Abstract
Various systematic reviews have recently shown that intra-articular platelet-rich plasma (IA-PRP) can lead to symptomatic relief of knee osteoarthritis for up to 12 months. There exist limited data on its use in small joints, such as the trapeziometacarpal joint (TMJ) or carpometacarpal joint (CMCJ) of the thumb. A prospective, randomized, blind, controlled, clinical trial of 33 patients with clinical and radiographic osteoarthritis of the TMJ (grades: I-III according to the Eaton and Littler classification) was conducted. Group A patients (16 patients) received 2 ultrasound-guided IA-PRP injections, while group B patients (17 patients) received 2 ultrasound-guided intra-articular methylprednisolone and lidocaine injections at a 2-week interval. Patients were evaluated prior to and at 3 and 12 months after the second injection using the visual analogue scale (VAS) 100/100, shortened Disabilities of the Arm, Shoulder, and Hand Questionnaire (Q-DASH), and patient’s subjective satisfaction. No significant differences between the baseline clinical and demographic characteristics of the 2 groups were identified. After 12 months’ follow-up, the IA-PRP treatment has yielded significantly better results in comparison with the corticosteroids, in terms of VAS score (P = 0.015), Q-DASH score (P = 0.025), and patients’ satisfaction (P = 0.002). Corticosteroids offer short-term relief of symptoms, but IA-PRP might achieve a lasting effect of up to 12 months in the treatment of early to moderate symptomatic TMJ arthritis.
Keywords: intra-articular injection, platelet-rich plasma, corticosteroid injection, trapeziometacarpal, thumb carpometacarpal
Introduction
Arthritis of the carpometacarpal joint (CMCJ) of the thumb or trapeziometacarpal joint (TMJ) is the second most frequent site of hand osteoarthritis following the interphalangeal joints.1-4 The prevalence of radiographically proven thumb base arthritis, according to the current literature, is ranging between 15% and 36% in the female and between 5% and 11% in the male general population,3-10 demonstrating a 1 to 3 ratio of affected male to female patients. It usually manifests itself in middle-aged patients.5-7
There is a weak to modest association between radiographic arthritis and symptomatic disease3,11 with rates as high as 28%.8 Symptomatic TMJ arthritis is more frequent among women, older patients and patients with more advanced radiological features.3,7,9 Despite affecting only a small joint, symptomatic thumb base arthritis may cause significant disability, as it restricts thumb opposition, renders the joint weak and unstable, and reduces pinch and grip strength.10,12
Platelet-rich plasma (PRP) is an autologous concentrated cocktail of growth factors and inflammatory mediators.13 It contains increased levels of platelet-derived growth factor (PDGF)-AB, PDGF-BB, transforming growth factor–β1, insulin-like growth factor-I, fibroblast growth factor–basic (FGF-b), epidermal growth factor (EGF), vascular endothelial growth factor, and interleukin-12 (IL-12) (p40/70).14 Platelets in PRP are also a source of inflammatory mediators and modulators including IL-1 receptor antagonist (IL-1ra), soluble tumor necrosis factor (TNF) receptor (sTNF-R) I and II, IL-4, IL-10, IL-13, and interferon γ.15
Lately, there has been growing evidence from experimental studies demonstrating the protective effects of PRP on chondrocyte apoptosis, stem cell proliferation, and extracellular matrix anabolism16-18 and the potential to heal cartilage defects,13 thus creating promise on its potential use for treating arthritis. Treatment with PRP significantly attenuated cell apoptosis in chondrocytes and altered apoptosis-associated expression at the gene and protein level.18 Sundman et al.19 supported that PRP treatment resulted in a significant reduction of matrix metalloproteinase–13 (MMP-13) and tumor necrosis factor–a (TNF-a), an increase in hyaluronan synthase-2 (HAS-2) expression in synoviocytes, and an increase in cartilage synthetic activity. In addition, the fibrinogen in PRP may be activated to form a fibrin matrix to heal cartilage lesions, fulfilling the initial requirements of physiological wound healing.13
As a result, over the past 5 years, an increasing number of level I and level II studies have emerged, examining the effect of intra-articular PRP infiltration for the treatment of both hip and knee osteoarthritis.20-27 Particularly, a number of prospective therapeutic cohorts have demonstrated the positive effect of intra-articular PRP administration into arthritic hip and knee joints on pain and function scores compared with the preintervention baseline values. In most studies the improvement is evident by 4 to 6 weeks postinjection, is usually maintained at 6 months and seems to deteriorate by 12 months, although still present compared with the respective preintervention values.20-25 Two studies have reported on sustained results at 12 months postinjection, which can be prolonged up to 18 months with a repeat annual infiltration; however, there is significant deterioration by the end of the second year.26,27 On the contrary, there is limited data on its use in small joints, such as the TMJ.
Recent reviews on the subject of treatment options for thumb base arthritis are recommending intra-articular injections for early to moderate stages of the disease.1,28 Steroid injections are a useful conservative treatment modality prior to considering surgical treatment.29 Intra-articular injections of methylprednisolone and triamcinolone have been used for the treatment of thumb base arthritis.30,31 They are thought to reduce pain and inflammation in early to moderate stages of the disease, but their effect is usually temporary and quite variable.1,23
Our purpose was to investigate the superiority of ultrasound-guided intra-articular platelet-rich plasma (IA-PRP) injections compared with corticosteroid injections for the treatment of symptomatic TMJ arthritis. We hypothesized that the IA-PRP treatment will be more efficacious than the corticosteroids both in the short and long term.
Materials and Methods
This is a prospective, randomized, comparative, blind (with regard to the follow-up evaluation) clinical study, initially including 48 patients with clinical and radiographic osteoarthritis of the first CMCJ, grades I to III according to the Eaton and Littler classification system. These patients were examined in 2 orthopedic academic centers with special interest in upper limb surgery from July 2012 until December 2014.
The exclusion criteria used for our study were as follows: systemic rheumatic disease, self-reported comorbid hand conditions (such as carpal tunnel syndrome or De Quervain’s tenosynovitis), history of gout or pseudogout, bleeding predisposition, previous surgery to the affected thumb, previous injection to the involved thumb base within the past 12 months, severe X-ray osteoarthritis of grade IV (Eaton and Littler classification) and no evidence of CMC joint space narrowing on plain radiographs.
