Abstract
Objective
No studies currently exist with long-term follow-up of use of osteochondral allografting (OCA) for treatment of steroid-associated osteonecrosis of femoral condyles in young, active patients who wish to avoid total knee arthroplasty (TKA). We evaluate the extent to which fresh osteochondral allografts can (1) prevent or postpone need for prosthetic arthroplasty and (2) maintain long-term clinically meaningful decrease in pain and improvement in function at mean 11-year follow-up.
Design
Twenty-five patients (33 knees) who underwent OCA transplantation for osteonecrosis of the knee between 1984 and 2013 were evaluated, including 22 females and 11 males with average age of 25 years (range, 16-48 years). Mean total allograft surface area was 10.6 cm2 (range, 4.0-19.0 cm2). Evaluation included International Knee Documentation Committee (IKDC) scores, Knee Society function (KS-F) score, and modified (for the knee) Merle d’Aubigné-Postel (18-point) score.
Results
OCA survivorship was 90% at 5 years and 82% at 10 years. Twenty-eight of 33 knees (85%) avoided arthroplasty and 25 of 33 knees (73%) avoided other surgical intervention. Mean IKDC pain score improved (P = 0.001) from 7.2 preoperatively to 2.8 at latest follow-up, mean IKDC function score increased (P = 0.005) from 3.3 to 6.5, and mean IKDC total score improved (P = 0.001) from 31.9 to 61.1. Mean KS-F score improved (P = 0.003) from 61.7 to 87.5. Mean modified Merle d’Aubigné-Postel (18-point) score improved (P < 0.001) from 11.4 to 15.1.
Conclusions
Our findings suggest that OCA transplantation is a reasonable surgical treatment option for steroid-associated osteonecrosis of the femoral condyles, with durable long-term outcomes.
Keywords: osteochondral allografting, steroid-associated osteonecrosis, femoral condyles, survivorship
Introduction
Secondary avascular osteonecrosis of the knee is a rare but serious side effect of systemic high-dose corticosteroid therapy, often seen in young patients following steroid treatment for autoimmune disease or primary malignancy.1 The femoral condyles are the second most common site to be affected, after the femoral head. Steroid-induced lesions form in the subchondral bone, with eventual fracture and progression to overlying chondrosis, joint collapse, and arthritis.1 Treatment of steroid-associated osteonecrosis remains controversial, with proposed therapeutic approaches including activity modification and surgical intervention, including arthroscopic debridement, core decompression, osteotomy, osteochondral grafting, and partial and total knee arthroplasty (TKA).2-8 Regardless of the etiology for osteonecrosis of the femoral condyles, symptomatic, high-grade (modified Ficat/Arlet stages III-IV) osteonecrotic lesions of the distal femur generally require TKA for definitive treatment.8
Young patients, however, are more likely to continue placing high demands on their replaced joints and are thus more likely to require future revisions of TKA procedures due to aseptic loosening and polyethylene wear, as has been demonstrated in multiple national registry studies.9-11 Biological repair strategies such as osteochondral allograft (OCA) transplantation of the femoral condyles have emerged as a durable method to postpone the need for arthroplasty in young active patients.1 By replacing both osseous and chondral components of juxta-articular necrotic lesions, OCA may provide the most durable support possible to the damaged joint short of TKA, without precluding eventual successful TKA in case of eventual failure.12 Outcomes at median follow-up of 22 years have previously been obtained in patients with either direct trauma or osteochondritis dessicans as etiology for osteonecrosis of the knee13; however, patients with other etiologies such as steroid-associated osteonecrosis have not been characterized for such long-term follow-up. While earlier findings support a continued role for OCA transplantation in steroid-associated osteonecrosis of the femoral condyles, additional follow-up is necessary to characterize long-term graft performance and clinical outcomes.
Methods
Retrospective review of our institution’s OCA registry identified 26 patients (34 knees) who underwent OCA transplantation for knee osteonecrosis between 1984 and 2013, all at least 2 years postoperative. One patient (1 knee) was deceased attributable to his underlying condition and the status of his knee was not ascertained. The remaining 25 patients (33 knees) comprise the current study population ( Table 1 ), including 22 knees in females and 11 in males with average age of 25 years (range, 16-48 years) and mean body mass index of 21.8 kg/m2 (range, 17.1-28.1 kg/m2). All patients provided informed consent and were entered prospectively in an institutional review board–approved clinical database.
