Abstract
Inflammation is triggered by stimulation of innate sensors that recognize pathogens, chemical and physical irritants, and damaged cells subsequently initiating a well-orchestrated adaptive immune response. Immune cell activation is a strictly regulated and self-resolving process supported by an array of negative feedback mechanisms to sustain tissue homeostasis. The disruption of these regulatory pathways forms the basis of chronic inflammatory diseases, including periodontitis. Ubiquitination, a covalent posttranslational modification of target proteins with ubiquitin, has a profound effect on the stability and activity of its substrates, thereby regulating the immune system at molecular and cellular levels. Through the cooperative actions of E3 ubiquitin ligases and deubiquitinases, ubiquitin modifications are implicated in several biological processes, including proteasomal degradation, transcriptional regulation, regulation of protein-protein interactions, endocytosis, autophagy, DNA repair, and cell cycle regulation. A20 (tumor necrosis factor α–induced protein 3 or TNFAIP3) is a ubiquitin-editing enzyme that mainly functions as an endogenous regulator of inflammation through termination of nuclear factor (NF)–κB activation as part of a negative feedback loop. A20 interacts with substrates that reside downstream of immune sensors, including Toll-like receptors, nucleotide-binding oligomerization domain-containing receptors, lymphocyte receptors, and cytokine receptors. Due to its pleiotropic functions as a ubiquitin binding protein, deubiquitinase and ubiquitin ligase, and its versatile role in various signaling pathways, aberrant A20 levels are associated with numerous conditions such as rheumatoid arthritis, diabetes, systemic lupus erythematosus, inflammatory bowel disease, psoriasis, Sjögren syndrome, coronary artery disease, multiple sclerosis, cystic fibrosis, asthma, cancer, neurological disorders, and aging-related sequelae. Similarly, A20 has recently been implicated as an essential regulator of inflammation in the oral cavity. This review presents information on the ubiquitin system and regulation of NF-κB by ubiquitination using A20 as a representative molecule and highlights how the dysregulation of this system can lead to several immune pathologies, including oral cavity–related disorders mainly focusing on periodontitis.
Keywords: periodontitis, ubiquitination, TLR, NF-κB, cytokines, inflammation
Introduction
Inflammation is a universal phenomenon that functions during severe perturbations of homeostasis and is mediated by various cells. Inflammatory responses are dependent on numerous signaling pathways, including nuclear factor κ light-chain enhancer of activated B cells (NF-κB), mitogen-activated protein kinase (MAPK), and interferon regulatory factor (IRF). (Rothschild et al. 2018; Adelaja and Hoffmann 2019). As differences in strength of these signals or crosstalk between pathways can elicit distinct cell functions, regulation of each cascade is critical for appropriate immune responses. If regulatory mechanisms are disrupted, it can lead to molecular and cellular dysregulation, forming the basis of multiple immune and inflammatory conditions, including those affecting the oral cavity such as periodontal disease.
Among many regulatory circuits at work, ubiquitination has recently emerged as an essential player in controlling virtually every aspect of cellular homeostasis (Swatek and Komander 2016; Rape 2018; Kliza and Husnjak 2020). In addition, aberrant function of enzymes involved in ubiquitin regulation oftentimes contribute to the onset and progression of several disorders. Particularly, abnormal expression and/or function of ubiquitin-editing protein A20 (tumor necrosis factor α–induced protein 3 or TNFAIP3) has been associated with chronic inflammation and tissue damage, contributing to the immunopathology of multiple human autoimmune and inflammatory diseases, including diabetes and cancer, as well as pulmonary, neurological, skin, and gastrointestinal disorders (Fukaya et al. 2016; Zhou et al. 2016; Aeschlimann et al. 2018; Kadowaki et al. 2018; Devos et al. 2019; Momtazi et al. 2019). Recent evidence also reveals the ubiquitin system and A20 as a critical regulator in the oral cavity and particularly in periodontal disease pathogenesis (Hong et al. 2016; Zhou et al. 2016; Crump et al. 2017; Li et al. 2019; 2020; Yan et al. 2020).
In this review article, we present information on the ubiquitin system and regulation of NF-κB by ubiquitination using A20 as a representative ubiquitin-editing enzyme. We further provide insights on how the dysregulation of this system can lead to several immune pathologies, including oral cavity–related disorders, mainly focusing on periodontitis.
Pathogenesis of Periodontitis
As one of the most common inflammatory disorders, periodontal diseases affect nearly half of the adult population, resulting in destruction of tooth-supporting structures. Triggered by host-mediated microbial dysbiosis, periodontitis is also associated with a multitude of systemic conditions (Crump and Sahingur 2016; Beck et al. 2019). The inflammatory response in the periodontium involves host cells of myeloid and nonmyeloid origins (Figure 1). Cytosolic, membrane-associated, and secreted pattern recognition receptors (PRRs) on tissue-resident cells, including Toll-like receptors (TLRs) and NOD (nucleotide-binding oligomerization-domain protein)–like receptors (NLRs), sense and bind evolutionary conserved structures on pathogens (pathogen-associated molecular patterns [PAMPs]) or endogenous stress signals (danger-associated molecular patterns [DAMPs]). The corresponding interaction results in the initiation of diverse inflammatory signaling cascades, most of which converge through NF-κB. In addition to mediating proinflammatory gene induction, NF-κB signaling regulates the differentiation, activation, and recruitment of immune cells to infection sites, thereby shaping critical events that control innate and adaptive immune responses within the periodontium (Crump and Sahingur 2016; Rothschild et al. 2018; Adelaja and Hoffmann 2019).
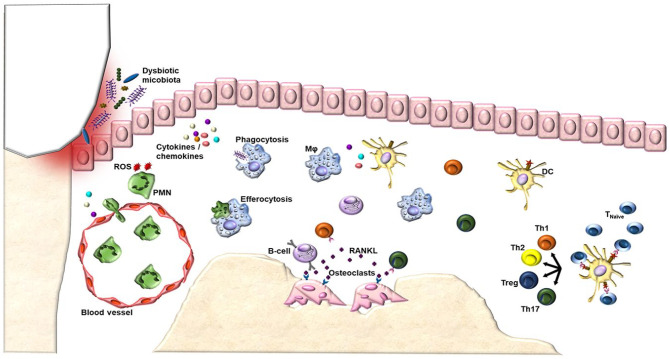
Figure 1.
The pathological host immune response in periodontitis. A dysbiotic oral microflora initiates the innate immune response by stimulating resident cells in the oral epithelium to produce mediators of inflammation. The secretion of these proinflammatory cytokines and chemokines drives the infiltration of various leukocytes of the innate and adaptive immune response into the compromised tissue. Among the first responders, neutrophils and macrophages act as professional phagocytes by engulfing and killing bacteria. Neutrophils attempt to kill invading agents by releasing potent effector molecules, such as reactive oxygen species (ROS). In addition to phagocytosis of microorganisms, macrophages clear apoptotic neutrophils in a process known as efferocytosis and activate lymphocyte-mediated adaptive immunity, bridging innate and adaptive responses. Dendritic cells uptake microbial antigenic material and present to lymphocytes in the lymphoid tissue, evoking the adaptive immune response. Failure of inflammation resolution now transitions the inflammatory response to a chronic state, altering bone homeostasis and leading to subsequent osteoclast activation and destruction of alveolar bone. Specifically, dendritic cells can interact with naive T-helper cells, driving their differentiation into several subsets, including Th1, Th2, Th17, and regulatory T cells (Treg). In addition to activating macrophages, T cells are important regulators of bone turnover through their elaborate production of proresorptive cytokines, including interleukin 17, which directly support osteoclastogenesis. Due to the importance of T-cell subsets, characteristics of prolonged adaptive immunity can differ depending on whichever class of T cells is present. In addition to prominent T-cell infiltration, B cells are also abundant in the chronic lesions. The dual functionality of B cells is highlighted by their protective ability in facilitating bacterial clearance and their destructive roles in promoting inflammation, bone resorption, and matrix dissolution. These observations suggest that the clinical progression from gingivitis to periodontitis is characterized by the shift to T- and B-cellular infiltrates. For a complete list of references used for this figure, please see the Appendix references for Figure 1 legend. DC, dendritic cell; Mφ, macrophage; PMN, polymorphonuclear neutrophil; ROS, reactive oxygen species.
