Abstract
Objective
Osteochondral allograft transplantation is a procedure to treat focal osteochondral lesions (OCLs), but is limited by tissue availability, the quality of transplanted tissue, and inconsistent storage protocols. The objective of this study was to assess the clinical outcomes of a novel tissue procurement, storage, and quality control protocol in treating OCLs.
Design
Prospective case series. Donor cadaveric tissue was processed, stored, and the tissue quality analyzed using the unique tissue preservation protocol developed at our institution. Advanced cross-sectional imaging was used to size match donor tissue with recipient patients. Osteochondral allografts were transplanted using the Arthrex Allograft OATS. Patients were evaluated with the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC), Knee injury and Osteoarthritis Outcome Score (KOOS), visual analog scale (VAS), and 36-Item Short Form Survey (SF-36) preoperatively and at 1 year and 2 years postoperatively.
Results
Twenty patients (17 knees, 3 shoulders) were included in the study. There was a significant improvement in the following scores: overall WOMAC score, WOMAC function and pain subcategories; KOOS pain, knee-related symptoms, activities of daily living, sports and recreation, and quality of life; SF-36 physical functioning, physical role, pain, and social functioning subcategories; and VAS at all time points postoperatively. There was a significant improvement in WOMAC stiffness at 2 years postoperatively. There were 2 failures, defined by graft subsidence and persistent pain requiring reoperation.
Conclusion
The protocol developed at our institution for OAT resulted in significant clinical improvement in patients with OCLs and is an improvement on existing tissue storage techniques.
Keywords: osteochondral allograft transplantation, osteochondral lesion, cartilage transplantation, osteoarthritis, X-VIVO 10
Treatment of osteochondral lesions (OCLs) remains a challenging problem facing young and active patients. Cartilage has a poor intrinsic healing capacity and without treatment, many patients remain symptomatic from these lesions.1 Full-thickness OCLs (defined by lesions with exposed subchondral bone) often result in significant morbidity and reduction in function and quality of life.2 Older patients who sustain osteochondral injuries often have some evidence of degenerative osteoarthritis (OA) and can be treated with joint replacement to relieve the pain and debilitation that result from these lesions. However, patients younger than 50 years with osteochondral injuries are not candidates for joint replacement due to their increased activity demands and otherwise normal joint health. Treatment of OCLs in young patients is necessary for reducing the pain and debilitation caused by these acute lesions, and also to slow the progression of OA in the long term.1 OCLs are common, with 1 retrospective review of 25,124 knee arthroscopies revealing that 60% of patients had a chondral lesion with 67% of these being focal OCLs.3 If left untreated, osteochondral injuries can have a significant impact on function and lead to early degenerative arthritis.4 Cell- and tissue-based methods for treating OCLs have been developed for younger patients, including microfracture, mosaicplasty, autologous chondrocyte implantation (ACI), and osteochondral allograft transplantation. Despite multiple studies comparing these different methods of cartilage regeneration and joint restoration, a singular approach for treating OCLs is yet to emerge.1,5
Osteochondral allograft transplantation (OAT) involves transplanting a cadaveric graft of cartilage and underlying subchondral bone into a recipient defect. The donor graft is matched in size, shape, and location to the recipient defect to restore the native articular surface as closely as possible. Articular cartilage is a complex tissue that is dependent on a specific arrangement of chondrocytes within the extracellular matrix to sustain its mechanical properties.1 OAT is the only cartilage restoration technique that restores normal hyaline cartilage tissue architecture to an OCL. There have been several studies evaluating the longer term success of OAT that demonstrate promising clinical outcomes when performed in an appropriate patient population.6-8 However, issues with tissue availability, donor and recipient joint congruency matching, and graft storage techniques often conspire to limit the use of this procedure. Multiple studies exist of OAT of tissue stored for 20 to 30 days, however pre-implantation chondrocyte viability and histological tissue architecture is not routinely assessed or measured.9-11 The biopreservative X-VIVO 10 culture medium has been previously shown to preserve human articular osteochondral grafts with high cell viability.12 As a result, we have developed a unique protocol to preserve fresh osteochondral allograft tissue with a novel preimplantation tissue quality assessment and have performed OAT in 20 patients with outcomes up to 2 years.