Table 1.
Patient Data.
| Patient | Knee | Gender | Age (Years) | Underlying Diagnosis | Steroid Use at Time of OCA | No. of Grafts | Condyle | Total Graft Area (cm2) | Graft Technique | Bone Grafting | Follow-up (Years) | Modified Merle d’Aubigné-Postel Score (Category) or Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 01 | 01 | Female | 19 | Sickle cell anemia | No | 1 | Medial | Shell | No | 3.9 | Deceased | |
| 02 | 02 | Male | 17 | Leukemia | No | 1 | Lateral | 9.9 | Shell | Yes | 13.7a | TKA |
| 03 | Male | 17 | Leukemia | No | 1 | Lateral | 11.6 | Shell | Yes | 13.7a | TKA | |
| 03 | 04 | Female | 16 | Leukemia | No | 1 | Lateral | 11.8 | Shell | Yes | 3.8a | Revision allograft |
| 05 | Female | 17 | Leukemia | No | 2 | Both | 11.0 | Shell | No | 11.4 | 17 (good) | |
| 04 | 06 | Female | 25 | SLE | No | 2 | Both | 9.25 | Shell | No | 10.6a | TKA |
| 05 | 07 | Female | 47 | Crohn’s disease | No | 2 | Both | 17.2 | Shell | No | 16.1 | 12 (poor) |
| 08 | Female | 48 | Crohn’s disease | No | 1 | Lateral | 9.2 | Shell | No | 15.5 | 11 (poor) | |
| 06 | 09 | Male | 25 | SLE | No | 1 | Medial | 17.5 | Shell | Yes | 22.6 | 14 (fair)/deceased |
| 07 | 10 | Male | 29 | Leukemia | No | 2 | Lateral | 6.3 | Plug | Yes | 11.3 | 18 (excellent) |
| 08 | 11 | Female | 25 | SLE | Yes | 1 | Lateral | 5.25 | Shell | Yes | 3.1 | 17 (good)/deceased |
| 09 | 12 | Male | 32 | Ulcerative colitis | No | 1 | Medial | 7.4 | Shell | Yes | 1.6a | Revision allograft |
| 13 | Male | 35 | Ulcerative colitis | No | 2 | Both | 9.0 | Plug | Yes | 8.9a | TKA | |
| 10 | 14 | Female | 19 | Leukemia | No | 1 | Medial | 10.0 | Shell | No | 14.9 | 16 (good) |
| 11 | 15 | Female | 37 | Closed head injury | No | 1 | Lateral | 7.8 | Shell | No | 29 | 18 (excellent) |
| 12 | 16 | Female | 17 | Hodgkin’s lymphoma | No | 3 | Medial | 11.2 | Plug | Yes | 10.7 | 13 (fair) |
| 17 | Female | 17 | Hodgkin’s lymphoma | No | 3 | Medial | 12.0 | Plug | Yes | 10.7 | 13 (fair) | |
| 13 | 18 | Female | 21 | Leukemia | No | 3 | Both | 19.0 | Plug | Yes | 5.3 | 16 (good) |
| 14 | 19 | Female | 18 | Renal transplant | No | 1 | Lateral | 11.0 | Shell | Yes | 18.4 | 14 (fair) |
| 15 | 20 | Female | 30 | Transient allergies | No | 2 | Both | 13.75 | Shell | Yes | 6.5a | TKA |
| 16 | 21 | Female | 23 | SLE | No | 2 | Lateral | 10.5 | Shell | Yes | 22.6 | 11 (poor) |
| 17 | 22 | Female | 18 | Leukemia | No | 1 | Lateral | 5.0 | Plug | No | 10.5 | 17 (good) |
| 23 | Female | 18 | Leukemia | No | 1 | Medial | 9.7 | Shell | Yes | 10.5 | 17 (good) | |
| 18 | 24 | Female | 20 | Ulcerative colitis | No | 2 | Medial | 6.3 | Plug | Yes | 11.2 | 17 (good) |
| 19 | 25 | Female | 18 | SLE | No | 3 | Both | 19.0 | Plug | Yes | 3.3a | Revision allograft |
| 20 | 26 | Female | 44 | Renal infection | No | 1 | Lateral | 10 | Shell | No | 10.1 | 11 (poor) |
| 21 | 27 | Male | 27 | Ulcerative colitis | No | 2 | Medial | 8.5 | Plug | Yes | 11.6 | 18 (excellent) |
| 22 | 28 | Male | 16 | Myositis | Yes | 1 | Lateral | 12.6 | Shell | No | 10.7 | 16 (good) |
| 23 | 29 | Male | 24 | Leukemia | No | 4 | Both | 16.0 | Plug | Yes | 2.9 | 14 (fair) |
| 30 | Male | 24 | Leukemia | No | 2 | Medial | 12.5 | Plug | No | 3.5 | 14 (fair) | |
| 24 | 31 | Male | 23 | Heart transplant | Yes | 2 | Lateral | 9.0 | Plug | No | 3.0 | 16 (good) |
| 25 | 32 | Female | 22 | Ulcerative colitis | No | 1 | Medial | 4.0 | Plug | No | 3.3 | 17 (good) |
| 33 | Female | 22 | Ulcerative colitis | No | 2 | Medial | 5.5 | Plug | No | 3.3 | 17 (good) |