In early lesions, neutrophils accumulate at insult sites in a highly regulated process known as the leukocyte adhesion cascade. In addition to forming an initial barrier located between biofilm and host tissues, neutrophils kill invading agents by releasing noxious contents of their granules, which do not distinguish between microbial and host targets, so oftentimes collateral tissue damage is unavoidable (Hajishengallis and Korostoff 2017). Following this initial response is a proliferative phase characterized by monocyte and macrophage expansion. However, the most potent antigen-presenting cell of the initial response is the dendritic cell (DC). In addition to detecting invading pathogens, they also capture, process, and present antigens to lymphocytes, subsequently controlling the differentiation and quality of T- and B-cell proliferation (Meghil and Cutler 2020). At this acute stage, the cumulative functions of innate cells are necessary to control microbial insult, resolve inflammation, and establish homeostasis with no damage to oral cavity tissues. Periodontal inflammation transitions to a chronic state if the initial inflammatory response fails to terminate in a timely fashion. While this response provides the host with new specialized defenses, an impaired balance in distinct T-cell subsets, including Th1, Th2, Th17, and regulatory T cells, as well as aberrant expression of B cells and extravascular immunoglobulins, can contribute to periodontitis pathogenesis (Hajishengallis and Korostoff 2017).
In summary, acute inflammation triggered by the activation of innate sensors successfully supports the careful orchestration of cellular innate and adaptive immune responses to promote tissue homeostasis restoration. A failure to timely terminate inflammation leads to not only periodontitis but also a plethora of chronic inflammatory diseases. In the milieu of periodontitis, the biological functions initiating the inflammatory responses mainly converge through NF-κB signaling, but there are still critical gaps in our knowledge about downstream regulatory pathways that terminate the initial response and facilitate resolution. Aberrant function of any of the cellular networks due to impaired activity of regulatory pathways, such as the ubiquitin system, can result in exaggerated production of proinflammatory mediators (TNF, interleukin 1, and prostaglandins), tissue-damaging matrix metalloproteinases (MMPs), reactive oxygen species (ROS), impaired antigen presentation, and defects in lymphocyte development, all of which contribute periodontitis severity.
The Ubiquitin System and NF-κB Signaling
Ubiquitination is a highly dynamic, enzymatically catalyzed posttranslational modification, which regulates cellular and immune homeostasis through structurally and functionally distinct polyubiquitin signals (Swatek and Komander 2016; Kliza and Husnjak 2020; Mendes et al. 2020). Ubiquitin can be covalently attached to targeted substrates as monomers (monoubiquitination) or polymers (polyubiquitination) through the coordinated activity of 3 main enzymes: E1 (ubiquitin activating), E2 (ubiquitin conjugating), and E3 (ubiquitin ligating). The topology of ubiquitin chains dictates the fate of proteins, and ubiquitination can be reversed by the activity of deubiquitinating enzymes (Rape 2018). In contrast to phosphorylation, multiple types of ubiquitin chains can be constructed and added to target proteins, which significantly increases the amount of signaling information that can be encoded by ubiquitination (Fig. 2). Originally, the ubiquitin system had been recognized for its role in proteasomal degradation, whereas recently, other regulatory functions have been uncovered, including regulation of mitophagy, autophagy, receptor internalization and downregulation, intracellular trafficking, DNA repair, and protein-protein interactions (Fig. 2) (Swatek and Komander 2016; Kliza and Husnjak 2020; Mendes et al. 2020). Due to its expansive roles, deregulation of ubiquitination has broad consequences from aberrant activation of inflammatory pathways to insufficient assembly of protein complexes, all which underlie divergent disease outcomes (Hu and Sun 2016; Adelaja and Hoffmann 2019; Kliza and Husnjak 2020). Therefore, there is growing impetus to understand the ubiquitin machinery responsible for addition or removal of ubiquitin molecules, which are essential for preserving cellular homeostasis and preventing disease pathogenesis.
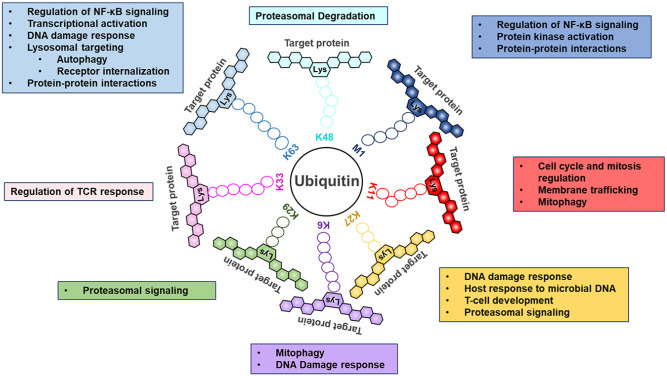
Figure 2.
Individual ubiquitin linkage types and their associated biological roles. Ubiquitin, a small 76–amino acid protein, can be attached to a targeted substrate or a ubiquitin molecule that is already attached to a substrate, resulting in specific polyubiquitin linkage types. In a ubiquitin chain, ubiquitin moieties can be conjugated through one of their lysine resides (Lys11, Lys27, Lys6, Lys29, Lys33, Lys63, and Lys48) or the N-terminal methionine residue (Met1). Each chain is recognized by different ubiquitin-binding domains, targeting proteins in specific signaling pathways. Most extensively studied, Lys48- and Lys63-linked chains are the 2 most abundant chain types and regulate proteasomal degradation and a variety of proteolytic and nonproteolytic events, respectively. Recently, innovative technologies revealed the role of the remaining ubiquitin chain types in controlling cellular processes ranging from cell cycle control to cytokine signaling (Swatek and Komander 2016; Mendes et al. 2020).
E3 ubiquitin ligases represent the most heterogenous class of enzymes in the ubiquitin system. Over 600 E3 ligases are encoded by the human genome, which can be classified into 4 families based on the presence of characteristic domains and the mechanism of ubiquitin transfer to the targeted substrate (Rape 2018; Kliza and Husnjak 2020). Conversely, only around 100 deubiquitinases exist in current literature, which primarily function to preserve ubiquitin homeostasis by maintaining a free ubiquitin pool in cells, editing and trimming ubiquitin chains, and removing ubiquitin from modified substrates (Swatek and Komander 2016; Rape 2018; Kliza and Husnjak 2020). All deubiquitinases can be subdivided into 6 families according to their sequence and structural similarity, and similar to E3 ligases, they regulate several cellular processes.
There is extensive research to characterize the intermolecular interactions between E3 ubiquitin ligases, deubiquitinases, and their cognate substrates, and each layer of the ubiquitin system is now being recognized as novel targets for molecular-based therapies (Huang and Dixit 2016; Kliza and Husnjak 2020). In addition, as E3 ligases and deubiquitinases use distinct catalytic mechanisms, targeting them is anticipated to yield therapies with better specificity and less toxicity. With this burgeoning knowledge, the development of agents to specifically antagonize or complement their functions could serve as promising modulators of cellular physiology and means to regulate aberrant signaling.
Given the extent to which ubiquitin can modulate signaling cascades, it comes as no surprise that ubiquitin plays a regulatory role in nearly every major inflammatory signaling pathway in periodontitis and similar disease pathologies. Among several ubiquitin-regulated pathways, ubiquitin regulation of NF-κB signaling is perhaps the best studied, offering novel insights into one of the archetypical drivers of inflammation involved in disease pathogenesis (Hu and Sun 2016; Rothschild et al. 2018; Adelaja and Hoffmann 2019). Since its discovery over 30 y ago, NF-κB has been implicated in the initiation and propagation of the host innate and adaptive immune response (Rothschild et al. 2018; Adelaja and Hoffmann 2019). There are 5 members of the NF-κB family of transcription factors: Rel (c-Rel), Rel A (p65), Rel B, NF-κB1 (p105/50), and NF-κB2 (p100/52). Together, these proteins target many genes to activate their expression, which include chemokines/cytokines, immunoreceptors, proteins involved in antigen presentation, cell adhesion molecules, acute-phase proteins, stress response genes, and cell death receptor ligands and their modulators (Rothschild et al. 2018).
