Abstract
The plasma membrane is a lipid bilayer of < 10 nm width that separates intra- and extra-cellular environments and serves as the site of cell-cell communication, as well as communication between cells and the extracellular environment. As such, biophysical phenomena at and around the plasma membrane play key roles in determining cellular physiology and pathophysiology. Thus, the selective visualization and characterization of the plasma membrane are crucial aspects of research in wide areas of biology and medicine. However, the specific characterization of the plasma membrane has been a challenge using conventional imaging techniques, which are unable to effectively distinguish between signals arising from the plasma membrane and those from intracellular lipid structures. In this regard, interface-specific second harmonic generation (SHG) and sum-frequency generation (SFG) imaging demonstrate great potential. When combined with exogenous SHG/SFG active dyes, SHG/SFG can specifically highlight the plasma membrane as the most prominent interface associated with cells. Furthermore, SHG/SFG imaging can be readily extended to multimodal multiphoton microscopy with simultaneous occurrence of other multiphoton phenomena, including multiphoton excitation and coherent Raman scattering, which shed light on the biophysical properties of the plasma membrane from different perspectives. Here, we review traditional and current applications, as well as the prospects of long-known but unexplored SHG/SFG imaging techniques in biophysics, with special focus on their use in the biophysical characterization of the plasma membrane.
Keywords: Second harmonic generation, Sum frequency generation, Plasma membrane, Multimodal multiphoton microscopy, Biophysics, Imaging, Nonlinear optics, Dye, Fluorescence, Coherent anti-stokes Raman scattering
Biophysical characterization of the plasma membrane
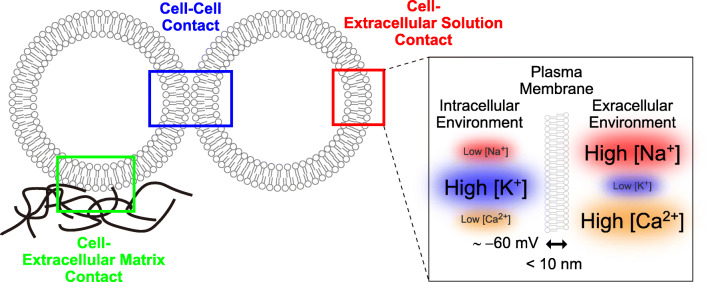
The plasma membrane is the outermost layer of a cell, separating the intracellular and extracellular environments and thereby defining the border of the cell. As this membrane is a defining feature for all cells, the integrity of the plasma membrane is considered one of the most important indicators of cellular health. At this site, cells communicate with one another through direct cell-to-cell interactions, while also communicating with extracellular matrices, or with individual components of the extracellular environment (Fig. 1). Strikingly, the plasma membrane separates these completely distinct intracellular and extracellular environments by a width of < 10 nm. Through this narrow yet effective barrier, cells maintain chemical and electrical differences that are essential to all aspects of cellular physiological activities (Fig. 1). Therefore, any change in the biophysical properties of the plasma membrane directly affects the physiology and pathophysiology of cells.
Fig. 1.
Biophysical properties of the plasma membrane. The plasma membrane is the sites of cellular contacts with other cells, extracellular matrices as well as extracellular solutions. The plasma membrane separates intracellular and extracellular environments that are distinct in ionic compositions as well as membrane potential
The importance of the plasma membrane makes it crucial to characterize its biophysical properties across wide areas of biological and medical research. Many techniques have been developed in this regard, including electrophysiological and various imaging techniques. Imaging techniques have advantages over electrophysiology in that they are much less invasive, require fewer skills, and have high spatial resolution, all of which are beneficial for the characterization of the plasma membrane. The applicability of imaging techniques has been further facilitated by the development of a plethora of fluorescent dyes and proteins, which increases the options for color and functional analysis (Maier et al. 2002). Following the recognition of the importance of the plasma membrane, various membrane-targeted fluorescent imaging methods have been developed (Cairo et al. 2010; Kwiatek et al. 2013). One such method is small organic dye-based imaging, which involves the insertion of partially or wholly lipophilic dyes into the lipid bilayer. In protein-based imaging, fluorescent proteins are fused either to membrane integral proteins or to signal sequences, which are then recognized for subsequent attachment of lipids. In all cases, the underlying mechanisms are the same and involve fluorophores attaching to lipid structures. However, these approaches have one major pitfall: the fluorophores not only stain the plasma membrane but also all the lipid structures within the cells, which is further facilitated by high levels of dynamic lipid structure exchanges within cells. Therefore, although not widely recognized, these fluorescence-based membrane imaging techniques are well suited for imaging of the lipid structures of cells as a whole but cannot be used to selectively image the plasma membrane. This poses a fundamental issue, as these intracellular lipid structures are often located immediately below the plasma membrane, at a distance much smaller than the spatial resolution of optical microscopy. Therefore, acquisition of the optical signals associated solely with the plasma membrane cannot be realized, even with the recent advancements in super-resolution microscopy (Shim et al. 2012). Total internal reflection fluorescence (TIRF) imaging is another useful imaging technique used in the biophysical characterization of the plasma membrane (Mattheyses et al. 2010). However, it is still difficult to identify the position of the plasma membrane via the optical resolution of evanescent light. In addition, the detection area of TIRF imaging is limited to areas attached to the artificial glass substrate, which is distinct from the physiological environment. Thus, the field of the plasma membrane biophysics has long been in need of a tool to specifically image and characterize the plasma membrane. In this regard, the non-fluorescent imaging technique of second harmonic generation (SHG) and sum-frequency generation (SFG) holds great potential (Cox 2011).