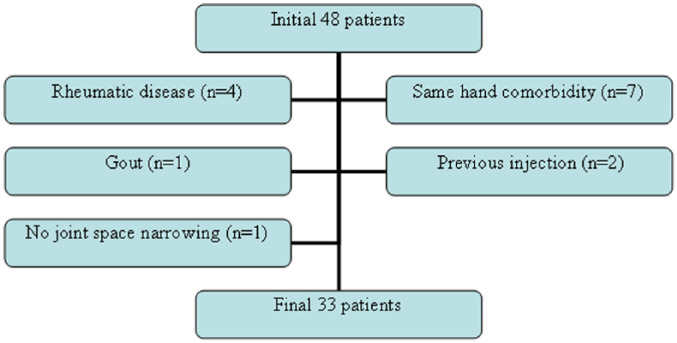
As a result, from the initial group of 48 patients we have excluded 4 patients with systemic rheumatic disease, 7 patients who were suffering from carpal tunnel syndrome or De Quervain’s tenosynovitis, 2 patients with a previous intra-articular injection, 1 patient with history of gout, and 1 patient with no evidence of joint space narrowing, thus leaving the final study cohort with 33 patients ( Fig. 1 ).
Figure 1.
Flowchart showing excluded patients from the initial sample until the conduction of the final group.
These patients were informed in a verbal and written manner regarding their therapeutic options and have received thorough information on the treatment with PRP, which was proposed to them as an alternative to corticosteroids. An approved consent form was signed by each patient prior to inclusion in the study. They were then divided into 2 treatment groups using the sealed envelope method for randomization. The members of group A received 2 ultrasound-guided IA-PRP injections (16 patients), while group B (17 patients) were subjected to 2 ultrasound-guided intra-articular methylprednisolone (125 mg/2 mL methylprednisolone sodium succinate inj. sol., Solu medrol, Pfizer Ltd.) and lidocaine (lidocaine hydrochloride 2% inj. sol. Astra Zeneca Ltd.) injections with the second injection for each treatment group performed 15 days after the first one.
Under sterile conditions, 20 mL of autologous venous blood were taken and centrifuged at 2 consecutive centrifugations (of a total time of 10 minutes at 3100 rounds, at the laboratory of our hospital) in the hematological department of our institution (manually, without any commercial kit).32 At the first centrifugation, the red blood cells were separated and, at the second centrifugation, 2 mL of autologous, leukocyte-poor, nonactivated PRP were separated from the platelet-poor plasma (PPP) and were put in sterile tubes.32 The mean platelets’ final concentration into the PRP was estimated 2.6 times higher than the baseline value.
The ultrasonography was performed with a portable gray scale ultrasound (frequency of 10-12 MHz, A6 Portable Ultrasonic Diagnostic System, Sonoscape Company Limited, Shenzen, People’s Republic of China) by the same operator, an orthopedic surgeon proficient in musculoskeletal ultrasonography. The infiltration was undertaken under sterile conditions (sterilization of the skin, coverage of the ultrasound probe with a sterile pad, use of appropriate gel) by a physician who simultaneously managed the ultrasound device (free hand one man’s technique: a single operator holds the syringe with one hand, while he scans moving the probe with his other hand). Under continuous imaging the tip of the needle was inserted inside the joint, where infiltration with PRP or corticosteroid was performed ( Fig. 2 ). Special care was taken for the correct placement of the needle tip within the joint space, without penetrating the articular cartilage. The synovial swelling during and after infiltration was recorded to confirm the correct application of the technique.
Figure 2.
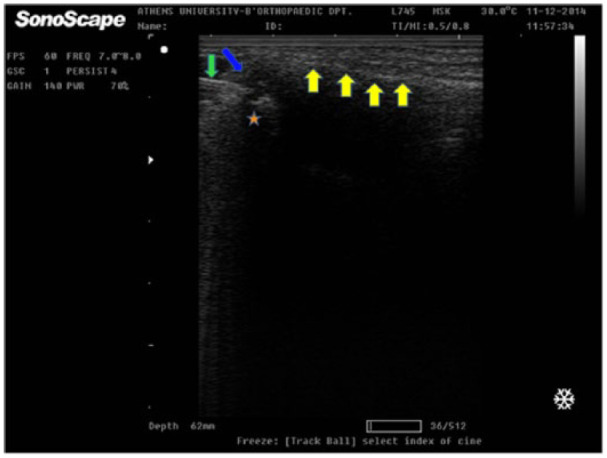
Ultrasound-guided intra-articular carpometacarpal (CMC) injection. The needle (green arrow) is recorded, as it is inserted into the joint (anechoic area, blue arrow). The star illustrates the trapezium bone and above the yellow arrows lies the first metacarpal bone (For interpretation of the references to colours in this figure legend, refer to the online version of this article).
To evaluate the patients’ clinical course (follow-up at 3 and 12 months), the visual analogue scale 0-100/100, shortened Disabilities of the Arm, Shoulder, and Hand Questionnaire (Q-DASH) score and subjective patient’s satisfaction were used. One doctor performed the procedure in all patients and evaluated them prior to the injections through the visual analogue scale (VAS) 100/100 and Q-DASH questionnaires. A second doctor evaluated the patients during the follow-up period (VAS, Q-DASH). This physician was blinded to the procedure (PRP or corticosteroid injection) and to the preinjection scores of each patient. Instructions for return to work (or usual activities in elderly people) from the following day without the additional use of wrist splints were identical for all patients. Despite there are some no-high level evidence studies supporting orthoses, patient education in joint protection, and exercise as treatment options,28 we avoided to use these conservative means in order to evaluate only the therapeutic impact of IA-PRP.
Statistical analysis was performed using the statistical package R.33-35 Numerical variables are expressed in their mean value and standard deviation (SD) if continuous and in their median value and interquartile range (IQR) if discrete. Continuous numerical variables were tested for normality with the Shapiro-Wilk test. Normally distributed continuous numerical variables were further analyzed with the t test, while nonnormally distributed continuous and discrete numerical variables with the Wilcoxon (Mann-Whitney U test) rank sum test. Categorical variables are expressed as percentages and were compared with the chi-square of Fischer’s exact test. Statistical significance was set at the level of P < 0.05.
