign mass in only 6% of cases (7/123), substantially lower than the approximately 13% rate of benign disease quoted in the literature [28]. While most ccLS≤2 cT1a lesions are malignant (49/56, 88%), most of these lesions are pRCCs (41/56, 73%), which are more indolent tumors [29]. Only two lesions (2/56, 4%) were ccRCC in this group. Importantly, these lesions are followed with serial imaging as per routine AS protocols at most institutions (i.e., initial follow-up in 6 months followed by annual imaging). Subsequently, less common, rapidly growing, tumors representing aggressive histology in this group can be promptly treated.
Second, ccLS substantially reduces the need for RMB in management decision and patient disposition. In our cohort, only 13% of masses (57/454) would be eligible for a biopsy, sparing the remaining 87% the risk and morbidity of RMB.
Third, ccLS may add diagnostic information for frail or significantly comorbid patients with cT1b-T2 lesions in whom ‘extended criteria’ AS could be considered [6]. ccLS may play a complementary role in those patients with larger, heterogeneous masses where RCC subtyping based on RMB may be indeterminate. Ultimately, the ability to replace RMB with mpMRI in most cases prior to systemic therapy is desired but further investigation in larger cohorts are necessary. Depending of institutional practice, many such patients may undergo MRI for clinical staging (e.g. evaluate for renal vein thrombus) where ccLS may be included in the interpretation.
Fourth, some may argue that identification of ccRCC versus other subtypes is insufficient as International Society of Urological Pathology (ISUP)/World Health Organization (WHO) grade is the critical feature denoting tumor aggressiveness. We acknowledge that this is an important consideration. However, most tumors, including both extirpative and RMB cases, are not ISUP grade 1 or 4 making the distinction of low- vs high-grade less clear [30-32]. Further, RMB to extirpative pathology Fuhrman grade concordance is low (52-76%) [12]. Wang, et al. identified higher concordance (96%) but omitted carcinoma subtype in many biopsies [33]. This is consistent with reported concordance at our institution[34]. Ball et al. reported on intratumoral heterogeneity in renal masses, identifying that nearly 40% of high-grade tumors had substantial amounts of low-grade tissue [35], underscoring the previously mentioned poor RMB to extirpative pathology concordance. Given the unreliable nature of tumor grade in most cases, management decision making generally relies upon the pathologic subtype to which ccLS can be beneficial. In the authors’ experience, the high PPV of ccLS≥4 for ccRCC in cT1a (103/123, 84%) facilitates the decision for definitive treatment.
Routine use of RMB was proposed recently [11] but the diagnostic ability of RMB also has limitations. He, et al. reported sensitivity of 95% and specificity of 100% for detecting malignancy but excluded non-diagnostic biopsies [36]. Patel, et al. demonstrated 4.0% false-positive and 3.1% false-negative rate for malignancy, but the NPV was low (63%) [12]. Inherent in all these reports is selection bias, as hilar and anterior tumors are often not amenable to RMB. Further, renal mass subtyping with RMB can be challenging due to scant material. In this study, no patients undergoing mpMRI were excluded due to technical reasons.
Further, RMB carries the risk of procedural complications, such as hematoma (~5%), pain (~1-2%), pneumothorax (~0.5%), and hemorrhage (~0.4%) [12]. High grade complications (Clavien III or greater) are uncommon (0.5-1%) [12,33]. In the event of a complication, definitive treatment of the tumor is often delayed, and surgery may be more complicated.
Our study has some limitations. Our cohort was only moderately sized although remains the largest series evaluating ccLS diagnostic performance. The ccLS has some subjectivity in its determination, and interpretation could vary based upon the experience of the radiologist. MRI studies were interpreted by many fellowship-trained abdominal radiologists in clinical practice, a scenario that replicates the practice of many academic centers but may not be reproducible in other settings. However, a previous study on multiple radiology readers of varied experience level suggested that inter-reader variability for ccLS may be small [17]. Furthermore, specific rules to guide interpretation have been incorporated since its original inception to improve reproducibility across practices [22]. mpMRI is not the first line imaging modality (e.g. many patients undergo CT). Therefore, not all renal masses underwent mpMRI. Further, there are no strict institutional practice guidelines with regards to renal masses evaluation. Accordingly, our cohort may suffer from selection bias. For example, the incidence of benign disease (8% overall, 11% in cT1a) is lower than previously reported [37]. Our analysis of patients without histologic confirmation indicates that patients with lower ccLS scores and smaller size were less likely to have confirmatory histopathology. Therefore, it is possible that patients with suspected benign or non-ccRCC disease based on mpMRI undergo AS more frequently at our institution without confirmatory biopsy. If this were to be the case, our reported specificity would be underestimated. Although ccLS performance was evaluated in 2 separate patient cohorts from 2 institutions with different MRI equipment, the protocols and interpretative radiologists were similar. Multi-institutional validation of ccLS with different radiologists is needed. Finally, as with any new diagnostic modality, a cost analysis should be performed relative to RMB.