NF-κB signaling is regulated through posttranscriptional mechanisms (e.g., microRNA regulation) or posttranslational mechanisms (e.g., phosphorylation and ubiquitination) (Rothschild et al. 2018; Adelaja and Hoffmann 2019). Among those regulatory mechanisms, it is now evident that ubiquitination and ubiquitin-editing enzymes play an integral part in the regulation of NF-κB signaling downstream of PRRs, cytokine receptors, and T- and B-cell receptors (Appendix Table 1, Fig. 3) (Hu and Sun 2016; Rothschild et al. 2018; Adelaja and Hoffmann 2019). A few of the most well-studied deubiquitinases that can specifically inhibit NF-κB signaling include A20, CYLD (cylindromatosis tumor suppressor protein), and Cezanne (cellular zinc finger anti–NF-κB) (Table). These ubiquitin-editing enzymes primarily function by disassembling Lys63-linked polyubiquitin chains to restrict NF-κB signaling (Fig. 3) (Hu and Sun 2016; Rothschild et al. 2018; Adelaja and Hoffmann 2019). Although they share overlapping substrates and partially redundant functions, they display differences in ubiquitin chain specificity and mechanisms in which they are regulated. Due to these differences, different phenotypes have been observed in both mice and humans carrying mutations of these enzymes (Table) (Hu and Sun 2016; Rothschild et al. 2018).
Table.
Structure and Function of Deubiquitinases in NF-κB Signaling and Associated Disorders.
| Ubiquitin-Editing Enzymes | Structure | Targeted Substrates | Function | Related Human Diseases | References |
|---|---|---|---|---|---|
| A20 | N-terminal ovarian tumor domain responsible for deubiquinating activity. C-terminal domain composed of 7 zinc fingers, which mediate its E3 ubiquitin ligase activity and ubiquitin-binding activity. | RIP1, RIP2, RIP3, TRAF2/TRAF6, NEMO, MALT1, UBC13 | Restricts NF-κB signaling downstream of TNFR, TCR, TLR, NLR, and IL-1R | Periodontitis, SLE, rheumatoid arthritis, psoriasis, type 1 diabetes, Crohn disease, coronary artery disease in type 2 diabetes, systemic sclerosis, Behçet disease, Sjögren syndrome, diffuse large B-cell lymphoma, mucosa-associated lymphoid tissue-type lymphoma, Hodgkin lymphoma, neurological disorders, cystic fibrosis, asthma, haploinsufficiency of A20, cancers | Ma and Malynn (2012), Zhou et al. (2016), Crump et al. (2017), Aeschlimann et al. (2018), Malynn and Ma (2019), Martens and van Loo (2020) |
| CYLD | Three cytoskeletal-associated protein-glycine-conserved (CAP-Gly) repeats and 1 ubiquitin-specific protease domain that mediates its deubiquitinating activity | TRAF2/TRAF6, NEMO, RIP1, TAK1 | Negatively regulates NF-κB signaling upstream of IKK | Cylindromatosis, multiple familial trichoepithelioma, Brook-Spiegler syndrome, multiple myeloma, cancers | Hu and Sun (2016), Rothschild et al. (2018) |
| Cezanne | N-terminal domain responsible for its deubiqutinating activity and a C-terminal domain that mediates its ubiquitin-binding activity | RIP1, TRAF3, TRAF6 | Restricts NF-κB activation upstream of IKK complex | Cancers | Hu and Sun (2016), Rothschild et al. (2018) |
IKK, IκB kinase complex; IL-1R, interleukin 1 receptor; MALT1, mucosa-associated lymphoid tissue lymphoma translocation protein; NEMO, NF-κB essential modulator; RIP, receptor interacting protein; SLE, systemic lupus erythematosus; TAK1, TGFβ-activated kinase 1; TCR, T-cell receptor; TLR, Toll-like receptor; TNFR, tumor necrosis factor receptor; TRAF, TNF receptor-associated factor; UBC13, ubiquitin-conjugating 13.
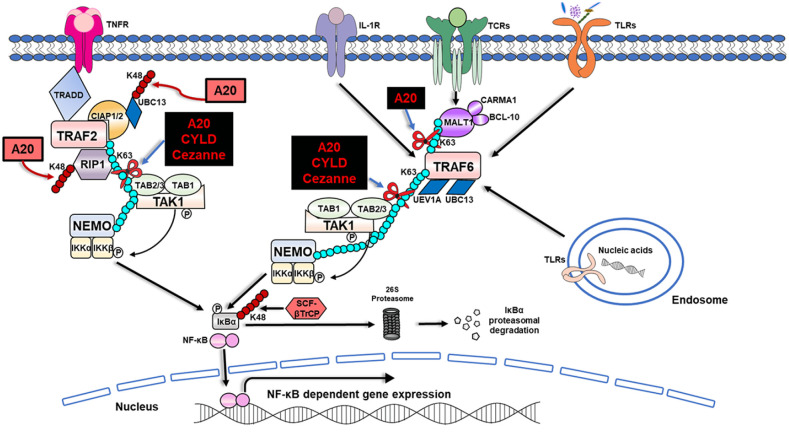
Figure 3.
Regulation of NF-κB by ubiquitination and the deubiquitinases: A20, CYLD, and Cezanne. In the initiation of NF-κB signaling, ligand binding to a variety of immune sensors such as TNFR, IL-1R, TCR, and TLR results in the recruitment of TRAFs to the receptors. TRAF2 is recruited with a protein complex, which consists of multiple adaptor proteins, including TRADD, RIP1, and ubiquitin ligases: cIAP1 and cIAP2. cIAP promotes Lys63-linked polyubiquitination on both RIP1 and TRAF2 but also on themselves to generate a binding platform, allowing the subsequent attachment of further distal components. Polyubiquitinated RIP1 is recognized by the binding partners TAB1 and TAB2/TAB3. The Lys63-linked polyubiquitin chains also can bind the IKK regulatory subunit, NEMO. Due to the new proximity between the TAK1 and IKK, TAK1 phosphorylates IKKβ at 2 serine residues, resulting in the activation of IKK and rapid phosphorylation of IκBα. Subsequent ubiquitination of IκBα can now be carried out by E3 ligase, SCF-βTrCP. Although polyubiquitinated IκBα remains attached to NF-κB dimers, it is selectively degraded by the 26S proteasome, allowing the nuclear translocation of NF-κB, where it induces the activation of NF-κB through the transcription of NF-κB responsive genes. Conversely, once TRAF6 is activated, it functions as an E3 ubiquitin ligase and, together with the ubiquitin E2 complex, UBC13 and UEV1A, catalyzes the synthesis of Lys63-linked polyubiquitin chains onto itself as well as NEMO. Similar enzymatic events mentioned above follow, resulting in the ultimate degradation of IκBα by the 26S proteasome, allowing the nuclear translocation and activation of NF-κB–dependent genes (Rothschild et al. 2018). Lys63-linked polyubiquitin chains also are found to be bound to MALT1, which is a part of the CBM (CARMA1/BCL-10/MALT1) complex downstream of TCR, aiding in the activation of NF-κB signaling (Hu and Sun 2016). Deubiquitinating enzymes (A20, Cezanne, and CYLD) negatively regulate the NF-κB pathway by cleaving the Lys63-linked polyubiquitin chains from various target molecules, as denoted by black squares (Ma and Malynn 2012; Hu and Sun 2016; Rothschild et al. 2018). In the case of TNFR signaling, RIP1 can be subjected to the deubiquitinase function of all these enzymes, interfering with ubiquitin-mediated protein-protein interactions, thereby inhibiting downstream NF-κB activation. Furthermore, A20 and CYLD were also shown to inhibit NF-κB activation by the Lys63-linked deubiquitination and subsequent inactivation of TRAF6. Uniquely, A20 also can remove Lys63-linked polyubiquitin chains from MALT1 downstream of T- and B-cell antigen receptors. In addition, via its specialized E3 ligase activity, A20 adds Lys48-linked polyubiquitin chains to RIP1 and UBC13 for their subsequent degradation, as shown in pink squares (Martens and van Loo 2020). BCL-10, B-cell lymphoma 10; CARMA1, caspase recruitment domain-containing membrane-associated guanylate kinase protein 1; cIAP1 and 2, cellular inhibitor of apoptosis 1 and 2; IKK, IκB kinase complex; IL-1R, interleukin 1 receptor; MALT1, mucosa-associated lymphoid tissue lymphoma translocation protein; NEMO, NF-κB essential modulator; RIP1, receptor interacting protein 1; SCF-βTrCP, Skp1-Cull-F-box ligase containing the F-box protein βTrCP; TAB1,2,3, TAK1-binding protein 1,2,3; TAK1, TGFβ-activated kinase 1; TCR, T-cell receptor; TNFR, tumor necrosis factor receptor; TRADD, tumor necrosis factor receptor type 1–associated DEATH domain; TRAF, TNF receptor-associated factor; UBC13, ubiquitin-conjugating 13; UEV1A, ubiquitin-conjugating enzyme E2 variant 1A.
