Abstract
The glymphatic system is network of perivascular spaces through which cerebrospinal fluid and interstitial fluid can move through the brain, clearing metabolic waste, such as amyloid beta, lactate and more, from the parenchyma. This cleaning system is regulated by sleep and norepinephrine, with increased levels of norepinephrine during wakefulness inhibiting fluid movement. Norepinephrine is also essential for transition from acute to chronic pain, and sufferers of chronic neuropathic pain frequently present with sleep disruption. These connections among glymphatic clearance, sleep, and pain are very intriguing, and might lead to nonpharmaceutical interventions for pain treatment. This short perspective provides a rationale for the hypothesis that mind–body interventions—such as acupuncture—can reduce norepinephrine and increase glymphatic function, ultimately relieving chronic neuropathic pain.
Keywords: glymphatic, norepinephrine, chronic neuropathic pain, acupuncture, neuroinflammation
Introduction
Pain is an uncomfortable warning sign intended to draw attention to a body part at risk. It is an adaptive mechanism initiated to avoid additional injury. However, chronic neuropathic pain, such as back pain, persists long after the initial insult and is often maladaptive, negatively impacting the life quality of a large number of people worldwide.1,2 The conventional prescribed analgesics are often not effective or are associated with unacceptable side-effects. The current opioid overdose epidemic is—at least in part—a result of providing uncritical prescriptions to patients in need of temporary pain relief, for example, following surgeries. Here, this article presents a new understanding of brain homeostasis and cleaning, combined with conventional views on chronic neuropathic pain development, to propose the idea of treating multiple aspects of pain, perhaps via acupuncture, as a new approach to chronic neuropathic pain.
Discussion
In tissue throughout the body, cellular debris and toxic molecules in the interstitial fluid (ISF) are drained via lymphatic vessels. However, the brain and spinal cord lack a conventional lymphatic system and only a few waste products, such as amyloid-β (Aβ), a protein, can be transported across the blood–brain barrier.3 Furthermore, the buildup of protein aggregates is a common feature in many age-related neurodegenerative disorders, such as Alzheimer's disease and Parkinson's disease. Although the brain and central nervous system are characterized by disproportionately high metabolic rates, compared to the rest of the body, conventional lymphatic vessels are not responsible for elimination of their metabolic wastes. The mechanisms underlying solute clearance in the brain have long puzzled researchers. It was previously believed that, due to the lack of a conventional lymphatic system, the human being's most vital organ—the brain—was responsible for recycling its own wastes. Attempting to understand this paradox ultimately led to the current authors' interest in the novel brain-clearance mechanism—the glymphatic pathway.
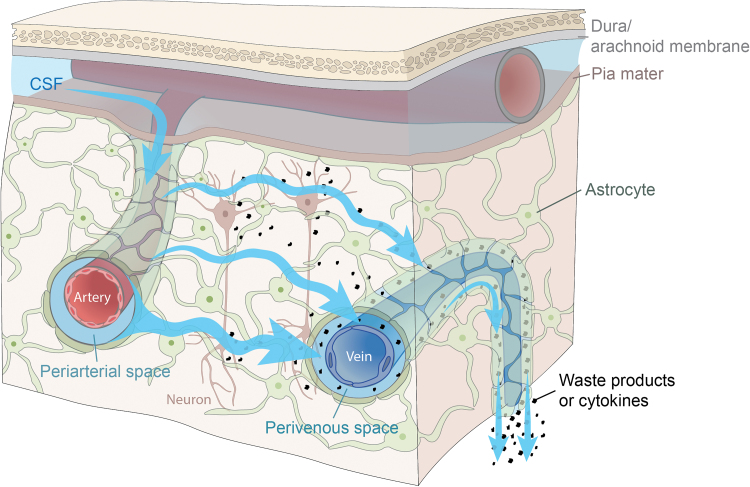
The glymphatic system is a glial-dependent perivascular network that plays a pseudolymphatic role in the brain (Fig. 1). Within the glymphatic pathway, CSF enters the brain via periarterial spaces, passes into the interstitium, and ISF, containing waste products, exits via the perivenous spaces and along cranial and spinal nerves.4 This process is facilitated by perivascular astrocytic aquaporin-4.5 CSF transport is set in motion as a polarized, directed fluid flow driven by arterial pulsations, respiration, and slow vasomotion.6–10 This is achieved through pumping the CSF into the brain along the periarterial spaces via the pulsatility of the vessel wall.6 The perivascular spaces form a complex brain-fluid transport system that supports fast exchange with interstitial fluid and clearance of waste products from the brain from the intricate environment of the neuropil. In addition to protein waste, such as Aβ, the glymphatic system also transports lactate, glucose, lipids, and cytokines.11 Glymphatic dysfunction has been shown in animal models of traumatic brain injury, Alzheimer's disease, and stroke, indicating that waste clearance is essential to keep the brain healthy.11
FIG. 1.