The purpose of this study was 2-fold: (a) to determine the preoperative tissue quality and viability of human osteochondral grafts in vitro using a novel application of X-VIVO 10 and (b) to evaluate the clinical outcomes of patients who received osteochondral allografts stored using this novel preservation protocol who have failed treatment of their OCLs using other surgical techniques. This prospective case series reports and evaluates the subjective and objective clinical outcomes of bulk and dowel osteochondral allograft transplantation in the knee and shoulder joints.
Materials and Methods
This research was reviewed and approved by the Conjoint Health Research Ethics Board (University of Calgary) REB15-0132. Written consent was obtained from each patient prior to enrollment in the study. The use of X-VIVO 10 in this application is not approved by the Food and Drug Administration or by Health Canada.
Recipient Selection Criteria
Patients were recruited through referral to 4 orthopaedic surgeons who are fellowship-trained in cartilage transplantation procedures based on the following criteria: age 18 to 50 years, presence of 80% of meniscus, no inflammatory arthropathy, no evidence of osteoarthritis, and no Workers Compensation Board cases. Lesions of all sizes were considered for transplantation with the maximum single dowel size being 35 mm in diameter (limited by the donor harvester of the Arthrex Allograft OATS system). At the time of this study, lesions larger than 35 mm required superimposition of 2 dowel grafts to fill the defect. A study-specific radiographic protocol was developed and allowed for customized matching of donor and recipient allografts. Three-foot standing alignment radiographs were obtained to ensure a normal mechanical axis prior to implantation of allografts in the lower extremity. The size of the OCL was measured on magnetic resonance imagining (MRI) prior to transplantation. Based on the size of OCL postresection, the predicted size of graft was determined. When a viable donor joint became available, anterior-posterior and medial-lateral dimensions of the whole joint were measured and compared with existing potential recipients. Donors and recipients were then matched based on expected joint congruency posttransplantation. Donor and recipient joints for size matching of bulk and dowel allografts were required to be within 1 to 2 mm in size with as similar joint contour as possible. Patient outcomes were collected prior to transplantation and at 1 and 2 years postoperative by using the Short Form 36 (SF-36),13 Western Ontario and McMaster Universities Arthritis Index (WOMAC),14 Western Ontario Shoulder Instability Index (WOSI),15 visual analog scale (VAS),16 and Knee injury and Osteoarthritis Outcome Score (KOOS)17 surveys. Data were analyzed using StataIC13 using a Wilcoxon signed-rank test with a level of statistical significance of 0.05.
Tissue Procurement, Donor Selection, Tissue Preservation, and Quality Control
Grafts were procured according to standard operating procedures by technicians at the Tissue Program from donors without extremity trauma or whose cause of death was systemic illness such as sepsis or organ failure. Musculoskeletal tissue was harvested following removal of organs from the donor occurring within six to twelve hours after asystole. En bloc knee and shoulder joints were sectioned in the metaphyseal regions (including 5-7 cm of metaphyseal bone), stripped of soft tissue attachments and immediately placed in Biowhittaker’s X-VIVO 10 culture media. The tissue was stored at 4°C with media changed every 7 days, based on pH. Four-millimeter cartilage biopsy was obtained at the time of initial storage and histological and viability analyses were performed. Cartilage biopsies were stained with paravital dyes (Molecular Probes: Syto 13 for live cells, Syto X for dead cells) to estimate chondrocyte viability and numerical density on a Zeiss 710 fluorescent confocal microscope. Tissue with an initial chondrocyte viability of >90%, density within a healthy range and normal collagen fibril orientation, was then hypothermically preserved ( Fig. 1 ). Tissue that did not meet this threshold was not banked for transplantation. Osteochondral tissue was stored at in X-VIVO 10 for up to 30 days. Initial serological and microbiological testing was performed at the time of donor tissue recovery. Serology results for human immunodeficiency virus and hepatitis C were determined after 48 hours. Microbiological testing reported at 7 days and confirmed by day 14. Tissue is deferred and disposed of following the Tissue Programs standards, if any results are positive. Three patients had transplants before the 7-day reports were obtained and these patients were carefully monitored clinically for signs of septic arthritis while the testing was pending. On the day of each transplant, a second articular cartilage biopsy was taken from an area just adjacent to where the dowels were harvested from so as not to damage the cartilage on the dowels but to also obtain a representative sample for analysis. Chondrocyte viability, density, as well as collagen fiber structure was assessed. This process validated our storage protocol.