Although CYLD and Cezanne only function as deubiquitinases, A20 also uniquely functions as an E3 ligase mediating Lys48-linked ubiquitination of several substrates in NF-κB signaling, resulting in their subsequent proteasomal degradation (Fig. 3) (Malynn and Ma 2019; Martens and van Loo 2020; Priem et al. 2020). Due to its diverse functions, A20 has frequently been described as a key regulator of inflammation through termination of NF-κB activation, which makes it a plausible target for therapeutic interventions (Ma and Malynn 2012; Martens and van Loo 2020; Priem et al. 2020). In addition, recent discoveries identified A20 as a susceptibility gene for a plethora of immune pathologies, including those related to the oral cavity, underscoring its significance in the resolution of inflammation and the prevention of human disease (Table) (Ma and Malynn 2012; Martens and van Loo 2020; Priem et al. 2020).
A20 in Health and Diseases
A20 is a 790–amino acid zinc finger protein that was originally identified as a potent inhibitor of inflammation downstream of TNF receptor. However, it has been increasingly characterized as a pleiotropically expressed regulator of ubiquitin-dependent signals interfering with a wide array of biological processes (Ma and Malynn 2012; Martens and van Loo 2020; Priem et al. 2020). Similar to other tissues, A20 is constitutively expressed in the gingiva, albeit expression is low or absent in healthy conditions (Crump et al. 2017). Its transcription can be rapidly induced in a NF-κB–dependent manner in response to stimuli (e.g., microbial species or ROS), providing negative feedback control of NF-κB signaling. In addition, A20 expression and function may be regulated by transcriptional, posttranscriptional, and posttranslational mechanisms, including microRNA regulation, phosphorylation, glycosylation, and protein interactions (Ma and Malynn 2012; Martens and van Loo 2020). Loss of A20 function through single-nucleotide polymorphisms (SNPs) and/or promoter methylation has been frequently observed in human lymphomas and autoimmune and inflammatory disorders (Boonyasrisawat et al. 2007; Honma et al. 2009; Kato et al. 2009; Nair et al. 2009; Ma and Malynn 2012; Tessier-Cloutier et al. 2019). The potent anti-inflammatory role of A20 is further exemplified by the phenotype of A20-deficient (A20−/−) mice, which succumb to premature death from systemic inflammation and severe cachexia (Malynn and Ma 2019). Moreover, mice with cell-specific deletions of A20 recapitulate several human pathologies (Das et al. 2018; Malynn and Ma 2019).
The role of A20 in inflammation and its contribution to microbial homeostasis have been most arduously studied in cell-specific A20-deficient models. Mice with keratinocyte-specific A20 deletion develop epidermal hyperplasia, splenomegaly, and increased numbers of splenic neutrophils and monocytes (Devos et al. 2019). Furthermore, these mice show exacerbated disease severity upon induction of experimental psoriasis, atopic dermatitis, or skin barrier disruption (Devos et al. 2019). In addition, mice with A20 deficiency in myeloid cells develop spontaneous polyarthritis with massive cartilage and bone destruction due to elevated synovial and periarticular inflammation (Matmati et al. 2011). Consistent with its role as a negative feedback regulator of inducible NF-κB–dependent gene expression, cultured A20-deficient peritoneal macrophages from these mice display sustained degradation of IκBα in response to lipopolysaccharide (LPS) challenge. Aberrant NF-κB activation resulting in increased inflammation was also observed in A20-deficient human and murine macrophages upon infection with oral bacterium, Porphyromonas gingivalis (Li et al. 2019). Furthermore, subsequent experiments using a murine ligature-induced periodontitis model revealed that even a partial A20 deficiency caused increased alveolar bone loss due to elevated inflammation in the gingiva.
A20 has also been observed to preserve commensal microbial homeostasis. Decreased intestinal microbial richness and composition were detected in mice with a myeloid-specific deletion of A20, which is likely related to the rheumatoid arthritis–like phenotype of these mice (Vereecke et al. 2014). In fact, lowered bacterial richness and less stable microbiota are often associated with pronounced inflammatory phenotypes. Similarly, young mice lacking A20 in dendritic cells display a dysbiotic microbiome that confers their susceptibility to intestinal inflammation (Talpin et al. 2019). This regulatory mechanism is also seen in the lung epithelial cells, as A20 deficiency in these cells sensitized these mice to allergic asthma upon chronic exposure to low-dose bacterial endotoxin (Schuijs et al. 2015).
Aberrant A20 function has been also linked to autoimmune conditions. Patients with systemic lupus erythematosus (SLE) carrying an SNP in the A20 deubiquitinase domain exhibit elevated presence of antibodies against citrullinated proteins in their serum (Odqvist et al. 2019). In fact, neutrophils isolated from these patients display increased neutrophil extracellular trap (NET) formation. These results highlight the likely contribution of A20 deubiquitinase domain polymorphisms to abnormal neutrophil function as crucial factors in SLE pathogenesis.
A20 is also implicated to modulate cardiovascular responses in murine experimental in vivo models of cardiovascular dysfunction. Specifically, A20 expression in CD11c+ DCs was shown to attenuate the severity of the hypertensive response by limiting the activation of T cells in the kidney and draining lymph nodes (Lu et al. 2019). In the oral mucosa, DCs play a critical role in immune defense by inducing antigen-specific T-cell activation, differentiation, and proliferation (Meghil and Cutler 2020). However, the role of A20 in regulating the function of DCs in the oral cavity has not been previously investigated but can certainly offer insights into the causal link between dendritic cell function and periodontal breakdown.
A constant state of low-grade systemic inflammation likely links obesity with chronic inflammatory diseases, including cardiovascular disease, diabetes, and periodontitis, although the exact biological mechanisms are poorly understood (Beck et al. 2019; Konkel et al. 2019). Mechanistic studies revealed that A20 overexpression in mice with obesity-induced heart injuries reversed myocardial dysfunction, hypertrophy, and fibrosis through reducing cardiac inflammation and apoptosis (Xu et al. 2018). In addition, genetic variants in the A20 locus resulting in lower A20 messenger RNA (mRNA) levels in patients with type 2 diabetes are shown to be associated with increased cardiovascular risk, while higher A20 mRNA levels are associated with protection against coronary artery disease (Boonyasrisawat et al. 2007). Moreover, the presence of an SNP in the TNFAIP3 gene contributes to compromised residual β-cell function and impaired glycemic control in type 1 diabetic children (Fukaya et al. 2016) . Similarly, increased A20 expression in adipose tissue was shown to ameliorate adipose tissue inflammation (Hand et al. 2015). Collectively, targeting A20 may likely offer a novel approach to mitigating obesity- and diabetes-related pathologies within the oral cavity, thereby improving health as well.
