Abstract
2,3-Dihydrobenzofurans and indolines are common substructures in medicines and natural products. Herein, we describe a method that enables direct access to these core structures from non-conjugated alkenyl amides and ortho-iodoanilines/phenols. Under palladium(II) catalysis this [3 + 2] heteroannulation proceeds in an anti-selective fashion and tolerates a wide variety of functional groups. N-Acetyl, -tosyl, and -alkyl substituted ortho-iodoanilines, as well as free –NH2 variants, are all effective. Preliminary results with carbon-based coupling partners also demonstrate the viability of forming indane core structures using this approach. Experimental and computational studies on reactions with phenols support a mechanism involving turnover-limiting, endergonic directed oxypalladation, followed by intramolecular oxidative addition and reductive elimination.
Subject terms: Catalytic mechanisms, Homogeneous catalysis, Synthetic chemistry methodology, Stereochemistry
Dihydrobenzofurans and indolines are common substructures in medicines and natural products. Herein, the authors report a palladium-catalyzed [3 + 2] (hetero)annulation proceeding in an anti-selective fashion and enabling direct access to these valuable heterocycles with broad reaction scope.
Introduction
Catalytic (hetero)annulation1–4 of C–C π-systems is a powerful method for constructing carbocyclic and heterocyclic core structures from comparatively simple progenitor compounds. Of special importance within the modern synthetic repertoire is the Pd(0)-catalyzed Larock indole synthesis, first described in 19915, which unites alkynes and 2-haloanilines (Fig. 1a). Later in 1995 and 2005, the Larock group reported analogous methods for benzo[b]furan and indene synthesis6,7. Mechanistically, such reactions are generally believed to proceed via oxidative addition, migratory insertion of the resultant arylpalladium(II) species, and C(sp2)–N/O/C reductive elimination.
Fig. 1. Background and project synopsis.
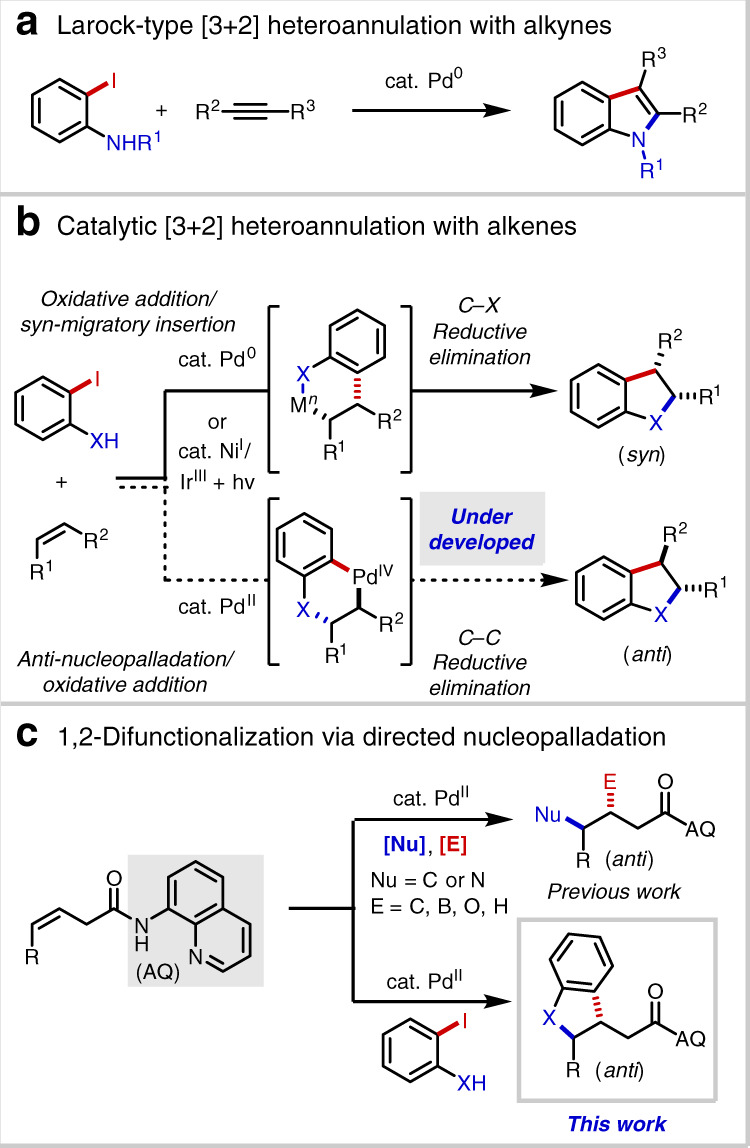
a Larock-type [3 + 2] heteroannulation with alkynes. b Catalytic [3 + 2] heteroannulation with alkenes. c 1,2-difunctionalization via directed nucleopalladation.
While palladium-catalyzed alkyne annulation reactions are well developed and widely deployed in preparative chemistry, employment of alkenes in analogous processes is comparatively rare and limited to activated substrates like styrenes8–10, 1,3-dienes11, norbornenes12, and enol ethers/esters13–17 (Fig. 1b). Extension of this mode of reactivity to unactivated alkenes has proven to be challenging for several interrelated reasons: (1) competitive β-hydride elimination following migratory insertion; (2) inherently challenging C(sp3)–N/O/C reductive elimination18; and (3) competitive alkene isomerization pathways19. In 2015, the Jamison group reported an elegant method to access indolines from unactivated alkenes by taking advantage of nickel/photoredox dual catalysis20, though the reaction required use of an N-acetyl protecting group and was only demonstrated with terminal alkenes, thereby limiting the product structures that can be accessed. To our knowledge, analogous processes between O-based and C-based coupling partners and unactivated alkenes remain unknown.