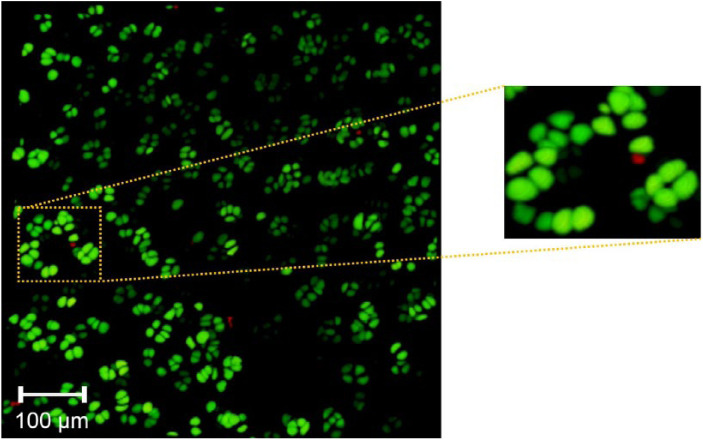
Figure 1.
Syto 13 viability staining of cartilage biopsies of human osteochondral tissue. Initial postharvest viability staining of en bloc joint tissue prior to storage in X-VIVO 10 (a, b). Representative images of articular cartilage biopsies performed after storage in X-VIVO 10, just prior to bulk or dowel transplantation of osteochondral allografts (c, d). Preimplantation viability of all grafts was confirmed before transplantation and only tissue with greater than 90% viability was transplanted.
Bulk grafts were defined by the entire joint with underlying metaphyseal subchondral bone and were used for large defects where the traumatic lesion extended from the articular surface to the supporting metaphyseal pedestal. Dowel grafts were used when the traumatic lesion was contained and was limited to the articular cartilage with no underlying injury to the supporting subchondral bone. When lesions treated with dowel grafts were larger than 35 mm or when the anterior to posterior length of a graft exceeded the width (a rectangular defect), overlapping “snowman” grafts were used to cover the defect.
Each specimen was stained with Calcein AM and ethidium homodimer-1 for live and dead cell identification, respectively (Molecular Probes/Invitrogen, Carlsbad, CA, USA). After a 30-minute incubation period in complete darkness, each specimen was washed 3 times for 5 minutes with a fresh phosphate buffered saline (PBS) solution. Cartilage specimens were fixed in a petri dish and immersed in PBS. A multiphoton microscope (FVMPE-RS, Olympus, Japan) was used to scan 2 areas on the cartilage surface. Cartilage images were performed using a 25 × 1.05 NA water-immersion objective (Olympus Inc., Japan) coupled with 2 independent multiphoton infrared pulsed lasers (InSight DS and Mai Tai DeepSee, Spectra-Physics Inc., Santa Clara, CA, USA) enabling simultaneous excitation at different wavelengths. The first laser was tuned to 940 nm to excite live cell stain while the second laser was tuned to 800 nm to excite the dead cell stains. A stack consisted of serial images of 1 μm thickness up to a depth of 120 μm into the cartilage tissue. The field of view was 509 × 509 μm2 (pixel size: 0.994 µm × 0.994 µm; pixel dwell time: 2 µs; frame scan time: 1.084 seconds). Three-dimensional shapes of these stacks were reconstructed using open source software (ImageJ, NIH, Bethesda, MD, USA). Live and dead cells were counted manually over the entire 3-dimensional volume. The cell viability was calculated as C/C0 × 100%, where C is the number of live cells and C0 is the total number of cells.