The physiological function of A20 along with its potent tumor suppressor functions has been exemplified by its ability to modulate cell death programs. A20-deficient B cells are hyperresponsive to various stimuli and display increased numbers of germinal centers, autoantibodies, and glomerular immunoglobulin deposits (Tavares et al. 2010; Chu et al. 2011; Hovelmeyer et al. 2011). The increase in germinal center B cells is likely due to the resistance of A20-deficient B cells to apoptosis mediated by enhanced expression of NF-κB–dependent antiapoptotic proteins, Bcl-x and Bcl-2. The deregulation of these processes is heavily implicated in human disease, and loss of A20 expression is found in a variety of lymphomas (Chu et al. 2011; Malynn and Ma 2019). Conversely, increased A20 expression has been associated with more aggressive subtypes in breast cancer patients through its effect on transforming growth factor β (TGF-β) signaling, which promotes increased epithelial to mesenchymal transition (Yoon et al. 2019). Overall, the studies highlight the importance of elucidating factors contributing to tumor microenvironment heterogeneity to determine pathogenesis and treatment options. At minimum, it is evident that A20 levels must be tightly regulated, and either A20 overexpression or deficiency can contribute to divergent disease outcomes in cancer biology.
A20 also displays an antiapoptotic function, often related to its inhibition of NF-κB signaling (Ma and Malynn 2012). In fact, hepatocyte-specific A20 deficiency was shown to render mice more susceptible to spontaneous liver inflammation and enhanced apoptosis (Catrysse et al. 2016). In the oral mucosa, the stringent regulation of immune cell life span and turnover is an important dimension of immune homeostasis, and a disruption of apoptosis can contribute to chronic inflammation seen in periodontitis (Meghil and Cutler 2020). In fact, insufficient A20 levels sensitize gingival keratinocytes to bacteria and TNF-induced apoptosis, supporting the notion that A20-targeted therapies can help maintain the integrity of the gingival epithelium and restore impaired periodontal tissue homeostasis (Li et al. 2020).
A20 was also shown to prevent inflammasome-dependent arthritis by inhibiting RIPK3-MLKL–mediated necroptosis in macrophages (Polykratis et al. 2019). Similarly, deletion of A20 in microglia cells exacerbates neuroinflammation in mice characterized by altered microglia morphology, inflammasome hyperactivation, and pyroptosis induction (Voet et al. 2018; Mohebiany et al. 2020). A20 downregulation in cortical neurons, astrocytes, and microglial cells also results in increased necroptosis in vitro, and in a rat model of traumatic brain injury, silencing of A20 results in aggravated controlled cortical-induced necroptosis and a slowed recovery of motor neuron function (Bao et al. 2019). In the light of emerging associations between neuroinflammatory disorders such as Alzheimer disease and periodontitis, future investigations are warranted to determine if A20 can be a link between these conditions.
A20 has also been shown to interact with autophagic proteins p62 and ATG16L1, regulating autophagic, inflammatory, and cell death responses (Kanayama et al. 2015; Slowicka et al. 2019). Autophagy is another critical intracellular pathway involved in the maintenance of oral tissue homeostasis and periodontium integrity (Meghil and Cutler 2020). In fact, A20 was shown to exert antiosteoclastogenic effects via the inhibition of TRAF6-dependent autophagy in human periodontal ligament cells under hypoxic conditions, which mimics the microenvironment frequently seen in periodontitis (Yan et al. 2020). These results further substantiate the critical role of A20 in the pathophysiology of periodontitis.
Overall, A20 exerts diverse influences on the physiological functions of a variety of cell types. Although the anti-inflammatory role of A20 is well established, its role in cell death responses seems to be highly context dependent, resulting in sensitization or desensitization to apoptosis, necroptosis, or autophagy. In addition, while we predominantly focus on how A20 expression and function can affect disease outcome through NF-κB–mediated regulation, it is important to note that A20 regulates immune and nonimmune functions through both NF-κB–dependent and NF-κB–independent mechanisms. Specifically, A20 has been shown to regulate several signaling pathways, including TGF-β, STAT (signal transducer and activator of transcription proteins), JNK (c-Jun N-terminal kinase), IRF, and MAPK signaling cascades (Ma and Malynn 2012; Jung et al. 2013; Bhattacharyya et al. 2016; Das et al. 2018; Malynn and Ma 2019; Yoon et al. 2019).
Insights for A20 Function in the Oral Cavity
An increased appreciation of ubiquitin-mediated regulation of key biological processes in health and disease states has led to the discovery of A20 as a critical modulator of cellular homeostasis (Hu and Sun 2016; Malynn and Ma 2019; Martens and van Loo 2020; Priem et al. 2020). Genome-wide association studies identified somatic mutations, deletions, and/or aberrant expression of A20 in rheumatoid arthritis, diabetes, SLE, inflammatory bowel disease, psoriasis, Sjögren syndrome, coronary artery disease, multiple sclerosis, cystic fibrosis, and asthma (Boonyasrisawat et al. 2007; Ma and Malynn 2012; Momtazi et al. 2019; Martens and van Loo 2020). While A20 function is fairly well-characterized in several tissues, only recently did it begin to receive attention as a regulator of oral mucosa homeostasis. Nonetheless, a better understanding of A20 function and A20-related disorders can offer new insights into the pathogenesis of diseases related to the oral cavity.
In support of plausible biological functions of A20 in promoting oral cavity health, A20 has been specifically shown to regulate inflammation, autophagy, and cell death responses in the oral mucosa (Hong et al. 2016; Crump et al. 2017; Li et al. 2019; 2020; Yan et al. 2020). A20 insufficiency was associated with a more severe disease phenotype in a murine periodontitis model (Li et al. 2019). Similarly, clinical evidence suggests that A20 may not reach sufficient levels in the periodontal lesions (Crump et al. 2017). Most recently, genetic alterations in the deubiquitinase domain of A20 have been shown to be associated with an increased risk of SLE via increased neutrophil dysfunction and NET formation (Odqvist et al. 2019). Deregulated neutrophil function and subsequent NET formation are of significance for periodontitis pathology, and it will be critical to determine how A20-directed biological responses modulate tissue homeostasis in the oral mucosa through their effect on neutrophil physiology (Hajishengallis and Korostoff 2017).
In periodontitis pathogenesis, a microbial dysbiosis can fuel inflammatory tissue damage in a vicious cycle. A20 deficiency promotes a dysbiotic microbiome in the intestine, as well as sensitizes lung epithelial cells to LPS, underscoring its critical function in commensal microbial homeostasis (Vereecke et al. 2014; Schuijs et al. 2015; Talpin et al. 2019). It is therefore plausible that A20 insufficiency may increase predisposition to exaggerated inflammation through modulation of the oral microbiome, which warrants further investigation.
Several factors, including genetics, epigenetics (e.g., microRNAs, DNA methylation), a dysbiotic microbiota, systemic perturbations (e.g., aging, obesity, diabetes), and lifestyle preferences (e.g., smoking, diet), affect the local environment and host response, leading to the progression of periodontitis, and oftentimes result in poor treatment response (Kebschull and Papapanou 2015; Beck et al. 2019). As new generations of therapeutics evolve, targeting proresolving mediators provides a nontoxic approach by increasing the body’s natural production of endogenous anti-inflammatory and resolution mediators (Bartold and Van Dyke 2017). Among those, lipoxin 15-epi-LXA4 has been shown to be an endogenous agonist for A20 expression, subsequently promoting inflammation resolution (Sham et al. 2018). This evidence further provides support for the plausibility of A20-targeted therapies in the management of periodontitis. Supporting this notion, A20 targeted interventions are reported to reverse diabetes and obesity associated sequel through reducing inflammation and apoptosis (Boonyasrisawat et al. 2007; Fukaya et al. 2016; Xu et al. 2018). As the association between diabetes and periodontal health is well established, targeting A20 may likely mitigate diabetes-related pathologies within the oral cavity as well. In addition, A20 protein levels can be regulated by microRNAs, including let-7f, miR-29, miR-125a, and miR-125b (Kim et al. 2012; Balkhi et al. 2013; Kumar et al. 2015). Interestingly, several of the microRNAs that were shown to regulate A20 expression are differentially expressed in periodontitis lesions compared to healthy tissues (Kebschull and Papapanou 2015). It is therefore likely that microRNA-based therapies can likely act through modulating A20 and/or A20-regulated pathways to sustain periodontal tissue homeostasis.
