Abstract
Objective
Autologous chondrocyte implantation is a necessary procedure for the repair of articular cartilage defects; however, isolated chondrocyte implantation requires a 2-step procedure (for harvesting and implantation) and is limited by cytotoxicity due to enzymatic digestion. Therefore, in this in vitro study, we evaluated the possible benefit of using minced cartilage embedded in a 3-dimensional culture scaffold and fixed with fibrin glue, in comparison with isolated chondrocytes in atelocollagen, to induce cell migration, proliferation, and matrix production, using cartilage from patients with knee joint osteoarthritis.
Design
Cartilage fragments were obtained from 7 female patients with knee osteoarthritis (OA) and embedded in atelocollagen gels. As a control, chondrocytes were isolated and embedded in gels in the same manner. These composites were cultured for 3 weeks, and cell proliferation and matrix production were evaluated using histology and immunochemistry.
Results
Histologically, minced cartilage showed cell migration from the cartilage fragments into the gel, with the Bern score and cell count in the minced cartilage group being significantly higher than those in the control group. Immunohistochemistry revealed that the number of Ki67-positive cells, the expression of LECT-1 and TGF-β, and the glycosaminoglycan content were significantly higher in the minced cartilage than in the control group. Minced cartilage exhibited superior cell migration, proliferation, and glycosaminoglycan content than isolated chondrocytes.
Conclusion
Our findings support that minced cartilage has a favorable potential for cell proliferation and matrix production compared with the isolated chondrocytes after enzymatic treatment.
Keywords: osteoarthritis, minced cartilage, chondrocytes, cartilage repair
Introduction
Although articular cartilage is an important tissue in weight bearing on a joint and ensuring smooth motion of articular surfaces, it is well known that articular cartilage has a limited capacity for self-repair once it is damaged.1,2 Damage to articular cartilage leads to osteoarthritic changes within the joint, which often result in joint dysfunction and pain. Therefore, appropriate interventions are needed to repair damaged cartilage3,4 with the goal of replacing the cartilage defect with tissue that closely approximates the histology of the surrounding native hyaline cartilage, returning the patient to previous activity and providing long term durability of the repair. To achieve these goals, cell-based repair techniques, such as autologous chondrocyte implantation (ACI), have been used to repair large diameter cartilage defects that exceed an area of 2.5 to 3.0 cm2. Good clinical results after ACI have been reported since Britteberg and colleagues first described the breakthrough method of transplanting human autologous chondrocytes to treat defects of the articular cartilage.5-8 Ochi et al.9 developed a third-generation ACI method, using a tissue engineering technology that creates cartilage-like tissue in a 3-dimensional culture system, with atelocollagen gels used to resolve some concerns, such as the risk of chondrocytes leakage from the recipient site and an uneven distribution of chondrocytes, with good clinical results having been reported.9-12 However, several issues with cartilage repair techniques remain to be improved, such as the need for 2 surgical procedures to first harvest the cartilage and second to implant the cultured chondrocytes, chondrocytes toxicity by enzymatic digestion with the use of trypsin and collagenase, and destruction of normal cartilage tissue.13
Recently, alternative 1-step procedures, using cartilage fragments have been developed to address these issues. Although first described in 1982, interest in 1-step procedures increased in 2006 being described in several animal studies,14,15 with satisfactory clinical results having been reported for minced cartilage implantation.16,17 In this approach, about 200 mg of hyaline cartilage is harvested from a low load-bearing area and minced into 1- to 2-mm pieces. The fragmented cartilage is dispersed onto a biodegradable scaffold with the cartilage-scaffold composite fixed by fibrin sealant. Subsequently, this composite is transferred onto the cartilage defect via mini-arthrotomy. Cole et al.16 reported good clinical results using this procedure (known as the cartilage autograft implantation system or CAIS) in a prospective clinical safety trial with a 2-year follow-up. However, the area of cartilage defect in Cole et al.’s case series was around 2.75 cm2, which means that this procedure may not be appropriate for the repair of large cartilage defects. Therefore, a more elaborate one-step approach for using minced cartilage and scaffold for the repair of a large cartilage defect is needed. Moreover, considering the increasing need to treat large cartilage defects, there is a further need to develop cartilage repair procedures for patients.