Surgical Technique
The Arthrex Allograft OATS system was used to perform the transplantation for dowel transplants. Patients were prepped and draped in the usual fashion. A midline skin incision and medial or lateral parapatellar arthrotomy was used to access the patella, femoral condyles, and tibial plateau. For proximal humerus transplantation, a deltopectoral or deltoid-splitting approach was used. The recipient defect was measured using the sizing cylinder and marked with a marking pen. A K-wire was then drilled into the subchondral bone in the center of the defect ( Fig. 2 ). The Allograft OATS instrumentation was selected as it allows for dowels to be created ranging in size from 15 to 35 mm in diameter. The appropriately sized recipient harvester was used to score the cartilage down to bone. The recipient defect was then drilled using the graduated Allograft OATS recipient counterbore down to a depth of 6 to 8 mm in the subchondral bone until bleeding bone was encountered. The donor tissue was then pulse-lavaged before being secured to the workstation. The Allograft OATS Donor Harvester was then used to obtain a size-matched cylindrical osteochondral graft. The donor allograft was trimmed to obtain an appropriate press fit. The recipient site was dilated by 0.5 mm using the Allograft OATS dilator and the donor allograft was press-fit into the recipient site. No supplementary fixation was used to secure the grafts into the underlying subchondral bone. All edges of the allograft were carefully palpated to ensure a smooth, continuous joint surface with no steps in the articular surface greater than 1 mm. Wounds were closed in the standard fashion.
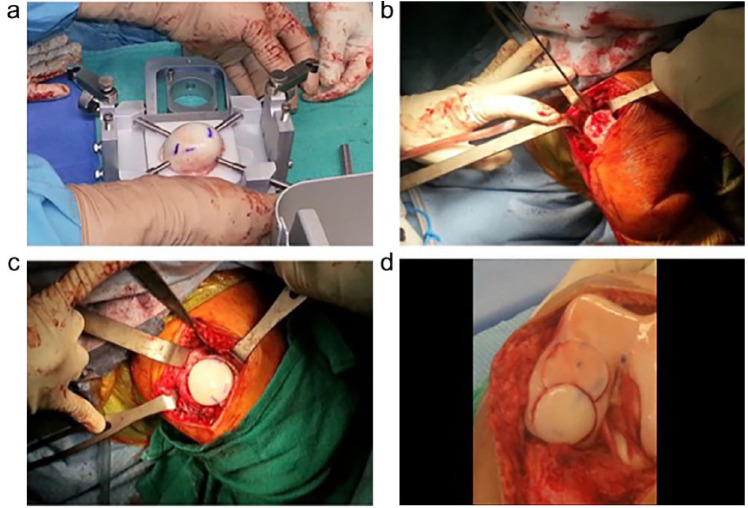
Figure 2.
Intraoperative photographs of osteochondral allograft transplantation using the Arthrex OATS technique in a knee. The donor tissue is secured to the workstation in preparation for harvesting (a). The recipient site is prepared using the recipient harvester (b). The allograft is press-fit into the recipient site (c). Example of a large defect treated with the “snowman” technique of overlapping 2 dowels to fill the defect (d).