In order to bridge this important synthetic gap, we sought to develop an all-encompassing annulation process that could unite non-conjugated alkenes and a diverse collection of O-based, N-based, and C-based coupling partners. Specifically, we envisioned that the issues highlighted above could potentially be circumvented by engaging an alternative mechanism, namely a sequence of Pd(II)-mediated Wacker-type nucleopalladation, followed by oxidative addition of the Ar–X bond to form a high-valent Pd(IV) intermediate, and finally C(sp3)–Ar reductive elimination. If successfully developed, such a process would potentially enable access to net anti-annulation, thereby complementing existing methods, which proceed in a syn-selective fashion. Of relevance to this proposal, the Mazet and Zhang groups have described Pd(0)/Pd(II) heteroannulation systems in which syn-oxy/aminopalladation is proposed to take place from a Ln•PdII(Ar)(X) intermediate, such that C(sp3)–O/N bond formation precedes C(sp3)–Ar bond formation14–17.
During the past 5 years, our laboratories have developed a variety of transition-metal-catalyzed, three-component directed alkene 1,2-difunctionalization reactions facilitated by the 8-aminoquinoline (AQ) auxiliary, a strongly coordinating bidentate directing group. Using this strategy we and others have reported examples of hydrofunctionalization21–33, dicarbofunctionalization34–39, carboamination40,41, and carbo-/aminoboration42–45, among other transformations46,47 (Fig. 1c). We recognized several issues that would need to be addressed to bring a directed (hetero)annulation process to fruition: (1) phenols and anilines have previously proven to be ineffective coupling partners in AQ-directed alkene additions in our hands, leading to the hypothesis that nucleopalladation in these cases is endergonic; (2) due to their intramolecular nature, the oxidative addition and reductive elimination transitions states involving Pd(IV) would likely be highly strained; and (3) competitive pathways arising from Heck-type or Wacker-type oxidative alkene addition or hydrofunctionalization could predominate. At the outset, our hypothesis was that the presence of an ortho-halogen would allow for rapid trapping of a potentially short-lived nucleopalladated intermediate.
In this work, by employing ambiphilic coupling partners we successfully achieve the (hetero)annulation of AQ-directed non-conjugated alkenes48, thereby providing direct access to useful 2,3-dihydrobenzofurans, indolines, and indanes49–52. Mechanistic experiments and DFT studies shed light on the origins of diastereoinduction, anti-selectivity, and other aspects of this Pd(II)/Pd(IV) annulation process.
Results
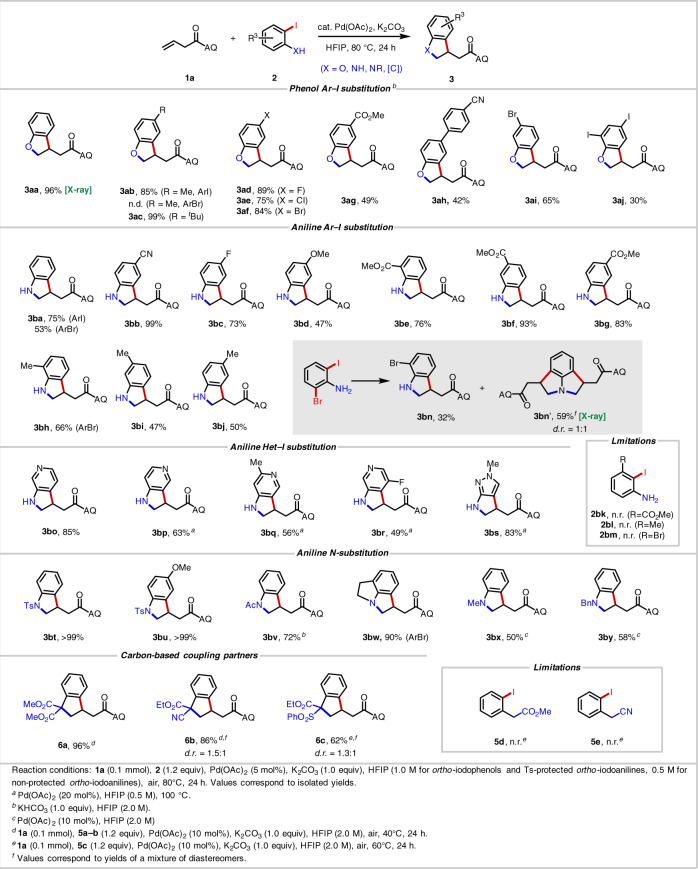
Scope of phenols
We began by using AQ-containing alkenyl amide 1a as the model alkene and 2-iodophenol (2aa) as the coupling partner. After brief optimization (see Supplementary Information for details), we identified effective conditions using Pd(OAc)2 (5 mol%) as the catalyst, K2CO3 (1.0 equiv) as the base, and HFIP (1.0 M) as the solvent, at 80 °C, under an air atmosphere, allowing for isolation of desired product 3aa in 96% yield.
Having identified a high-yielding and operationally simple procedure, we next examined different ortho-iodophenols (Table 1). Phenols containing weakly electron-donating groups, namely 4-Me (3ab) and 4-tBu (3ac), furnished the corresponding products in 85% and 99% yield, respectively. 4-F (3ad), 4-Cl (3ae), 4-Br (3af), and 5-Br (3ai) phenols were also well tolerated and gave moderate to high yields, demonstrating the reactions compatibility with various halogen atoms. The presence of strongly electron-withdrawing groups at the 4-position, namely 4-CO2Me (3ag) and 4-(4-CN-C6H4) (3ah), led to lower yields.
Table 1.
Scope of ortho-iodophenols, ortho-iodoanilines and carbon-based coupling partners.
Scope of anilines
Next, we turned to evaluating the aniline scope (Table 1). With free NH2 anilines, lowering the concentration to 0.5 M led to higher yields, and substrates with electron-withdrawing groups performed better in this case. With ortho-iodoaniline, 75% of the desired product 3ba was obtained, and pleasingly ortho-bromoaniline also provided product 3ba in 53% yield. The ability to use bromoanilines is advantageous in many circumstances since they are easier to prepare and more readily available from commercial suppliers than the corresponding iodoanilines. 4-, 5-, and 6-CO2Me-substituted 2-iodoanilines (3be–3bg) were all effective reaction partners, among which 5-CO2Me (3bf) gave the highest yield of 93%. 4-CN-substituted 2-iodoaniline (3bb) delivered quantitative yield. Halogen atoms (3bc), alkyl groups (3bi, 3bj), and electron-donating substituents (3bd) on the aromatic ring led to decreased yields. With electron-donating groups, the product is prone to autooxidation to the corresponding indole, particularly in the case of 3bd. 6-Me-substituted 2-bromoaniline (3bh) was also compatible in this reaction. 3-Substituted 2-iodoanilines were incompatible due to the steric hinderance (2bk–2bm).