In this study, we hypothesized that implantation of minced cartilage embedded in atelocollagen gel could have favorable properties such as chondrocytes proliferation, migration, and production of cartilage matrix in atelocollagen gel. Therefore, the purpose of this in vitro study was to investigate if minced cartilage could play a role on the future treatment of cartilage defects, through a comparison of cell migration, proliferation and matrix production between the atelocollagen technique and the use of isolated chondrocytes in a 3-dimensional culture, using cartilage from patients with knee OA.
Methods
Seven female patients (mean age 79.1 years; range, 69-88 years) who were undergoing a total knee arthroplasty were enrolled into our study for the purpose of harvesting articular cartilage. All included patients had a clinical diagnosis of primary OA, Kellgren-Lawrence grade 3 or 4, and a varus alignment of the knee. Patients with secondary OA, such as posttraumatic OA, and/or a systemic joint disease, such as rheumatoid arthritis, were not considered.
Our study was approved by the local ethical committee of our university, and informed consent was obtained from all individual participants included in the study.
Preparation of Cartilage/Atelocollagen Composite
After bone resection, articular cartilage was harvested from the patellar groove, lateral femoral and condyle, in a sterile fashion. The cartilage was washed in 0.9% sodium chloride and subsequently minced manually using a scalpel to obtain cartilage fragments of <1 mm3. To isolate chondrocytes, cartilage specimens were treated with 0.25% trypsin (Gibco, Carlsbad, CA, USA) in sterile saline for 30 minutes, followed by 0.25% collagenase type 2 (Gibco, Carlsbad, CA, USA) in Dulbecco’s modified Eagle’s medium (DMEM, Gibco, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (FBS) (Sigma, Taufkirchen, Germany) and antibiotics (penicillin [10,000 units], streptomycin [10,000 μg/mL]) (Nacalai tesque, Japan) for 4 hours at 37°C in a culture tube, according to methods previously described.1 The chondrocytes were washed 3 times with culture medium and then filtered through a 70-mm sterile nylon mesh (cell strainer, BD Biosciences Discovery Labware, Franklin Lakes, NJ, USA).
Culture of Cartilage/Atelocollagen Composite
Isolated chondrocytes (2.0 × 105 cells) were dispersed and mixed in 100 μL of atelocollagen gel (Koken, Tokyo, Japan) to form the chondrocyte group (isolated chondrocyte; group IC). In 2 other groups, 12.5 mg (group M1) or 25 mg (group M2) of minced cartilage were mixed in 100 μL of atelocollagen gel. All cell mixtures were placed in 6-well culture plates and incubated in a mixture of 5% CO2 and 95% air at a temperature of 37°C for 30 minutes to form atelocollagen composites ( Fig. 1 ). A culture medium containing 5% FBS and antibiotics was subsequently added into each well. The mixture was then incubated, again, in a mixture of 5% CO2 and 95% air at 37°C for 3 weeks. The culture medium was changed every 3 days, and L-ascorbic acid (50 μg/mL) was added every 2 days. After 3 weeks, each composite was divided into 2 parts, with one half of each sample fixed in 4% paraformaldehyde (PFA), and the other half used for biochemical analysis.
Figure 1.
(A) Isolated chondrocytes embedded in atelocollagen gel. (B) Minced cartilage embedded in atelocollagen gel. Arrow head indicates the cartilage fragment.
Histological Analysis
Samples for histological analysis were fixed in 4% PFA, at 4°C overnight and subsequently embedded in paraffin. Four micrometer sections were prepared and stained using hematoxylin and eosin (H&E) and safranin-O Fast-Green. Each sample was evaluated using the Bern score, according to the methods previously described.18 The Bern score (minimum score, 0; maximum score, 9) is based on 3 items—uniformity and intensity of safranin-O staining, distance between cells/amount of matrix produced, and cell morphologic characteristics—with each item scored from 0 to 3.
For the evaluation of chondrocyte migration and proliferation in the atelocollagen gel, 6 areas (500 μm × 500 μm grid) were randomly selected in each section and chondrocytes were counted under a magnification of ×400.