Postoperative Protocol
Patients began passive range of motion exercises immediately. Patients who received humeral grafts were nonweightbearing for 6 weeks postoperative while patients who received grafts in the knee were nonweightbearing for 8 weeks. They were followed with serial radiographs at 2, 6, 12, and 52 weeks postoperatively to assess for donor graft subsidence (defined by an articular step of >2 mm between host cartilage and donor cartilage), graft resorption, as well as overall joint alignment. All patients followed the same customized rehabilitation protocol. Patients who received proximal humerus transplantation began with active-assisted range of motion exercises with progressive strengthening over a period of 12 weeks. All patients were monitored for complications including major medical complications and failure of the graft. Failure was defined by continued symptoms postoperatively requiring reoperation.
Results
Mean patient age was 33 years (range, 17-45 years). As this was a prospective case series, all patients followed up at the 1- and 2-year postoperative time point. Fifteen of the 20 patients (75%) had previous operations on their affected joint with 40% of these patients having multiple operations ( Table 1 ). Four patients received bulk grafts with 16 receiving dowel grafts. Five patients received snowman dowels. Seventeen transplants in the knee (14 femoral and 3 tibial plateau) and 3 transplants in the proximal humerus were performed ( Table 1 ). A total of 14 cadaveric donors were used to transplant the 20 patients in this series ( Table 2 ). Tissue was transplanted only when the histology demonstrated adequate cartilage tissue architecture and the viability was greater than 85% ( Table 2 ). Mean storage time prior to transplantation was 10.6 days (range, 3-18 days). All serologic and microbiological testing was negative.
Table 1.
Summary of Transplanted Patients.
| Patient | Gender | Age | Graft Type | Transplanted Location | Graft Size | Complications | Previous Surgeries |
|---|---|---|---|---|---|---|---|
| 1 | F | 33 | Bulk | Left LTP | — | ORIF × 2 | |
| 2 | F | 33 | Dowel | Left LFC | 25 mm | Microfracture | |
| 3 | F | 31 | Dowel | Right MFC | 34 × 24 mm | Microfracture; HTO | |
| 4 | M | 41 | Snowman | Left LFC | 25 mm and 20 mm | Graft failure | Arthroscopic joint debridement |
| 5 | F | 19 | Dowel | Right LFC | 25 mm | ||
| 6 | F | 26 | Dowel | Left MFC | 30 mm | ||
| 7 | M | 29 | Snowman | Right LFC | 25 mm and 25 mm | Graft failure | Microfracture |
| 8 | M | 45 | Bulk | Right MFC | — | ORIF; HTO | |
| 9 | M | 27 | Dowel | Right MFC | 25 mm | DVT and PE | Arthroscopic joint debridement |
| 10 | F | 17 | Bulk | Left LTP | — | ACL reconstruction | |
| 11 | M | 36 | Snowman | Left MFC | 25 mm and 30 mm | Arthroscopy × 2; ACL reconstruction | |
| 12 | M | 39 | Dowel | Left LFC | 20 mm | Microfracture | |
| 13 | M | 36 | Dowel | Right LFC | 30 mm | ORIF; HTO | |
| 14 | F | 42 | Dowel | Left MFC | 20 mm | ||
| 15 | M | 22 | Snowman | Right MFC | 25 mm and 20 mm | Microfracture | |
| 16 | F | 27 | Snowman | Right LFC | 25 mm and 25 mm | Arthroscopic partial menisectomy; HTO | |
| 17 | F | 52 | Bulk | Left LTP | — | ||
| 18 | M | 45 | Dowel | Right proximal humerus | 30 mm | Arthroscopic labral repair | |
| 19 | M | 29 | Dowel | Left proximal humerus | 35 mm | ||
| 20 | F | 24 | Dowel | Left proximal humerus | 35 mm | Arthroscopic joint debridement |
LTP = lateral tibial plateau; LFC = lateral femoral condyle; MFC = medial femoral condyle; DVT = deep vein thrombosis; PE = pulmonary embolism; ORIF = open reduction internal fixation; HTO = high tibial osteotomy; ACL = anterior cruciate ligament.