OCA = osteochondral allografting; TKA = total knee arthroplasty; SLE = systemic lupus erythematosus.
Time to revision allograft/conversion to TKA.
Patients’ underlying diagnoses were primarily related to an autoimmune disorder (44% of patients with underlying diagnosis of systemic lupus erythematosus, ulcerative colitis, Crohn’s disease or myositis) or malignancy (32% of patients with underlying diagnosis of leukemia or Hodgkin’s lymphoma), with the remainder of underlying diagnoses being less common causes to receive high-dose corticosteroid therapy—one each of sickle cell disease, closed head injury, renal transplant, transient allergies, renal infection, and heart transplant. Steroid use at the time of OCA was reported in 2 out of 11 (18%) of patients with underlying autoimmune disorder diagnosis, in 0 out of 8 (0%) with underlying malignancy, and in 1 out of 6 (17%) with other underlying diagnosis.
All patients underwent OCA transplantation for osteoarticular stage III-IV (modified Ficat/Arlet stage) lesions sustained secondary to steroid-associated osteonecrosis of the femoral condyles. History was notable for a medical diagnosis requiring prednisone use exceeding 20 mg per day, with 3 patients in the study continuing to receive corticosteroid therapy at time of surgery ( Table 1 ). All patients were younger than 50 years and had symptoms that did not respond to other treatment modalities. Patients were candidates for arthroplasty but had declined due to young age and were referred to allografting as an alternative treatment option. Patients were evaluated preoperatively with 54-inch standing radiographs for limb malalignment to rule out realignment osteotomy before consideration of OCA. Meniscus status and ligament stability were normal in all patients preoperatively. Fifteen of 33 (45.5%) knees had an average of 1.5 previous surgeries (range, 1-5 surgeries), including arthroscopic debridement (10), loose body removal (5), drilling (4), bone grafting (3), bone cement packing (1), and distal femoral osteotomy (1), but never OCA transplantation. No previous surgeries had been performed in the remaining 18 knees.
Thirteen surgeries involved only the left knee, 4 involved only the right, and 16 were bilateral. Twenty-five knees had unicondylar lesions (13 lateral, 12 medial), whereas 8 knees had bicondylar involvement (medial and lateral femoral condyles in the same knee) and received allografts to both condyles. Mean total allograft surface area was 10.6 cm2 (range, 4.0-19.0 cm2). Seventeen of 33 (51.5%) knees had multiple grafts; these included cases of bicondylar involvement, large lesions using dowel technique, or additional nonstructural particulate bone allografting of necrotic areas beneath the grafts. Overall, patients required an average of 1.7 osteochondral allografts per knee (range 1-4).
Standard AP radiographs, corrected for magnification, were used to measure mediolateral tibial plateau dimensions for recipients, and matched to donors by direct measurement of donor dimensions. Blood typing, tissue typing and immunosuppression were not used. Donor tissue was recovered within 24 hours of donor death, and grafts implanted between 5 and 28 days of donor death. Processing of fresh allograft tissue involved storage at 4°C in tissue culture media from a commercial tissue bank. All allografts were obtained from healthy donors, aged 15 to 40 years, and who met the criteria of the American Association of Tissue Banks.