Model of glymphatic fluid movement. Cerebrospinal fluid (CSF) enters the subarachnoid space, ultimately traveling down periarterial spaces. From there, the bulk flow of fluid causes CSF and interstitial fluid (ISF) mixing. The “dirty” ISF, containing waste and perhaps cytokines/inflammatory markers, exits the brain via the perivenous spaces. Graphic courtesy of Dan Xue. Color images are available online.
Perhaps the most interesting aspect of the glymphatic system is that fluid transport is regulated by sleep12 and sleeplike brain activity.13 Indeed, human positron emission tomography studies have revealed that Aβ accumulates in the healthy brain after a single night of sleep deprivation, suggesting that the human glymphatic pathway might also be primarily active during sleep.14 The initial findings demonstrated that12:
-
(1)
Clearance of metabolites increases twofold during sleep relative to the waking state
-
(2)
Natural sleep was associated with enhanced periarterial CSF-tracer influx along with improved interstitial solute clearance
-
(3)
The arousal-mediator norepinephrine is chiefly responsible for turning the glymphatic system off during wakefulness, decreasing the size of the interstitial space and thereby increasing the resistance to fluid transport in the interstitial space.
Arousal causes a burst of norepinephrine release, maintaining a continued higher level of norepinephrine during wakefulness. This phenomenon is responsible for the low glymphatic activity in the wakened state. Norepinephrine release decreases during sleep, resulting in an expansion of the interstitial space and a subsequent potentiation of glymphatic-fluid transport. Along with wakefulness, pain is linked to increased adrenergic signaling15,16 and an impairment of sleep quality.17–19 It is still an open question regarding how pain affects the glymphatic system.
Based on what is known about the glymphatic system, the current authors suggest that pain might suppress the glymphatic system. The disruptions in sleep that often accompany chronic neuropathic pain create a malfunctioning of the brain state. Additionally, pain affects brain homeostasis negatively, tilting the system toward excitation20 and inflammation,21 which would reduce glymphatic clearance. Would this lack of glymphatic clearance cause accumulation of proinflammatory agents that aggravate pain? Conversely, could manipulations that increase glymphatic clearance, such as mind–body therapy or improvements in sleep, be effective approaches to reduce chronic pain? Does mind–body therapy, such as acupuncture, which has an analgesic effect correlated to local neuromodulators of pain,22–24 affect the glymphatic system? Acupuncture might, by reducing the severity of pain concurrently with improving sleep quality, act to normalize glymphatic clearance. The current authors speculate that acupuncture reduces adrenergic tone by multiple mechanisms, accelerating glymphatic clearance and thereby reducing the severity of neuroinflammation and the sensation of pain.
Conclusions
Sleep is necessary for brain homeostasis, cleaning waste via the glymphatic system. Pain might block glymphatic-system function by disrupting sleep, altering neuronal function, and inducing neuroinflammation. Therapies for addressing chronic neuropathic pain and glymphatic dysfunction should reduce pain, reduce inflammation, and improve sleep. The overall question for this proposal is: Are nonaddictive mind–body interventions, such as acupuncture, viable alternative approaches to chronic pain relief?
Acknowledgments
The authors would like to thank Dan Xue, BA for the graphics, and Libin Jia, MD for input and editing of this article.
Author Disclosure Statement
No financial conflicts of interest exist.
Funding Information
This work was supported by the National Institute of Neurological Disorders and Stroke grant R01NS100366 (the National Institutes of Health, United States); the National Institute on Aging RF1AG057575 (the National Institutes of Health, United States), the US Army Research Office grant MURI W911NF1910280HT (United States).