Significance of A20 in the maintenance of oral cavity health is further corroborated by studies reporting A20 haploinsufficiency (HA20) syndrome (Zhou et al. 2016; Aeschlimann et al. 2018). This condition results in a proinflammatory state beginning in childhood, ultimately leading to a Behçet disease phenotype and recurrent oral ulcers. As we learn more about this recently identified condition, monitoring oral health status of HA20 patients would provide valuable tools to elucidate the clinical effects of aberrant A20 levels in the oral cavity. In addition, patients with primary Sjögren syndrome (pSS) have been shown to exhibit germline and somatic abnormalities of TNFAIP3 (Nocturne et al. 2013). While pSS is predominately characterized as an autoimmune condition, it is also associated with localized salivary gland and mucosa-associated lymphoid tissue (MALT) lymphomas. The resultant mutations highlight a novel concept in which continuous autoimmunity enhances lymphoma risk and offers a possible intervention strategy in pSS patients (Nocturne et al. 2013). Aberrant A20 expression has also been observed in nasopharyngeal carcinomas and poorly differentiated head and neck cancers, but limited data underscore the importance for further investigation (Codd et al. 1999).
Overall, emerging evidence recognizes A20 as a key regulator of immune and inflammatory pathways in the oral cavity and at distant tissues (Fig. 4). It is evident that within all tissues, A20 levels must be tightly regulated to preserve homeostasis and health. Most systemic conditions associated with aberrant A20 levels share a common pathophysiology with periodontitis, and defining the effect of ubiquitin-mediated events and A20 in cellular and molecular pathways will ultimately lead to better oral and systemic health.
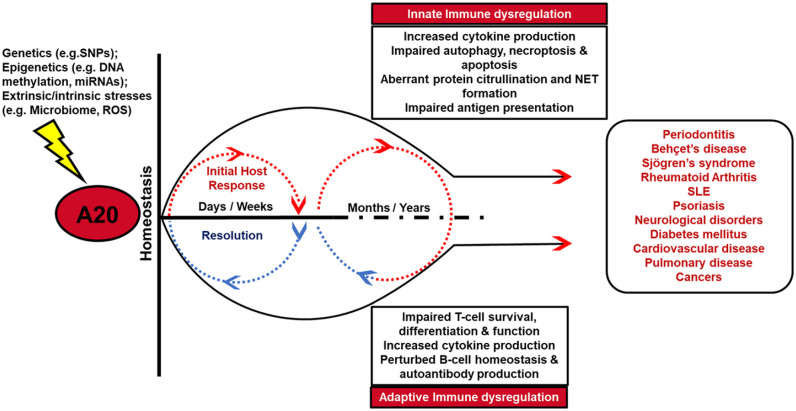
Figure 4.
A20 function and its contribution to cellular homeostasis and disease pathology. A20 is recognized as one of the central regulators of inflammation, apoptosis, autophagy, and necroptosis in a variety of innate and adaptive immune cells. Tightly regulated A20 levels are required to preserve tissue homeostasis and restrain inflammation following initial host response to tissue injury or infection. Aberrant A20 expression and activity may be caused by a variety of mechanisms, including genetics (single-nucleotide polymorphisms [SNPs]), epigenetics (DNA methylation, microRNAs [miRNAs]), and environmental or intrinsic stimuli (microbiome, danger signals, reactive oxygen species [ROS]). Impaired A20 activity for a prolonged period promotes an environment of sustained inflammation and delayed resolution and affects a variety of innate and adaptive immune responses, consequently leading to several pathologies in the oral cavity and at distant tissues. NET, neutrophil extracellular trap; SLE, systemic lupus erythematosus.
Conclusion and Future Perspectives
The oral cavity is one of the most multifaceted microenvironments in the human body where interactions between the host and microbiome define health and disease states. Regulation of inflammatory mechanisms, cell death pathways, and basic immune function is critical for sustaining tissue homeostasis and periodontium integrity. Although there has been some success with current anti-inflammatory therapies, there are also considerable limitations (Bartold and Van Dyke 2017). New advances in the understanding of downstream regulation of inflammatory signaling and its links to restoration of homeostasis offer promise in translational clinical research. There has been growing interest in exploiting components of ubiquitination machinery as therapeutic targets (Huang and Dixit 2016; Rothschild et al. 2018; Kliza and Husnjak 2020). Given their extreme diversity, E3 ligases and deubiquitinases offer great potential for targeted therapies. Structurally, deubiquitinases possess well-defined catalytic clefts, making them intrinsically attractive as potential drug targets (Huang and Dixit 2016). Among those enzymes, A20 is particularly unique through its dual action both as a ubiquitin ligase and a deubiquitinase. A20 has been recognized as one of the central regulators of cellular homeostasis and a major determinant of inflammatory status and disease progression in numerous conditions (Fig. 4). A20 is implicated in key biological processes, including inflammation, apoptosis, necroptosis, and autophagy, and contributes to microbial dysbiosis. A20 is also identified as a downstream target for resolvins. As we continue to learn more about the genetics (SNPs), epigenetics (e.g., microRNAs, DNA methylation), and extrinsic/intrinsic stresses (e.g., dysbiotic microbiome, danger signals, ROS) that regulate A20 expression and function in the context of diseases, targeting the ubiquitin system through A20 possibly creates a path to personalized medicine for the treatment of not only periodontitis but also several other immune and inflammatory conditions.
Author Contributions
E.C. Mooney, contributed to design, data acquisition, analysis, and interpretation, drafted and critically revised the manuscript; S.E. Sahingur, contributed to conception, design, data acquisition, analysis, and interpretation, drafted and critically revised the manuscript. Both authors gave final approval and agree to be accountable for all aspects of the work.
Supplemental Material
Supplemental material, DS_10.1177_0022034520949486 for The Ubiquitin System and A20: Implications in Health and Disease by E.C. Mooney and S.E. Sahingur in Journal of Dental Research
Footnotes
A supplemental appendix to this article is available online.
This work was supported by US Public Health Service grants R01DE025037 and R01DE027374 to S.E. Sahingur from the National Institute of Dental and Craniofacial Research/National Institutes of Health.
We apologize to our colleagues if their original contributions are missed in the references owing to space limitations.