SHG/SFG imaging
The history of SHG and SFG dates back to the early days of laser inventions. In fact, SHG is one of the earliest examples of nonlinear optical phenomena, which was experimentally demonstrated using a quartz crystal irradiated by a ruby laser (Franken et al. 1961). In both SHG and SFG, two photons, with a frequency of ω1 and ω2, simultaneously interact with the material of interest and are then converted to a single photon of frequency ω1 + ω2 without any energy loss (Fig. 2a). SFG is a general term for this frequency conversion, and SHG is a special case where the frequencies of the two incident photons are the same (ω1 = ω2).
Fig. 2.
Optical properties of SHG/SFG. (a) Two incoming photons (ω1 and ω2) interact with SHG/SFG active material and emit SHG/SFG signal. SHG is the special case when the frequencies of the two photons are the same (ω1 = ω2). (b) When orderly aligned and thereby break the centrosymmetry, the SHG/SFG active polar fluorescent dyes emit SHG/SFG signals. However, if the distributions of the dyes have centrosymmetric structures, no SHG/SFG signals will be generated. In contrast, fluorescent signals are generated regardless of the dye distributions
After the first demonstration of SHG using biological samples (Fine and Hansen 1971), SHG microscopy gradually began to develop. It has progressively been used in biological research since the late 1980s, with particular interest spiking after the development of ultra-short pulse laser and two-photon microscopy (Freund and Deutsch 1986). Since SHG/SFG involves more than one photon, they are included in the category of multiphoton phenomena (Fig. 3), the most well-known of which is two-photon excitation (TPE) of fluorescence (Oheim et al. 2006). TPE microscopy was first introduced to biology by Webb and colleagues (Denk et al. 1990). Since then, it has been applied to a variety of biophysical research areas and has brought unprecedented knowledge to the field (Williams et al. 2001; Yuste 2005; Carriles et al. 2009). The key advantages of TPE microscopy are deep tissue penetration through the use of near-infrared light (Helmchen and Denk 2005) and low toxicity to the tissue, because the excitation is limited to a small volume of the focal point (Zipfel et al. 2003b). Together with the development of fluorescent probes, TPE microscopy has gained much attention and has been applied widely to biological research.
Fig. 3.
Energy diagrams of multiphoton optical processes. Energy diagrams of single- and two-photon excited fluorescence and vibrational optical processes are shown. The horizontal solid lines are the stable energy levels and the horizontal dashed lines are the virtual energy levels. The solid straight arrows represent the excitation and emission lights. The solid wavy arrows represent the relaxation process of fluorescence
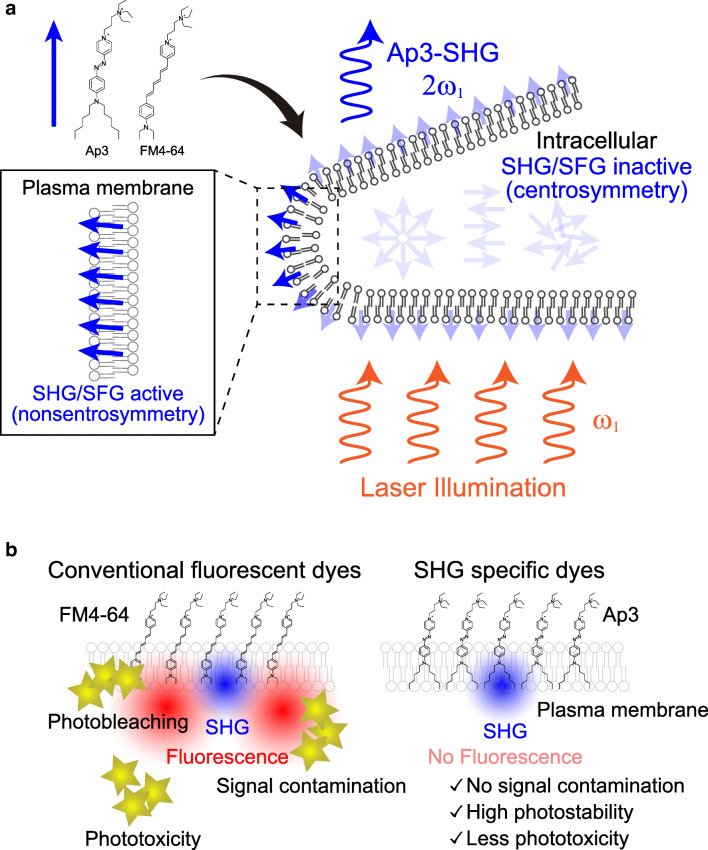
As a two-photon effect, SHG/SFG is endowed with all of the benefits of TPE microscopy. However, there is a critical difference between TPE and SHG/SFG in their requirements; SHG/SFG requires target molecules to be aligned in a highly ordered manner which breaks the centrosymmetry (Mertz and Moreaux 2001; Pavone and Campagnola 2013), whereas TPE can occur in disordered materials (Fig. 2b). Therefore, these two imaging modalities reveal distinct phenomena. Although not widely employed amongst biologists, SHG is frequently utilized in laser technology for wavelength conversion. The most familiar example is a commercially available green laser pointer, which emits a frequency-doubled green laser from an infrared laser source using an internal SHG crystal. In biological samples, some structures are so well-organized that they serve as native SHG crystals (Yokota et al. 2012), such as collagen fibers (Fine and Hansen 1971), myosin fibrils (Plotnikov et al. 2006), and several others (Freund and Deutsch 1986; Campagnola et al. 2002; Mohler et al. 2003; Cox et al. 2003; Zipfel et