Abstract
In the last years, the presence of multidrug-resistant (MDR) Gram-negative (like Klebsiella pneumoniae, Pseudomonas aeruginosa, Acinetobacter baumannii) and Gram-positive bacteria (mostly methicillin-resistant Staphylococcus aureus) was worldwide reported, limiting the options for an effective antibiotic therapy. For these reasons, inappropriate antimicrobial therapy and delayed prescription can lead to an unfavorable outcome, especially in patients with pneumonia. New antibiotics approved belong to classes of antimicrobials, like beta-lactams with or without beta-lactamase inhibitors, aminoglycosides, oxazolidinones, quinolones, and tetracyclines, or based on new mechanisms of action. These new compounds show many advantages, including a broad spectrum of activity against MDR pathogens, good lung penetration, safety and tolerability, and finally the possibility of intravenous and/or oral formulations. However, the new antibiotics under development represent an important possible armamentarium against difficult-to-treat strains. The safety and clinical efficacy of these future drugs should be tested in clinical practice. In this review, there are reported characteristics of newly approved antibiotics that represent potential future options for the treatment of respiratory tract infections, including those caused by multidrug-resistant bacteria. Finally, the characteristics of the drugs under development are briefly reported.
Keywords: Pneumonia, multidrug resistant bacteria, new antimicrobial options, clinical efficacy, drugs under development
Introduction
In recent years, also considering the progressive increase in antimicrobial resistance, there have been developed and approved new antibiotics for the therapy of pneumonia, including severe forms.
The treatment of pneumonia caused by multidrug-resistant (MDR) Gram-negative pathogens, like Klebsiella pneumoniae, Pseudomonas aeruginosa, and Acinetobacter baumannii, was based on an empiric antibiotic not displaying in vitro activity.1-3 Considering the lack of novel therapeutic options, many therapies and antibiotic combinations were widely used in the setting of carbapenem resistance, but the mortality rates remained very high.4 Of importance, over 30% of cases of hospital-acquired pneumonia (HAP) are caused by methicillin-resistant Staphylococcus aureus (MRSA)5,6; vancomycin still is considered the first option for the treatment of MRSA pneumonia, but its role is debated for the risk of nephrotoxicity and the limited lung penetration. For these reasons, therapeutic drug monitoring (TDM) is necessary to achieve an adequate plasma concentration.7,8 In the setting of severe MRSA pneumonia, the role of linezolid was widely assessed9 although some limits in its use are represented by side effects like hematological alterations and drug interactions with selective serotonin reuptake inhibitors and other drugs with serotonergic activity.10
Then, the high reported rates of treatment failure caused by administration of inadequate antibiotic treatment lead to increased morbidity and mortality, prolonged length of hospital stay, and, not less importantly, an increase in healthcare costs.11,12
The aim of this review is to analyze the characteristics of new approved antibiotics for the treatment of pneumonia due also to MDR pathogens. This review is focused on the spectrum of activities and the possible role in daily clinical practice. Finally, the characteristics of the drugs under development are briefly reported (see also Table 1).
Table 1.
Characteristics of new approved antibiotics for the treatment of pneumonia.
| Drug | Spectrum of activity | Administration | Mechanism of action | Drug comparator in pneumonia trials | Major side effects |
|---|---|---|---|---|---|
| Ceftobiprole | MRSA, H. influenzae (comprising b-lactamase-producing strains), M. catarrhalis, K. pneumoniae, E. coli, P. aeruginosa. In vitro activity against S. aureus strains resistant to vancomycin and linezolid. | 500 mg every 8 h, intravenous | Inhibition of transpeptidase activity and binding to PBPs | ceftazidime plus linezolid for HAP ceftriaxone ± linezolid for severe CAP | Diarrhea, infusion site reactions, nausea, vomiting, hepatic enzyme elevation, hyponatremia. |
| Ceftaroline | MRSA, methicillin-resistant coagulase-negative staphylococci, penicillin-resistant Streptococcus pneumoniae, vancomycin-resistant Enterococcus faecalis. Gram-negative pathogens no ESBL-producing. | 600 mg every 12 h, intravenous | Inhibition of transpeptidase activity and binding to PBPs | Ceftriaxone (in 2 phase III trials) for CAP | Diarrhea, nausea, rash, infusion-site reactions |
| Ceftazidime/avibactam | ESBL and KPC, AmpC, and OXA-48 enzymes | 2000 mg/500 mg every 8 h, intravenous | The bactericidal action of ceftazidime is mediated through binding to PBPs. The avibactam inactivates beta-lactamases and protects ceftazidime from degradation. | Meropenem for VAP | Abdominal pain, nausea, vomiting, constipation, infusion-site reactions |
| Ceftolozane/tazobactam | AmpC β-lactamases in P. aeruginosa. Enhanced activity against ESBL-producing Enterobacterales | 3 g every 8 h, intravenous | Inhibition of bacterial cell wall synthesis, binding PBPs. Ceftolozane is an inhibitor of PBP3 with higher affinity for PBP1b | Meropenem for HAP | Headache, constipation, hypertension, nausea, diarrhea |
| Meropenem/vaborbactam | Gram-negative pathogens, especially Enterobacterales (ESBL and KPC). AmpC β-lactamases in P. aeruginosa |
2 g/2 g every 8 h, intravenous | Vaborbactam inhibits serine β-lactamases with the formation of a covalent complex and inhibition of beta-lactamases enzymes | Piperacillin/tazobactam for HAP and VAP | Headache, infusion-site reactions |
| Omadacycline | Methicillin-resistant staphylococci, penicillin-resistant streptococci, Gram-negative strains, and atypical bacterial pathogens | 100 mg once daily, intravenous 200 mg once daily, oral |
Action on efflux pumps and ribosomal protection associated with modifications in the chemical structure | Moxifloxacin for CAP | Gastrointestinal side effects |
| Solithromycin | S. pneumoniae, H. influenzae, and atypical pathogens | 400 mg once daily, intravenous and oral with 800 mg loading dose as the first dose | Inhibits bacterial translation by binding to the 23S ribosomal RNA | Moxifloxacin for CAP | Hepatotoxicity |
| Telavancin | Gram-positive strains, including VRE and MRSA | 10 mg/kg every 24 h, intravenous | Inhibition of bacterial wall synthesis (transglycosylation and transpeptidation) and disruption of bacterial membrane function | Vancomycin for HAP | Moderate-to-severe renal impairment |
| Lefamulin | Gram-positive strains, including VRE and MRSA, atypical pathogens | 150 mg twice daily, intravenous or an oral dose of 600 mg twice daily | Inhibition of protein synthesis by binding to the peptidyl transferase center of the 50s bacterial ribosome, thus preventing the binding of tRNA for peptide transfer. | Moxifloxacin ± linezolid for CAP | Diarrhea, nausea, pain at the site of injection, liver inflammation |
Abbreviations: ESBL, extended-spectrum beta-lactamases; PBP, penicillin binding proteins; CRE, carbapenem-resistant Enterobacterales; MRSA, methicillin-resistant Staphylococcus aureus; VRE, vancomycin-resistant enterococci; KPC, Klebsiella pneumoniae carbapenemase; CAP, community-acquired pneumonia; HAP, hospital-acquired pneumonia; VAP, ventilator-associated pneumonia.