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- Adelaja A, Hoffmann A. 2019. Signaling crosstalk mechanisms that may fine-tune pathogen-responsive NFκB. Front Immunol. 10:433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aeschlimann FA, Batu ED, Canna SW, Go E, Gül A, Hoffmann P, Leavis HL, Ozen S, Schwartz DM, Stone DL, et al. 2018. A20 haploinsufficiency (HA20): clinical phenotypes and disease course of patients with a newly recognised NF-κB-mediated autoinflammatory disease. Ann Rheum Dis. 77(5):728–735. [DOI] [PubMed] [Google Scholar]
- Balkhi MY, Iwenofu OH, Bakkar N, Ladner KJ, Chandler DS, Houghton PJ, London CA, Kraybill W, Perrotti D, Croce CM, et al. 2013. miR-29 acts as a decoy in sarcomas to protect the tumor suppressor A20 mRNA from degradation by HuR. Sci Signal. 6(286):ra63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao Z, Fan L, Zhao L, Xu X, Liu Y, Chao H, Liu N, You Y, Liu Y, Wang X, et al. 2019. Silencing of A20 aggravates neuronal death and inflammation after traumatic brain injury: a potential trigger of necroptosis. Front Mol Neurosci. 12:222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartold PM, Van Dyke TE. 2017. Host modulation: controlling the inflammation to control the infection. Periodontol 2000. 75(1):317–329. [DOI] [PubMed] [Google Scholar]
- Beck JD, Papapanou PN, Philips KH, Offenbacher S. 2019. Periodontal medicine: 100 years of progress. J Dent Res. 98(10):1053–1062. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya S, Wang W, Graham LV, Varga J. 2016. A20 suppresses canonical Smad-dependent fibroblast activation: novel function for an endogenous inflammatory modulator. Arthritis Res Ther. 18(1):216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boonyasrisawat W, Eberle D, Bacci S, Zhang Y-Y, Nolan D, Gervino EV, Johnstone MT, Trischitta V, Shoelson SE, Doria A. 2007. Tag polymorphisms at the A20 (TNFAIP3) locus are associated with lower gene expression and increased risk of coronary artery disease in type 2 diabetes. Diabetes. 56(2):499–505. [DOI] [PubMed] [Google Scholar]
- Catrysse L, Ghahremani MF, Vereecke L, Youssef SA, Mc Guire C, Sze M, Weber A, Heikenwalder M, de Bruin A, Beyaert R, et al. 2016. A20 prevents chronic liver inflammation and cancer by protecting hepatocytes from death. Cell Death Dis. 7(6):e2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu Y, Vahl JC, Kumar D, Heger K, Bertossi A, Wójtowicz E, Soberon V, Schenten D, Mack B, Reutelshöfer M, et al. 2011. B cells lacking the tumor suppressor TNFAIP3/A20 display impaired differentiation and hyperactivation and cause inflammation and autoimmunity in aged mice. Blood. 117(7):2227–2236. [DOI] [PubMed] [Google Scholar]
- Codd JD, Salisbury JR, Packham G, Nicholson LJ. 1999. A20 RNA expression is associated with undifferentiated nasopharyngeal carcinoma and poorly differentiated head and neck squamous cell carcinoma. J Pathol. 187(5):549–555. [DOI] [PubMed] [Google Scholar]
- Crump KE, Oakley JC, Xia-Juan X, Madu TC, Devaki S, Mooney EC, Sahingur SE. 2017. Interplay of toll-like receptor 9, myeloid cells, and deubiquitinase A20 in periodontal inflammation. Infect Immun. 85(1):e00814–e00816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crump KE, Sahingur SE. 2016. Microbial nucleic acid sensing in oral and systemic diseases. J Dent Res. 95(1):17–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das T, Chen Z, Hendriks RW, Kool M. 2018. A20/tumor necrosis factor α–induced protein 3 in immune cells controls development of autoinflammation and autoimmunity: lessons from mouse models. Front Immunol. 9:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devos M, Mogilenko DA, Fleury S, Gilbert B, Becquart C, Quemener S, Dehondt H, Tougaard P, Staels B, Bachert C, et al. 2019. Keratinocyte expression of A20/TNFAIP3 controls skin inflammation associated with atopic dermatitis and psoriasis. J Invest Dermatol. 139(1):135–145. [DOI] [PubMed] [Google Scholar]
- Fukaya M, Brorsson CA, Meyerovich K, Catrysse L, Delaroche D, Vanzela EC, Ortis F, Beyaert R, Nielsen LB, Andersen ML, et al. 2016. A20 inhibits β-cell apoptosis by multiple mechanisms and predicts residual β-cell function in type 1 diabetes. Mol Endocrinol. 30(1):48–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajishengallis G, Korostoff JM. 2017. Revisiting the Page & Schroeder model: the good, the bad and the unknowns in the periodontal host response 40 years later. Periodontol 2000. 75(1):116–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hand LE, Usan P, Cooper GJ, Xu LY, Ammori B, Cunningham PS, Aghamohammadzadeh R, Soran H, Greenstein A, Loudon AS, et al. 2015. Adiponectin induces A20 expression in adipose tissue to confer metabolic benefit. Diabetes. 64(1):128–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong JY, Bae WJ, Yi JK, Kim GT, Kim EC. 2016. Anti-inflammatory and anti-osteoclastogenic effects of zinc finger protein A20 overexpression in human periodontal ligament cells. J Periodontal Res. 51(4):529–539. [DOI] [PubMed] [Google Scholar]
- Honma K, Tsuzuki S, Nakagawa M, Tagawa H, Nakamura S, Morishima Y, Seto M. 2009. TNFAIP3/A20 functions as a novel tumor suppressor gene in several subtypes of non-Hodgkin lymphomas. Blood. 114(12):2467–2475. [DOI] [PubMed] [Google Scholar]
- Hovelmeyer N, Reissig S, Xuan NT, Adams-Quack P, Lukas D, Nikolaev A, Schluter D, Waisman A. 2011. A20 deficiency in B cells enhances B-cell proliferation and results in the development of autoantibodies. Eur J Immunol. 41(3):595–601. [DOI] [PubMed] [Google Scholar]
- Hu H, Sun SC. 2016. Ubiquitin signaling in immune responses. Cell Res. 26(4):457–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Dixit VM. 2016. Drugging the undruggables: exploring the ubiquitin system for drug development. Cell Res. 26(4):484–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung SM, Lee JH, Park J, Oh YS, Lee SK, Park JS, Lee YS, Kim JH, Lee JY, Bae YS, et al. 2013. Smad6 inhibits non-canonical TGF-β1 signalling by recruiting the deubiquitinase A20 to TRAF6. Nat Commun. 4:2562. [DOI] [PubMed] [Google Scholar]
- Kadowaki T, Ohnishi H, Kawamoto N, Hori T, Nishimura K, Kobayashi C, Shigemura T, Ogata S, Inoue Y, Kawai T, et al. 2018. Haploinsufficiency of A20 causes autoinflammatory and autoimmune disorders. J Allergy Clin Immunol. 141(4):1485–1488e11. [DOI] [PubMed] [Google Scholar]
- Kanayama M, Inoue M, Danzaki K, Hammer G, He YW, Shinohara ML. 2015. Autophagy enhances NFκB activity in specific tissue macrophages by sequestering A20 to boost antifungal immunity. Nat Commun. 6:5779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato M, Sanada M, Kato I, Sato Y, Takita J, Takeuchi K, Niwa A, Chen Y, Nakazaki K, Nomoto J, et al. 2009. Frequent inactivation of A20 in B-cell lymphomas. Nature. 459(7247):712–716. [DOI] [PubMed] [Google Scholar]
- Kebschull M, Papapanou PN. 2015. Mini but mighty: microRNAs in the pathobiology of periodontal disease. Periodontol 2000. 69(1):201–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SW, Ramasamy K, Bouamar H, Lin AP, Jiang D, Aguiar RC. 2012. MicroRNAs miR-125a and miR-125b constitutively activate the NF-κB pathway by targeting the tumor necrosis factor alpha-induced protein 3 (TNFAIP3, A20). Proc Natl Acad Sci. 109(20):7865–7870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliza K, Husnjak K. 2020. Resolving the complexity of ubiquitin networks. Front Mol Biosci. 7:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konkel JE, O’Boyle C, Krishnan S. 2019. Distal consequences of oral inflammation. Front Immunol. 10:1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar M, Sahu SK, Kumar R, Subuddhi A, Maji RK, Jana K, Gupta P, Raffetseder J, Lerm M, Ghosh Z, et al. 2015. MicroRNA let-7 modulates the immune response to Mycobacterium tuberculosis infection via control of A20, an inhibitor of the NF-κB pathway. Cell Host Microbe. 17(3):345–356. [DOI] [PubMed] [Google Scholar]