al. 2003a; Campagnola and Loew 2003; Fu et al. 2007; Campagnola 2011; Chen et al. 2012). Although useful, particularly with potential clinical applications, including non-label tissue diagnosis, this intrinsic imaging is restricted by naturally occurring structures. Similar to TPE, however, such limitations can be overcome by the incorporation of exogenous dyes. In the case of SHG/SFG, when highly charged dyes are aligned in a very ordered manner, these dyes can serve as SHG substances. Such alignment can be achieved at some sort of interface region (Chen 1989), arguably the most prominent of which is the lipid-water interface of the plasma membrane (Reeve et al. 2009, 2010). Therefore, this type of interface-selective SHG/SFG may be the best fit for the plasma membrane imaging (Fig. 4a).
Fig. 4.
Schematic illustration of dye-based SHG/SFG imaging. (a) Chemical structure of SHG/SFG active dyes (Ap3 and FM4–64) and schematics of SHG imaging of the plasma membrane are shown. When applied to cells, these amphipathic dyes (solid blue arrow) align themselves at the plasma membrane breaking centrosymmetry and show SHG/SFG signals. However, the dyes in an intracellular/extracellular solution are SHG/SFG inactive because these dyes have centrosymmetric structure as a whole. (b) Characteristics of conventional SHG-active fluorescent dye and SHG-specific dyes. Conventional fluorescent dyes potentially cause photobleaching, phototoxicity, and signal contamination arising from their photochemical reactions with excitation lasers. SHG-specific dyes without fluorescence not only exclude the potential signal contaminations but also result in high photostability and less phototoxicity
SHG/SFG imaging of the plasma membrane
Investigation into biological membrane imaging using SHG/SFG began soon after the introduction of multiphoton imaging to the field of biology. These techniques were initially attempted using the model system of giant vesicles (Moreaux et al. 2000a, 2000b; Pons et al. 2003) to examine the characteristics of lipid membranes by SHG/SFG and later applied to cultured cells for biological applications (Moreaux et al. 2001). Although the plasma membrane itself is not a native SHG/SFG source, studies have demonstrated that dyes developed for fluorescent membrane imaging also emit SHG/SFG signals at the membrane (Campagnola et al. 2001). Reflecting the need to develop techniques for characterizing the plasma membrane, attention has been paid to the use of SHG/SFG signals as reporters of biophysical changes of the plasma membrane, particularly regarding membrane potential changes (Bouevitch et al. 1993; Peleg et al. 1999; Campagnola et al. 1999; Zochowski et al. 2000a, 2000b; Dombeck et al. 2005; Sacconi et al. 2006; Nuriya et al. 2006; Pons and Mertz 2006; Peterka et al. 2011). In fact, the use of SHG imaging enabled quantitative membrane potential imaging of otherwise unreachable finite synaptic structures at neuronal communication sites, dendritic spines, and axons (Nuriya et al. 2006; Nuriya and Yasui 2010). In addition to membrane potential imaging, SHG imaging has also been utilized in the characterization of other biophysical properties of the plasma membrane, such as membrane integrity and lipid composition (Zochowski et al. 2000b; Moen et al. 2014; Kato 2019; Momotake et al. 2020). Importantly, no SHG/SFG signals are observed inside cells, presumably because the small intracellular vesicles and closely stacked membrane structures create centrosymmetric dye distributions within an optical resolution (Fig. 4a). This unique property of SHG/SFG allows background-free signal acquisition from the plasma membrane, which makes it possible to obtain quantitative measurement of the membrane potential changes mentioned above (Nuriya et al. 2006; Nuriya and Yasui 2010). Finally, in contrast to TIRF imaging, SHG/SFG can visualize the side edge of the cell where the plasma membrane exists in a more physiological environment.