Table 2.
Summary of Donor Cadaveric Tissue Used for Osteochondral Allograft Transplantation with Associated Viability Results When the Tissue Was First Harvested (% Viability Initial) and Just Prior to Transplantation (% Viability Second Biopsy) after the Tissue Had Been Stored for a Period of Time.
| Donor | Patient Number | % Viability (Initial) | % Viability (2nd Biopsy) | Time Interval (Days) | Comments |
|---|---|---|---|---|---|
| 1 | 1 | >95 | >95 | 7 | Transplanted |
| 2 | >95 | 9 | Transplanted | ||
| 2 | 3 | >95 | >95 | 12 | Transplanted |
| 4 | >95 | 13 | Transplanted | ||
| 3 | 5 | >95 | >90 | 18 | Transplanted |
| 4 | 6 | >90 | 90 | 7 | Transplanted |
| 5 | <75 | — | 21 | Poor initial viability; not transplanted | |
| 6 | 7 | >95 | >90 | 7 | Transplanted (single donor 6) |
| 8 | >90 | 9 | Transplanted (single donor 6) | ||
| 9 | >90 | 11 | Transplanted (single donor 6) | ||
| 7 | 10 | >95 | >90 | 17 | Transplanted |
| 8 | >85 | — | 5 | Poor tissue histology; not transplanted | |
| 9 | 11 | >95 | >90 | 13 | Transplanted |
| 10 | 12 | > 95 | >90 | 7 | Transplanted (single donor 10) |
| 13 | >90 | 11 | Transplanted (single donor 10) | ||
| 14 | >85 | 12 | Transplanted (single donor 10) | ||
| 15 | >85 | 14 | Transplanted (single donor 10) | ||
| 11 | 16 | >95 | >90 | 14 | Transplanted |
| 12 | 17 | >95 | >90 | 3 | Transplanted |
| 13 | 18 | >90 | >90 | 10 | Transplanted |
| 19 | >90 | 13 | Transplanted | ||
| 14 | 20 | >95 | >95 | 6 | Transplanted |
Western Ontario and McMaster Universities Arthritis Index Results
All patients who had received OAT in the knee completed the WOMAC (n = 17). There were statistically and clinically significant improvements in overall WOMAC score, as well as improvements in the WOMAC function, WOMAC pain, and WOMAC stiffness subcategories ( Table 3 ). There was a statistically and clinically significant improvement in the overall WOMAC score when comparing preoperative scores at both 1- and 2-year postoperative time points. Analysis of WOMAC subcategories revealed a statistically and clinically significant improvement when comparing preoperative function after 1 year postoperative and a clinical and significant improvement at 2 years postoperative. There was no improvement in WOMAC stiffness at 1 year postoperative, but there was a significant improvement in stiffness at 2 years postoperative. There was also a statistically significant improvement in WOMAC pain at both 1 and 2 years postoperative compared with preoperative scores.
Table 3.
Western Ontario and McMaster University (WOMAC) scores and Western Ontario Shoulder Instability (WOSI) Scores for All Patients Undergoing Osteochondral Allograft Transplantation in the Knee.a
| Preoperative | 1-Year Postoperative | P | 2 Years Postoperative | P | |
|---|---|---|---|---|---|
| WOMAC Overall | 65.1 ± 24.8 | 87.6 ± 11.0 | 0.002* | 82.3 ± 19.9 | 0.02* |
| WOMAC Pain | 91.0 ± 5.7 | 97.0 ± 2.6 | 0.001* | 96.1 ± 4.6 | 0.002* |
| WOMAC Stiffness | 83.2 ± 15.3 | 90.0 ± 7.3 | 0.09 | 93.1 ± 6.8 | 0.04* |
| WOMAC Function | 68.1 ± 23.8 | 89.7 ± 11.5 | 0.001* | 83.1 ± 20.4 | 0.03* |
| WOSI Overall | 36.8% | 9.0% | — | 17.2% | — |
| WOSI Sports/Recreation/Work | 41.0% | 7.5% | — | 13.1% | — |
| WOSI Lifestyle | 37.0% | 9.0% | — | 16.8% | — |
| WOSI Emotions | 54.3% | 10.3% | — | 29.7% | — |
Patients who received transplants in knee completed the WOMAC score (n = 17) while the patients who received transplants in the shoulder completed the WOSI (n = 3). Preoperative values comparing 1- and 2-year postoperative scores.