In conclusion, we confirm the good accuracy and diagnostic performance of mpMRI to classify a renal mass as ccRCC in a cohort of patients with solid renal masses in a bi-institutional study. While ccLS does not provide reliable information about the likelihood of malignant histology or oncologic aggressiveness in a renal mass, it provides valuable information for management decision making. The high PPV of ccLS≥4 and high NPV of ccLS≤2 for ccRCC can help inform more selective use of invasive biopsies in patients with renal masses of any stage.
Supplementary Material
Supplemental Figure 1. A) Distribution of lesions with and without pathology (path) by clear cell likelihood score (ccLS). B) Distribution of lesions with and without pathology by ranges of tumor size and evidence invasive features on MRI: <4 cm (i.e., T1a), 4-7 cm (i.e., T1b), >7 cm or T3-4 (i.e., T2-4).
Key Points:
The positive predictive value of the clear cell likelihood score (ccLS) for detecting clear cell renal cell carcinoma was 5% (ccLS1), 6% (ccLS2), 35% (ccLS3), 78% (ccLS4), and 93% (ccLS5). Sensitivity of ccLS≥4 and specificity of ccLS≤2 were 91% and 56%, respectively
When controlling for confounding variables, ccLS is an independent risk factor for identifying clear cell renal cell carcinoma
Utilization of the ccLS can help guide clinical care, including the decision for renal mass biopsy, reducing the morbidity and risk to patients
1.
Funding information
This study has received funding by the NIH grants #U01CA207091, #P50CA196516 and #5RO1CA154475.
Abbreviations:
- ADC
Apparent diffusion coefficient
- AS
active surveillance
- ccLS
clear cell likelihood score
- ccRCC
clear cell renal cell carcinoma
- chrRCC
chromophobe RCC
- mpMRI
multiparametric magnetic resonance imaging
- pRCC
papillary RCC
- RMB
renal mass biopsy
Footnotes
Guarantor:
The scientific guarantor of this publication is Ivan Pedrosa.
Conflict of Interest:
Ivan Pedrosa served in an Advisory Scientific Board for Bayer Healthcare.
Institutional Research Agreement, Philips Healthcare
Institutional Research Agreement, Siemens Healthineers
Institutional Research Agreement, GE Healthcare
Statistics and Biometry:
One of the authors (YX) has significant statistical expertise.
Informed Consent:
Written informed consent was waived by the Institutional Review Board.
Ethical Approval:
Institutional Review Board approval was obtained.
Study subjects or cohorts overlap:
Some study subjects or cohorts have been previously reported in Johnson BA, Kim S, Steinberg RL, Diaz de Leon A, Pedrosa I, Cadeddu JA, “Diagnostic performance of prospectively assigned clear cell Likelihood scores (ccLS) in small renal masses at multiparametric magnetic resonance imaging,” Urologic Oncology: Seminars and Original Investigations, 2019.