Ceftobiprole
Ceftobiprole is a cephalosporin of a new generation with broad-spectrum action, showing in vitro activity against Gram-positive and Gram-negative strains; it was approved in Europe for the treatment of adults with HAP, but it did not receive approval for the treatment of ventilator-associated pneumonia (VAP). Moreover, ceftobiprole was approved for the treatment of community-acquired pneumonia (CAP).13 The in vitro activity includes MRSA, H. influenzae (comprising b-lactamase-producing strains), M. catarrhalis, K. pneumoniae, and E. coli. It is also reported good activity against P. aeruginosa. Of note, the in vitro activity against S. aureus strains resistant to vancomycin and linezolid has been reported. Finally, ceftobiprole shows no activity against Acinetobacter spp. and extended-spectrum beta-lactamases (ESBLs)-producing Enterobacterales. Ceftobiprole exerts its antibacterial activity through the inhibition of transpeptidase activity and binding to penicillin binding proteins (PBPs),14 an essential component for synthesis of the peptidoglycan layer of bacterial cell walls.
Of interest, studies about PK/PD showed that 500 mg every 8 hours dosage is considered the optimal dosage to provide activity against Gram-positive strains.15 Two trials evaluated its clinical efficacy for the treatment of patients with HAP or CAP.16,17 Ceftobiprole was non-inferior to ceftazidime plus linezolid in patients with HAP and to ceftriaxone ± linezolid in patients with severe CAP in phase III trials. Compared to ceftazidime plus linezolid, ceftobiprole did not show non-inferiority in the subgroup of patients with VAP, with the exclusion of this indication. Ceftobiprole achieved a cure rate of 76.4% compared with that of 79.3% for ceftriaxone/linezolid group [95% CI: −9.3% to 3.6%].
Finally, ceftobiprole showed good safety as demonstrated by adverse events (AEs) related to treatment and occurring in patients with HAP or CAP. Most frequent AEs included diarrhea, infusion site reactions, nausea, vomiting, hepatic enzyme elevation, and hyponatremia. However, ceftobiprole showed a good profile of tolerance in all clinical experiences reported in the literature.
Of importance, dose adjustment is required for patients with moderate or severe renal impairment and for patients with end-stage renal disease: 500 mg every 12 hours for creatinine clearance from 30 to 50 ml/min, 250 mg every 12 hours for creatinine clearance <30 ml/min. Dose adjustment is required also for hemodialysis.
Ceftaroline
Ceftaroline is a novel cephalosporin displaying in vitro activity against the most common bacterial pathogens associated with pneumonia. The prodrug ceftaroline fosamil has been approved for the treatment of CAP and acute bacterial skin and skin structure infections (ABSSSI). Of interest, it shows activity against MRSA, meticillin-resistant coagulase-negative staphylococci, penicillin-resistant Streptococcus pneumoniae, and vancomycin-resistant Enterococcus faecalis. Of importance, activity is reported against Gram-negative pathogens, no ESBL-producing. No activity was reported against E. faecium.
Its approval in CAP treatment was based on 2 Phase 3 multinational, randomized controlled trials: FOCUS 1 (Clinicaltrials.gov, Identifier NCT00621504) and FOCUS 2 (Clinicaltrials.gov, Identifier NCT00509106), in which ceftaroline fosamil 600 mg was administered every 12 hours. In these trials, ceftaroline demonstrated non-inferiority compared to ceftriaxone 1 g every 24 hours, with a better outcome (in both trials) in clinical cure rates at the test-of-cure (TOC) visit for patients treated with ceftaroline fosamil.18,19 Ceftaroline fosamil 600 mg every 12 hours was tested and demonstrated superiority to ceftriaxone (2 g every 24 hours)20 in another randomized controlled trial with PORT class 3-4 CAP (Clinicaltrials.gov, Identifier NCT01371838). Of importance, in these trials no definitive data about MRSA pneumonia were discussed, considering also that MRSA patients in intensive care units were not included.21 However, 2 recent meta-analyses highlighted the superior role of ceftaroline to ceftriaxone for the treatment of MRSA.22,23
In the CAPTURE study, real-world observational data supported the use of ceftaroline as an alternative to ceftriaxone for the empirical treatment of adult patients hospitalized with CAP, considering also its safety profile.24,25 In the CAPTURE study was evaluated the outcome of patients who were excluded in the original Phase 3 trials in a noncomparative fashion, providing valuable informations about ceftaroline use in special populations such as MRSA CAP, elderly, critically ill patients, and those with renal failure.