- Li Y, Mooney EC, Holden SE, Xia XJ, Cohen DJ, Walsh SW, Ma A, Sahingur SE. 2019. A20 orchestrates inflammatory response in the oral mucosa through restraining NF-κB activity. J Immunol. 202(7):2044–2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Mooney EC, Xia XJ, Gupta N, Sahingur SE. 2020. A20 restricts inflammatory response and desensitizes gingival keratinocytes to apoptosis. Front Immunol. 11:365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X, Rudemiller NP, Wen Y, Ren J, Hammer GE, Griffiths R, Privratsky JR, Yang B, Sparks MA, Crowley SD. 2019. A20 in myeloid cells protects against hypertension by inhibiting dendritic cell-mediated T-cell activation. Circ Res. 125(12):1055–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma A, Malynn BA. 2012. A20: linking a complex regulator of ubiquitylation to immunity and human disease. Nat Rev Immunol. 12(11):774–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malynn BA, Ma A. 2019. A20: a multifunctional tool for regulating immunity and preventing disease. Cell Immunol. 340:103914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martens A, van Loo G. 2020. A20 at the crossroads of cell death, inflammation, and autoimmunity. Cold Spring Harb Perspect Biol. 12(1):a036418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matmati M, Jacques P, Maelfait J, Verheugen E, Kool M, Sze M, Geboes L, Louagie E, Mc Guire C, Vereecke L, et al. 2011. A20 (TNFAIP3) deficiency in myeloid cells triggers erosive polyarthritis resembling rheumatoid arthritis. Nat Genet. 43(9):908–912. [DOI] [PubMed] [Google Scholar]
- Meghil MM, Cutler CW. 2020. Oral microbes and mucosal dendritic cells, “spark and flame” of local and distant inflammatory diseases. Int J Mol Sci. 21(5):1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendes ML, Fougeras MR, Dittmar G. 2020. Analysis of ubiquitin signaling and chain topology cross-talk. J Proteomics. 215:103634. [DOI] [PubMed] [Google Scholar]
- Mohebiany AN, Ramphal NS, Karram K, Di Liberto G, Novkovic T, Klein M, Marini F, Kreutzfeldt M, Hartner F, Lacher SM, et al. 2020. Microglial A20 protects the brain from CD8 T-cell-mediated immunopathology. Cell Rep. 30(5):1585–1597e6. [DOI] [PubMed] [Google Scholar]
- Momtazi G, Lambrecht BN, Naranjo JR, Schock BC. 2019. Regulators of A20 (TNFAIP3): new drug-able targets in inflammation. Am J Physiol Lung Cell Mol Physiol. 316(3):L456–L469. [DOI] [PubMed] [Google Scholar]
- Nair RP, Duffin KC, Helms C, Ding J, Stuart PE, Goldgar D, Gudjonsson JE, Li Y, Tejasvi T, Feng BJ, et al. 2009. Genome-wide scan reveals association of psoriasis with IL-23 and NF-kappaB pathways. Nat Genet. 41(2):199–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nocturne G, Boudaoud S, Miceli-Richard C, Viengchareun S, Lazure T, Nititham J, Taylor KE, Ma A, Busato F, Melki J, et al. 2013. Germline and somatic genetic variations of TNFAIP3 in lymphoma complicating primary Sjogren’s syndrome. Blood. 122(25):4068–4076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odqvist L, Jevnikar Z, Riise R, Öberg L, Rhedin M, Leonard D, Yrlid L, Jackson S, Mattsson J, Nanda S, et al. 2019. Genetic variations in A20 DUB domain provide a genetic link to citrullination and neutrophil extracellular traps in systemic lupus erythematosus. Ann Rheum Dis. 78(10):1363–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polykratis A, Martens A, Eren RO, Shirasaki Y, Yamagishi M, Yamaguchi Y, Uemura S, Miura M, Holzmann B, Kollias G, et al. 2019. A20 prevents inflammasome-dependent arthritis by inhibiting macrophage necroptosis through its ZnF7 ubiquitin-binding domain. Nat Cell Biol. 21(6):731–742. [DOI] [PubMed] [Google Scholar]
- Priem D, van Loo G, Bertrand MJM. 2020. A20 and cell death-driven inflammation. Trends Immunol. 41(5):421–435. [DOI] [PubMed] [Google Scholar]
- Rape M. 2018. Ubiquitylation at the crossroads of development and disease. Nat Rev Mol Cell Biol. 19(1):59–70. [DOI] [PubMed] [Google Scholar]
- Rothschild DE, McDaniel DK, Ringel-Scaia VM, Allen IC. 2018. Modulating inflammation through the negative regulation of NF-κB signaling. J Leukoc Biol. 103(6):1131–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuijs MJ, Willart MA, Vergote K, Gras D, Deswarte K, Ege MJ, Madeira FB, Beyaert R, van Loo G, Bracher F, et al. 2015. Farm dust and endotoxin protect against allergy through A20 induction in lung epithelial cells. Science. 349(6252):1106–1110. [DOI] [PubMed] [Google Scholar]
- Sham HP, Walker KH, Abdulnour RE, Krishnamoorthy N, Douda DN, Norris PC, Barkas I, Benito-Figueroa S, Colby JK, Serhan CN, et al. 2018. 15-epi-Lipoxin A4, Resolvin D2, and Resolvin D3 induce NF-κB regulators in bacterial pneumonia. J Immunol. 200(8):2757–2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slowicka K, Serramito-Gomez I, Boada-Romero E, Martens A, Sze M, Petta I, Vikkula HK, De Rycke R, Parthoens E, Lippens S, et al. 2019. Physical and functional interaction between A20 and ATG16L1-WD40 domain in the control of intestinal homeostasis. Nat Commun. 10(1):1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swatek KN, Komander D. 2016. Ubiquitin modifications. Cell Res. 26(4):399–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talpin A, Kattah MG, Advincula R, Fadrosh D, Lynch K, LaMere B, Fujimura KE, Nagalingam NA, Malynn BA, Lynch SV, et al. 2019. A20 in dendritic cells restrains intestinal anti-bacterial peptide expression and preserves commensal homeostasis. PLoS One. 14(7):e0218999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavares RM, Turer EE, Liu CL, Advincula R, Scapini P, Rhee L, Barrera J, Lowell CA, Utz PJ, Malynn BA, et al. 2010. The ubiquitin modifying enzyme A20 restricts B cell survival and prevents autoimmunity. Immunity. 33(2):181–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessier-Cloutier B, Twa DD, Baecklund E, Gascoyne R, Johnson NA, Backlin C, Kamen DL, Clarke AE, Ramsey-Goldman R, Lee JL, et al. 2019. Cell of origin in diffuse large B-cell lymphoma in systemic lupus erythematosus: molecular and clinical factors associated with survival. Lupus Sci Med. 6(1):e000324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vereecke L, Vieira-Silva S, Billiet T, van Es JH, Mc Guire C, Slowicka K, Sze M, van den Born M, De Hertogh G, Clevers H, et al. 2014. A20 controls intestinal homeostasis through cell-specific activities. Nat Commun. 5:5103. [DOI] [PubMed] [Google Scholar]
- Voet S, Mc Guire C, Hagemeyer N, Martens A, Schroeder A, Wieghofer P, Daems C, Staszewski O, Vande Walle L, Jordao MJC, et al. 2018. A20 critically controls microglia activation and inhibits inflammasome-dependent neuroinflammation. Nat Commun. 9(1):2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, Wang C, Liang M, Chen L, Fu Q, Zhang F, Wang Y, Huang D, Huang K. 2018. A20 prevents obesity-induced development of cardiac dysfunction. J Mol Med (Berl). 96(2):159–172. [DOI] [PubMed] [Google Scholar]
- Yan K, Wu C, Ye Y, Li L, Wang X, He W, Ren S, Xu Y. 2020. A20 inhibits osteoclastogenesis via TRAF6-dependent autophagy in human periodontal ligament cells under hypoxia. Cell Prolif. 53(3):e12778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon CI, Ahn SG, Bae SJ, Shin YJ, Cha C, Park SE, Lee J-H, Ooshima A, Lee HS, Yang K-M, et al. 2019. High A20 expression negatively impacts survival in patients with breast cancer. PLoS One. 14(8):e0221721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Wang H, Schwartz DM, Stoffels M, Park YH, Zhang Y, Yang D, Demirkaya E, Takeuchi M, Tsai WL, et al. 2016. Loss-of-function mutations in TNFAIP3 leading to A20 haploinsufficiency cause an early-onset autoinflammatory disease. Nat Genet. 48(1):67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, DS_10.1177_0022034520949486 for The Ubiquitin System and A20: Implications in Health and Disease by E.C. Mooney and S.E. Sahingur in Journal of Dental Research