Despite its unique potential, however, membrane imaging by SHG/SFG has not been extensively utilized by biologists. One of the main reasons for this is the lack of probes specifically designed for SHG/SFG imaging. In fact, all available SHG/SFG imaging studies to date utilized dyes which were developed for use in fluorescence imaging, such as FM4–64 dye (Nuriya et al. 2006; Nuriya and Yasui 2010). However, while these dyes can generate SHG/SFG signals, they also generate much higher intensities of fluorescence with a broad spectrum, which severely limits their use and imposes multiple disadvantages (Fig. 4b). For example, fluorescence signals from the dyes used for SHG prevent simultaneous imaging of other fluorescent molecules having any spectral overlap. Furthermore, excitation of the dye molecules not only generates fluorescence, but its transition to other energy states results in disruption of the dye molecules themselves and/or nearby molecules at the plasma membrane, which then leads to photobleaching and membrane damage. To overcome these fundamental limitations of fluorescent dye-based SHG imaging, we recently developed the Ap3 dye, specifically designed for SHG/SFG imaging that shows SHG/SFG signals without fluorescence (Nuriya et al. 2016). By using this SHG/SFG specific dye, it became possible, for the first time in the history of its application to biology, to compare the plasma membrane imaging by SHG and TPE of green fluorescence protein (GFP). Consequently, we confirm that dye-based SHG is the plasma membrane selective but conventional fluorescent imaging techniques are not (Fig. 5) (Mizuguchi et al. 2018).
Fig. 5.
Comparison between SHG and fluorescence-based membrane imaging. (a) Schematic of comparison between SHG- and fluorescence-based imaging of the cellular membranes. SHG specifically visualizes the plasma membrane, whereas fluorescence visualizes all of the membrane structures including those inside cells. (b) The images of simultaneously visualized membrane-anchored GFP-TPE and Ap3-SHG signals in Chinese Hamster Ovary (CHO) cells. In the merged image, GFP-TPE and Ap3-SHG are shown in green and magenta, respectively. Reprinted from iScience 2018, 9, T. Mizuguchi, et al., High-Resolution Plasma Membrane-Selective Imaging by Second Harmonic Generation, 359–366, Copyright 2020, with permission from Elsevier
Multimodal multiphoton imaging
In biological research, it is rare to investigate just a single phenomenon at a time. In most cases, characterization of multiple phenomena needs to be performed simultaneously to unravel complicated networks of signaling within cells. In this regard, the power and applicability of SHG/SFG imaging are dramatically enhanced when they are combined with other imaging modalities, enabling multimodal multiphoton imaging. Although it may sound complicated, it is in fact a very simple extension because when SHG/SFG imaging is being performed, other nonlinear optical phenomena also occur naturally under the same optical setup. All what it takes is to recognize the capacity of simultaneous imaging with additional detectors and utilize them.
The most straightforward extension of SHG/SFG microscopy is the TPE of fluorescence molecules. If fluorescent dyes or proteins with enough two-photon cross section are to be illuminated with laser intensities that are sufficient to emit SHG/SFG signals, they are most likely excited and emit TPE signals simultaneously. Although these signals are generated simultaneously, they can be readily separated using band-pass filters; TPE is inevitably accompanied by energy loss, whereas SHG/SFG is not, so the TPE signals are always Stokes shifted compared with SHG/SFG (Fig. 3). This benefit is compromised when SHG/SFG is performed using fluorescent dyes, which is one of the reasons why these imaging techniques are not fully utilized in biology (Fig. 4b). With the introduction of non-fluorescent SHG/SFG dyes, researchers can now enjoy the intrinsic advantages of SHG/SFG imaging and perform multifaceted analyses together with other fluorescent dye of their choice. One of the unique applications of dye-based SHG/SFG imaging is the distance analysis between the plasma membrane and intracellular organelles which are in close vicinity to the membrane. For example, we successfully examined the organization of GFP-tagged actin and tubulin fibers beneath the plasma membrane in a physiological context (Fig. 6) (Mizuguchi et al. 2018).
Fig. 6.