Patients were placed in supine position for surgery under tourniquet control, using a full arthrotomy through midline incision as described previously,1 with key considerations to technique summarized as follows: The location, size, and shape of debrided lesions determined feasibility of dowel versus shell technique for allograft placement. Particulate bone graft was used to fill any lesions requiring curettage to depth >12 mm, and a host bed of 50% or more viable bleeding bone was considered acceptable for graft placement. Copious intraoperative lavage was employed to remove debris and reduce immunogenicity of graft material. Need for supplemental screw fixation was assessed on a case-by-case basis, generally when geometry of the allograft prevented a press fit. With increased operator experience, exclusive use of shell technique in earlier patients eventually progressed to the technically simpler and more easily reproducible dowel technique.
Patients underwent formal physical therapy after the procedure, including supervised range-of-motion exercises and quadriceps strengthening. Patients progressed to cycling or other closed-chain exercises at one month postoperatively. Only limited (toe touch) weightbearing was permitted for the first 6 weeks, after which patients were progressed gradually to full weightbearing at 3 months if radiographs demonstrated full osseous integration of the allograft at that time. Patients were counseled on risks of high-impact loading activities and were permitted to resume unrestricted physical activity at 6 months postoperatively.
Patients returned for clinical evaluation at standard follow-up intervals as described in the previous report, at 6 weeks, 3 months, 6 months, and then annually.1 Patients who did not live locally were sent a questionnaire via mail. Further surgery after OCA transplantation was documented. Allograft failure was defined as revision OCA transplantation or conversion to arthroplasty. Pain and function were assessed preoperatively and postoperatively (for patients with the allograft remaining in situ) using International Knee Documentation Committee (IKDC) scores, Knee Society function (KS-F) score, and the modified Merle d’Aubigné-Postel (18-point) score. Patient satisfaction was captured at each follow-up interval using a 5-item categorical scale. The most recently available postoperative outcomes scores were analyzed for the study.
Statistics
Preoperative and postoperative IKDC, KS-F, and modified Merle d’Aubigné-Postel (18-point) scores were compared using Wilcoxon signed-rank tests. Allograft survivorship was determined using Kaplan-Meier method with failure as the endpoint (revision OCA or arthroplasty conversion). SPSS version 13.0 was used for all analyses. All analyses were performed on a per-knee basis. Patient sample sizes were too small for formal consideration of t test or chi-square analysis to compare subgroup performances.
Results
Nine of 33 knees (27%) had further surgery following the OCA transplantation. Of these, 1 knee underwent 2 arthroscopic debridements and a loose body removal within 5 years after OCA, but had no further surgery after that, and is now 18 years post-OCA with graft in situ. The remaining eight knees (24% of entire cohort) underwent further surgery that involved graft removal and were classified as OCA failures (3 revision OCA transplantations and 5 conversions to TKA). All 3 patients undergoing repeat allografting had recurrent pain and radiographic evidence of allograft resorption, collapse, and fragmentation. One had bicondylar involvement and had multiple grafts revised at 40 months. The second patient underwent distal femoral varus osteotomy to correct residual valgus deformity at 26 months and subsequently underwent revision allografting of the lateral femoral condyle at 45 months from the initial allograft procedure. The third patient underwent revision allografting 1.6 years after primary OCA. The 5 TKA conversions occurred at 6.5, 8.9, 10.6, and 13.7 (2 knees) years after OCA transplantation. Mean time to OCA failure (including OCA revisions and conversions to TKA) was 7.8 years (range 1.6-13.7 years). Graft survivorship was 90% at 5 years and 82% at 10 years ( Fig. 1 ).
Figure 1.