Indicates significance with P < 0.05.
Knee Injury and Osteoarthritis Outcome Score Results
The patients who received OAT in the knee also completed the KOOS (n = 17). Analysis of the KOOS data revealed statistically significant improvements in all modalities measured, including pain, knee-related symptoms, activities of daily living, sports and recreation, and quality of life ( Table 4 ). There was a statistically significant improvement in pain, knee-related symptoms, activities of daily living, sports and recreation scores, and quality of life scores at both 1 and 2 years postoperative compared with preoperative pain scores.
Table 4.
Knee Injury and Osteoarthritis Outcome Score (KOOS) Scores of Patients Undergoing Osteochondral Allograft Transplantation in the Knee at 1 and 2 Years Postoperative.a
| Preoperative | 1-Year Postoperative | P | 2-Year Postoperative | P | |
|---|---|---|---|---|---|
| Pain | 52.5 ± 24.3 | 82.6 ± 11.7 | 0.001* | 79.0 ± 20.53* | <0.001* |
| Symptoms | 52.1 ± 21.3 | 72.4 ± 16.4 | 0.002* | 70.8 ± 22.3* | 0.01* |
| Activities of Daily Living | 68.5 ± 24.3 | 90.3 ± 10.6 | 0.002* | 84.8 ± 19.9* | 0.004* |
| Sports and Recreation | 21.2 ± 22.0 | 52.7 ± 27.7 | 0.007* | 54.4 ± 31.9* | 0.002* |
| Quality of Life | 20.6 ± 15.4 | 50.4 ± 21.1 | 0.001* | 51.2 ± 31.1* | 0.001* |
Significance indicated by an asterisk comparing 1- and 2-year postoperative scores with preoperative scores with P < 0.05 (n = 17).
Short Form–36 Results
All patients who underwent OAT in either the glenohumeral joint or the knee completed the SF-36 (n = 20). Overall, there were statistically significant improvements in physical functioning, physical role, bodily pain, and social functioning at both 1- and 2-year postoperative as analyzed by the SF-36 ( Table 5 ). There were no significant improvements in general health, vitality, emotional role, or mental health.
Table 5.
Short Form–36 (SF-36) Scores of All Patients Undergoing Osteochondral Allograft Transplantation at 1 and 2 Years Postoperative.a
| Preoperative | 1 Year Postoperative | P | 2-Year Postoperative | P | |
|---|---|---|---|---|---|
| Physical Functioning | 49.3 ± 27.5 | 72.2 ± 23.2 | 0.001* | 72.9 ± 26.5 | 0.003* |
| Role Physical | 43.8 ± 46.5 | 75.0 ± 36.5 | 0.006* | 72.4 ± 44.8 | 0.03* |
| Bodily Pain | 35.9 ± 24.1 | 68.4 ± 17.0 | 0.001* | 63.4 ± 26.1 | 0.001* |
| General Health | 72.9 ± 25.9 | 78.8 ± 22.3 | 0.17 | 74.4 ± 25.1 | 0.6 |
| Vitality | 61.8 ± 21.2 | 67.5 ± 20.7 | 0.09 | 67.4 ± 19.3 | 0.08 |
| Social Functioning | 65.6 ± 30.0 | 79.7 ± 27.7 | 0.04* | 82.2 ± 24.4 | 0.03* |
| Role Emotional | 83.3 ± 31.5 | 83.3 ± 32.2 | 0.35 | 86.0 ± 30.1 | 0.95 |
| Mental Health | 75.4 ± 19.7 | 76.0 ± 22.2 | 0.49 | 79.6 ± 16.8 | 0.1 |
There was a statistically significant improvement (indicated by asterisk) in physical functioning, role physical, bodily pain, and social functioning at 1- and 2-year postoperative scores when compared with preoperative scores with P < 0.05 (n = 20).