Results
The preintervention descriptive values as well as the postintervention outcomes for the two treatment groups are summarized in Table 1 . One patient from group B was lost during the follow-up period. As a result, 16 patients were treated with each intervention modality. No statistically significant difference was identified with regards to the preintervention age (P = 0.95) and gender (P = 0.827) distribution between the patients of the 2 treatment groups. As expected the female patients in each group outnumbered their male counterparts by approximately 4 times, with an average age at initiation of treatment of 63 years.
Table 1.
Descriptive Preintervention Values and Postintervention Outcomes per Treatment Group.
| Variable | Descriptives | PRP | Steroid and LA | P |
|---|---|---|---|---|
| Gender | Male:Female | 3:13 (19%:81%) | 3:13 (19%:81%) | 0.827a |
| Age (years) | Mean ± SD | 62.8 ± 10.6 | 63 ± 11.8 | 0.95b |
| VAS score (mm) | ||||
| Preoperative | Median (IQR) | 75 (57.5-80.0) | 70 (60.0-82.5) | 0.76c |
| 3 months | Median (IQR) | 40 (17.5-70.0) (P = 0.004 vs. preoperatively)d |
20 (10.0-62.5) (P = 0.001 vs. preoperatively)d |
0.46c |
| 12 months | Median (IQR) | 20 (10.0-52.5) (P = 0.005 vs. preoperatively)d (P = 0.28 vs. 3 months)d |
65 (50-80) (P = 0.105 vs. preoperatively)d (P = 0.002 vs. 3 months)d |
0.015c |
| Q-DASH score | ||||
| Preoperative | Mean ± SD | 50.4 ± 21.6 | 57.9 ± 25.6 | 0.38b |
| 3 months | Mean ± SD | 32.8 ± 29.2 (P = 0.002 vs. preoperatively)d |
32.6 ± 31.8 (P = 0.014 vs. preoperatively)d |
0.65c |
| 12 months | Mean ± SD | 20.4 ± 27.7 (P = 0.002 vs. preoperatively)d (P = 0.076 vs. 3 months)d |
43.0 ± 27.6 (P = 0.06 vs. preoperatively)d (P = 0.034 vs. 3 months)d |
0.025c |
| Patient’s satisfaction | ||||
| 3 months | Yes:No | 7:9 (44%:56%) | 9:7 (56%:44%) | 0.48a |
| 12 months | Yes:No | 11:5 (69%:31%) | 2:14 (12.5%:87.5%) | 0.002a |
SD = standard deviation; IQR = interquartile range; PRP = platelet rich plasma; LA = local anesthetic; VAS = visual analogue scale; Q-DASH = Disabilities of Arm, Shoulder and Hand Questionnaire (Quick).
Chi-square or Fischer’s exact test
t test.
Wilcoxon rank sum test.
Paired Wilcoxon signed rank test.
VAS Score
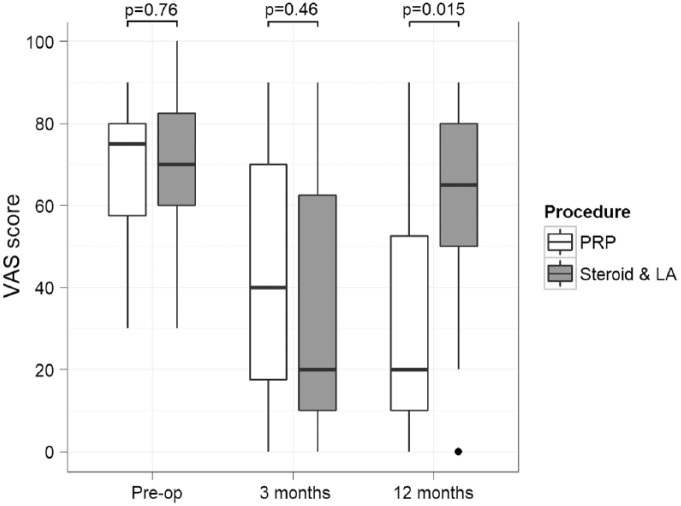
The patients’ pain level prior to treatment, as depicted in the VAS score, was not found to significantly differ between the 2 treatment groups (P = 0.76), with the median VAS score value ranging between 70 and 75. Paired comparisons have shown that both treatment modalities significantly improved pain management at 3 months compared with their respective preintervention VAS score values (P = 0.004 for PRP vs. P = 0.001 for steroid and LA); however, at 12 months this effect was maintained only for the PRP treatment (P = 0.005 vs. P = 0.105, respectively). VAS scores for the PRP group have actually improved further between the 3- and 12-month follow-up at a nonsignificant level (P = 0.28), while for the steroid- and LA-treated patients the median pain score had significantly deteriorated during the same time period (P = 0.002).
The median VAS score at 3 months achieved by the steroid and LA injection was lower than that achieved by the IA-PRP at a nonsignificant level (20 vs. 40, P = 0.46). However, the PRP injection has yielded significantly better results after 12 months follow-up, with a median VAS score of 20/100 compared with 65/100 achieved by the alternative treatment (P = 0.015). These findings are summarized in Figure 3 .
Figure 3.
Changes in pain levels as depicted in the visual analogue scale (VAS) score per intervention group at the consecutive follow-up points. The box represents the interquartile range (IQR), the transverse line within represents the median value, and the whiskers represent the upper and lower adjacent values that lie within 1.5 times the IQR. PRP, platelet-rich plasma; LA, local anesthetic.
We have also estimated the percentage of patients in each intervention group reporting a VAS score below a value of 20/100 (mild pain) at the 2 consecutive follow-up points. A total of 31% of the patients receiving an IA-PRP injection reported mild pain levels at the 3 months follow-up compared with 56% of those receiving a steroid and LA one. However, this difference was not proven to be statistically significant (P = 0.154). The difference was reverted by 12 months, with 62.5% of the patients in the PRP group versus 12.5% of those in the steroid and LA group reporting mild pain (P = 0.005).