Given the importance of azaindole derivatives as building blocks in medicinal chemistry53,54, we next tested aminoiodopyridines. Notably, compared to azaindoles, the synthesis of azaindolines has been less thoroughly studied. We were wary that the basic and coordinating azine nitrogen atoms could pose a significant challenge, as is often the case in Pd(II)-catalyzed reactions34,55. To our delight, however, various pyridine-containing substrates were indeed compatible. For 3bo, we obtained 85% yield under the standard conditions; for 3bp–3bs, higher Pd loadings and higher temperatures were employed, but these also gave satisfactory yields. Of note we were able to prepare 3br, which contains a fluorine atom at the sterically challenging site ortho to the reactive iodide group.
Anilines containing a variety of N-substituents were then tested, with different groups requiring different optimal reaction concentrations (see Supplementary Information). Tosyl (Ts) proved to be a highly effective protecting group. The yield of the simplest Ts-protected 2-iodoaniline was >99% (3bt). In cases where the product is susceptible to autooxidation (e.g., OMe-containing compound 3bd), Ts protection leads to improved yield (>99% yield, 3bu). Changing the protecting group to acetyl (Ac) (3bv) at higher concentration (2.0 M) gave a 72% yield. Tricyclic products (3bw) could also be formed in the reaction conditions, and we were intrigued to observe 59% of the 1:1 cis/trans-configured diaddition product 3bn′ along with 32% monoaddition product 3bn when using 2-bromo-6-iodoaniline. Different N-alkyl 2-iodoanilines also proved to be effective in this reaction. By increasing the palladium loading and performing the reaction at higher concentration, N-Me (3bx) and N-Bn (3by) products were obtained in 50% and 58% yield, respectively.
Scope of carbon-based coupling partners
A small collection of carbon-based coupling partners were also compatible in this transformation, and the corresponding products 6a–6c could be obtained in 62–96% yield with 10 mol% Pd(OAc)2 and HFIP (2.0 M) (Table 1). Doubly activated pronucleophiles were required for efficient coupling (5d and 5e), and in the case of coupling partners bearing two different electron-withdrawing groups (6b and 6c), diastereoinduction was modest. This carboannulation process56,57 enables preparation of indane structures that are found in numerous natural products and pharmaceutical compounds58–60.
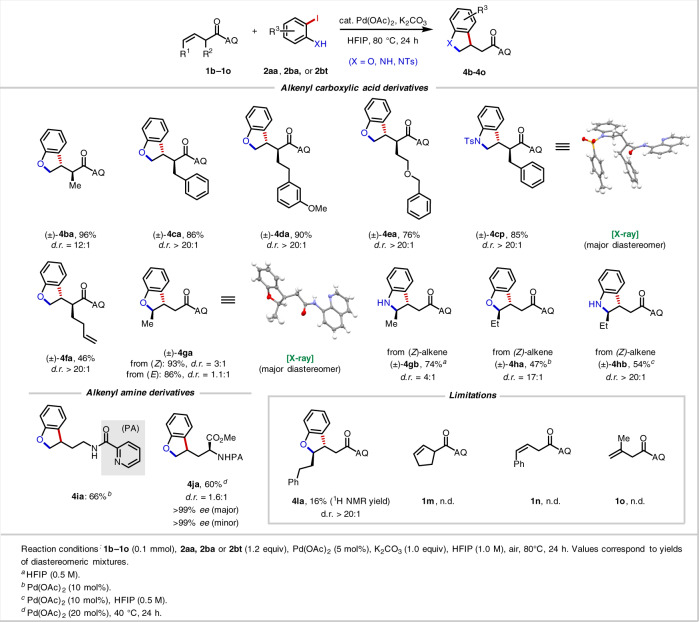
Scope of alkenes
We moved on to explore the alkene scope (Table 2). To our delight, reactions with α-substituted alkenyl amides proceeded efficiently and with high diastereoselectivity. Even with a small moiety, in this case a Me group (4ba), we obtained 96% yield and 12:1 d.r. With a larger group at the α-position (4ca–4ea), >20:1 d.r. and 76–90% yield were obtained. An X-ray crystal structure of the Ts-protected products 4cp confirmed that the major product arises from C(sp3)–Ar bond formation on the face of the alkene opposite of the α-substituent (vide infra). A diene substrate (4fa) reacted exclusively at the β, γ-alkene rather than the δ,ε-alkene, showcasing a unique example of chemoselectivity through substrate directivity. Notably, acyclic, non-conjugated internal alkenes, which are a difficult substrate class in nondirected systems due to issues with both reactivity and selectivity, engaged in productive [3 + 2] heteroannulation. Both (Z)-alkenes and (E)-alkenes were compatible, with (Z)-alkenes leading to higher d.r. (3:1 vs 1.1:1). According to previous studies of related transformations, internal alkenes are prone to E/Z isomerization (see Supplementary Information), and (Z)-alkenes react much faster in nucleopalladation than (E)-alkenes29,43. Both ortho-iodophenol (4ga, 4ha) and ortho-iodoaniline (4gb, 4hb) worked well for internal alkenes, and the X-ray crystal structure of 4ga shows that the major product is in the trans configuration. Moreover, alkenyl amine substrates containing a 2-picolinyl amide (NHPA) directing group were also competent substrates. For the simple alkene and simple ortho-iodophenol (4ia), we isolated 66% yield by using 20 mol% Pd(OAc)2. Using an optically pure allylglycine starting material, we obtained 60% yield (4ja), 1:1.6 d.r. and >99% e.e without any enantiomerization by reducing the reaction temperature to 40 °C.
Table 2.
Alkene scope.