Immunohistochemistry
Immunostaining of each section was performed using anti-Ki67 antibody (Richard-Allan Scientific, Kalamazoo, MI, USA), anti-LECT1 antibody (Abcam, Cambridge, UK), anti-transforming growth factor (TGF)-β antibody (Abcam, Cambridge, UK), anti-collagen type I antibody (Novus Biologicals, Littleton, CO, USA), and anti-collagen type II antibody (Kyowa Pharma Chemical, Toyama, Japan). As the secondary antibody for Ki67, we used Alexa Flour 568-conjugated anti-rabbit IgG (Molecular Probes, Invitrogen, Carlsband, CA). Alexa Flour 488-conjugated anti-rabbit IgG was used for LECT1 and TGF-β. The negative control was treated with IgGs isotypes to replace primary antibodies. A DAPI (4′,6-diamidino-2-phenylindole; Dojindo Laboratories, Kumamoto, Japan) solution was applied for nuclear staining. For collagen types I and II, the sections were visualized using the avidin-biotin system (Vectastain Elite ABC Mouse IgG kit, Vector Laboratories, Inc., Burlingame, CA) and 3,3′-diaminobenzidine (Peroxidase Substrate Kit, Vector Laboratories, Inc.), according to the manufacturer’s instructions. For the evaluation of cell proliferation ability, the ratio of Ki-67 positive cells to the total cell count was calculated in each section.
Evaluation of Glycosaminoglycan Contents
After the 3-week period incubation, half of the composites for each group were used for biochemical assays for glycosaminoglycan (GAG)/DNA quantification, using the Blyscan Glycosaminoglycan assay kit (Biocolor, Carrickfergus, UK), according to the manufacturer’s protocol.
Statistical Analysis
The data were expressed as a mean and standard deviation (SD). One-way analysis of variance, followed by Turkey’s post hoc analysis, was used to evaluate between-group differences, with a P value <0.05 considered statistically significant.
Results
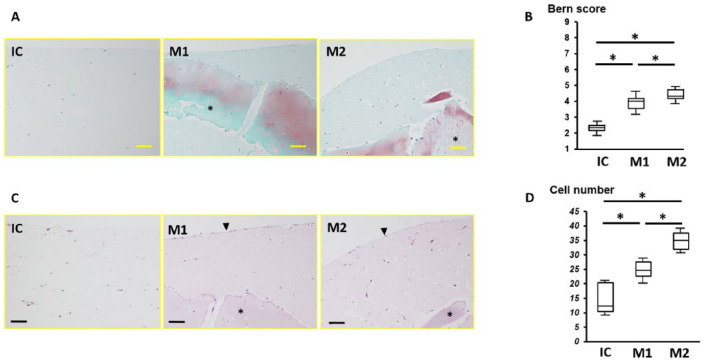
In the IC group, dispersed chondrocytes were observed in the atelocollagen gel, with many cells accumulated at the edge of the gel. In the M1 and M2 groups, chondrocytes outside of the cartilage fragments were observed, indicative of cell migration from the cartilage fragment. The cartilage fragments were stained with safranin-O in groups M1 and M2 ( Fig. 2 ). The mean ± SD number of chondrocytes in the atelocollagen gel in groups IC, M1, and M2 was 15.2 ± 10.8, 24.9 ± 5.4, and 35.4 ± 17.8 cells, respectively, which was significant between all groups (P < 0.01). The average Bern score in groups IC, M1, and M2 was 2.3 ± 0.5, 3.9 ± 0.6, and 4.4 ± 0.8, respectively, which was significant between all groups (P < 0.01).
Figure 2.
(A) Safranin-O staining in the IC (isolated chondrocyte), M1, and M2 groups; *minced cartilage. (B) The Bern score for each group; *P < 0.05. (C) Hematoxylin and eosin staining in each group. Arrowheads indicate the cell lining on the surface of the atelocollagen gel; *minced cartilage. (D) Cell number in each group; bar = 100 μm.
Positive Ki67 cells, indicative of the proliferation capacity of chondrocytes in atelocollagen gel, were identified in all groups ( Fig. 3 ). The average proportion of Ki67-positive cells in groups IC, M1, and M2 was 58.1% ± 30.9%, 89.6% ± 11.4%, and 94.9% ± 6.0%, respectively. This proportion was significantly difference just between IC and M1, as well as IC and M2 (P < 0.01).