References
- 1. Phillips CJ. The cost and burden of chronic pain. Rev Pain. 2009;3(1):2–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mills SEE, Nicolson KP, Smith BH. Chronic pain: A review of its epidemiology and associated factors in population-based studies. Br J Anaesth. 2019;123(2):e273–e283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wardlaw JM, Benveniste H, Nedergaard M, et al. . Perivascular spaces in the brain: Anatomy, physiology and pathology. Nat Rev Neurol. 2020;16(3):137–153 [DOI] [PubMed] [Google Scholar]
- 4. Iliff JJ, Wang M, Liao Y, et al. . A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid beta. Sci Transl Med. 2012;4(147):147ra111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mestre H, Hablitz LM, Xavier AL, et al. . Aquaporin-4–dependent glymphatic solute transport in the rodent brain. Elife. 2018;7:e40070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mestre H, Tithof J, Du T, et al. . Flow of cerebrospinal fluid is driven by arterial pulsations and is reduced in hypertension. Nat Commun. 2018;9(1):4878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Iliff JJ, Wang M, Zeppenfeld DM, et al. . Cerebral arterial pulsation drives paravascular CSF–interstitial fluid exchange in the murine brain. J Neurosci. 2013;33(46):18190–18199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rennels ML, Gregory TF, Blaumanis OR, Fujimoto K, Grady PA. Evidence for a “paravascular” fluid circulation in the mammalian central nervous system, provided by the rapid distribution of tracer protein throughout the brain from the subarachnoid space. Brain Res. 1985;326(1):47–63 [DOI] [PubMed] [Google Scholar]
- 9. van Veluw SJ, Hou SS, Calvo-Rodriguez M, et al. . Vasomotion as a driving force for paravascular clearance in the awake mouse brain. Neuron. 2020;105(3):549–561.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kiviniemi V, Wang X, Korhonen V, et al. . Ultra-fast magnetic resonance encephalography of physiological brain activity—glymphatic pulsation mechanisms? J Cereb Blood Flow Metab. 2016;36(6):1033–1045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rasmussen MK, Mestre H, Nedergaard M. The glymphatic pathway in neurological disorders. Lancet Neurol. 2018;17(11):1016–1024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Xie L, Kang H, Xu Q, et al. . Sleep drives metabolite clearance from the adult brain. Science. 2013;342(6156):373–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hablitz LM, Vinitsky HS, Sun Q, et al. . Increased glymphatic influx is correlated with high EEG delta power and low heart rate in mice under anesthesia. Sci Adv. 2019;5(2):eaav5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shokri-Kojori E, Wang G-J, Wiers CE, et al. . Beta-amyloid accumulation in the human brain after one night of sleep deprivation. Proc Natl Acad Sci U S A. 2018;115(7):4483–4488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jansen AS, Nguyen XV, Karpitskiy V, Mettenleiter TC, Loewy AD. Central command neurons of the sympathetic nervous system: Basis of the fight-or-flight response. Science. 1995;270(5236):644–646 [DOI] [PubMed] [Google Scholar]
- 16. Taylor BK, Westlund KN. The noradrenergic locus coeruleus as a chronic pain generator. J Neurosci Res. 2017;95(6):1336–1346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Haack M, Simpson N, Sethna N, Kaur S, Mullington J. Sleep deficiency and chronic pain: Potential underlying mechanisms and clinical implications. Neuropsychopharmacology. 2020;45(1):205–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ferini-Strambi L. Neuropathic pain and sleep: A review. Pain Ther. 2017;6(suppl1):19–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Finan PH, Goodin BR, Smith MT. The association of sleep and pain: An update and a path forward. J Pain. 2013;14(12):1539–1552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Latremoliere A, Woolf CJ. Central sensitization: A generator of pain hypersensitivity by central neural plasticity. J Pain. 2009;10(9):895–926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ji R-R, Nackley A, Huh Y, Terrando N, Maixner W. Neuroinflammation and central sensitization in chronic and widespread pain. Anesthesiology. 2018;129(2):343–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Goldman N, Chen M, Fujita T, et al. . Adenosine A1 receptors mediate local anti-nociceptive effects of acupuncture. Nat Neurosci. 2010;13(7):883–888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhang R, Lao L, Ren K, Berman BM. Mechanisms of acupuncture–electroacupuncture on persistent pain. Anesthesiology. 2014;120(2):482–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tang Y, Yin H-Y, Rubini P, Illes P. Acupuncture-induced analgesia: A neurobiological basis in purinergic signaling. Neuroscientist. 2016;22(6):563–578 [DOI] [PubMed] [Google Scholar]