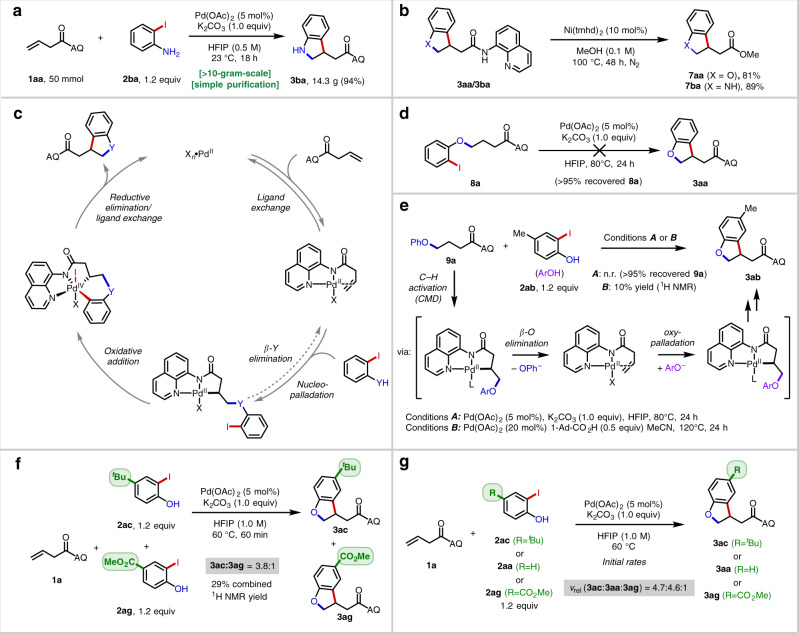
Large-scale reaction and directing group removal
This methodology could be conveniently scaled-up (Fig. 2a) and allowed for isolation of 14.3 g of 3ba with minimal impurities after an operationally simple work-up and purification procedure (see Supplementary Information). Using the Morimoto–Ohshima AQ amide alcoholysis method with catalytic Ni(tmhd)261, we obtained 81% (7aa) and 89% yield (7ba) yields for representative 2,3-dihydrobenzofuran and indoline products, respectively (Fig. 2b).
Fig. 2. Large-scale reaction, directing group removal and mechanistic studies.
a 50 mmol-scale reaction. b Directing group removal. c Proposed mechanism. d Viability evaluation of an alternative pathway. e Evaluation of the potential reversibility of C(sp3)–O bond formation. f Competition experiments. g Initial rate studies.
Mechanistic studies
In analogy to earlier work34–47, we surmised that the reaction may proceed by the general mechanism depicted in Fig. 2c, involving a sequence of alkene coordination, nucleopalladation, intramolecular oxidative addition, and reductive elimination. To evaluate the viability of an alternative pathway comprised of alkene hydrofunctionalization followed by intramolecular C(sp3)–H arylation62,63, we first performed a control experiment in which compound 8a was subjected to the standard reaction conditions (Fig. 2d). In this case, no product was formed (with quantitative starting material recovery), establishing that 8a is not a competent intermediate. This result is inconsistent with the two-step hydrofunctionalization/C(sp3)–H arylation mechanism. Next, to evaluate the potential reversibility of the C(sp3)–O bond formation step, we used compound 9a as a starting material (Fig. 2e). Under the standard conditions, no reaction was observed. However, in the presence of 1-Ad-CO2H, which presumably facilitates C(sp3)–H activation via a concerted metalation/deprotonation (CMD) mechanism64, we were able to observe small amounts of the [3 + 2] product 3ab. The result suggests that C(sp3)–O bond formation is reversible from the alkylpalladium(II) species—at least when accessed through this alternative C(sp3)–H activation pathway. An analogous experiment with 8a furnished an approximate 1:1 mixture of 3aa and 3ab in <10% combined yield (see Supplementary Information), consistent with the notion that C(sp3)–O bond formation is reversible and that oxidative addition and β-O elimination have similar energy barriers from the putative alkylpalladium(II) intermediate.
Next, a series of competition experiments were conducted. A one-pot experiment with electron-poor 2ag and electron-rich 2ac revealed the product-determining step is accelerated by electron-donating groups (Fig. 2f). This trend was recapitulated when global rates in single-component experiments were measured (Fig. 2g), which is consistent with either oxypalladation or C(sp3)–Ar reductive elimination (with the Ar fragment acting as the nucleophilic moiety40) as the turnover-limiting and product-determining steps for phenol substrates.
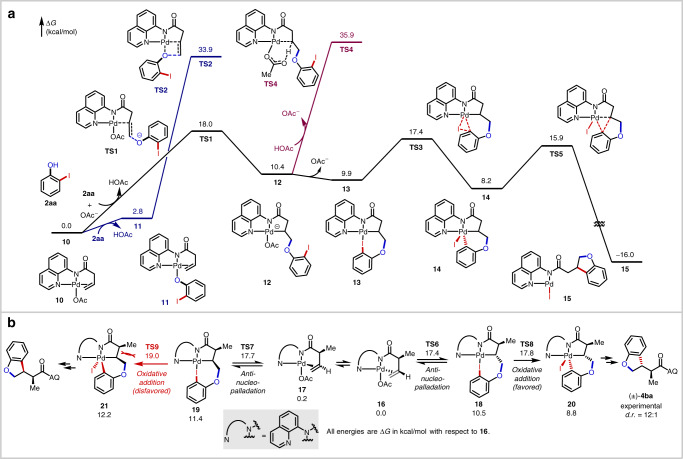
Computational studies
To clarify the reaction mechanism, density functional theory (DFT) calculations were performed (Supplementary Data 1) on the reaction of alkene 1a and iodophenol 2aa (Fig. 3a). The reaction starts with the complexation of the Pd(II) catalyst with 1a to form π-alkene complex 10. Several syn-nucleopalladation and anti-nucleopalladation pathways were evaluated computationally. The most favorable pathway proceeds through the anti-addition of deprotonated phenolate anion to 10 (via TS1), requiring a barrier of 18.0 kcal/mol. The competing inner-sphere syn-addition pathway (TS2) from phenolate complex 11 is disfavored and requires a barrier of 33.9 kcal/mol. The palladacycle intermediate 12 is 10.4 kcal/mol less stable than 10. The endergonicity is consistent with control experiments that indicate reversible nucleopalladation when this intermediate is accessed through an independent route (Fig. 2d). Dissociation of the acetate anion from 12 and the coordination of the ortho-iodo group to Pd forms complex 13, which promotes the intramolecular oxidative addition (OA) (TS3, ΔG‡ = 17.4 kcal/mol). The resulting Pd(IV) complex 14 can undergo a relatively facile reductive elimination (TS5) with a barrier of 7.7 kcal/mol with respect to 14. The competing protodepalladation of palladacycle 12 with acetic acid (TS4) is kinetically inaccessible, requiring an activation energy of 35.9 kcal/mol. In the overall annulation reaction from complex 10, the nucleopalladation is the turnover-limiting step. Although the formation of palladacycle 12 is endergonic, the intramolecular oxidative addition/reductive elimination is entropically facilitated and proceeds without significant distortions in either transition state. It is notable that in this model reaction between 1a and 2aa, nucleopalladation and oxidative addition are computed to have very similar barriers (TS1 vs. TS3). This raises the possibility that in reactions with other alkene substrates and coupling partners, the rate and selectivity could be controlled by either of these two steps (vide infra).