Of interest, recent experience on ceftaroline in the setting of severe CAP was reported in Italy and Spain.26
Ceftazidime/avibactam
Ceftazidime is an old third-generation cephalosporin, administered intravenously and bound to a variety of PBPs including the PBP3 of Gram-negative bacteria, including Pseudomonas aeruginosa. Avibactam is a non-beta-lactam semisynthetic with beta-lactamase inhibitor action that differs from other beta-lactamase inhibitors (like sulbactam, clavulanic acid, and tazobactam) in structure, mechanism and spectrum of inhibition.27 The main mechanism of action is represented by its in vitro activity against ESBL and Klebsiella pneumoniae carbapenemase (KPC), AmpC, and OXA-48 enzymes; however, no activity is reported against MBLs strains or against OXA-type carbapenemases expressed by Acinetobacter spp.28
Ceftazidime/avibactam (CAZ-AVI) is licensed for the treatment of HAP and VAP29 caused by carbapenem-resistant Enterobacterales (CRE), both in empiric or targeted therapy, and in critically ill patients. CAZ-AVI can therefore be considered as an important alternative to the use of colistin in the treatment of infections caused by KPC strains, comprising patients with primary and secondary bacteremia.30,31 In addition, data about PK/PD of CAZ-AVI confirmed the good lung penetration as demonstrated by the levels reported in the epithelial lung fluid (ELF), representing about 30-35% of the plasma concentration. In clinical trials, CAZ-AVI at the dosage of 2000 mg/500 mg every 8 hours achieved the optimal PK/PD target in patients with HAP. Thus, CAZ-AVI represents an important option in critically ill patients with HAP caused by MDR Gram-negative strains.32
In clinical trials, CAZ-AVI was considered overall well tolerated with the most common adverse events represented by abdominal pain, nausea, vomiting, and constipation, and infusion-site reactions.33,34
Ceftolozane/tazobactam
Ceftolozane/tazobactam is a new beta-lactam/beta-lactamase inhibitor combination that shows its action by bactericidal activity through inhibition of bacterial cell wall synthesis, binding PBPs. Ceftolozane is an inhibitor of PBP3 with higher affinity for PBP1b if compared with other beta-lactam agents; its property gives to ceftolozane/tazobactam a peculiar action against AmpC β-lactamases in P. aeruginosa.35 In combination with tazobactam, ceftolozane showed enhanced activity against ESBL-producing Enterobacterales.36
Ceftolozane/tazobactam was recently licensed for the treatment of pneumonia at a dosage of 3 g every 8 hours, expanding its previous indications. Ceftolozane/tazobactam confirmed its specific action in severe infections caused by MDR and extensively drug-resistant (XDR)37,38 P. aeruginosa. Studies showed also that ceftolozane/tazobactam has a high cure rate in patients with pulmonary infections and cystic fibrosis, with excellent safety and tolerability.39 Furthermore, ceftolozane/tazobactam showed a good ELF penetration like CAZ-AVI, confirming its role for the treatment of severe pneumonia.40 A Phase III trial (ASPECT-NP) assessed the efficacy of ceftolozane/tazobactam (3 g every 8 hours) compared with meropenem (1 g every 8 hours) for the treatment of HAP. In this trial were also included VAP caused by P. aeruginosa (Clinicaltrials.gov, Identifier NCT02070757). At 28 days, 87 (24%) patients in the ceftolozane/tazobactam group and 92 (25.3%) in the meropenem group died [95% CI −5.1 to 7.4]. At the test-of-cure visit, 197 (54%) patients in the ceftolozane/tazobactam group and 194 (53%) in the meropenem group were clinically cured [95% CI −6.2 to 8.3]. Ceftolozane/tazobactam was thus noninferior to meropenem in terms of both 28-day all-cause mortality and clinical cure at the test of cure.
Of importance, recent studies reported the clinical experience of ceftolozane/tazobactam for the treatment of MDR-P aeruginosa infections.41 Considering data from clinical trials, ceftolozane/tazobactam (like other cephalosporins) showed a good safety profile.
Of interest, in a Phase 3 trial of patients with intra-abdominal infections was reported a moderate renal impairment (CrCl 30-50 ml/minute), and was reported a lower cure rate in the ceftolozane/tazobactam plus metronidazole group compared to the meropenem group (48% vs 69.2%, respectively). This decreased cure rate, especially in the subgroup of patients ⩾65 years, compared to younger ones (69% vs 82%, respectively), was also considered directly secondary to changes in renal clearance. To date, FDA included a warning for the use of ceftolozane/tazobactam in patients with impaired renal function, monitoring creatinine’s levels.42
Meropenem/vaborbactam
Vaborbactam (VAB) is a β-lactamase inhibitor that belongs to a novel class A and class C; this drug in combination with meropenem (MER) has been evaluated for the therapy of patients with severe infections caused by resistant Gram-negative pathogens, especially Enterobacterales.43 Of interest, VAB is a cyclic boronic acid pharmacophore with a potent inhibition of serine β-lactamases due to the high affinity between the serine-based active sites of beta-lactamases and boronates; its characteristic leads to the formation of a covalent complex and inhibition of beta-lactamases enzymes.44,45 Particularly, VAB was found to be very effective in reducing MIC50 of meropenem from 32 to 0.06 µg/ml and MIC90 from 32 to 1 µg/ml in a study encompassing 991 isolates of KPC-producing Enterobacterales collected between 2014 and 2015.44
A phase 1 study in healthy adult subjects evaluated the safety, tolerability, and pharmacokinetics of VAB following single and multiple ascending doses (Clinicaltrials.gov, Identifier NCT01751269). Of importance, VAB showed a favorable safety profile in the absence of serious AEs. The most common mild AEs were mainly represented by headache and infusion-site reactions.46
In 2017, FDA approved MER/VAB for the treatment of cUTIs based on the results of TANGO-1 trial: MER/VAB (2 g/2 g every 8 hours) was compared with piperacillin/tazobactam (4 g/0.5 g every 8 hours) and showed non-inferiority for the treatment of cUTI and acute pyelonephritis in adult patients (Clinicaltrials.gov, Identifier NCT02166476).
Finally, in a new trial MER/VAB is compared to the best available therapy for the treatment of infections due to CRE (Clinicaltrials.gov, Identifier NCT02168946) about its efficacy, safety and tolerability.
Omadacycline
Among the new compounds, omadacycline (OMC) represents the first aminomethylcycline that was developed for human use. Aminomethylcyclines are semisynthetic antibiotics strictly derivated from tetracycline.47 For this reason, as for tetracycline, efflux pump and ribosomal protection associated with modifications in the chemical structure are considered the main important characteristics of OMC. About the microbiological profile, OMC demonstrated in vitro activity against common etiologies of CAP like methicillin-resistant staphylococci, penicillin-resistant streptococci, Gram-negative strains, and atypical bacterial pathogens.48
The most important study for OMC evaluation was the Omadacyline for Pneumonia Treatment In the Community (OPTIC) study. In this trial, intravenous once-daily oral OMC was compared for efficacy and safety to oral moxifloxacin (MOX) administered once daily in patients with CAP. In the Phase 3 OPTIC study, OMC resulted noninferior to moxifloxacin for the treatment of patients with CAP.49 Based on FDA endpoints, the clinical response rates were very high and OMC resulted generally safe and well tolerated. Omadacycline was noninferior to moxifloxacin for an early clinical response (81.1% and 82.7%, respectively; difference, −1.6 percentage points; 95% CI, −7.1 to 3.8), and the rates of investigator-assessed clinical response at the post-treatment evaluation were 87.6% and 85.1%, respectively (difference, 2.5 percentage points; 95% CI, −2.4 to 7.4).