Figure 3.
(A) Immunohistochemistry of Ki67; *minced cartilage; bar = 100 μm. (B) Number of Ki67-positive cells; *P < 0.05; NS, no significant difference.
Immunohistochemistry for TGF-β and LECT-1 was performed to examine whether these three groups had anabolic factors. LECT-1 was expressed in all groups, with greater intensity in groups M1 and M2 than in group IC, as well as greater intensity in group M2 than in group M1 ( Fig. 4 ). Similarly, TGF-β expression was greater in groups M1 and M2 than IC, as well as in group M2 than M1 ( Fig. 5 ). In groups M1 and M2, TGF-β was intensely expressed at the edge of the gel.
Figure 4.
Immunohistochemistry of LECT-1; bar = 100 μm. LECT-1 was expressed to a greater intensity in groups M1 and M2 than in group IC (isolated chondrocyte).
Figure 5.
Immunohistochemistry of TGF-β; bar = 100 μm. TGF-β was expressed to a greater intensity in groups M1 and M2 than in group IC (isolated chondrocyte).
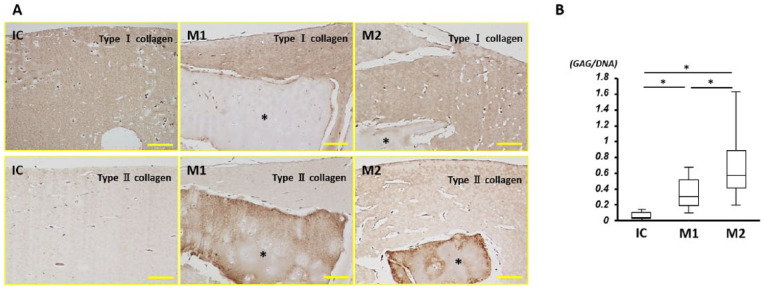
With regard to immunohistochemistry for type I collagen, the atelocollagen gel was well stained in all groups, with no staining of cartilage fragments. In contrast, cartilage fragments were well-stained for type II collagen. Chondrocytes in the gel also had positive staining for type II collagen ( Fig. 6 ).
Figure 6.
(A) Immunohistochemistry of types I and II collagen; *minced cartilage; bar = 100 μm. (B) Glycosaminoglycan content; *P < 0.05.
On biochemical analysis, the GAG-to-DNA ratio in group M2 was the highest among the 3 groups, as well as being higher in group M1 than group IC, with a significant difference in the ratio between all groups (P < 0.01).
Discussion
In this study, we found that implantation of minced cartilage embedded in atelocollagen gel could be more effective for chondrocytes proliferation, migration, and production of cartilage matrix in atelocollagen gel than isolated chondrocytes in atelocollagen gel.
Application of cartilage fragments can be used as a 1-step procedure for the repair of articular cartilage defects, without the need for cell culture. The intra-articular environment after implantation of minced cartilage embedded in atelocollagen gel might be beneficial, as a 1-step procedure, to promote outgrowth of chondrocytes and to induce the culture environment for chondrogenesis.19,20 This approach might be useful for the treatment of large cartilage defects which will be an important future task to be solved. To cover a large cartilage defect, a large amount of cartilage fragments is required. However, there is a limit to the extent of cartilage that can be harvested in patients due to donor site morbidity. Therefore, a suitable scaffold to support the three-dimensional proliferation of chondrocytes and matrix production is needed. Hence, in this in vitro study, we evaluated the possible benefits of using minced cartilage embedded in a 3-dimensional culture scaffold, and compared this to isolated chondrocytes in atelocollagen, to induce cell migration, proliferation, and matrix production, using cartilage from patients with knee joint osteoarthritis. We found that implanting minced cartilage embedded in atelocollagen gel has the better characteristics to repair cartilage defect than isolated chondrocytes after culture.