Q-DASH Score
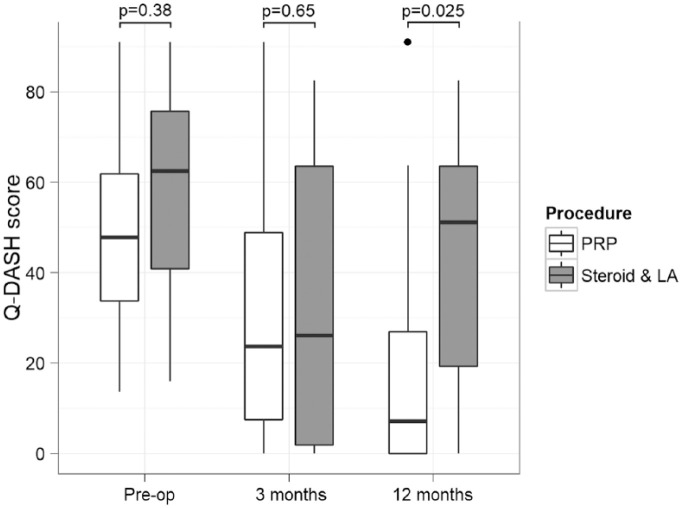
No significant difference was identified in the preintervention Q-DASH scores between the 2 treatment groups (P = 0.38). This effect was maintained at 3 months with equal average Q-DASH values in the range of 32.6 to 32.8 (P = 0.65). By 12 months, however, the PRP treatment significantly reduced the average Q-DASH score compared with its steroid and LA injection counterpart (20.4 vs 43, P = 0.025). Both interventions improved the QDASH scores within each treatment group at 3 months compared with their respective preintervention values (P = 0.002 for PRP vs. P = 0.014 for steroid and LA). By 12 months, the steroid and LA injection group appears to have lost the improvements in hand function observed at the mid-term follow-up (P = 0.06 vs. preintervention function scores, P = 0.034 vs. function scores at 3 months), while the PRP treatment has preserved and further improved its positive effect on the function scores (P = 0.002 vs. preintervention function scores, P = 0.076 vs. function scores at 3 months). These findings are illustrated in Figure 4 .
Figure 4.
Disabilities of Arm, Shoulder and Hand Questionnaire (Quick) (Q-DASH) score changes per intervention group at the consecutive follow-up points. The box represents the interquartile range (IQR), the transverse line within represents the the median value, and the whiskers represent the upper and lower adjacent values that lie within 1.5 times the IQR. PRP, platelet-rich plasma; LA, local anesthetic.
Patients’ Satisfaction
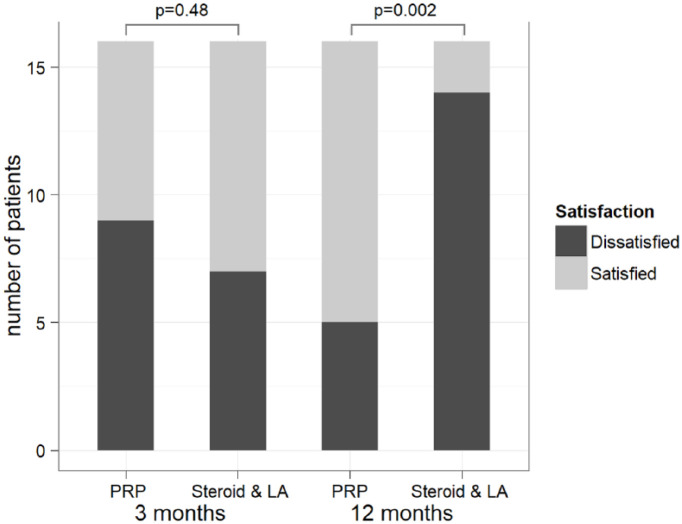
Less than half of the patients treated with a PRP injection (44%) have declared themselves as satisfied 3 months after the initial procedure, compared to 56% of those, who were injected with steroid and LA (P = 0.48). By 12 months, the PRP treatment has yielded significantly higher patients’ satisfaction rates compared with the alternative option (69% vs. 12.5%, P = 0.002) ( Fig. 5 ).
Figure 5.
Patients’ satisfaction per intervention group at the consecutive follow-up points. PRP, platelet-rich plasma; LA, local anesthetic.
As expected, the patients’ satisfaction at both follow-up points was associated with significantly lower VAS and Q-DASH scores (P < 0.001 for both VAS and Q-DASH scores at 3 and 12 months compared with dissatisfied patients). A higher percentage of patients scoring within the mild pain range on the VAS scale have declared themselves as overall satisfied with their intervention at both the 3 months (75% vs. 25%, P < 0.001) and 12 months (92% vs. 8%, P < 0.001) follow-up points compared with those scoring within the more severe pain ranges.
Discussion
The Kellgren-Lawrence and the Eaton and Littler classifications are more commonly used to describe the radiographic severity of the disease.2-4,36,37 The latter allows rationalization of treatment decisions38 and hence it was selected as the default classification system for our study. Day et al.37 in their prospective trial have shown that intra-articular steroid treatment had little to no effect on joints in advanced stages of the disease. We have therefore chosen to exclude patients with stage IV changes according to the Eaton and Littler classification from our trial.
Accuracy is a concern with thumb CMCJ injections. Pollard et al.39 have demonstrated a 25% rate of dye extravasation outside the CMCJ even with the use of fluoroscopy. On the other hand, musculoskeletal ultrasound (MSK U/S) has a proven sensitivity in the evaluation of cartilage pathology and osteophyte morphology in patients with hand osteoarthritis40 and can provide reproducible, quantitative data on thumb CMCJ effusion.41 For the above reasons, we have adopted MSK U/S as the appropriate imaging modality to verify the correct needle positioning and successful joint infiltration.
The DASH questionnaire is a self-administered region-specific outcome instrument developed as a measure of self-rated upper extremity disability and among the most commonly used in the United States to determine the outcomes after hand surgery.4 It has been shown that the use of DASH leads to better evaluation of the impairment due to hand conditions, while it discriminates between patients with CMC joint thumb arthritis and those with involvement of other joints of the hand, when compared with other patient-reported questionnaires.42 Both the DASH and its shortened version the Q-DASH are characterized by high intrarater reliability.43 The VAS and the Q-DASH score have been used in studies evaluating the effects of surgery to the thumb CMC joint to quantify postoperative pain and functional outcomes, respectively.44-48
Day et al.37 have studied the short- and long-term effects of thumb CMCJ steroid injections and splinting on patients suffering from progressively worse stages of the disease. They have demonstrated an average 23 months of pain relief for all patients at the first stage, long-term efficacy in only 40% of those with stages II and III and lack of either short- or long-term relief in those with stage IV. Meenegah et al.30 were unable to identify any significant differences in the pain levels and functional outcomes at 6 months after injecting moderately arthritic thumb CMCJ with steroids. On the other hand, Bahadir et al.49 have demonstrated significant pain relief for patients with stages II and III at 12 months after a single injection of triamcinolone. The studies by Fuchs et al.,50 Stahl et al.,31 and Heyworth et al.51 have shown a fast onset of symptoms’ relief at 4 weeks after injecting stage II arthritic thumb CMC joints with a single shot of triamcinolone, methylprednisolone, and betamethasone, respectively, followed by decreasing efficacy at 6 months post injection for all 3 treatment options.