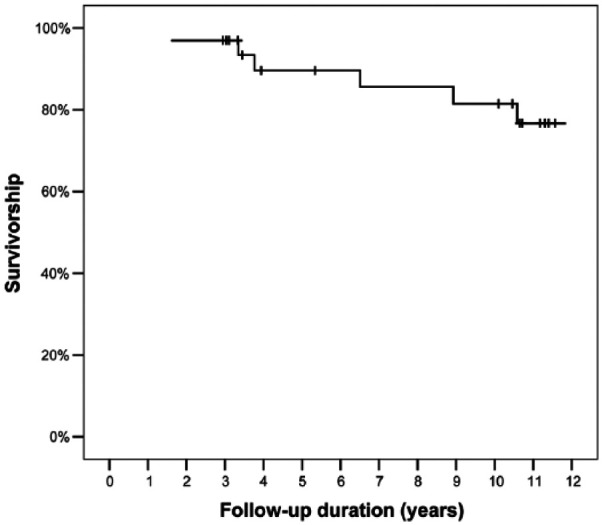
Kaplan Meier curve showing graft survivorship of 90% at 5 years and 82% at10 years.
Among the 25 knees that had the allograft in situ, the mean follow-up duration was 11.0 years (range, 2.9-29 years). Pain and function scores decreased from early follow-up to long-term follow-up ( Table 2 ), but all scores were statistically better at latest follow-up than preoperatively. Of the 23 knees with postoperative IKDC and KS-F scores available, mean IKDC pain score improved from 7.2 preoperatively to 2.8 at latest follow-up (P = 0.001) and mean IKDC function score increased from 3.3 to 6.5 (P = 0.005). Mean KS-F score improved from 61.7 to 87.5 (P = 0.003). Mean modified Merle d’Aubigné-Postel (18-point) score improved from 11.4 to 15.1 (P < 0.001); of the 22 knees that had postoperative modified Merle d’Aubigné-Postel (18-point) scores available, 12 (54%) scored 15 or greater (representing a score of “good” or “excellent”), 7 (32%) were classified as “fair,” and 3 (14%) as “poor.” At latest follow-up among 23 knees that had data regarding satisfaction, 10 (43%) reported being “extremely satisfied” with the results of the OCA transplantation, 8 (35%) were “satisfied,” 3 (13%) were “somewhat satisfied,” and 2 (9%) were “somewhat dissatisfied.”
Table 2.
Subjective Outcome Difference Scores (Change From Preoperative to Early- and Long-Term Follow-up) and Satisfaction Rates Among Patients With Grafts Remaining In Situ.
| Measure | Early-Term Follow-up: Median (Range) or % | Long-Term Follow-up: Median (Range) or % | P a |
|---|---|---|---|
| IKDC pain | −4 (−7 to 2) | −5 (−9 to 3) | 0.001 |
| IKDC function | 5 (−1 to 7) | 3 (−2 to 7) | 0.005 |
| IKDC total | 28 (6-34) | 23 (−3 to 70) | 0.001 |
| KS-F | 30 (10-50) | 30 (0-50) | 0.003 |
| Modified Merle d’Aubigné-Postel | 5 (1-16) | 4 (−4 to 7) | <0.001 |
| Excellent (18 points) | 27.8 | 13.6 | — |
| Good (15-17 points) | 50.0 | 40.9 | — |
| Fair (12-14 points) | 11.1 | 31.8 | — |
| Poor (<12 points) | 11.1 | 13.6 | — |
| Satisfaction | |||
| Extremely satisfied | 57.9 | 43.5 | — |
| Satisfied | 26.3 | 34.8 | — |
| Somewhat satisfied | 15.8 | 13.0 | — |
| Somewhat dissatisfied | — | 8.7 | — |
| Dissatisfied | — | — | — |
IKDC = International Knee Documentation Committee; KS-F = Knee Society function.
P value for comparison of scores from preoperative to long-term follow-up.
Statistical analysis (t test and chi-square) for subgroups to evaluate if any difference in outcomes could be found based on patients’ demographics, underlying diagnosis, previous surgeries, amount and duration of steroid use, and continued steroid use were limited by the small patient sample and did not yield significant differences between any of the above patient subgroups.
Discussion
In this study, we report a case series of OCA transplantation for steroid-associated osteonecrosis of femoral condyles with a mean follow-up of 11.0 years, the longest follow-up available in patients undergoing OCA for this indication.1 We note an increase in the rate of new arthroplasties (15%) or other surgical intervention (27%) on affected knees in the present study compared with previous findings at mean 5.6 years’ follow-up in 2010 (4% and 15%, respectively). Of the 8 knees requiring additional surgical intervention, 4 involved bicondylar lesions, 4 involved above-average necrotic area (range 11.6-19.0 vs. 10.6 cm2), and 6 were performed using shell allograft technique.