The safety profile was similar to moxifloxacin but with a lower incidence of diarrhea (of importance no cases of C. difficile infection were reported). Based on the aforementioned, OMC is considered clinically effective and attractive as once-daily option for the treatment of patients with community onset of pneumonia.
Solithromycin
Solithromycin is a fourth-generation macrolide antibiotic, precisely a fluoroketolide. In phase 2 and 3 trials, solithromycin was tested for its use in patients with CAP documented by S. pneumoniae, H. influenzae, and atypical pathogens. Of interest, these trials evaluated also strains showing resistance to other macrolide antibiotics. Five days of solithromycin (800 mg once on day 1, 400 mg once daily thereafter) were compared to 5 days of levofloxacin (750 mg once daily) in patients with CAP in a phase 2, randomized, double-blind, controlled trial50; moreover, the SOLITAIRE-ORAL phase 3 trial was performed comparing solithromycin for 5 days to 7 days of moxifloxacin 400 mg once daily.51 Early clinical response in the intention-to-treat (ITT) population was observed in 333/426 (78.2%) patients in the solithromycin group and 338/434 (77.9%) patients in the moxifloxacin group. Moreover, a non-inferiority of solithromycin group was reported in early clinical response (solithromycin 326 [80.9%], moxifloxacin 330 [81.1%], difference −0.19, 95% CI −5.8 to 5.5). In this trial, a significant rate of aminotransferase elevation with ALT levels greater than 3 times the upper limit of normal was reported in 22 (5.4%) patients of the solithromycin group and only in 14 (3.3%) patients receiving moxifloxacin, and AST greater than 3 times upper limit of normal in 10 (2.5%) patients treated with solithromycin compared to 8 (1.9%) patients of moxifloxacin arm.
Of interest, in another Phase III, randomized study (named SOLITAIRE-IV) solithromycin was compared to moxifloxacin52 with the option to switch to oral therapy based on predefined criteria. These drugs were intravenously administered at the dose of 400 mg once daily, while oral therapy was in line with the dosage of SOLITAIRE-ORAL trial that included the 800 mg loading dose as the first dose. Of importance, in the solithromycin group was not reported ALT and AST elevation in treated patients. For this reason, safety concerns about solithromycin and hepatotoxicity were resolved, and solithromycin may find a place as a first-line therapy for the treatment of patients with CAP or as a second-line therapy for patients with previous antibiotic failure.
Telavancin
Telavancin belongs to the class of new lipoglycopeptides (such as dalbavancin) with peculiar PK/PD characteristics showing a rapid, concentration-dependent activity with bactericidal action against Gram-positive strains, including VRE and MRSA.53,54 Telavancin action is based on 2 different mechanisms of action: inhibition of bacterial wall synthesis (transglycosylation and transpeptidation) and disruption of bacterial membrane function.55 Of interest, the most important PK/PD studies showed a very good concentration in ELF of this drug, with a AUCELF approximately of 75% of the free AUCplasma.56
Telavancin is currently approved by EMA for the treatment of adult patients with suspected or documented MRSA etiology in HAP and VAP, mainly in the setting of patients showing previous treatment failure or where other therapies are not suitable.
In 2 Phase 3, randomized, double-blinded studies (ATTAIN studies) were reported non-inferiority of telavancin (10 mg/kg every 24 hours) versus vancomycin (1 g every 12 hours) for the treatment of HAP.57 The mortality difference was 21.5% versus 16.6% (95% CI −0.7% to 10.6%) for the NCT00107952 trial and 18.5% versus 20.6% (95% CI −7.8% to 3.5%) for the NCT00124020 trial. However, in a systematic review and meta-analysis of data about patients with ABSSSI and HAP reported for telavancin a higher risk of nephrotoxicity and potential serious AEs, if compared to vancomycin.58 Particularly, a higher mortality rate was observed in patients with HAP with moderate-to-severe renal impairment compared to vancomycin.59
Finally, it is important to underline that in a post hoc analysis of data from the 2 Phase 3 ATTAIN trials, analyzing the subset of patients without severe renal impairment or preexisting acute renal failure, telavancin results were similar to vancomycin in the clinical and safety outcomes of treatment groups.60 Then, the indication now is to use telavancin only for patients with normal renal function, monitoring creatinine’s clearance.61
Lefamulin
Lefamulin belongs to the class of pleuromutilin antibiotics, approved by FDA as the therapy of patients with CAP, as intravenous and oral formulations.62-64 Lefamulin exhibits a peculiar mechanism of action through inhibition of protein synthesis by binding to the peptidyl transferase center of the 50s bacterial ribosome, thus preventing the binding of tRNA for peptide transfer.
The microbiological profile of lefamulin is expressed by activity against Gram-positive and pathogens typically associated with community-onset pneumonia, like Streptococcus pneumoniae, Haemophilus influenzae, and atypical pathogens like Mycoplasma pneumonia, Legionella pneumophila, and Chlamydophila pneumoniae. Of importance, lefamulin shows a therapeutic activity that includes MRSA and VRE.
Lefamulin was tested in Phase 2 trials and resulted well tolerated at an IV dose of 150 mg twice daily or an oral dose of 600 mg twice daily. In the multinational Phase 3 trial named Lefamulin Evaluation Against Pneumonia 1 (LEAP 1) was compared about efficacy and safety with moxifloxacin associated or not with linezolid for the treatment of CAP (Clinicaltrials.gov, Identifier NCT02559310). Lefamulin was noninferior to moxifloxacin for early clinical response (87.3% vs 90.2%, respectively; difference −2.9%, 95%CI −8.5 to 2.8) and investigator assessment of clinical response (mITT, 81.7% vs 84.2%, respectively; difference −2.6%, 95% CI −8.9 to 3.9; CE, 86.9% vs 89.4%, respectively; difference −2.5%, 95% CI −8.4 to 3.4).