We report similar results for the patients in our cohort treated with steroid injections. Although we did not collect short-term data at 4 weeks and our patients had received a double injection to eliminate bias when compared to the PRP group, we have identified a statistically significant improvement in mid-term pain and function scores at 3 months compared with their respective preintervention values, an effect that has decreased in the long term to nonsignificant levels for both pain and function at 12 months after the initial treatment. This result was also demonstrated subjectively in the significant reduction, from 56% to 12.5%, in the percentage of patients feeling satisfied with their treatment between the 2 follow-up points.
We have identified only a single noncomparative study evaluating the effect of intraarticular PRP on thumb base arthritis.52 In this pilot study, 10 patients have been treated with 2 IA-PRP injections within 4 weeks, and at 6 months have demonstrated significantly lower VAS and significantly higher Mayo wrist scores compared with their pretreatment values. However, the DASH scores remained unaffected.52 In our study, thumb base IA-PRP injections have significantly improved clinical and functional scores at the short- to mid-term follow-up. These findings are supported by the already published evidence. Furthermore, we have identified a further nonsignificant improvement in both pain and function scores by the end of the first year after treatment, when the PRP-treated patients have enjoyed the maximum benefit from their intervention. The exclusion of patients with end stage arthritis, less amenable to the reparative effect of biological treatments, could be a contributing factor to this result.
With regard to studies comparing IA-PRP injections with pharmacological agents for the treatment of arthritic joints, there is ample evidence comparing PRP with hyaluronate and placebo for the treatment of hip and knee arthritis.53-55 However, we were able to identify only 1 study comparing single injections of PRP and steroid for the treatment of moderate knee osteoarthritis.56 In this study, the intra-articular administration of PRP decreased joint pain more and for a longer duration. In addition, the IA-PRP injection improved activities of daily living and quality of life in the short term at a higher level, when compared with the steroid injection. The above findings were verified by the results of our study, which demonstrated a progressively increasing discrepancy in the clinical and functional outcomes as well as the patients’ overall satisfaction in favor of the PRP treatment.
There is conflicting evidence concerning the optimal number of initial applications. There are several protocols regarding the interval between consecutive intra-articular PRP or corticosteroid injections, varying from 1 to 4 weeks, and all are considered acceptable.57,58 In addition, it has been shown that a single PRP injection illustrates inferior results in comparison with more than one injections.59 One study recommended at least 2 injections at 1 week interval,60 while another study reported no significant difference between a single attempt and 2 injections performed 3 weeks apart.61
To our knowledge this is the first study to compare intra-articular PRP and steroid injections for the symptomatic treatment of mild to moderate thumb base arthritis and the only study comparing PRP injections with any other conservative treatment modality for the above condition. We have performed multiple PRP injections per patient, as this protocol appears to be more favored in the emerging literature.57,60-64 This approach was matched with an equal number of steroid injections to reduce observational bias. Ultrasound guidance was used to verify the correct and complete administration of each therapeutic agent. All injections were performed by the same experienced MSK U/S operator thus minimizing confounding bias. Furthermore, we chose to use established and specific outcome questionnaires, such as the Q-DASH, to evaluate changes in both pain and function. We sought to establish long-term outcomes at 12 months follow-up, as we have identified a relative paucity of long-term evidence in the existing literature compared to ever increasing recent reports on short to mid-term results.
Potential limitations of our study include the small number of patients treated. As previously mentioned, despite the high prevalence of radiographically proven TMJ arthritis in the general population, only a small percentage of the affected patients complain of symptoms thus rendering recruitment to our study somewhat problematic. With regard to our statistical non-significant results, our study could have been underpowered to detect such a difference. Another limitation could be argued to be the absence of a placebo treated group. We chose to omit this option, because the evidence from studies on hip and knee arthritis has shown significantly better outcomes of both intra-articular steroid and PRP injections compared with the placebo treatment. We have also omitted repeat X-ray imaging both at the mid- and long-term follow-up, as we could not find any evidence from the studies on hip and knee arthritis to suggest otherwise. Finally, we did not measure the body mass index (BMI) in the initial baseline characteristics, which might affect the clinical outcomes of the 2 groups.
Spaans et al.,28 in their recent review on the subject of conservative treatment of thumb base arthritis, have concluded that intra-articular hyaluronate injections seem to be a better alternative compared with the steroid ones because of a longer lasting effect of at least 6 months. On the other hand, a more recent systematic review reported that corticosteroid injections not only act faster, but they also lead to greater pain relief than hyaluronic acid in TMJ OA.65 Papalia et al.65 concluded that available data from included studies show that there is no clear evidence to suggest a treatment with hyaluronate injections as the best advisable nonoperative treatment for TM OA.65 Furthermore, a survey concerning active members of the American Society for Surgery of the Hand reported that the vast majority of surgeons use nowadays corticosteroid injections for TMJ arthritis.66
We support that a randomized controlled trial comparing PRP with hyaluronate injections would contribute in the ongoing debate regarding the most efficient injectable agent for the symptomatic treatment of mild to moderate thumb CMCJ arthritis. Plain X-ray imaging at the long-term follow-up could also be considered for evidence of halting or even reversal of the arthritic process.
Our study has shown that IA-PRP injections significantly improve pain and function from mild to moderate thumb TMJ (CMCJ) arthritis in both the mid- and long-term and achieve significantly better results in the long-term compared with the traditional treatment with intraarticular steroid injections, thus proving them a more dependable single treatment option to provide continuous, stable relief in the short, mid-, and long terms.
Footnotes
Authors’ Note: Investigations performed at the Attica General Hospital KAT and the Konstantopoulio Hospital of Nea Ionia “Agia Olga”.
Acknowledgments and Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval: This study has been approved by the Scientific and Ethical Committees of Attica General Hospital KAT and Konstantopoulio Hospital of Nea Ionia “Agia Olga”. The study protocol, including our written consent form, was approved by the Scientific and Ethical Committees of both institutions.
Informed Consent: Written informed consent was obtained from all subjects before the study.
Trial Registration: Not applicable.