Fig. 3. Computational studies.
a Computed energy profiles of the Pd-catalyzed [3 + 2] heteroannulation of 1a and 2aa. Calculations were performed using Gaussian 16 at the M06/SDD-6-311++G(d,p)/SMD(HFIP)//B3LYP-D3/SDD-6-31G(d) level of theory. b DFT calculations on the origin of diastereoselectivity with alkene 1b.
Last, we investigated the origin of diastereoselectivity in reactions with α-substituted alkenyl amides. We modeled the reaction of alkene 1b bearing a small α-methyl substituent and iodophenol 2aa (Fig. 3b). The two diastereomeric products are formed via nucleopalladation of two nearly isoenergetic complexes 16 and 17 with two opposite π-faces of the alkene bound to the Pd. The nucleopalladation of 16 (TS6, ΔG‡ = 17.4 kcal/mol) is only slightly more favorable than that of 17 (TS7, ΔG‡ = 17.7 kcal/mol), due to the relatively early transition states. The diastereoselectivity is determined in the subsequent oxidative addition step (TS8 vs. TS9), which requires higher activation energies than nucleopalladation for this substrate. The oxidative addition from palladacycle 19 (TS9) requires a 1.2 kcal/mol higher activation barrier than that from 18 due to the steric repulsion between the α-methyl substituent and the methylene, which are placed cis to each other. The predicted major diastereomeric product and the selectivity (ΔΔG‡ = 1.2 kcal/mol) are in agreement with the experimental results.
In conclusion, we have developed a highly versatile and selective method for [3 + 2] (hetero)annulation of non-conjugated alkenes via a directed nucleopalladation strategy. The transformation tolerates a diverse collection of ortho-iodophenols and ortho-iodoanilines, as well as α-(ortho-iodo-aryl)-carbonyl compounds, enabling access to substituted 2,3-dihydrobenzofurans, indolines, and indanes. This reaction is effective with challenging α-substituted alkenyl carbonyl substrates as well as alkenes bearing 1,2-dialkyl-substitution and proceeds in regioselective and diastereoselective fashion. The reaction tolerates both air and moisture and does not require any special precautions to perform. Mechanistic studies shed light on the origins of anti-selectivity, diastereoinduction, and other aspects of the mechanism of this Pd(II)/Pd(IV) annulation process.
Methods
General procedure for [3 + 2] (hetero)annulation
To a 4-mL scintillation vial equipped with a Teflon-coated magnetic stir bar were added the Pd(OAc)2 (0.005 mmol, 5 mol%), alkene substrate (0.1 mmol, 1 equiv), the appropriate coupling partner (0.12 mmol, 1.2 equiv), K2CO3 (0.1 mmol, 1 equiv), and HFIP (0.1 or 0.2 mL). The vial was sealed with a screw-top septum cap and placed in a heating block that was pre-heated to 80 °C. The reaction was allowed to run for 24 h and then was cooled to room temperature. The reaction mixture was filtered through a short plug of Celite, which was washed with EtOAc. The filtrate was concentrated under reduced pressure to afford a brown residue, which, upon purification by preparative TLC or column chromatography, afforded the pure product.
Supplementary information
Description of Additional Supplementary Files
Acknowledgements
Dr. Gary J. Balaich, Dr. Milan Gembicky, Maximilian G. Bernbeck, and Dr. Jake B. Bailey (USCD) are acknowledged for assistance with X-Ray crystallography. We thank Prof. Zachary K. Wickens and his group at UW Madison for helpful discussion. This work was financially supported by the National Institutes of Health (5R35GM125052-03; 1R35GM128779), Pfizer, Inc., the Alfred P. Sloan Fellowship Program, the Camille Dreyfus Teacher-Scholar Program, and the Cottrell Scholars Program. Calculations were performed at the Center for Research Computing at the University of Pittsburgh and the Extreme Science and Engineering Discovery Environment (XSEDE) supported by the NSF. Dr. Jason S. Chen, Brittany B. Sanchez, and Emily J. Sturgell (Scripps Research Automated Synthesis Facility) are acknowledged for assistance with analysis and purification.
Author contributions
H.-Q.N. and K.M.E. conceived the project. H.-Q.N. optimized the reaction conditions. H.-Q.N., P.G.B., J.S.B., S.Y., M.T.D., A.M.R., H.-X.L. examined the scope of the transformation. H.-Q.N. performed mechanistic experiments. I. K. and P.L. carried out DFT studies. I.J.M., P.L. and K.M.E. directed the research. H.-Q.N., I.K., P.L., and K.M.E. wrote the manuscript with input from all authors.
Data availability
The data supporting the findings of this study are available within the article and its Supplementary Information. CCDC 1999888, 1999889, 2031197, and 2033387 contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
Competing interests
The authors declare no competing interests.
Footnotes
Peer review information Nature Communications thanks the anonymous reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Indrawan J. McAlpine, Email: indrawan.mcalpine@pfizer.com
Peng Liu, Email: pengliu@pitt.edu.