Applications of SHG imaging for biophysical characterization of the plasma membrane. (a) Schematic illustration of the organization of actin and tubulin cytoskeletons near the plasma membrane. (b) The images of simultaneously visualized plasma membrane by Ap3-SHG and GFP-tagged actin and tubulin cytoskeleton in CHO cells. In the merged image, GFP-TPE and Ap3-SHG are shown in green and magenta, respectively. Reprinted from iScience 2018, 9, T. Mizuguchi, et al., High-Resolution Plasma Membrane-Selective Imaging by Second Harmonic Generation, 359–366, Copyright 2020, with permission from Elsevier
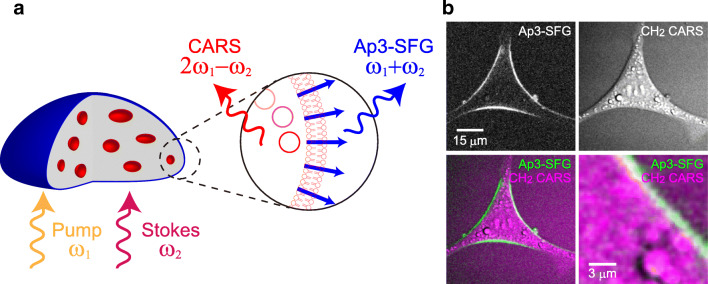
Another exciting extension is the combination of SFG imaging with coherent Raman scattering microscopy methods, such as coherent anti-Stokes Raman scattering (CARS) and stimulated Raman scattering (SRS) (Fig. 3). Although the spontaneous Raman signals are much weaker than fluorescence due to their small scattering cross section, these coherent Raman scattering microscopies strongly enhance Raman signals and realize sensitive and fast acquisition of chemical information (Cheng et al. 2002; Cheng and Xie 2004; Evans and Xie 2008; Freudiger et al. 2008; Camp Jr and Cicerone 2015). For example, the distributions of lipid structures and water molecules in cells and tissues can be rapidly imaged as C-H (Wurpel et al. 2002; Wang et al. 2005, 2008; Nan et al. 2006) and O-H vibrations (Nuriya et al. 2019). To achieve coherent Raman scattering, synchronous illumination of two different wavelengths of lasers whose energy difference matches the vibrational energy of the target molecule is required. Since these photons are illuminated simultaneously, they meet the requirements of SFG at the same time. Thus, if SFG-active materials exist, the CARS/SRS microscope realizes simultaneous imaging of SFG signals. For example, a well-organized collagen fiber in biological tissues shows clear SFG signals combined with the detection of CARS signals, which can be applied for the label-free diagnosis of healthy and cancer tissues (Wang et al. 2008). However, this was not the case with the plasma membrane imaging, due to the lack of SHG/SFG-specific dyes. Once again, if SFG is generated from fluorescent molecules, their fluorescent signals interfere with other modalities, including CARS signals, but this problem can be avoided by non-fluorescent SFG materials. Taking advantage of the non-fluorescent SFG-active Ap3 dye, we were able to image the lipid structures of living cells using CARS in combination with specific plasma membrane imaging by SFG (Fig. 7) (Mizuguchi et al. 2020). Therefore, applications of multimodal multiphoton microscopy in biophysical studies are expanding with the introduction of SHG/SFG dyes that specifically label and characterize the plasma membrane without any optical interference with other modalities.
Fig. 7.
Applications of multimodal multiphoton microscopy for biophysical characterization of the cells. (a) Schematic illustration of the irradiation of pump and Stokes beams that have frequencies of ω1 and ω2 respectively, which allows multimodal imaging of the plasma membrane by SFG (the frequency of ω1 + ω2) and intracellular lipid structures by CH2 CARS (the frequency of 2ω1 - ω2). (b) The images of simultaneously visualized plasma membrane by Ap3-SFG and intracellular lipid structures by CH2 CARS in N27 rat dopaminergic neural cells. In the merged image, Ap3-SFG and CH2 CARS are shown in green and magenta, respectively. Reprinted (adapted) with permission from Anal. Chem. 2020, 92(8), T. Mizuguchi, et al., Multimodal Multiphoton Imaging of the Lipid Bilayer by Dye-Based Sum-Frequency Generation and Coherent Anti-Stokes Raman Scattering, 5656–5660. Copyright 2020 American Chemical Society
Conclusions and future perspectives
Despite their long history, applications of SHG/SFG imaging in biophysics are still far from being fully explored. With the recognition that SHG/SFG is a powerful research tool in biophysical characterization of cell interface structures, the most significant of which is the plasma membrane, SHG/SFG provides researchers with an additional tool that sheds new lights on these phenomena from its own angle. Incorporation of SHG/SFG does not require much effort or additional equipment to the existing multiphoton microscopy setup; the technology is there to be utilized. With the future development of diverse SHG/SFG dyes with different properties and/or functions, similar to those of fluorescent dyes, we predict further fruitful expansion of SHG/SFG imaging in biophysics.
Acknowledgments
The authors gratefully acknowledge Olympus Corporation for their continuous support in the development of the multimodal multiphoton microscopy technique. Funding was provided by JST PRESTO (Grant JPMJPR17G6) and JSPS KAKENHI (ResonanceBio Grant 16H01434).
Compliance with ethical standards
Conflict of interest
M.N. has filed an international patent application on the development and use of SHG-specific dyes (PCT/JP2014/063754).