ORCID iDs: Leonidas Roumeliotis  https://orcid.org/0000-0001-6864-0918
https://orcid.org/0000-0001-6864-0918
Vasileios S. Nikolaou  https://orcid.org/0000-0001-7422-4195
https://orcid.org/0000-0001-7422-4195
References
- 1. Swigart CR. Arthritis of the base of the thumb. Curr Rev Musculoskelet Med. 2008;1(2):142-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bijsterbosch J, Visser W, Kroon HM, Stamm T, Meulenbelt I, Huizinga TW, et al. Thumb base involvement in symptomatic hand osteoarthritis is associated with more pain and functional disability. Ann Rheum Dis. 2010;69(3):585-7. [DOI] [PubMed] [Google Scholar]
- 3. Dahaghin S, Bierma-Zeinstra SM, Ginai AZ, Pols HA, Hazes JM, Koes BW. Prevalence and pattern of radiographic hand osteoarthritis and association with pain and disability (the Rotterdam study). Ann Rheum Dis. 2005;64(5):682-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gillis J, Calder K, Williams J. Review of thumb carpometacarpal arthritis classification, treatment and outcomes. Can J Plast Surg. 2011;19(4):134-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ghavami A, Oishi SN. Thumb trapeziometacarpal arthritis: treatment with ligament reconstruction tendon interposition arthroplasty. Plast Reconstr Surg. 2006;117:116e-128e. [DOI] [PubMed] [Google Scholar]
- 6. Kriegs-Au G, Petje G, Fojtl E, Ganger R, Zachs I. Ligament reconstruction with or without tendon interposition to treat primary thumb carpometacarpal osteoarthritis. Surgical technique. J Bone Joint Surg Am. 2005;87(Suppl 1 Pt 1):78-85. [DOI] [PubMed] [Google Scholar]
- 7. Kellgren JH, Moore R. Generalized osteoarthritis and nodes. BMJ. 1952;1:181-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Armstrong AL, Hunter JB, Davis TR. The prevalence of degenerative arthritis of the base of the thumb in post-menopausal women. J Hand Surg Br. 1994;19(3):340-1. [DOI] [PubMed] [Google Scholar]
- 9. Sonne-Holm S, Jacobsen S. Osteoarthritis of the first carpometacarpal joint: a study of radiology and clinical epidemiology. Results from the Copenhagen Osteoarthritis Study. Osteoarthritis Cartilage. 2006;14(5):496-500. [DOI] [PubMed] [Google Scholar]
- 10. Bakri K, Moran SL. Thumb carpometacarpal arthritis. Plast Reconstr Surg. 2015;135(2):508-20. [DOI] [PubMed] [Google Scholar]
- 11. Hoffler CE, 2nd, Matzon JL, Lutsky KF, Kim N, Beredjiklian PK. Radiographic stage does not correlate with symptom severity in thumb basilar joint osteoarthritis. J Am Acad Orthop Surg. 2015;23(12):778-82. [DOI] [PubMed] [Google Scholar]
- 12. Patel TJ, Beredjiklian PK, Matzon JL. Trapeziometacarpal joint arthritis. Curr Rev Musculoskelet Med. 2013;6(1):1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Xie X, Zhang C, Tuan RS. Biology of platelet-rich plasma and its clinical application in cartilage repair. Arthritis Res Ther. 2014;16(1):204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. El-Sharkawy H, Kantarci A, Deady J, Hasturk H, Liu H, Alshahat M, et al. Platelet-rich plasma: growth factors and pro- and anti-inflammatory properties. J Periodontol. 2007;78(4):661-9. [DOI] [PubMed] [Google Scholar]
- 15. Woodell-May J, Matuska A, Oyster M, Welch Z, O’Shaughnessey K, Hoeppner J. Autologous protein solution inhibits MMP-13 production by IL-1β and TNFα-stimulated human articular chondrocytes. J Orthop Res. 2011;16:1320-6. doi: 10.1002/jor.21384 [DOI] [PubMed] [Google Scholar]
- 16. van Buul GM, Koevoet WL, Kops N, Bos PK, Verhaar JA, Weinans H, et al. Platelet-rich plasma releasate inhibits inflammatory processes in osteoarthritic chondrocytes. Am J Sports Med. 2011;39(11):2362-70. [DOI] [PubMed] [Google Scholar]
- 17. Smyth NA, Murawski CD, Fortier LA, Cole BJ, Kennedy JG. Platelet-rich plasma in the pathologic processes of cartilage: review of basic science evidence. Arthroscopy. 2013;29(8):1399-409. [DOI] [PubMed] [Google Scholar]
- 18. Yang J, Lu Y, Guo A. Platelet-rich plasma protects rat chondrocytes from interleukin-1β-induced apoptosis. Mol Med Rep. 2016;14(5):4075-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sundman EA, Cole BJ, Karas V, Della Valle C, Tetreault MW, Mohammed HO, et al. The anti-inflammatory and matrix restorative mechanisms of platelet-rich plasma in osteoarthritis. Am J Sports Med. 2014;42(1):35-41. doi: 10.1177/0363546513507766 [DOI] [PubMed] [Google Scholar]
- 20. Kon E, Buda R, Filardo G, Di Martino A, Timoncini A, Cenacchi A, et al. Platelet-rich plasma: intra-articular knee injections produced favorable results on degenerative cartilage lesions. Knee Surg Sports Traumatol Arthrosc. 2010;18(4):472-9. [DOI] [PubMed] [Google Scholar]
- 21. Torrero JI, Aroles F, Ferrer D. Treatment of knee chondropathy with platelet rich plasma. Preliminary results at 6 months of follow-up with only one injection. J Biol Regul Homeost Agents. 2012;26(2 Suppl 1):71S-78S. [PubMed] [Google Scholar]
- 22. Sánchez M, Guadilla J, Fiz N, Andia I. Ultrasound-guided platelet-rich plasma injections for the treatment of osteoarthritis of the hip. Rheumatology (Oxford). 2012;51(1):144-50. [DOI] [PubMed] [Google Scholar]
- 23. Patel S, Dhillon MS, Aggarwal S, Marwaha N, Jain A. Treatment with platelet-rich plasma is more effective than placebo for knee osteoarthritis: a prospective, double-blind, randomized trial. Am J Sports Med. 2013;41(2):356-64. [DOI] [PubMed] [Google Scholar]