In previous studies regarding the use of minced cartilage for repair of a defect, minced cartilage was fixed to the area using fibrin glue.16,17 Fibrin is used in practice because of its biocompatible and biodegradable properties. However, several animal studies have demonstrated the negative effects of fibrin on cell migration and tissue repair.21,22 Moreover, there is the possibility that exogenous fibrin may induce an immune response.23 It is to address these limitations of fibrin that we used atelocollagen gel as a scaffold, according to previous reports and our experiences.9-12
Atelocollagen, with the inclusion of type I collagen, has the capacity to remove telopeptides, which are antigenic determinants on the peptides chains of type I collagen.24,25 Previous studies have confirmed the effectiveness of atelocollagen gel in maintaining chondrocyte phenotype in 3-dimensional in vitro chondrocyte cultures with good results for cartilage repair in in vivo experiments.26-28 Unlike other cartilage repair procedures using minced cartilage, we identified migration and proliferation of chondrocytes from cartilage fragments in the atelocollagen gel.
Although the benefit of cartilage repair using chondrocytes has been reported, the source of chondrocytes also needs to be considered to improve chondrocyte mobility at the donor site for repair of a large cartilage defect. As such, the use of cartilage fragments has been proposed as a cell source, without isolation and culture.15,29,30 In our present study, migration of chondrocytes in the atelocollagen composites was identified even though the cell source was OA minced cartilage. Similarly, compared to composites of suspended chondrocytes from enzymatic digestion, the composites of suspended minced cartilage contained significantly more chondrocytes and more Ki67-positive cells in the gel. These findings are indicative that the enzymatic digestion procedure damaged chondrocytes. By comparison, in the minced cartilage group, trypsin and collagenase were not used for enzymatic digestion, which yielded better cell proliferation and would be helpful to cover a large cartilage defect. From our preliminary data, 100 mg of cartilage fragment, harvested from patients who were undergoing total knee arthroplasty, contained about 2 × 105 chondrocytes. The composite of isolated chondrocytes group contained 2 × 105 chondrocytes in 100 μL atelocollagen gel in a manner of previous study.27 Therefore, the isolated chondrocytes composite contained cells isolated from 100 mg cartilage fragment in 100 μL gel. The best cell proliferation ability and Bern score were identified in the group containing 25 mg of minced cartilage in 100 μL of atelocollagen gel, which means that the amount of cartilage in the minced cartilage procedure is only one-fourth that of isolated chondrocytes. Hence, minced cartilage embedded in atelocollagen gel would have a potential to cover a cartilage defect 4 times larger than conventional ACI and need less cartilage which can repair the same size lesion with less donor site morbidity. In addition, minced cartilage obtained from normal cartilage tissue and embedded in atelocollagen gel, which does not require enzymatic digestion to isolate chondrocytes, has the advantage of preserving the cartilage matrix at the time of implantation. In fact, minced cartilage composites in our study had more abundant GAG content compared with isolated chondrocyte composites. Moreover, a recent study showed that type II collagen, which is the specific collagen type in articular cartilage, has an extremely limited turnover in an individual’s lifetime.31 We demonstrated that it was favorable to use cartilage fragments that contain cartilage matrix, collagen, and chondrocytes for cartilage repair without digestion.
Tissue-engineered cartilage has been implanted into cartilage defects with good clinical results. However, the effect of the implanted tissue-engineered cartilage on the subchondral bone and surrounding cartilage has not been clearly evaluated. To address this issue, we evaluated the anabolic factor of atelocollagen gel. Our results showed that minced cartilage had an abundance of LECT-1 expression, which encodes chondromodulin-1. Chondromodulin-1 plays an important role in inhibiting chondrocyte hypertrophy and vascular invasion.32,33 Moreover, atelocollagen gel containing minced cartilage also had an abundance of TGF-β, compared with the chondrocytes group, indicative of the advantage of minced cartilage, embedded in the atelocollagen gel.