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Bouevitch O, Lewis A, Pinevsky I, Wuskell JP, Loew LM. Probing membrane potential with nonlinear optics. Biophys J. 1993;65:672–679. doi: 10.1016/S0006-3495(93)81126-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairo CW, Key JA, Sadek CM. Fluorescent small-molecule probes of biochemistry at the plasma membrane. Curr Opin Chem Biol. 2010;14:57–63. doi: 10.1016/j.cbpa.2009.09.032. [DOI] [PubMed] [Google Scholar]
- Camp CH, Jr, Cicerone MT. Chemically sensitive bioimaging with coherent Raman scattering. Nat Photonics. 2015;9:295–305. [Google Scholar]
- Campagnola P. Second harmonic generation imaging microscopy: applications to diseases diagnostics. Anal Chem. 2011;83:3224–3231. doi: 10.1021/ac1032325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campagnola PJ, Loew LM. Second-harmonic imaging microscopy for visualizing biomolecular arrays in cells, tissues and organisms. Nat Biotechnol. 2003;21:1356–1360. doi: 10.1038/nbt894. [DOI] [PubMed] [Google Scholar]
- Campagnola PJ, Wei M, Lewis A, Loew LM. High-resolution nonlinear optical imaging of live cells by second harmonic generation. Biophys J. 1999;77:3341–3349. doi: 10.1016/S0006-3495(99)77165-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campagnola PJ, Clark HA, Mohler WA, Lewis A, Loew LM. Second-harmonic imaging microscopy of living cells. J Biomed Opt. 2001;6:277. doi: 10.1117/1.1383294. [DOI] [PubMed] [Google Scholar]
- Campagnola PJ, Millard AC, Terasaki M, Hoppe PE, Malone CJ, Mohler WA. Three-dimensional high-resolution second-harmonic generation imaging of endogenous structural proteins in biological tissues. Biophys J. 2002;82:493–508. doi: 10.1016/S0006-3495(02)75414-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carriles R, Schafer DN, Sheetz KE, Field JJ, Cisek R, Barzda V, Sylvester AW, Squier JA (2009) Invited review article: imaging techniques for harmonic and multiphoton absorption fluorescence microscopy. Rev Sci Instrum 80:081101 [DOI] [PMC free article] [PubMed]
- Chen YR. Surface properties probed by second-harmonic and sum-frequency generation. Nature. 1989;337:519. [Google Scholar]
- Chen X, Nadiarynkh O, Plotnikov S, Campagnola PJ. Second harmonic generation microscopy for quantitative analysis of collagen fibrillar structure. Nat Protoc. 2012;7:654–669. doi: 10.1038/nprot.2012.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng J-X, Xie XS. Coherent anti-stokes Raman scattering microscopy: instrumentation, theory, and applications. J Phys Chem B. 2004;108:827–840. [Google Scholar]
- Cheng JX, Jia YK, Zheng G, Xie XS. Laser-scanning coherent anti-stokes Raman scattering microscopy and applications to cell biology. Biophys J. 2002;83:502–509. doi: 10.1016/S0006-3495(02)75186-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox G. Biological applications of second harmonic imaging. Biophys Rev. 2011;3:131–141. doi: 10.1007/s12551-011-0052-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox G, Kable E, Jones A, Fraser I, Manconi F, Gorrell MD. 3-dimensional imaging of collagen using second harmonic generation. J Struct Biol. 2003;141:53–62. doi: 10.1016/s1047-8477(02)00576-2. [DOI] [PubMed] [Google Scholar]
- Denk W, Strickler J, Webb W. Two-photon laser scanning fluorescence microscopy. Science (80-) 1990;248:73–76. doi: 10.1126/science.2321027. [DOI] [PubMed] [Google Scholar]
- Dombeck DA, Sacconi L, Blanchard-Desce M, Webb WW. Optical recording of fast neuronal membrane potential transients in acute mammalian brain slices by second-harmonic generation microscopy. J Neurophysiol. 2005;94:3628–3636. doi: 10.1152/jn.00416.2005. [DOI] [PubMed] [Google Scholar]
- Evans CL, Xie XS. Coherent anti-stokes Raman scattering microscopy: chemical imaging for biology and medicine. Annu Rev Anal Chem. 2008;1:883–909. doi: 10.1146/annurev.anchem.1.031207.112754. [DOI] [PubMed] [Google Scholar]
- Fine S, Hansen WP. Optical second harmonic generation in biological systems. Appl Opt. 1971;10:2350. doi: 10.1364/AO.10.002350. [DOI] [PubMed] [Google Scholar]
- Franken PA, Hill AE, Peters CW, Weinreich G. Generation of optical harmonics. Phys Rev Lett. 1961;7:118–119. [Google Scholar]
- Freudiger CW, Min W, Saar BG, Lu S, Holtom GR, He C, Tsai JC, Kang JX, Xie XS. Label-free biomedical imaging with high sensitivity by stimulated Raman scattering microscopy. Science (80- ) 2008;322:1857–1861. doi: 10.1126/science.1165758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund I, Deutsch M. Second-harmonic microscopy of biological tissue. Opt Lett. 1986;11:94. doi: 10.1364/ol.11.000094. [DOI] [PubMed] [Google Scholar]