- 24. Kavadar G, Demircioglu DT, Celik MY, Emre TY. Effectiveness of platelet-rich plasma in the treatment of moderate knee osteoarthritis: a randomized prospective study. J Phys Ther Sci. 2015;27(12):3863-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Filardo G, Kon E, Buda R, Timoncini A, Di Martino A, Cenacchi A, et al. Platelet-rich plasma intra-articular knee injections for the treatment of degenerative cartilage lesions and osteoarthritis. Knee Surg Sports Traumatol Arthrosc. 2011;19(4):528-35. [DOI] [PubMed] [Google Scholar]
- 26. Gobbi A, Lad D, Karnatzikos G. The effects of repeated intra-articular PRP injections on clinical outcomes of early osteoarthritis of the knee. Knee Surg Sports Traumatol Arthrosc. 2015;23(8):2170-7. [DOI] [PubMed] [Google Scholar]
- 27. Loibl M, Lang S, Dendl LM, Nerlich M, Angele P, Gehmert S, et al. Leukocyte-reduced platelet-rich plasma treatment of basal thumb arthritis: a pilot study. Biomed Res Int. 2016;2016:9262909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Spaans AJ, van Minnen LP, Kon M, Schuurman AH, Schreuders AR, Vermeulen GM. Conservative treatment of thumb base osteoarthritis: a systematic review. J Hand Surg Am. 2015;40(1):16-21.e1-e6. [DOI] [PubMed] [Google Scholar]
- 29. Creamer P, Flores R, Hochberg MC. Management of osteoarthritis in older adults. Clin Geriatr Med. 1998;14(3):435-54. [PubMed] [Google Scholar]
- 30. Meenagh GK, Patton J, Kynes C, Wright GD. A randomized controlled trial of intra-articular corticosteroid injection of the carpometacarpal joint of the thumb in osteoarthritis. Ann Rheum Dis. 2004;63(10):1260-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Stahl S, Karsh-Zafrir I, Ratzon N, Rosenberg N. Comparison of intraarticular injection of depot corticosteroid and hyaluronic acid for treatment of degenerative trapeziometacarpal joints. J Clin Rheumatol. 2005;11(6):299-302. [DOI] [PubMed] [Google Scholar]
- 32. Malahias MA, Johnson EO, Babis GC, Nikolaou VS. Single injection of platelet-rich plasma as a novel treatment of carpal tunnel syndrome. Neural Regen Res. 2015;10(11):1856-9. doi: 10.4103/1673-5374.165322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. R Development Core Team. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2015. http://www.R-project.org/.
- 34. Helbig M, Urbanek S, Fellows I. JGR: JGR—Java GUI for R. R package version 1.7-16. https://cran.r-project.org/web/packages/JGR/index.html
- 35. Ockendon M, Cool P. DeducerSurvival: add survival dialogue to Deducer. R package version 0.1-0. http://CRAN.R-project.org/package=DeducerSurvival
- 36. Eaton RG, Littler JW. Ligament reconstruction for the painful thumb carpometacarpal joint. J Bone Joint Surg Am. 1973;55:1655-66. [PubMed] [Google Scholar]
- 37. Day CS, Gelberman R, Patel AA, Vogt MT, Ditsios K, Boyer MI. Basal joint osteoarthritis of the thumb: a prospective trial of steroid injection and splinting. J Hand Surg Am. 2004;29(2):247-51. [DOI] [PubMed] [Google Scholar]
- 38. Glickel SZ. Clinical assessment of the thumb trapeziometacarpal joint. Hand Clin. 2001;17(2):185-95. [PubMed] [Google Scholar]
- 39. Pollard MA, Cermak MB, Buck WR, Williams DP. Accuracy of injection into the basal joint of the thumb. Am J Orthop (Belle Mead NJ). 2007;36:204-6. [PubMed] [Google Scholar]
- 40. Hammer HB, Iagnocco A, Mathiessen A, Filippucci E, Gandjbakhch F, Kortekaas MC, et al. Global ultrasound assessment of structural lesions in osteoarthritis: a reliability study by the OMERACT ultrasonography group on scoring cartilage and osteophytes in finger joints. Ann Rheum Dis. 2016;75(2):402-7. [DOI] [PubMed] [Google Scholar]
- 41. Iagnocco A, Coari G. Usefulness of high resolution US in the evaluation of effusion in osteoarthritic first carpometacarpal joint. Scand J Rheumatol. 2000;29(3):170-3. [DOI] [PubMed] [Google Scholar]
- 42. MacDermid JC, Wessel J, Humphrey R, Ross D, Roth JH. Validity of self-report measures of pain and disability for persons who have undergone arthroplasty for osteoarthritis of the carpometacarpal joint of the hand. Osteoarthritis Cartilage. 2007;15:524-30. [DOI] [PubMed] [Google Scholar]
- 43. Franchignoni F, Vercelli S, Giordano A, Sartorio F, Bravini E, Ferriero G. Minimal clinically important difference of the disabilities of the arm, shoulder and hand outcome measure (DASH) and its shortened version (QuickDASH). J Orthop Sports Phys Ther. 2014;44(1):30-9. [DOI] [PubMed] [Google Scholar]
- 44. Adi M, Miyamoto H, Taleb C, Zemirline A, Gouzou S, Facca S, et al. Percutaneous fixation of first metacarpal base fractures using locked K-wires: a series of 14 cases. Tech Hand Up Extrem Surg. 2014;18(2):77-81. [DOI] [PubMed] [Google Scholar]
- 45. Mohan A, Shenouda M, Ismail H, Desai A, Jacob J, Sarkhel T. Patient functional outcomes with trapeziectomy alone versus trapeziectomy with TightRope®. J Orthop. 2015;12(Suppl 2):S161-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Harston A, Manon-Matos Y, McGill S, Jones R, Duerinckx J, Wolff TW. The follow-up of trapeziometacarpal arthrodesis using V-shaped osteotomy for osteoarthritis of the first carpometacarpal joint. Tech Hand Up Extrem Surg. 2015;19(1):18-22. [DOI] [PubMed] [Google Scholar]
- 47. Spacek E, Poiraudeau S, Fayad F, Lefèvre-Colau MM, Beaudreuil J, Rannou F, et al. Disability induced by hand osteoarthritis: are patients with more symptoms at digits 2-5 interphalangeal joints different from those with more symptoms at the base of the thumb? Osteoarthritis Cartilage. 2004;12(5):366-73. [DOI] [PubMed] [Google Scholar]