There were several limitations in this study that need to be acknowledged. First, only cartilage from OA patients undergoing total knee arthroplasty was used. It has been reported that the structural and mechanical properties, and even molecular aspects, of cartilage are changed by aging,34-37 with human cartilage fragments showing a slower migration rate of chondrocytes compared to juvenile cartilage.38 Our procedure using atelocollagen gel and cartilage fragment might be advantageous for repairing larger cartilage defect, such as OA, using a smaller amount of cartilage at the donor site than required for ACI. It would be important to evaluate cell proliferation, migration, and extracellular matrix production in the atelocollagen gel using osteoarthritic cartilage. Second, in Japan, most of cases who are performed total knee arthroplasty are female. Muraki et al.39 reported that the prevalence of radiographic knee OA and knee pain in the Japanese elderly using a large-scale population of a nationwide cohort study as Research on Osteoarthritis Against Disability (ROAD). In this study, radiographic OA was 47.0% and 70.2% in men and women, respectively, which was much higher than that of previous epidemiologic studies in elderly Caucasians in the United States and Europe. They also reported that the prevalence of knee pain was age-dependent only in women and this might be due to the mechanical stress caused by the Japanese traditional lifestyle and women’s less muscle strength than men.39 In our study period, same as these data, the cases that performed total knee arthroplasty were mostly female, and unfortunately we could obtain only female total knee arthroplasty cartilage. Third, other scaffolds were not compared with atelocollagen gel. Finally, an in vivo component was not included. However, our review of the literature identified prior cases reporting clinical outcomes of using atelocollagen embedded chondrocytes implantation for the repair of articular cartilage.9-12 Our findings confirm the feasibility of using cartilage fragments, even from patients with OA, as a cell source in the atelocollagen gel, with good clinical results expected using minced cartilage instead of cultured chondrocytes.
In conclusion, minced cartilage embedded in atelocollagen gel exhibited good cell migration and proliferation, with abundant GAG, compared with the isolated chondrocytes, even if harvested from OA cartilage. These findings support that there is the possibility to repair cartilage defects in a one-step procedure using implantation of minced cartilage embedded in atelocollagen gel.
Footnotes
Acknowledgments and Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by MEXT KAKENHI Grant-in-Aid for Scientific Research (B), Grant No. 17H04314 (N.A.).
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval: Our study was approved by the local ethical committee of our university.
Informed Consent: Written informed consent was obtained from all subjects before the study.
Trial Registration: Not applicable.
References
- 1. Buckwalter JA. Articular cartilage injuries. Clin Orthop Relat Res. 2002;(402):21-37. [DOI] [PubMed] [Google Scholar]
- 2. Hunziker EB. Articular cartilage repair: basic science and clinical progress. A review of the current status and prospects. Osteoarthritis Cartilage. 2002;10:462-3. [DOI] [PubMed] [Google Scholar]
- 3. Mithoefer K, McAdams TR, Scopp JM, Mandelbaum BR. Emerging options for treatment of articular cartilage injury in the athlete. Clin Sports Med. 2009;28:25-40. [DOI] [PubMed] [Google Scholar]
- 4. van Dijk CN, Reilingh ML, Zengerink M, van Bergen CJA. The natural history of osteochondral lesions in the ankle. Instr Course Lect. 2010;59:375-86. [PubMed] [Google Scholar]
- 5. Lacy KW, Cracchiolo A, Yu S, Goitz H. Medial femoral condyle cartilage defect biomechanics: effect of obesity, defect size, and cartilage thickness. Am J Sports Med. 2016;44:409-16. [DOI] [PubMed] [Google Scholar]