Methodology
- retrospective
- diagnostic or prognostic study
- performed at one institution
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
REFERENCES:
- 1.Smith-Bindman R, Miglioretti DL, and Larson EB, Rising Use Of Diagnostic Medical Imaging In A Large Integrated Health System. Health Aff (Millwood), 2008. 27(6): p. 1491–1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Znaor A, Lortet-Tieulent J, Laversanne M, Jemal A, and Bray F, International Variations and Trends in Renal Cell Carcinoma Incidence and Mortality. Eur Urol, 2015. 67(3): p. 519–530. [DOI] [PubMed] [Google Scholar]
- 3.Mason RJ, Abdolell M, Trottier G, et al. , Growth Kinetics of Renal Masses: Analysis of a Prospective Cohort of Patients Undergoing Active Surveillance. Eur Urol, 2011. 59(5): p. 863–867. [DOI] [PubMed] [Google Scholar]
- 4.Campbell SC, Novick AC, Belldegrun A, et al. , Guideline for management of the clinical T1 renal mass. J Urol, 2009. 182(4): p. 1271–9. [DOI] [PubMed] [Google Scholar]
- 5.Escudier B, Porta C, Schmidinger M, et al. , Renal cell carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol, 2016. 27(suppl 5): p. v58–v68. [DOI] [PubMed] [Google Scholar]
- 6.Mehrazin R, Smaldone MC, Kutikov A, et al. , Growth Kinetics and Short-Term Outcomes of cT1b and cT2 Renal Masses under Active Surveillance. J Urol, 2014. 192(3): p. 659–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leibovich BC, Lohse CM, Cheville JC, et al. , Predicting Oncologic Outcomes in Renal Cell Carcinoma After Surgery. Eur Urol, 2018. 73(5): p. 772–780. [DOI] [PubMed] [Google Scholar]
- 8.Delahunt B, Cheville JC, Martignoni G, et al. , The International Society of Urological Pathology (ISUP) grading system for renal cell carcinoma and other prognostic parameters. Am J Surg Pathol, 2013. 37(10): p. 1490–504. [DOI] [PubMed] [Google Scholar]
- 9.Campbell S, Uzzo RG, Allaf ME, et al. , Renal Mass and Localized Renal Cancer: AUA Guideline. J Urol, 2017. 198(3): p. 520–529. [DOI] [PubMed] [Google Scholar]
- 10.Kutikov A, Smaldone MC, Uzzo RG, Haifler M, Bratslavsky G, and Leibovich BC, Renal Mass Biopsy: Always, Sometimes, or Never? Eur Urol, 2016. 70(3): p. 403–406. [DOI] [PubMed] [Google Scholar]
- 11.Richard PO, Lavallee LT, Pouliot F, et al. , Is Routine Renal Tumor Biopsy Associated with Lower Rates of Benign Histology following Nephrectomy for Small Renal Masses? J Urol, 2018. 200(4): p. 731–736. [DOI] [PubMed] [Google Scholar]
- 12.Patel HD, Johnson MH, Pierorazio PM, et al. , Diagnostic Accuracy and Risks of Biopsy in the Diagnosis of a Renal Mass Suspicious for Localized Renal Cell Carcinoma: Systematic Review of the Literature. J Urol, 2016. 195(5): p. 1340–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tomaszewski JJ, Uzzo RG, and Smaldone MC, Heterogeneity and renal mass biopsy: a review of its role and reliability. Cancer Biol Med, 2014. 11(3): p. 162–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Allen BC, Tirman P, Jennings Clingan M, Manny J, Del Gaizo AJ, and Leyendecker JR, Characterizing solid renal neoplasms with MRI in adults. Abdom Imaging, 2014. 39(2): p. 358–87. [DOI] [PubMed] [Google Scholar]
- 15.Hotker AM, Mazaheri Y, Wibmer A, et al. , Differentiation of Clear Cell Renal Cell Carcinoma From Other Renal Cortical Tumors by Use of a Quantitative Multiparametric MRI Approach. AJR Am J Roentgenol, 2017. 208(3): p. W85–W91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cornelis F, Tricaud E, Lasserre AS, et al. , Routinely performed multiparametric magnetic resonance imaging helps to differentiate common subtypes of renal tumours. Eur Radiol, 2014. 24(5): p. 1068–1080. [DOI] [PubMed] [Google Scholar]
- 17.Canvasser NE, Kay FU, Xi Y, et al. , Diagnostic Accuracy of Multiparametric Magnetic Resonance Imaging to Identify Clear Cell Renal Cell Carcinoma in cT1a Renal Masses. J Urol, 2017. 198(4): p. 780–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson BA, Kim S, Steinberg RL, de Leon AD, Pedrosa I, and Cadeddu JA, Diagnostic performance of prospectively assigned clear cell Likelihood scores (ccLS) in small renal masses at multiparametric magnetic resonance imaging. Urol Oncol, 2019. 37(12): p. 941–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Silverman SG, Pedrosa I, Ellis JH, et al. , Bosniak Classification of Cystic Renal Masses, Version 2019: An Update Proposal and Needs Assessment. Radiology, 2019. 