In LEAP 2 trial (completed in 2018) oral lefamulin was compared about safety and efficacy with oral moxifloxacin used in monotherapy (Clinicaltrials.gov, Identifier NCT02813694).65,66 Early clinical response rates were 90.8% with lefamulin and 90.8% with moxifloxacin (difference, 0.1% [1-sided 97.5% CI, −4.4% to ∞]). Rates of investigator assessment of clinical response success were 87.5% with lefamulin and 89.1% with moxifloxacin in the modified ITT population (difference, −1.6% [1-sided 97.5% CI, −6.3% to ∞]) and 89.7% and 93.6%, respectively, in the clinically evaluable population (difference, −3.9% [1-sided 97.5% CI, −8.2% to ∞]) at test of cure.
Antimicrobial compounds under development
A high number of new compounds for the treatment of patients with CAP and/or HAP are in the final stages of development (see Table 2 and Figure 1). The most important characteristics of these new drugs are represented by novel mechanisms of action and microbiological efficacy, including a broad spectrum activity against multidrug resistant pathogens mainly represented by ESBL-producing strains, CRE, and carbapenem-resistant Acinetobacter baumannii, which still represent a major challenge for management and treatment in clinical practice due to the lack of therapeutic options.
Table 2.
Characteristics of antibiotics under development for the treatment of pneumonia.
| Drug | Spectrum of activity | Administration |
|---|---|---|
| Cefiderocol | ESBL strains CRE also including MBL MDR Pseudomonas spp Carbapenem-resistant Acinetobacter spp S. maltophilia |
2 g every 8 h, intravenous |
| Tedizolid | Gram-positive also including MRSA and linezolid-resistant strains | 200 mg every 24 h, intravenous or oral |
| Imipenem/relebactam | Gram negatives pathogens including AmpC, ESBL, KPC. No activity is reported against MBL and OXA enzymes |
500 mg/250-125 mg every 6 h, intravenous |
| Plazomicin | Gram positives including MRSA Gram negatives strains including ESBL, CRE (KPCs, OXA) MDR Pseudomonas spp MDR Acinetobacter spp No activity is reported against MBL |
15 mg/kg every 24 h, intravenous |
| Iclaprim | MRSA Gram-negative bacteria including Haemophilus influenzae and Moraxella catarrhalis |
80 mg every 12 h, intravenous |
| Aztreonam/avibactam | Enterobacterales including OXA48- and MBL-producing strains. Limited activity against A. baumannii and P. aeruginosa |
6500 mg ATM/2167 mg AVI every 24 h on Day 1 followed by 6000 mg ATM/2000 mg AVI every 24 h, intravenous |
| Ceftaroline/avibactam | MRSA, ESBL- and KPC-producing Enterobacterales | 600/600 mg q24h, IV |
| Eravacycline | MRSA vancomycin-resistant enterococci Enterobacterales including ESBL, KPC and OXA enzymes |
1 mg/kg every 12 h, intravenous |
| Delafloxacin | MRSA penicillin-resistant and levofloxacin-resistant S. pneumoniae, Streptococcus pyogenes Enterococci Gram-negative pathogens including quinolone- susceptible P. aeruginosa Anaerobes |
300 mg every 12 h, intravenous 450 mg every 12 h, oral |
| Murepavadin | Pseudomonas aeruginosa
Stenotrophomonas maltophilia Burkholderia cepacia Enterobacterales A. baumannii Gram-positive bacteria |
Not defined |
Abbreviations: CRE, carbapenem-resistant Enterobacterales; ESBL, extended-spectrum beta-lactamases; KPC, Klebsiella pneumoniae carbapenemase; MDR, multidrug-resistant; MRSA, methicillin-resistant Staphylococcus aureus; NDM, new Delhi metallo-beta-lactamase.
Figure 1.
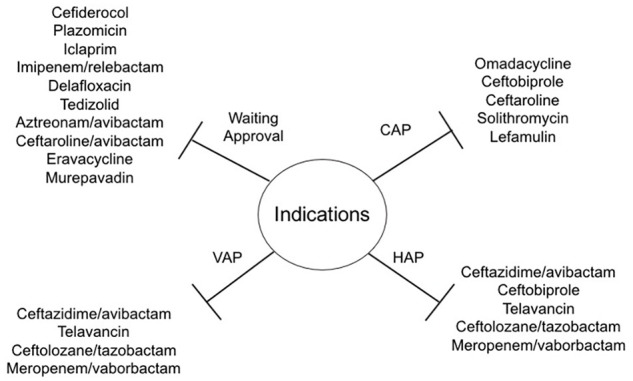
Indications of new approved antibiotics for pneumonia and list of compounds under development.
Moreover, these drugs are probably characterized by favorable toxicity profiles compared with old drugs currently used in clinical practice. Furthermore, the availability of oral formulations could be another important advantage in the use of these drugs, with the potential for an early oral switch even in infections due to resistant pathogens. This list includes: new mechanisms of action, like cefiderocol, iclaprim, and murepavadin, or evolution of well-known mechanisms, like plazomicin (aminoglycoside), ceftaroline/avibactam, aztreonam/avibactam and imipenem/relebactam (Beta-lactams/beta-lactamases inhibitors), delafloxacin (fluoroquinolones), tedizolid (oxazolidinone), eravacycline (fluorocycline).67,68
Conclusion
A high number of new drugs were recently approved for the treatment of pneumonia, including severe forms of community, hospital and ventilator-associated.
The most attractive characteristic of new drugs is represented by the broad spectrum of activity against MDR pathogens, in particular ESBL-producing Enterobacterales and CRE, which still represent a major threat in clinical practice, considering the lack of therapeutic options. Moreover, these new antibiotics in most cases are characterized by favorable toxicity profiles compared with old drugs that are currently used in clinical practice. Some of the new antimicrobials will be also available as oral formulations, with the potential for oral shift even in infections due to resistant pathogens.
Footnotes
Funding:The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contribution: The manuscript was written entirely by the author (Alessandro Russo).