- 6. Niemeyer P, Albrecht D, Andereya S, Angele P, Ateschrang A, Aurich M, et al. Autologous chondrocyte implantation (ACI) for cartilage defects of the knee: a guideline by working group “Clinical Tissue Regeneration” of the German Society of Orthopaedics and Trauma (DGOU). Knee. 2016;23:425-35. [DOI] [PubMed] [Google Scholar]
- 7. Campbell AB, Pineda M, Harris JD, Flanigan DC. Return to sport after articular cartilage repair in athletes’ knees: a systematic review. Arthroscopy. 2016;32:651-68.e1. [DOI] [PubMed] [Google Scholar]
- 8. Britteberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331:889-95. [DOI] [PubMed] [Google Scholar]
- 9. Ochi M, Uchio Y, Tobita M, Kuriwaka M. Current concepts in tissue engineering technique for repair of cartilage defect. Artif Organs. 2001;25:172-9. [DOI] [PubMed] [Google Scholar]
- 10. Tohyama H, Yasuda K, Minami A, Majima T, Iwasaki N, Muneta T, et al. Atelocollagen-associated autologous chondrocytes implantation for the repair of chondral defects of the knee: a prospective multicenter clinical trial in Japan. J Orthop Sci. 2009;14:579-88. [DOI] [PubMed] [Google Scholar]
- 11. Takazawa K, Adachi N, Deie M, Kamei G, Uchio Y, Iwasa J, et al. Evaluation of magnetic resonance imaging and clinical outcome after tissue-engineered cartilage implantation: prospective 6-year follow-up study. J Orthop Sci. 2012;17:413-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Adachi N, Ochi M, Deie M, Nakamae A, Kamei G, Uchio Y, et al. Implantation of tissue-engineered cartilage-like tissue for the treatment for full-thickness cartilage defects of the knee. Knee Surg Sports Traumatol Arthosc. 2014;22:1241-8. [DOI] [PubMed] [Google Scholar]
- 13. Jakob M, Démarteau O, Schäfer D, Stumm M, Heberer M, Martin I. Enzymatic digestion of adult human articular cartilage yields a small fraction of the total available cells. Connect Tissue Res. 2003;44:173-80. [DOI] [PubMed] [Google Scholar]
- 14. Albrecht FH. Closure of joint cartilage defects using cartilage fragments and fibrin glue [in German]. Fortschr Med. 1983;101:1650-2. [PubMed] [Google Scholar]
- 15. Lu Y, Dhanaraj S, Wang Z, Bradley DM, Bowman SM, Cole BJ, et al. Minced cartilage without cell culture serves as an effective intraoperative cell source for cartilage repair. J Orthop Res. 2006;24:1261-70. [DOI] [PubMed] [Google Scholar]
- 16. Cole BJ, Farr J, Winalski CS, Hosea T, Richmond J, Mandelbaum B, et al. Outcomes after a single-stage procedure for cell-based cartilage repair: a prospective clinical safety trial with 2-year follow-up. Am J Sports Med. 2011;39:1170-9. [DOI] [PubMed] [Google Scholar]
- 17. Christensen BB, Foldager CB, Jensen J, Lind M. Autologous dual-tissue transplantation for osteochondral repair: early clinical and radiological results. Cartilage. 2015;6:166-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Grogan SP, Barbero A, Winkelmann V, Rieser F, Fitzsimmons JS, O’Driscoll S, et al. Visual histological grading system for the evaluation of in vitro generated neocartilage. Tissue Eng. 2006;12:2141-9. [DOI] [PubMed] [Google Scholar]
- 19. Mistry H, Connock M, Pink J, Shyangdan D, Clar C, Royle P, et al. Autologous chondrocyte implantation in the knee: systemic review and economic evaluation. Health Technol Assess. 2017;21:1-294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wang N, Grad S, Stoddart MJ, Niemeyer P, Reising K, Schmal H, et al. Particulate cartilage under bioreactor-induced compression and shear. Int Orthop. 2014;38:1105-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Brittberg M, Sjogren-Jansson E, Lindahl A, Peterson L. Influence of fibrin sealant (Tisseel) on osteochondral defect repair in the rabbit knee. Biomaterials. 1997;18:235-42. [DOI] [PubMed] [Google Scholar]
- 22. van Susante JL, Buma P, Schuman L, Homminga GN, van den Berg WB, Veth RP. Resurfacing potential of heterologous chondrocytes suspended in fibrin glue in large full-thickness defects of femoral articular cartilage: an experimental study in the goat. Biomaterials. 1999;20:1167-75. [DOI] [PubMed] [Google Scholar]