Previous study results in patients undergoing OCA for more favorable etiologies leading to osteochondral defects of the femoral condyles, such as for unipolar posttraumatic osteochondral or osteochondritis dissecans defects in the distal aspect of the femur, have demonstrated survivorship at 10 years of 91%.13 In comparison, patients in the current study demonstrate a graft survivorship of only 82% at 10 years. Patients in our study additionally are more likely to have large lesions requiring multiple grafts, or to require allografting of both femoral condyles. Reduced graft survivorship in this study population likely reflects the higher level of lesion complexity in patients with steroid-associated etiology, combined with underlying disease burden from patients’ primary diagnoses. Comparing results of this study only to other patient populations with osteonecrosis as an included etiology for defects in the femoral condyles, outcomes compare favorably to meta-analyses on OCA transplantation in the broader population.14 While outcomes for patients with steroid-associated osteonecrosis of the femoral condyles are not as durable as for patients of a similar age with more benign etiologies; however, the overall graft survivorship is still helpful at preventing progression to TKA in a strong majority of patients.13
The second major endpoint for this case series was to evaluate the impact of OCA for long-term maintenance of a clinically meaningful decrease in pain and improvement in function. We noted a persistently high level of satisfaction with the procedure among patients, even among those whose pain and function scores have declined compared with previously reported interval follow-up at median 5.6 years.1 Compared with previous results, clinical performance of surviving allografts has declined at latest follow-up—of the 19 patients who had not required surgical intervention either in our original case series publication or in latest follow-up, 10 demonstrated a decline in the modified Merle-d’Aubigné-Postel (18-point) scale score compared to prior follow-up and 9 patients scores remained unchanged or improved. Nonetheless, all patient scores remained improved compared with preoperative scores. Postoperative IKDC pain and function scores were slightly lower in the latest follow-up compared to our previous interval report, while remaining significantly above preoperative scores. KS-F scores do not differ significantly from previous interval follow-up.
Clinical outcomes in our study continue to outperform previous results by Bayne et al.,6 where 4 out of 4 patients undergoing OCA for steroid-associated osteonecrosis had poor outcomes, and this difference in results may be due to low frequency of continued steroid use in our patient population postoperatively. These results confirm the utility of fresh OCA transplantation as a treatment option for steroid induced osteonecrosis of the knee. This longer term follow-up period demonstrated further failures and decrease in clinical outcome scores in some patients. Steroid-associated osteonecrosis is a multifocal disease process15-17 and at the time of OCA transplantation, only lesions impacting the chondral surface were treated. Continued degeneration from untreated lesions covered by healthy cartilage may have contributed to decline in graft performance over time.18 Collectively, these findings support that OCA transplantation continues to provide a long-term advantage compared with preoperative function and can contribute to postponing the need for future arthroplasty or other surgical intervention.