- 48. Wajon A, Ada L, Edmunds I. Surgery for thumb (trapeziometacarpal joint) osteoarthritis. Cochrane Database Syst Rev. 2005;(4):CD004631. [DOI] [PubMed] [Google Scholar]
- 49. Bahadir C, Onal B, Dayan VY, Gürer N. Comparison of therapeutic effects of sodium hyaluronate and corticosteroid injections on trapeziometacarpal joint osteoarthritis. Clin Rheumatol. 2009;28(5):529-33. [DOI] [PubMed] [Google Scholar]
- 50. Fuchs S, Mönikes R, Wohlmeiner A, Heyse T. Intra-articular hyaluronic acid compared with corticoid injections for the treatment of rhizarthrosis. Osteoarthritis Cartilage. 2006;14(1):82-8. [DOI] [PubMed] [Google Scholar]
- 51. Heyworth BE, Lee JH, Kim PD, Lipton CB, Strauch RJ, Rosenwasser MP. Hylan versus corticosteroid versus placebo for treatment of basal joint arthritis: a prospective, randomized, double blinded clinical trial. J Hand Surg Am. 2008;33(1):40-8. [DOI] [PubMed] [Google Scholar]
- 52. Loibl M, Lang S, Dendl LM, Nerlich M, Angele P, Gehmert S, et al. Leukocyte-reduced platelet-rich plasma treatment of basal thumb arthritis: a pilot study. Biomed Res Int. 2016;2016:9262909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Campbell KA, Saltzman BM, Mascarenhas R, Khair MM, Verma NN, Bach BR, Jr, et al. Does intra-articular platelet-rich plasma injection provide clinically superior outcomes compared with other therapies in the treatment of knee osteoarthritis? A systematic review of overlapping meta-analyses. Arthroscopy. 2015;31(11):2213-21. [DOI] [PubMed] [Google Scholar]
- 54. Kanchanatawan W, Arirachakaran A, Chaijenkij K, Prasathaporn N, Boonard M, Piyapittayanun P, et al. Short-term outcomes of platelet-rich plasma injection for treatment of osteoarthritis of the knee. Knee Surg Sports Traumatol Arthrosc. 2016;24(5):1665-77. [DOI] [PubMed] [Google Scholar]
- 55. Meheux CJ, McCulloch PC, Lintner DM, Varner KE, Harris JD. Efficacy of intra-articular platelet-rich plasma injections in knee osteoarthritis: a systematic review. Arthroscopy. 2016;32(3):495-505. [DOI] [PubMed] [Google Scholar]
- 56. Forogh B, Mianehsaz E, Shoaee S, Ahadi T, Raissi GR, Sajadi S. Effect of single injection of platelet-rich plasma in comparison with corticosteroid on knee osteoarthritis: a double-blind randomized clinical trial. J Sports Med Phys Fitness. 2016;56(7-8):901-8. [PubMed] [Google Scholar]
- 57. Raeissadat SA, Rayegani SM, Hassanabadi H, Fathi M, Ghorbani E, Babaee M, et al. Knee osteoarthritis injection choices: platelet- rich plasma (PRP) versus hyaluronic acid (a one-year randomized clinical trial). Clin Med Insights Arthritis Musculoskelet Disord. 2015;8:1-8. doi: 10.4137/CMAMD.S17894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ahmad HS, Farrag SE, Okasha AE, Kadry AO, Ata TB, Monir AA, et al. Clinical outcomes are associated with changes in ultrasonographic structural appearance after platelet-rich plasma treatment for knee osteoarthritis. Int J Rheum Dis. 2018;21(5):960-6. doi: 10.1111/1756-185X.13315 [DOI] [PubMed] [Google Scholar]
- 59. Görmeli G, Görmeli CA, Ataoglu B, Çolak C, Aslantürk O, Ertem K. Multiple PRP injections are more effective than single injections and hyaluronic acid in knees with early osteoarthritis: a randomized, double-blind, placebo-controlled trial. Knee Surg Sports Traumatol Arthrosc. 2017;25(3):958-65. doi: 10.1007/s00167-015-3705-6 [DOI] [PubMed] [Google Scholar]
- 60. Kavadar G, Demircioglu DT, Celik MY, Emre TY. Effectiveness of platelet-rich plasma in the treatment of moderate knee osteoarthritis: a randomized prospective study. J Phys Ther Sci. 2015;27(12):3863-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Patel S, Dhillon MS, Aggarwal S, Marwaha N, Jain A. Treatment with platelet-rich plasma is more effective than placebo for knee osteoarthritis: a prospective, double-blind, randomized trial. Am J Sports Med. 2013;41(2):356-64. [DOI] [PubMed] [Google Scholar]
- 62. Cerza F, Carnì S, Carcangiu A, Di Vavo I, Schiavilla V, Pecora A, et al. Comparison between hyaluronic acid and platelet-rich plasma, intra-articular infiltration in the treatment of gonarthrosis. Am J Sports Med. 2012;40(12):2822-7. [DOI] [PubMed] [Google Scholar]
- 63. Filardo G, Di Matteo B, Di Martino A, Merli ML, Cenacchi A, Fornasari P, et al. Platelet-rich plasma intra-articular knee injections show no superiority versus viscosupplementation: a randomized controlled trial. Am J Sports Med. 2015;43(7):1575-82. [DOI] [PubMed] [Google Scholar]
- 64. Smith PA. Intra-articular autologous conditioned plasma injections provide safe and efficacious treatment for knee osteoarthritis: an FDA-sanctioned, randomized, double-blind, placebo-controlled clinical trial. Am J Sports Med. 2016;44(4):884-91. [DOI] [PubMed] [Google Scholar]
- 65. Wolf JM, Delaronde S. Current trends in nonoperative and operative treatment of trapeziometacarpal osteoarthritis: a survey of US hand surgeons. J Hand Surg Am. 2012;37(1):77-82. doi: 10.1016/j.jhsa.2011.10.010 [DOI] [PubMed] [Google Scholar]
- 66. Papalia R, Albo E, Russo F, Tecame A, Torre G, Sterzi S, et al. The use of hyaluronic acid in the treatment of ankle osteoarthritis: a review of the evidence. J Biol Regul Homeost Agents. 2017;31(4 Suppl 2):91-102. [PubMed] [Google Scholar]