Keary M. Engle, Email: keary@scripps.edu
Supplementary information
Supplementary information is available for this paper at 10.1038/s41467-020-20182-4.
References
- 1.Liu D, Zhao G, Xiang L. Diverse strategies for the synthesis of the indoline scaffold. Eur. J. Org. Chem. 2010;2010:3975–3984. doi: 10.1002/ejoc.201000323. [DOI] [Google Scholar]
- 2.Silva TS, et al. Recent advances in Indoline synthesis. Tetrahedron. 2019;75:2063–2097. doi: 10.1016/j.tet.2019.02.006. [DOI] [Google Scholar]
- 3.Bertolini F, Pineschi M. Recent progress in the synthesis of 2,3-dihydrobenzofurans. Org. Prep. Proc. Int. 2009;41:385–418. doi: 10.1080/00304940903240836. [DOI] [Google Scholar]
- 4.Garlets ZJ, White DR, Wolfe JP. Recent developments in Pd0-catalyzed alkene-carboheterofunctionalization reactions. Asian J. Org. Chem. 2017;6:636–653. doi: 10.1002/ajoc.201600577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Larock RC, Yum EK. Synthesis of indoles via palladium-catalyzed heteroannulation of internal alkynes. J. Am. Chem. Soc. 1991;113:6689–6690. doi: 10.1021/ja00017a059. [DOI] [Google Scholar]
- 6.Larock RC, Yum EK, Doty MJ, Sham KKC. Synthesis of aromatic heterocycles via palladium-catalyzed annulation of internal alkynes. J. Org. Chem. 1995;60:3270–3271. doi: 10.1021/jo00116a003. [DOI] [Google Scholar]
- 7.Zhang D, Yum EK, Liu Z, Larock RC. Synthesis of indenes by the palladium-catalyzed carboannulation of internal alkynes. Org. Lett. 2005;7:4963–4966. doi: 10.1021/ol051907a. [DOI] [PubMed] [Google Scholar]
- 8.Emrich DE, Larock RC. Palladium-catalyzed heteroannulation of cyclic alkenes by functionally substituted aryl iodides. J. Organomet. Chem. 2004;689:3756–3766. doi: 10.1016/j.jorganchem.2004.06.028. [DOI] [Google Scholar]
- 9.Buarque CD, et al. Palladium-catalyzed oxyarylation of olefins using silver carbonate as the base. Probing the mechanism by electrospray ionization mass spectrometry. J. Organomet. Chem. 2010;695:2062–2067. doi: 10.1016/j.jorganchem.2010.05.014. [DOI] [Google Scholar]
- 10.de Freitas de Moraes P, et al. Ligand-free palladium-catalyzed oxyarylation of dihydronaphthalenes and chromenequinone with o-iodophenols and 3-iodolawsone in PEG-400: An efficient synthesis of 5-carbapterocarpans and pterocarpanquinones. Synthesis. 2015;47:3505–3512. doi: 10.1055/s-0034-1378745. [DOI] [Google Scholar]
- 11.Larock RC, Berrios-Peña N, Narayanan K. Palladium-catalyzed heteroannulation of 1,3-dienes by functionally substituted aryl halides. J. Org. Chem. 1990;55:3447–3450. doi: 10.1021/jo00298a011. [DOI] [Google Scholar]
- 12.Catellani M, Del Rio A. Catalytic arylation of carbon–carbon double bond followed by N- or O-cyclization. Russ. Chem. Bull. 1998;47:928–931. doi: 10.1007/BF02498163. [DOI] [Google Scholar]
- 13.Borrajo-Calleja GM, Bizet V, Mazet C. Palladium-catalyzed enantioselective intermolecular carboetherification of dihydrofurans. J. Am. Chem. Soc. 2016;138:4014–4017. doi: 10.1021/jacs.6b02158. [DOI] [PubMed] [Google Scholar]
- 14.Bizet V, Borrajo-Calleja GM, Besnard C, Mazet C. Direct access to furoindolines by palladium-catalyzed intermolecular carboamination. ACS Catal. 2016;6:7183–7187. doi: 10.1021/acscatal.6b02238. [DOI] [Google Scholar]
- 15.Borrajo-Calleja GM, Bizet V, Besnard C, Mazet C. Mechanistic investigation of the Pd-catalyzed intermolecular carboetherification and carboamination of 2,3-dihydrofuran: similarities, differences, and evidence for unusual reaction intermediates. Organometallics. 2017;36:3553–3563. doi: 10.1021/acs.organomet.7b00483. [DOI] [Google Scholar]
- 16.Tao M, et al. Pd/Xiang-Phos-catalyzed enantioselective intermolecular carboheterofunctionalization under mild conditions. Chem. Sci. 2020;11:6283–6288. doi: 10.1039/D0SC01391A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tao M, Li W, Zhang J. Pd/Xiang-Phos-catalyzed enantioselective intermolecular carboheterofunctionalization of norbornene and norbornadiene. Chem. Commun. 2020;56:13125–13128. doi: 10.1039/D0CC04996D. [DOI] [PubMed] [Google Scholar]
- 18.Pérez-Temprano MH, Racowski JM, Kampf JW, Sanford MS. Competition between sp3-C–N vs sp3-C–F reductive elimination from PdIV complexes. J. Am. Chem. Soc. 2014;136:4097–4100. doi: 10.1021/ja411433f. [DOI] [PubMed] [Google Scholar]
- 19.Vasseur A, Bruffaerts J, Marek I. Remote functionalization through alkene isomerization. Nat. Chem. 2016;8:209–219. doi: 10.1038/nchem.2445. [DOI] [PubMed] [Google Scholar]
- 20.Tasker SZ, Jamison TF. Highly regioselective indoline synthesis under nickel/photoredox dual catalysis. J. Am. Chem. Soc. 2015;137:9531–9534. doi: 10.1021/jacs.5b05597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gurak JA, Jr., Engle KM. Regioselective hydroamination using a directed nucleopalladation/protodepalladation strategy. Synlett. 2017;28:2057–2065. doi: 10.1055/s-0036-1589077. [DOI] [Google Scholar]
- 22.Gurak JA, Jr., Yang KS, Liu Z, Engle KM. Directed, regiocontrolled hydroamination of unactivated alkenes via protodepalladation. J. Am. Chem. Soc. 2016;138:5805–5808. doi: 10.1021/jacs.6b02718. [DOI] [PubMed] [Google Scholar]