Our study has several limitations, including small study population, lack of long-term radiographic data, and lack of validation for one of the scoring instruments (modified Merle d’Aubigné-Postel scale) in the knee. Radiographic outcomes might improve prognostication for continued OCA performance, including evidence of impending failure, which cannot be assessed clinically. The majority of procedures were performed before the current explosion in the literature on improved OCA transplant indications and techniques, in particular during a time when shell grafts were more commonly indicated. Based on relative ease of alignment and securing tight fit with dowel technique, our preference for all OCA patients is currently for the dowel technique where possible. Given the small patient population, however, we cannot definitively identify these criteria as risk factors for failure. In particular when considering the effect of shell vs. dowel technique on need for additional surgical intervention, we note that the average follow-up time for shell technique grafts is longer than that for dowel technique grafts, and when corrected for years at risk, any differences in risk of additional surgery based on graft technique disappear. Patients undergoing OCA today may experience even greater benefit in ability to postpone TKA with broader dissemination of successful techniques, although longer term follow-up in knees receiving the shell technique would be necessary to confirm this.19-21 Our study does not address performance of OCA for steroid-associated osteonecrosis of femoral condyles in pediatric or adolescent populations, which account for a substantial portion of candidate patients.22 To fully assess long-term performance of OCA in our patient population, mean age 25 years, compared with performance of TKA, we would ideally be able to compare outcomes with those of similarly-aged patients undergoing TKA. Indications for TKA in such a young patient population are few, however, and to our knowledge, the long-term outcomes for TKA in such a young population have not been reported.1,2
The small patient population in our study is further complicated by the heterogeneity of primary diagnoses contributing to primary steroid use, leading to many small patient subgroups that are even more difficult to analyze and compare against each other. Of particular interest for further analysis would be the possible impact of underlying diagnosis on long-term outcomes following fresh OCA transplantation, as this may shed light on the underlying mechanisms of response to OCA transplantation. While all patients in this study had a primary diagnosis requiring use of high-dose steroids, in 12 out of 25 cases (48%) with either autoimmune or sickle cell disease, the primary diagnosis itself is associated with an increased risk of joint disease, either through chronic joint inflammation or increased vaso-occlusive risk respectively. Although we did not find a difference in long-term outcomes between patients based on primary diagnosis, this would be highly valuable to investigate in the future with a larger patient population. Concerning patients with underlying diagnosis of malignancy, we hesitate to generalize our findings beyond the limited range of diagnoses included here, namely leukemia. The high representation of leukemia within our sample we believe reflects primarily on the relatively common and survivable nature of this disease compared with other malignancies in the younger patient population, leading to increased manifestations of steroid-associated complications in the following decades of life.
This is the largest reported series of OCA for steroid-associated osteonecrosis of the femoral condyles. Patients undergoing the procedure were younger than 50 years, presented with symptoms that did not respond to other treatment modalities, and were seeking an alternative treatment to TKA. Our findings support that fresh OCA transplantation can produce excellent long-term results in this patient population. Overall survivorship, clinical scores and satisfaction continued to be excellent at long-term follow-up. We conclude that for patients with a history of high-dose steroid use and osteonecrosis of the femoral condyles, fresh OCA transplantation can be a durable long-term alternative to TKA in a majority of patients, with similar performance to OCA treatment for other etiologies of osteonecrosis. Longer term follow-up and larger numbers of patients will be necessary to further characterize procedure indications and outcomes, and to determine the relative impact of other patient characteristics such as demographics, duration or amount of steroid use, and primary diagnosis to require steroid use.
Footnotes
Authors’ Note: This work was performed at Scripps Clinic and Scripps Green Hospital in La Jolla, CA.