292(2): p. 475–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kay FU and Pedrosa I, Imaging of Solid Renal Masses. Radiol Clin North Am, 2017. 55(2): p. 243–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kay FU, Canvasser NE, Xi Y, et al. , Diagnostic Performance and Interreader Agreement of a Standardized MR Imaging Approach in the Prediction of Small Renal Mass Histology. Radiology, 2018. 287(2): p. 543–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Diaz de Leon A, Davenport MS, Silverman SG, Schieda N, Cadeddu JA, and Pedrosa I, Role of Virtual Biopsy in the Management of Renal Masses. AJR Am J Roentgenol, 2019: p. 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun MR, Ngo L, Genega EM, et al. , Renal cell carcinoma: dynamic contrast-enhanced MR imaging for differentiation of tumor subtypes--correlation with pathologic findings. Radiology, 2009. 250(3): p. 793–802. [DOI] [PubMed] [Google Scholar]
- 24.Sasiwimonphan K, Takahashi N, Leibovich BC, Carter RE, Atwell TD, and Kawashima A, Small (<4 cm) renal mass: differentiation of angiomyolipoma without visible fat from renal cell carcinoma utilizing MR imaging. Radiology, 2012. 263(1): p. 160–8. [DOI] [PubMed] [Google Scholar]
- 25.Moch H, Cubilla AL, Humphrey PA, Reuter VE, and Ulbright TM, The 2016 WHO Classification of Tumours of the Urinary System and Male Genital Organs-Part A: Renal, Penile, and Testicular Tumours. Eur Urol, 2016. 70(1): p. 93–105. [DOI] [PubMed] [Google Scholar]
- 26.Gudbjartsson T, Hardarson S, Petursdottir V, Thoroddsen A, Magnusson J, and Einarsson GV, Renal oncocytoma: a clinicopathological analysis of 45 consecutive cases. BJU Int, 2005. 96(9): p. 1275–1279. [DOI] [PubMed] [Google Scholar]
- 27.Bindayi A, Hamilton ZA, McDonald ML, et al. , Neoadjuvant therapy for localized and locally advanced renal cell carcinoma. Urol Oncol, 2018. 36(1): p. 31–37. [DOI] [PubMed] [Google Scholar]
- 28.Frank I, Blute ML, Cheville JC, Lohse CM, Weaver AL, and Zincke H, Solid renal tumors: An analysis of pathological features related to tumor size. J Urol, 2003. 170(6): p. 2217–2220. [DOI] [PubMed] [Google Scholar]
- 29.Beck SD, Patel MI, Snyder ME, et al. , Effect of papillary and chromophobe cell type on disease-free survival after nephrectomy for renal cell carcinoma. Ann Surg Oncol, 2004. 11(1): p. 71–7. [DOI] [PubMed] [Google Scholar]
- 30.Patard JJ, Leray E, Rioux-Leclercq N, et al. , Prognostic value of histologic subtypes in renal cell carcinoma: a multicenter experience. J Clin Oncol, 2005. 23(12): p. 2763–71. [DOI] [PubMed] [Google Scholar]
- 31.Campbell SC, Novick AC, Herts B, et al. , Prospective evaluation of fine needle aspiration of small, solid renal masses: accuracy and morbidity. Urology, 1997. 50(1): p. 25–9. [DOI] [PubMed] [Google Scholar]
- 32.Leveridge MJ, Finelli A, Kachura JR, et al. , Outcomes of small renal mass needle core biopsy, nondiagnostic percutaneous biopsy, and the role of repeat biopsy. Eur Urol, 2011. 60(3): p. 578–84. [DOI] [PubMed] [Google Scholar]
- 33.Wang R, Wolf JS, Wood DP, Higgins EJ, and Hafez KS, Accuracy of Percutaneous Core Biopsy in Management of Small Renal Masses. Urology, 2009. 73(3): p. 586–590. [DOI] [PubMed] [Google Scholar]
- 34.Friedman P, Sayah M, Egharevba A, et al. , Evaluation of the Concordance of Histologic Subtype and Prognostic Indicators Between Renal Cell Carcinoma Biopsies and Their Subsequent Resections, in USCAP 104th Annual Meeting 2015, Mod Pathol: Boston, MA p. 202–271. [Google Scholar]
- 35.Ball MW, Bezerra SM, Gorin MA, et al. , Grade heterogeneity in small renal masses: potential implications for renal mass biopsy. J Urol, 2015. 193(1): p. 36–40. [DOI] [PubMed] [Google Scholar]
- 36.He QQ, Wang HZ, Kenyon J, et al. , Accuracy of Percutaneous Core Biopsy in the Diagnosis of Small Renal Masses (<= 4.0 cm): A Meta-analysis. Int Braz J Urol, 2015. 41(1): p. 15–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Johnson DC, Vukina J, Smith AB, et al. , Preoperatively misclassified, surgically removed benign renal masses: a systematic review of surgical series and United States population level burden estimate. J Urol, 2015. 193(1): p. 30–5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. A) Distribution of lesions with and without pathology (path) by clear cell likelihood score (ccLS). B) Distribution of lesions with and without pathology by ranges of tumor size and evidence invasive features on MRI: <4 cm (i.e., T1a), 4-7 cm (i.e., T1b), >7 cm or T3-4 (i.e., T2-4).