- 23.Yang KS, Gurak JA, Jr., Liu Z, Engle KM. Catalytic, regioselective hydrocarbofunctionalization of unactivated alkenes with diverse C–H nucleophiles. J. Am. Chem. Soc. 2016;138:14705–14712. doi: 10.1021/jacs.6b08850. [DOI] [PubMed] [Google Scholar]
- 24.Gurak JA, Jr., Tran VT, Sroda MM, Engle KM. N-Alkylation of 2-pyridone derivatives via palladium(II)-catalyzed directed alkene hydroamination. Tetrahedron. 2017;73:3636–3642. doi: 10.1016/j.tet.2017.03.091. [DOI] [Google Scholar]
- 25.Matsuura R, et al. Palladium(II)-catalyzed γ-selective hydroarylation of alkenyl carbonyl compounds with arylboronic acids. Chem. Sci. 2018;9:8363–8368. doi: 10.1039/C8SC03081B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nimmagadda SK, et al. Catalytic, enantioselective α-alkylation of azlactones with nonconjugated alkenes by directed nucleopalladation. Angew. Chem. Int. Ed. 2019;58:3923–3927. doi: 10.1002/anie.201814272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang X, et al. Controlling cyclization pathways in palladium(II)-catalyzed intramolecular alkene hydrofunctionalization via substrate directivity. Chem. Sci. 2020;11:11307–11314. doi: 10.1039/D0SC03409F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lv H, Xiao L-J, Zhao D, Zhou Q-L. Nickel(0)-catalyzed linear-selective hydroarylation of unactivated alkenes and styrenes with aryl boronic acids. Chem. Sci. 2018;9:6839–6843. doi: 10.1039/C8SC02101E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang H, et al. Palladium-catalyzed amide-directed enantioselective hydrocarbofunctionalization of unactivated alkenes using a chiral monodentate oxazoline ligand. J. Am. Chem. Soc. 2018;140:3542–3546. doi: 10.1021/jacs.8b00641. [DOI] [PubMed] [Google Scholar]
- 30.Wang C, et al. Palladium-catalyzed regiocontrollable reductive Heck reaction of unactivated aliphatic alkenes. J. Am. Chem. Soc. 2018;140:9332–9336. doi: 10.1021/jacs.8b03619. [DOI] [PubMed] [Google Scholar]
- 31.Shen H-C, et al. Enantioselective addition of cyclic ketones to unactivated alkenes enabled by amine/Pd(II) cooperative catalysis. ACS Catal. 2019;9:791–797. doi: 10.1021/acscatal.8b04654. [DOI] [Google Scholar]
- 32.Wei C, et al. Synergistic palladium/enamine catalysis for asymmetric hydrocarbon functionalization of unactivated alkenes with ketones. Org. Biomol. Chem. 2019;17:6607–6611. doi: 10.1039/C9OB01165J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bai Z, et al. Palladium-catalyzed amide-directed hydrocarbofunctionalization of 3-alkenamides with alkynes. ACS Catal. 2020;10:933–940. doi: 10.1021/acscatal.9b04285. [DOI] [Google Scholar]
- 34.Liu Z, Zeng T, Yang KS, Engle KM. β,γ-Vicinal dicarbofunctionalization of alkenyl carbonyl compounds via directed nucleopalladation. J. Am. Chem. Soc. 2016;138:15122–15125. doi: 10.1021/jacs.6b09170. [DOI] [PubMed] [Google Scholar]
- 35.Derosa J, Tran VT, Boulous MN, Chen JS, Engle KM. Nickel-catalyzed β,γ-dicarbofunctionalization of alkenyl carbonyl compounds via conjunctive cross-coupling. J. Am. Chem. Soc. 2017;139:10657–10660. doi: 10.1021/jacs.7b06567. [DOI] [PubMed] [Google Scholar]
- 36.Derosa J, van der Puyl VA, Tran VT, Liu M, Engle KM. Directed nickel-catalyzed 1,2-dialkylation of alkenyl carbonyl compounds. Chem. Sci. 2018;9:5278–5283. doi: 10.1039/C8SC01735B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jeon J, et al. Site-selective 1,1-difunctionalization of unactivated alkenes enabled by cationic palladium catalysis. J. Am. Chem. Soc. 2019;141:10048–10059. doi: 10.1021/jacs.9b04142. [DOI] [PubMed] [Google Scholar]
- 38.Pan R, et al. Nickel-catalyzed reductive 1,2-dialkynylation of alkenes bearing an 8-aminoquinoline directing group. Org. Lett. 2019;21:8915–8920. doi: 10.1021/acs.orglett.9b03147. [DOI] [PubMed] [Google Scholar]
- 39.Zhang Y, Chen G, Zhao D. Three-component vicinal-diarylation of alkenes via direct transmetalation of arylboronic acids. Chem. Sci. 2019;10:7952–7957. doi: 10.1039/C9SC02182E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu Z, et al. Catalytic intermolecular carboamination of unactivated alkenes via directed aminopalladation. J. Am. Chem. Soc. 2017;139:11261–11270. doi: 10.1021/jacs.7b06520. [DOI] [PubMed] [Google Scholar]
- 41.van der Puyl VA, Derosa J, Engle KM. Directed, nickel-catalyzed umpolung 1,2-carboamination of alkenyl carbonyl compounds. ACS Catal. 2019;9:224–229. doi: 10.1021/acscatal.8b04516. [DOI] [Google Scholar]
- 42.Liu Z, Ni H-Q, Zeng T, Engle KM. Catalytic carbo- and aminoboration of alkenyl carbonyl compounds via five- and six-membered palladacycles. J. Am. Chem. Soc. 2018;140:3223–3227. doi: 10.1021/jacs.8b00881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu Z, Li X, Zeng T, Engle KM. Directed, palladium(II)-catalyzed enantioselective anti-carboboration of alkenyl carbonyl compounds. ACS Catal. 2019;9:3260–3265. doi: 10.1021/acscatal.9b00181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu Z, et al. Palladium(0)-catalyzed directed syn-1,2-carboboration and -silylation: alkene scope, applications in dearomatization, and stereocontrol by a chiral auxiliary. Angew. Chem. Int. Ed. 2019;58:17068–17073. doi: 10.1002/anie.201910304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bai Z, et al. Palladium-catalyzed amide-directed enantioselective carboboration of unactivated alkenes using a chiral monodentate oxazoline ligand. ACS Catal. 2019;9:6502–6509. doi: 10.1021/acscatal.9b01350. [DOI] [PubMed] [Google Scholar]