Acknowledgments and Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: William D. Bugbee receives travel funds and an honorarium for board participation from JRF Ortho. Julie C. McCauley and William D. Bugbee are consultants for JRF Ortho. No other authors have any disclosures.
Ethical Approval: Ethical approval for this study was obtained from the Scripps Institutional Review Board.
Informed Consent: Written informed consent was obtained from all subjects before the study.
Trial Registration: Not applicable.
References
- 1. Görtz S, De Young AJ, Bugbee WD. Fresh osteochondral allografting for steroid-associated osteonecrosis of the femoral condyles. Clin Orthop Relat Res. 2010;468(5):1269-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Karim AR, Cherian JJ, Jauregui JJ, Pierce T, Mont MA. Osteonecrosis of the knee: review. Ann Transl Med. 2015;3(1):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Koshino T, Okamoto R, Takamura K, Tsuchiya K. Arthroscopy in spontaneous osteonecrosis of the knee. Orthop Clin North Am. 1979;10(3):609-18. [PubMed] [Google Scholar]
- 4. Marulanda G, Seyler TM, Sheikh NH, Mont MA. Percutaneous drilling for the treatment of secondary osteonecrosis of the knee. J Bone Joint Surg Br. 2006;88(6):740-6. [DOI] [PubMed] [Google Scholar]
- 5. Koshino T. The treatment of spontaneous osteonecrosis of the knee by high tibial osteotomy with and without bone-grafting or drilling of the lesion. J Bone Joint Surg Am. 1982;64(1):47-58. [PubMed] [Google Scholar]
- 6. Bayne O, Langer F, Pritzker KP, Houpt J, Gross AE. Osteochondral allografts in the treatment of osteonecrosis of the knee. Orthop Clin North Am. 1985;16(4):727-40. [PubMed] [Google Scholar]
- 7. Mont MA, Rifai A, Baumgarten KM, Sheldon M, Hungerford DS. Total knee arthroplasty for osteonecrosis. J Bone Joint Surg Am. 2002;84-A(4):599-603. [DOI] [PubMed] [Google Scholar]
- 8. Seldes RM, Tan V, Duffy G, Rand JA, Lotke PA. Total knee arthroplasty for steroid-induced osteonecrosis. J Arthroplasty. 1999;14(5):533-7. [DOI] [PubMed] [Google Scholar]
- 9. Australian Orthopaedic Association. National Joint Replacement Registry. Annual report 2016. Available from: https://aoanjrr.sahmri.com/annual-reports-2016
- 10. Furnes O, Espehaug B, Lie SA, Vollset SE, Engesaeter LB, Havelin LI. Early failures among 7174 primary total knee replacements: a follow-up study from the Norwegian Arthroplasty Register 1994-2000. Acta Orthop Scand. 2002;73(2):117-29. [DOI] [PubMed] [Google Scholar]
- 11. Old AB, Long WJ, Scott WN. Revision of total knee arthroplasties performed in young, active patients with posttraumatic and osteoarthritis. J Knee Surg. 2017;30(9):905-8. [DOI] [PubMed] [Google Scholar]
- 12. Steinhoff AK, Bugbee WD. Outcomes of total knee arthroplasty after osteochondral allograft transplantation. Orthop J Sports Med. 2014;2(9):2325967114550276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Raz G, Safir OA, Backstein DJ, Lee PT, Gross AE. Distal femoral fresh osteochondral allografts: follow-up at a mean of twenty-two years. J Bone Joint Surg Am. 2014;96(13):1101-7. [DOI] [PubMed] [Google Scholar]
- 14. Assenmacher AT, Pareek A, Reardon PJ, Macalena JA, Stuart MJ, Krych AJ. Long-term outcomes after osteochondral allograft: a systematic review at long-term follow-up of 12.3 years. Arthroscopy. 2016;32(10):2160-8. [DOI] [PubMed] [Google Scholar]
- 15. Lerebours F, ElAttrache NS, Mandelbaum B. Diseases of subchondral bone 2. Sports Med Arthrosc Rev. 2016;24(2):50-5. [DOI] [PubMed] [Google Scholar]
- 16. Jose J, Pasquotti G, Smith MK, Gupta A, Lesniak BP, Kaplan LD. Subchondral insufficiency fractures of the knee: review of imaging findings. Acta Radiol. 2015;56(6):714-9. [DOI] [PubMed] [Google Scholar]
- 17. Lee K, Goodman SB. Cell therapy for secondary osteonecrosis of the femoral condyles using the Cellect DBM System: a preliminary report. J Arthroplasty. 2009;24(1):43-8. [DOI] [PubMed] [Google Scholar]
- 18. Tanaka Y, Mima H, Yonetani Y, Shiozaki Y, Nakamura N, Horibe S. Histological evaluation of spontaneous osteonecrosis of the medial femoral condyle and short-term clinical results of osteochondral autografting: a case series. Knee. 2009;16(2):130-5. [DOI] [PubMed] [Google Scholar]
- 19. Zouzias IC, Bugbee WD. Osteochondral allograft transplantation in the knee. Sports Med Arthrosc Rev. 2016;24(2):79-84. [DOI] [PubMed] [Google Scholar]
- 20. Bugbee WD, Pallante-Kichura AL, Görtz S, Amiel D, Sah R. Osteochondral allograft transplantation in cartilage repair: graft storage paradigm, translational models, and clinical applications. J Orthop Res. 2016;34(1):31-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bernstein DT, O’Neill CA, Kim RS, Jones HL, Noble PC, Harris JD. et al. Osteochondral allograft donor-host matching by the femoral condyle radius of curvature. Am J Sports Med. 2017;45(2):403-9. [DOI] [PubMed] [Google Scholar]
- 22. Murphy RT, Pennock AT, Bugbee WD. Osteochondral allograft transplantation of the knee in the pediatric and adolescent population. Am J Sports Med. 2014;42(3):635-40. [DOI] [PubMed] [Google Scholar]