- Fu Y, Wang H, Shi R, Cheng J-X. Second harmonic and sum frequency generation imaging of fibrous Astroglial filaments in ex vivo spinal tissues. Biophys J. 2007;92:3251–3259. doi: 10.1529/biophysj.106.089011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmchen F, Denk W. Deep tissue two-photon microscopy. Nat Methods. 2005;2:932–940. doi: 10.1038/nmeth818. [DOI] [PubMed] [Google Scholar]
- Kato N. Optical second harmonic generation microscopy: application to the sensitive detection of cell membrane damage. Biophys Rev. 2019;11:399–408. doi: 10.1007/s12551-019-00546-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwiatek JM, Owen DM, Abu-Siniyeh A, Yan P, Loew LM, Gaus K. Characterization of a new series of fluorescent probes for imaging membrane order. PLoS One. 2013;8:e52960. doi: 10.1371/journal.pone.0052960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier O, Oberle V, Hoekstra D. Fluorescent lipid probes: some properties and applications (a review) Chem Phys Lipids. 2002;116:3–18. doi: 10.1016/s0009-3084(02)00017-8. [DOI] [PubMed] [Google Scholar]
- Mattheyses AL, Simon SM, Rappoport JZ. Imaging with total internal reflection fluorescence microscopy for the cell biologist. J Cell Sci. 2010;123:3621–3628. doi: 10.1242/jcs.056218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mertz J, Moreaux L. Second-harmonic generation by focused excitation of inhomogeneously distributed scatterers. Opt Commun. 2001;196:325–330. [Google Scholar]
- Mizuguchi T, Yasui M, Nuriya M. High-resolution plasma membrane-selective imaging by second harmonic generation. iScience. 2018;9:359–366. doi: 10.1016/j.isci.2018.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuguchi T, Momotake A, Hishida M, Yasui M, Yamamoto Y, Saiki T, Nuriya M. Multimodal multiphoton imaging of the lipid bilayer by dye-based sum-frequency generation and coherent anti-stokes Raman scattering. Anal Chem. 2020;92:5656–5660. doi: 10.1021/acs.analchem.0c00673. [DOI] [PubMed] [Google Scholar]
- Moen EK, Ibey BL, Beier HT. Detecting subtle plasma membrane perturbation in living cells using second harmonic generation imaging. Biophys J. 2014;106:L37–L40. doi: 10.1016/j.bpj.2014.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohler W, Millard AC, Campagnola PJ. Second harmonic generation imaging of endogenous structural proteins. Methods. 2003;29:97–109. doi: 10.1016/s1046-2023(02)00292-x. [DOI] [PubMed] [Google Scholar]
- Momotake A, Mizuguchi T, Hishida M, Yamamoto Y, Yasui M, Nuriya M. Monitoring the morphological evolution of giant vesicles by azo dye-based sum-frequency generation (SFG) microscopy. Colloids Surf B: Biointerfaces. 2020;186:110716. doi: 10.1016/j.colsurfb.2019.110716. [DOI] [PubMed] [Google Scholar]
- Moreaux L, Sandre O, Blanchard-Desce M, Mertz J. Membrane imaging by simultaneous second-harmonic generation and two-photon microscopy. Opt Lett. 2000;25:320. doi: 10.1364/ol.25.000320. [DOI] [PubMed] [Google Scholar]
- Moreaux L, Sandre O, Mertz J. Membrane imaging by second-harmonic generation microscopy. J Opt Soc Am B. 2000;17:1685. doi: 10.1364/ol.25.000320. [DOI] [PubMed] [Google Scholar]
- Moreaux L, Sandre O, Charpak S, Blanchard-Desce M, Mertz J. Coherent scattering in multi-harmonic light microscopy. Biophys J. 2001;80:1568–1574. doi: 10.1016/S0006-3495(01)76129-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nan X, Potma EO, Xie XS. Nonperturbative chemical imaging of organelle transport in living cells with coherent anti-stokes Raman scattering microscopy. Biophys J. 2006;91:728–735. doi: 10.1529/biophysj.105.074534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuriya M, Yasui M. Membrane potential dynamics of axons in cultured hippocampal neurons probed by second-harmonic-generation imaging. J Biomed Opt. 2010;15:020503. doi: 10.1117/1.3365135. [DOI] [PubMed] [Google Scholar]
- Nuriya M, Jiang J, Nemet B, Eisenthal KB, Yuste R. Imaging membrane potential in dendritic spines. Proc Natl Acad Sci. 2006;103:786–790. doi: 10.1073/pnas.0510092103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuriya M, Fukushima S, Momotake A, Shinotsuka T, Yasui M, Arai T. Multimodal two-photon imaging using a second harmonic generation-specific dye. Nat Commun. 2016;7:11557. doi: 10.1038/ncomms11557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuriya M, Yoneyama H, Takahashi K, Leproux P, Couderc V, Yasui M, Kano H. Characterization of intra/extracellular water states probed by Ultrabroadband multiplex coherent anti-stokes Raman scattering (CARS) spectroscopic imaging. J Phys Chem A. 2019;123:3928–3934. doi: 10.1021/acs.jpca.9b03018. [DOI] [PubMed] [Google Scholar]