ORCID iD: Alessandro Russo  https://orcid.org/0000-0003-3846-4620
https://orcid.org/0000-0003-3846-4620
References
- 1. Gross AE, Van Schooneveld TC, Olsen KM, et al. Epidemiology and pre- dictors of multidrug-resistant community-acquired and healthcare-associated pneumonia. Antimicrob Agents Chemother. 2014;58:5262-5268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Delle Rose D, Pezzotti P, Fortunato E, et al. Clinical predictors and microbiology of ventilator-associated pneumonia in the intensive care unit: a retrospective analysis in six Italian hospitals. Eur J Clin Microbiol Infect Dis. 2016;35:1531-1539. [DOI] [PubMed] [Google Scholar]
- 3. Claeys KC, Zasowski EJ, Trinh TD, Lagnf AM, Davis SL, Rybak MJ. Antimicrobial stewardship opportunities in critically Ill patients with Gram-negative lower respiratory tract infections: a multicenter cross-sectional analysis. Infect Dis Ther. 2018;7:135-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bassetti M, De Waele JJ, Eggimann P, et al. Preventive and therapeutic strategies in critically ill patients with highly resistant bacteria. Intensive Care Med. 2015; 41:776-795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Teshome BF Lee GC, Reveles KR, et al. Application of a methicillin-resistant Staphylococcus aureus risk score for community-onset pneumonia patients and outcomes with initial treatment. BMC Infect Dis. 2015;15:380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Koulenti D, Lisboa T, Brun-Buisson C, et al. EU-VAP/CAP Study Group. Spectrum of practice in the diagnosis of nosocomial pneumonia in patients requiring mechanical ventilation in European intensive care units. Crit Care Med. 2009; 37:2360-2368. [DOI] [PubMed] [Google Scholar]
- 7. Cruciani M, Gatti G, Lazzarini L, et al. Penetration of vancomycin into human lung tissue. J Antimicrob Chemother. 1996;38:865-869. [DOI] [PubMed] [Google Scholar]
- 8. Ye ZK, Li C, Zhai SD. Guidelines for therapeutic drug monitoring of vancomycin: a systematic review. PLoS One. 2014;9:e99044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wunderink RG, Niederman MS, Kollef MH, et al. Linezolid in methicillin-resistant Staphylococcus aureus nosocomial pneumonia: a randomized, controlled study. Clin Infect Dis. 2012;54:621-629. [DOI] [PubMed] [Google Scholar]
- 10. Bassetti M, Baguneid M, Bouza E, Dryden M, Nathwani D, Wilcox M. European perspective and update on the management of complicated skin and soft tissue infections due to methicillin-resistant Staphylococcus aureus after more than 10 years of experience with linezolid. Clin Microbiol Infect. 2014;20 (suppl 4):3-18. [DOI] [PubMed] [Google Scholar]
- 11. Kollef MH, Bassetti M, Francois B, et al. The intensive care medicine research agenda on multidrug-resistant bacteria, antibiotics, and stewardship. Intensive Care Med. 2017;43:1187-1197. doi: 10.1007/s00134-017-4682-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bassetti M, Welte T, Wunderink RG. Treatment of Gram-negative pneumonia in the critical care setting: is the beta-lactam antibiotic backbone broken beyond repair? Crit Care. 2016;29;20:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Basilea Pharmaceutica International Ltd. Basilea’s antibiotic ceftobiprole obtains regulatory approval in Europe for pneumonia [media release]. 21 March 2018. Accessed March 21, 2018 http://www.basilea.com/chameleon/public/584f9d1e-4298-e47c0475a5e5e5288ded/5825.
- 14. Hebeisen P, Heinze-Krauss I, Angehrn P, et al. In vitro and in vivo properties of Ro 63-9141, a novel broad-spectrum cephalosporin with activity against methicillin-resistant staphylococci. Antimicrob Agents Chemother. 2001;45:825-836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Murthy B, Schmitt-Hoffmann A. Pharmacokinetics and pharmacodynamics of ceftobiprole, an anti-MRSA cephalosporin with broad-spectrum activity. Clin Pharmacokinet. 2008;47:21-33. [DOI] [PubMed] [Google Scholar]
- 16. Awad SS, Rodriguez AH, Chuang YC, et al. A phase 3 randomized double-blind comparison of ceftobiprole medocaril versus ceftazidime plus linezolid for the treatment of hospital acquired pneumonia. Clin Infect Dis. 2014;59:51-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nicholson SC, Welte T, File TM, Jr, et al. A randomised, doubleblind trial comparing ceftobiprole medocaril with ceftriaxone with or without linezolid for the treatment of patients with community-acquired pneumonia requiring hospitalisation. Int J Antimicrob Agents. 2012;39:240-246. [DOI] [PubMed] [Google Scholar]
- 18. File TM, Low DE, Eckburg PB, et al. FOCUS 1: a randomized, double blinded, multicentre, Phase III trial of the efficacy and safety of ceftaroline fosamil versus ceftriaxone in community-acquired pneumonia. J Antimicrob Chemother. 2011; 66(suppl 3): iii19-iii32. [DOI] [PubMed] [Google Scholar]
- 19. Low DE, File TM, Eckburg PB, et al. FOCUS 2: a randomized, doubleblinded, multicentre, Phase III trial of the efficacy and safety of ceftaroline fosamil versus ceftriaxone in community-acquired pneumonia. J Antimicrob Chemother. 2011;66(suppl 3): iii33-iii44. [DOI] [PubMed] [Google Scholar]
- 20. Zhong NS, Sun T, Zhuo C, et al. Ceftaroline fosamil versus ceftriaxone for the treatment of Asian patients with community-acquired pneumonia: a randomised, controlled, double-blind, Phase 3, non-inferiority with nested superiority trial. Lancet Infect Dis. 2015;15:161-171. [DOI] [PubMed] [Google Scholar]
- 21. Rosanova MT, Aguilar PS, Sberna N, et al. Efficacy and safety of ceftaroline: systematic review and meta-analysis. Ther Adv Infect Dis. 2018;6:2049936118808655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Eljaaly K, Wali H, Basilim A, Alharbi A, Afour HZ. Clinical cure with ceftriaxone versus ceftaroline or ceftobiprole in the treatment of staphylococcal pneumonia: a systematic review and meta-analysis. Int J Antimicrob Agents. 2019;54:149-153. [DOI] [PubMed] [Google Scholar]
- 23. Lan SH, Chang SP, Lai CC, Lu LC, Chao CM. Efficacy and safety of ceftaroline for the treatment of community-acquired pneumonia: a systemic review and meta-analysis of randomized controlled trials. J Clin Med. 2019;8:824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Huang X, Jandourek A, Cole P, Friedland D. Current use of ceftaroline for community-acquired bacterial pneumonia (cabp) in us hospitals: length of stay and total cost from the capture study. Chest J. 2013;144:259. [Google Scholar]