- 46.Li Y, et al. Directed copper-catalyzed intermolecular aminative difunctionalization of unactivated alkenes. J. Am. Chem. Soc. 2019;141:18475–18485. doi: 10.1021/jacs.9b07607. [DOI] [PubMed] [Google Scholar]
- 47.Zeng T, Liu Z, Schmidt MA, Eastgate MD, Engle KM. Directed, palladium(II)-catalyzed intermolecular aminohydroxylation of alkenes using a mild oxidation system. Org. Lett. 2018;20:3853–3857. doi: 10.1021/acs.orglett.8b01440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ye Z, Adhikari S, Xia Y, Dai M. Expedient syntheses of N-heterocycles via intermolecular amphoteric diamination of allenes. Nat. Commun. 2018;9:721. doi: 10.1038/s41467-018-03085-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lopes MN, et al. Unusual benzofuranoid neolignans from Licaria Chrysophylla. Phytochemistry. 1986;25:2609–2612. doi: 10.1016/S0031-9422(00)84519-7. [DOI] [Google Scholar]
- 50.Negoro N, et al. Discovery of TAK-875: A potent, selective, and orally bioavailable GPR40 agonist. ACS Med. Chem. Lett. 2010;1:290–294. doi: 10.1021/ml1000855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pieters L, et al. Synthesis and biological evaluation of dihydrobenzofuran lignans and related compounds as potential antitumor agents that inhibit tubulin polymerization. J. Med. Chem. 1999;42:5475–5481. doi: 10.1021/jm990251m. [DOI] [PubMed] [Google Scholar]
- 52.Wilson JE, et al. Discovery of novel indoline cholesterol ester transfer protein inhibitors (CETP) through a structure-guided approach. ACS Med. Chem. Lett. 2016;7:261–265. doi: 10.1021/acsmedchemlett.5b00404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Popowycz F, Routier S, Joseph B, Mérour J-Y. Synthesis and reactivity of 7-azaindole (1H-pyrrolo[2,3-b]pyridine). Tetrahedron. 2007;63:1031–1064. doi: 10.1016/j.tet.2006.09.067. [DOI] [Google Scholar]
- 54.Song JJ, et al. Organometallic methods for the synthesis and functionalization of azaindoles. Chem. Soc. Rev. 2007;36:1120–1132. doi: 10.1039/b607868k. [DOI] [PubMed] [Google Scholar]
- 55.Wang P, et al. Ligand-promoted meta-C–H arylation of anilines, phenols, and heterocycles. J. Am. Chem. Soc. 2016;138:9269–9276. doi: 10.1021/jacs.6b04966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Larock AC, Fried CA. Palladium-catalyzed carboannulation of 1,3-dienes by aryl halides. J. Am. Chem. Soc. 1990;112:5882–5884. doi: 10.1021/ja00171a040. [DOI] [Google Scholar]
- 57.Wu X, Chen S-S, Zhang L, Wang H-J, Gong L-Z. Palladium-catalyzed enantioselective carboannulation of 1,3-dienes with aryl iodides enables access to chiral indanes. Chem. Commun. 2018;54:9595–9598. doi: 10.1039/C8CC04641G. [DOI] [PubMed] [Google Scholar]
- 58.Bianco GG, et al. (+)- and (−)-Mutisianthol: First total synthesis, absolute configuration, and antitumor activity. J. Org. Chem. 2009;74:2561–2566. doi: 10.1021/jo9000405. [DOI] [PubMed] [Google Scholar]
- 59.Yu H, et al. Synthesis and pharmacological evaluation of 3-(3,4-dichlorophenyl)-1-indanamine derivatives as nonselective ligands for biogenic amine transporters. J. Med. Chem. 2004;47:2624–2634. doi: 10.1021/jm0305873. [DOI] [PubMed] [Google Scholar]
- 60.Dorsey BD, et al. L-735,524: The design of a potent and orally bioavailable HIV protease inhibitor. J. Med. Chem. 1994;37:3443–3451. doi: 10.1021/jm00047a001. [DOI] [PubMed] [Google Scholar]
- 61.Deguchi T, Xin H-L, Morimoto H, Ohshima T. Direct catalytic alcoholysis of unactivated 8-aminoquinoline amides. ACS Catal. 2017;7:3157–3161. doi: 10.1021/acscatal.7b00442. [DOI] [Google Scholar]
- 62.Zhang X, et al. A general strategy for synthesis of cyclophane-braced peptide macrocycles via palladium-catalysed intramolecular sp3 C−H arylation. Nat. Chem. 2018;10:540–548. doi: 10.1038/s41557-018-0006-y. [DOI] [PubMed] [Google Scholar]
- 63.Feng Y, Wang Y, Landgraf B, Liu S, Chen G. Facile benzo-ring construction via palladium-catalyzed functionalization of unactivated sp3 C–H bonds under mild reaction conditions. Org. Lett. 2010;12:3414–3417. doi: 10.1021/ol101220x. [DOI] [PubMed] [Google Scholar]
- 64.Tran VT, Gurak JA, Yang K, Engle KM. Activation of diverse carbon–heteroatom and carbon–carbon bonds via palladium(II)-catalysed β-X elimination. Nat. Chem. 2018;10:1126–1133. doi: 10.1038/s41557-018-0110-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
The data supporting the findings of this study are available within the article and its Supplementary Information. CCDC 1999888, 1999889, 2031197, and 2033387 contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.