- Oheim M, Michael DJ, Geisbauer M, Madsen D, Chow RH. Principles of two-photon excitation fluorescence microscopy and other nonlinear imaging approaches. Adv Drug Deliv Rev. 2006;58:788–808. doi: 10.1016/j.addr.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Pavone FS, Campagnola PJ (2013) Second harmonic generation imaging. CRC Press, Boca Raton, Florida
- Peleg G, Lewis A, Linial M, Loew LM. Nonlinear optical measurement of membrane potential around single molecules at selected cellular sites. Proc Natl Acad Sci. 1999;96:6700–6704. doi: 10.1073/pnas.96.12.6700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterka DS, Takahashi H, Yuste R. Imaging Voltage in Neurons. Neuron. 2011;69:9–21. doi: 10.1016/j.neuron.2010.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotnikov SV, Millard AC, Campagnola PJ, Mohler WA. Characterization of the myosin-based source for second-harmonic generation from muscle sarcomeres. Biophys J. 2006;90:693–703. doi: 10.1529/biophysj.105.071555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pons T, Mertz J. Membrane potential detection with second-harmonic generation and two-photon excited fluorescence: a theoretical comparison. Opt Commun. 2006;258:203–209. [Google Scholar]
- Pons T, Moreaux L, Mongin O, Blanchard-Desce M, Mertz J. Mechanisms of membrane potential sensing with second-harmonic generation microscopy. J Biomed Opt. 2003;8:428. doi: 10.1117/1.1581871. [DOI] [PubMed] [Google Scholar]
- Reeve JE, Collins HA, De Mey K, Kohl MM, Thorley KJ, Paulsen O, Clays K, Anderson HL. Amphiphilic Porphyrins for second harmonic generation imaging. J Am Chem Soc. 2009;131:2758–2759. doi: 10.1021/ja8061369. [DOI] [PubMed] [Google Scholar]
- Reeve JE, Anderson HL, Clays K. Dyes for biological second harmonic generation imaging. Phys Chem Chem Phys. 2010;12:13484–13498. doi: 10.1039/c003720f. [DOI] [PubMed] [Google Scholar]
- Sacconi L, Dombeck DA, Webb WW. Overcoming photodamage in second-harmonic generation microscopy: real-time optical recording of neuronal action potentials. Proc Natl Acad Sci. 2006;103:3124–3129. doi: 10.1073/pnas.0511338103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim S-H, Xia C, Zhong G, Babcock HP, Vaughan JC, Huang B, Wang X, Xu C, Bi G-Q, Zhuang X. Super-resolution fluorescence imaging of organelles in live cells with photoswitchable membrane probes. Proc Natl Acad Sci. 2012;109:13978–13983. doi: 10.1073/pnas.1201882109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Fu Y, Zickmund P, Shi R, Cheng J-X. Coherent anti-stokes Raman scattering imaging of axonal myelin in live spinal tissues. Biophys J. 2005;89:581–591. doi: 10.1529/biophysj.105.061911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H-W, Le TT, Cheng J-X. Label-free imaging of arterial cells and extracellular matrix using a multimodal CARS microscope. Opt Commun. 2008;281:1813–1822. doi: 10.1016/j.optcom.2007.07.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams RM, Zipfel WR, Webb WW. Multiphoton microscopy in biological research. Curr Opin Chem Biol. 2001;5:603–608. doi: 10.1016/s1367-5931(00)00241-6. [DOI] [PubMed] [Google Scholar]
- Wurpel GWH, Schins JM, Müller M. Chemical specificity in three-dimensional imaging with multiplex coherent anti-stokes Raman scattering microscopy. Opt Lett. 2002;27:1093. doi: 10.1364/ol.27.001093. [DOI] [PubMed] [Google Scholar]
- Yokota H, Kaneshiro J, Uesu Y. Optical second harmonic generation microscopy as a tool of material diagnosis. Phys Res Int. 2012;2012:1–12. [Google Scholar]
- Yuste R. Fluorescence microscopy today. Nat Methods. 2005;2:902–904. doi: 10.1038/nmeth1205-902. [DOI] [PubMed] [Google Scholar]
- Zipfel WR, Williams RM, Christie R, Nikitin AY, Hyman BT, Webb WW. Live tissue intrinsic emission microscopy using multiphoton-excited native fluorescence and second harmonic generation. Proc Natl Acad Sci. 2003;100:7075–7080. doi: 10.1073/pnas.0832308100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zipfel WR, Williams RM, Webb WW. Nonlinear magic: multiphoton microscopy in the biosciences. Nat Biotechnol. 2003;21:1369–1377. doi: 10.1038/nbt899. [DOI] [PubMed] [Google Scholar]
- Zochowski M, Wachowiak M, Falk C, Cohen L, Lam Y, Antic S, Zecevic D. Imaging membrane potential with voltage-sensitive dyes. Biol Bull. 2000;198:1–21. doi: 10.2307/1542798. [DOI] [PubMed] [Google Scholar]
- Zochowski M, Zochowski M, Wachowiak M, Wachowiak M, Falk CX, Falk CX, Cohen LB, Cohen LB, Lam Y-W, Lam Y-W, Antic S, Antic S, Zecevic D, Zecevic D. Concepts in imaging and microscopy imaging membrane potential with voltage-sensitive dyes. Biol Bull. 2000;198:1. doi: 10.2307/1542798. [DOI] [PubMed] [Google Scholar]