- 25. Maggiore C, Pasquale T, Jandourek A, Smith A, Friedland HD. Experience with ceftaroline fosamil as monotherapy and combination therapy with vancomycin in acute bacterial skin and skin structure infections and community-acquired bacterial pneumonia. ASHP Midyear Meeting 2013. Orlando, FL: American Society of Health-System Pharmacists; 2013:5-112. [Google Scholar]
- 26. Bassetti M, Russo A, Cilloniz C, et al. Ceftaroline for severe community-acquired pneumonia: a real-world two-center experience in Italy and Spain: ceftaroline for severe pneumonia. Int J Antimicrob Agents. 2020;55:105921. [DOI] [PubMed] [Google Scholar]
- 27. Sader HS, Castanheira M, Flamm RK, et al. Antimicrobial activity of ceftazidime-avibactam against Gram-negative organisms collected from U.S. medical centers in 2012. Antimicrob Agents Chemother. 2014; 58: 1684-1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Keepers TR, Gomez M, Celeri C, et al. Bactericidal activity, absence of serum effect, and time–kill kinetics of ceftazidime-avibactam against b-lactamase-producing Enterobacterales and Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2014;58:5297-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Torres A, Zhong N, Pachl J, et al. Ceftazidime-avibactam versus meropenem in nosocomial pneumonia, including ventilator-associated pneumonia (REPROVE): a randomised, double-blind, Phase 3 non-inferiority trial. Lancet Infect Dis. 2018;18:285-295. [DOI] [PubMed] [Google Scholar]
- 30. van Duin D, Lok JJ, Earley M, et al. ; Antibacterial Resistance Leadership Group. Colistin versus ceftazidime-avibactam in the treatment of infections due to carbapenem-resistant enterobacterales. Clin Infect Dis. 2018;66:163-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Shields RK, Nguyen MH, Chen L, et al. Ceftazidime-avibactam is superior to other treatment regimens against carbapenem-resistant Klebsiella pneumoniae bacteremia. Antimicrob Agents Chemother. 2017;61:e00883-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bassetti M, Vena A, Castaldo N, Righi E, Peghin M. New antibiotics for ventilator-associated pneumonia. Curr Opin Infect Dis. 2018;31:177-186. [DOI] [PubMed] [Google Scholar]
- 33. Vazquez JA, González Patzán LD, Stricklin D, et al. Efficacy and safety of ceftazidime-avibactam versus imipenem-cilastatin in the treatment of complicated urinary tract infections, including acute pyelonephritis, in hospitalized adults: results of a prospective, investigator-blinded, randomized study. Curr Med Res Opin. 2012;28:1921-1931. [DOI] [PubMed] [Google Scholar]
- 34. Lucasti C, Popescu I, Ramesh MK, et al. Comparative study of the efficacy and safety of ceftazidime/avibactam plus metronidazole versus meropenem in the treatment of complicated intra-abdominal infections in hospitalized adults: results of a randomized, double-blind, Phase II trial. J Antimicrob Chemother. 2013;68:1183-1192. [DOI] [PubMed] [Google Scholar]
- 35. Tato M, García-Castillo M, Bofarull AM, Cantón R; CENIT Study Group. In vitro activity of ceftolozane/tazobactam against clinical isolates of Pseudomonas aeruginosa and Enterobacterales recovered in Spanish medical centres: Results of the CENIT study. Int J Antimicrob Agents. 2015;46:502-510. [DOI] [PubMed] [Google Scholar]
- 36. Armstrong ES, Farrell DJ, Palchak M, Steenbergen JN. In Vitro Activity of Ceftolozane-Tazobactam against Anaerobic Organisms Identified during the ASPECT-cIAI Study. Antimicrob Agents Chemother. 2015;60:666-668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Xipell M, Bodro M, Marco F, Martínez JA, Soriano A. Successful treatment of three severe MDR or XDR Pseudomonas aeruginosa infections with ceftolozane/tazobactam. Future Microbiol. 2017;12:1323-1326. [DOI] [PubMed] [Google Scholar]
- 38. Munita JM, Aitken SL, Miller WR, et al. Multicenter evaluation of Ceftolozane/Tazobactam for serious infections caused by carbapenem-resistant Pseudomonas aeruginosa. Clin Infect Dis. 2017;65:158-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Monogue ML, Pettit RS, Muhlebach M, Cies JJ, Nicolau DP, Kuti JL. Population pharmacokinetics and safety of ceftolozane-tazobactam in adult cystic fibrosis patients admitted with acute pulmonary exacerbation. Antimicrob Agents Chemother. 2016;60:6578-6584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Xiao AJ, Miller BW, Huntington JA, Nicolau DP. Ceftolozane/tazobactam pharmacokinetic/pharmacodynamic-derived dose justification for Phase 3 studies in patients with nosocomial pneumonia. J Clin Pharmacol. 2016;56:56-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Haidar G, Philips NJ, Shields RK, et al. Ceftolozane–tazobactam for the treatment of multidrug-resistant Pseudomonas aeruginosa infections: clinical effectiveness and evolution of resistance. Clin Infect Dis. 2017; 65:110-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Solomkin J, Hershberger E, Miller B, et al. Ceftolozane/tazobactam plus metronidazole for complicated intra-abdominal infections in an era of multidrug resistance: results from a randomized, double-blind, Phase 3 trial (ASPECT-cIAI). Clin Infect Dis. 2015; 60:1462-1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Castanheira M, Huband MD, Mendes RE, Flamm RK. Meropenem-vaborbactam tested against contemporary Gram-negative isolates collected worldwide during 2014, including carbapenem-resistant, KPC-producing, multidrug-resistant, and extensively drug-resistant enterobacterales. Antimicrob Agents Chemother. 2017;61:e00567-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hackel MA, Lomovskaya O, Dudley MN, Karlowsky JA, Sahm DF. In vitro activity of meropenem-vaborbactam against clinical isolates of KPC-positive enterobacterales. Antimicrob Agents Chemother. 2017;62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sun D, Rubio-Aparicio D, Nelson K, et al. Meropenem-vaborbactam resistance selection, resistance prevention, and molecular mechanisms in mutants of KPC-producing klebsiella pneumoniae. Antimicrob Agents Chemother. 