- 23. Kawabe N, Yoshinao M. The repair of full-thickness articular cartilage defects. Immune responses to reparative tissue formed by allogenic growth plate chondrocyte implants. Clin Orthop Relat Res. 1991;268:179-293. [PubMed] [Google Scholar]
- 24. Furthmayr H, Timpl R. Immunochemistry of collagens and procollagens. Int Rev Connect Tissue Res. 1976;7:61-99. [DOI] [PubMed] [Google Scholar]
- 25. Pontz B, Meigel W, Rauterberg J, Kühn K. Localization of two species specific antigenic determinants on the peptide chains of calf skin collagen. Eur J Biochem. 1970;16:50-4. [DOI] [PubMed] [Google Scholar]
- 26. Uchio Y, Ochi M, Matsusaki M, Kurioka H, Katsube K. Human chondrocyte proliferation and matrix synthesis cultured in atelocollagen gel. J Biomed Mater Res. 2000;50:138-43. [DOI] [PubMed] [Google Scholar]
- 27. Katsube K, Ochi M, Uchio Y, Maniwa S, Matsusaki M, Tobita M, et al. Repair of articular cartilage defects with cultured chondrocytes in atelocollagen gel. Comparison with cultured chondrocytes in suspension. Arch Orthop Trauma Surg. 2000;120:121-7. [DOI] [PubMed] [Google Scholar]
- 28. Iwasa J, Ochi M, Uchio Y, Katsube K, Adachi N, Kawasaki K. Effects of cell density on proliferation and matrix synthesis of chondrocytes embedded in atelocollagen gel. Artif Organs. 2003;27:249-55. [DOI] [PubMed] [Google Scholar]
- 29. Marmotti A, Bonasia DE, Bruzzone M, Rossi R, Castoldi F, Collo G, et al. Human cartilage fragments in a composite scaffold for single-stage cartilage repair: an in vitro study of the chondrocyte migration and the influence of TGF-β1 and G-CSF. Knee Surg Sports Traumatol Arthrosc. 2013;21:1819-33. [DOI] [PubMed] [Google Scholar]
- 30. Bonasia DE, Marmotti A, Mattia S, Cosentino A, Spolaore S, Governale G, et al. The degree of chondral fragmentation affects extracellular matrix production in cartilage autograft implantation: an in vitro study. Arthroscopy. 2015;31:2355-41. [DOI] [PubMed] [Google Scholar]
- 31. Heinemeier KM, Schjerling P, Heinemeier J, Møller MB, Krogsgaard MR, Grum-Schwensen T, et al. Radiocarbon dating reveals minimal collagen turnover in both healthy and osteoarthritic human cartilage. Sci Transl Med. 2016;8:346ra90. [DOI] [PubMed] [Google Scholar]
- 32. Shukunami C, Hiraki Y. Role of cartilage-derived anti-angiogenic factor, chondromodulin-1, during endochondral bone formation. Osteoarthritis Cartilage. 2001;9(Suppl A):S91-S101. [DOI] [PubMed] [Google Scholar]
- 33. Kusafuka K, Hiraki Y, Shukunami C, Kayano T, Takemura T. Cartilage-specific matrix protein, chondromodulin-1(ChM-1), is a strong angio-inhibitor in endochondral ossification of human neonatal vertebral tissues in vivo: relationship with angiogenic factors in the cartilage. Acta Histochem. 2002;104:167-75. [DOI] [PubMed] [Google Scholar]
- 34. Buckwalter JA, Roughley PJ, Rosenberg LC. Age-related changes in cartilage proteoglycans: Quantitative electron microscopic studies. Microsc Res Tech. 1994;28:398-408. [DOI] [PubMed] [Google Scholar]
- 35. Buckwalter JA, Woo SL, Goldberg VM, Hadley EC, Booth F, Oegema TR, et al. Soft-tissue aging and musculoskeletal function. J Bone Joint Surg Am. 1993;75:1533-48. [DOI] [PubMed] [Google Scholar]
- 36. Martin JA, Buckwalter JA. Telomere erosion and senescence in human articular cartilage chondrocytes. J Gerontol A Biol Sci Med Sci. 2001;56:B172-9. [DOI] [PubMed] [Google Scholar]
- 37. Martin JA, Buckwalter JA. The role of chondrocyte senescence in the pathogenesis of osteoarthritis and in limiting cartilage repair. J Bone Joint Surg Am. 2003;85-A(Suppl 2):106-10. [DOI] [PubMed] [Google Scholar]
- 38. Bonasia DE, Martin JA, Marmotti A, Amendola RL, Buckwalter JA, Rossi R, et al. Cocultures of adult and juvenile chondrocytes compared with adult and juvenile chondral fragments: in vitro matrix production. Am J Sports Med. 2011;39:2355-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Muraki S, Oka H, Akune T, Mabuchi A, En-yo Y, Yoshida M, et al. Prevalence of radiographic knee osteoarthritis and its association with knee pain in the elderly of Japanese population-based cohorts: the ROAD study. Osteoarthritis Cartilage. 2009;17:1137-43. [DOI] [PubMed] [Google Scholar]