2017;61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Griffith DC, Loutit JS, Morgan EE, et al. Phase 1 study of the safety, tolerability, and pharmacokinetics of the β-lactamase inhibitor vaborbactam (RPX7009) in healthy adult subjects. Antimicrob Agents Chemother. 2016;60:6326-6332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Honeyman L, Ismail M, Nelson ML, et al. Structure-activity relationship of the aminomethylcyclines and the discovery of omadacycline. Antimicrob Agents Chemother. 2015;59:7044-7053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Macone AB, Caruso BK, Leahy RG, et al. In vitro and in vivo antibacterial activities of omadacycline, a novel aminomethylcycline. Antimicrob Agents Chemother. 2014;58:1127-1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sun H, Ting L, Machineni S, et al. Randomized, open-label study of the pharmacokinetics and safety of oral and intravenous administration of omadacycline to healthy subjects. Antimicrob Agents Chemother. 2016;60:7431-7435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Oldach D, Clark K, Schranz J, et al. Randomized, Double-Blind, Multicenter Phase 2 Study comparing the efficacy and safety of oral solithromycin (CEM-101) to those of oral levofloxacin in the treatment of patients with community-acquired bacterial pneumonia. Antimicrob Agents Chemother. 2013;57:2526-2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Barrera CM, Mykietiuk A, Metev H, et al. ; SOLITAIRE-ORAL Pneumonia Team. Efficacy and safety of oral solithromycin versus oral moxifloxacin for treatment of community-acquired bacterial pneumonia: a global, double-blind, multicentre, randomised, active-controlled, non-inferiority trial (SOLITAIRE-ORAL). Lancet Infect Dis. 2016;16:421-430. [DOI] [PubMed] [Google Scholar]
- 52. File TM, Jr, Rewerska B, Vucinic-Mihailovic V, et al. SOLITAIRE-IV: a randomized, double-blind, multicenter study comparing the efficacy and safety of intravenous-to-oral solithromycin to intravenous-to-oral moxifloxacin for treatment of community-acquired bacterial pneumonia. Clin Infect Dis. 2016;63:1007-1016. [DOI] [PubMed] [Google Scholar]
- 53. Smith JR, Barber KE, Hallesy J, et al. Telavancin demonstrates activity against methicillin-resistant Staphylococcus aureus isolates with reduced suscept-ibility to vancomycin, daptomycin, and linezolid in broth microdilution MIC and one-compartment pharmacokinetic/pharmacodynamic models. Antimicrob Agents Chemother. 2015;59:5529-5534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Pfaller MA, Mendes RE, Sader HS, Jones RN. Telavancin activity against Gram-positive bacteria isolated from respiratory tract specimens of patients with nosocomial pneumonia. J Antimicrob Chemother. 2010;65:2396-2404. [DOI] [PubMed] [Google Scholar]
- 55. Zhanel GG, Calic D, Schweizer F, et al. New lipoglycopeptides: a comparative review of dalbavancin, oritavancin and telavancin. Drugs. 2010; 70:859-886; Erratum in: Drugs 2011;71:526. [DOI] [PubMed] [Google Scholar]
- 56. Lodise TP, Jr, Gotfried M, Barriere S, Drusano GL. Telavancin penetration into human epithelial lining fluid determined by population pharmacokinetic modeling and Monte Carlo simulation. Antimicrob Agents Chemother. 2008;52:2300-2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Rubinstein E, Lalani T, Corey GR, et al. , ATTAIN Study Group. Telavancin versus vancomycin for hospital-acquired pneumonia due to gram-positive pathogens. Clin Infect Dis. 2011; 52:31-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Polyzos KA, Mavros MN, Vardakas KZ, et al. Efficacy and safety of telavancin in clinical trials: a systematic review and meta-analysis. PLoS One. 2012; 7:e41870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Barriere SL. The ATTAIN trials: efficacy and safety of telavancin compared with vancomycin for the treatment of hospital-acquired and ventilator-asso- ciated bacterial pneumonia. Future Microbiol. 2014; 9:281-289. [DOI] [PubMed] [Google Scholar]
- 60. Torres A, Rubinstein E, Corey GR, et al. Analysis of Phase 3 telavancin nosocomial pneumonia data excluding patients with severe renal impairment and acute renal failure. J Antimicrob Chemother. 2014;69:1119-1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Masterton R, Cornaglia G, Courvalin P, et al. The clinical positioning of telavancin in Europe. Int J Antimicrob Agents. 2015; 45:213-220. [DOI] [PubMed] [Google Scholar]
- 62. File TM, Jr, Goldberg L, Das A, et al. Efficacy and safety of IV-to-oral lefamulin, a pleuromutilin antibiotic, for treatment of community-acquired bacterial pneumonia: the phase 3 LEAP 1 trial. Clin Infect Dis. 2019;69:1856-1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Wicha WW, Strickmann DB, Paukner S. Pharmacokinetics/pharmacodynamics of lefamulin in a neutropenic murine pneumonia model with Staphylococcus aureus and Streptococcus pneumoniae. J Antimicrob Chemother. 2019;74(suppl 3):iii11-iii18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Zhang L, Wicha WW, Bhavnani SM, et al. Prediction of lefamulin epithelial lining fluid penetration after intravenous and oral administration using Phase 1 data and population pharmacokinetics methods. J Antimicrob Chemother. 2019;74(suppl 3):iii27-iii34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Paukner S, Gelone SP, Arends SJR, et al. Antibacterial activity of lefamulin against pathogens most commonly causing community-acquired bacterial pneumonia: SENTRY antimicrobial surveillance program (2015-2016). Antimicrob Agents Chemother. 2019;63:e02161-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Veve MP, Wagner JL. Lefamulin: review of a promising novel pleuromutilin antibiotic. Pharmacotherapy. 2018;38:935-946. [DOI] [PubMed] [Google Scholar]
- 67. Bassetti M, Russo A, Carnelutti A, Wilcox M. Emerging drugs for treating methicillin-resistant staphylococcus aureus. Expert Opin Emerg Drugs. 2019;24:191-204. [DOI] [PubMed] [Google Scholar]
- 68. Bassetti M, Righi E, Russo A, Carnelutti A. New antibiotics for pneumonia. Clin Chest Med. 2018;39:853-869. [DOI] [PubMed] [Google Scholar]