Visual Analog Scale Results
All patients who underwent OAT in either the glenohumeral joint or the knee completed the VAS (n = 20). There was a statistically significant improvement in VAS score when comparing preoperative VAS scores (69.5 ± 17.0) with both 1-year postoperative (85.8 ± 7.5, P = 0.002) and 2-year postoperative scores (81.8 ± 14.0, P = 0.009).
Western Ontario Shoulder Instability Index Results
The patients who received OAT in the glenohumeral joint completed the WOSI ( Table 3 ). The sample size was too small for statistical comparison (n = 3). There were trends toward improved total WOSI score, as well as improvements in all subcategories, including Sports/Recreation/Work, Lifestyle, and Emotions at the 1-year postoperative time point. The total WOSI scores remained improved at the 2-year postoperative time point when compared with preoperative scores ( Table 3 ).
Complications
There was no evidence of infection in any of the transplants postoperatively. There were 2 failures (as defined by ongoing pain and debilitation) of the OATs in the 5 patients treated with double-dowel or “snowman” grafts of the lateral femoral condyle in the knee ( Fig. 2 ). Both had persistent pain and had MRIs that confirmed increased edema at the overlap of the 2 grafts. These 2 patients underwent a second arthroscopy and there was evidence of fragmentation at the interpositional interface of the 2 “snowman” grafts with early degeneration of the transplanted tissue. The articular cartilage had appeared to delaminate from the underlying collapsed bone. Joint incongruities, as defined by an articular step of greater than 2 mm, were present where the 2 dowel grafts overlapped. One patient sustained a deep vein thrombosis and pulmonary embolism postoperatively which was successfully treated with anticoagulation and did not significantly alter his postoperative rehabilitation.
Discussion
There have been many prospective randomized controlled trials comparing ACI and microfracture, ACI to mosaicplasty, microfracture to mosaicplasty, and studies comparing all 3 treatments.18-24 Despite a large number of studies, no consensus exists as to the optimal method of treating OCLs.1 Large case series of treating OCLs with OAT were initially published more than 3 decades ago and have produced encouraging results.6,7,25-27 The primary purpose of this study was to study the clinical outcomes of patients who received OAT using this specific tissue assessment protocol in patients with OCLs that have failed previous treatments in order to improve their joint function and reduce or eliminate pain.
The rapid fall in chondrocyte viability after in vitro culture of osteochondral allografts after 14 days severely limits opportunities to perform OAT and creates challenges in matching donor tissue to recipient joints.1 The protocol developed and reported here involved hypothermically storing the tissue in X-VIVO 10. This is the first application of X-VIVO 10 to successfully preserve human osteochondral tissue for transplantation. All 12 tissue samples that were deemed suitable for transplantation demonstrated greater than 85% cell viability at a maximum of 18 days of incubation ( Table 2 ). As described in Hunter et al.,12 human osteochondral allograft viability remained up to 90% at 6 weeks of culture. With this in mind, the tissue in this study was preserved for up to 18 days prior to transplantation. Graft storage with high viability permitted the flexibility of delivery of the procedure. Previous OAT protocols of transplanting the tissue within 48 to 72 hours often necessitated that the surgical team was capable of only using the donor tissue for a single recipient. By having a larger window to store the donor tissue, the surgical teams are also able to perform multiple surgeries on different patients to ensure that the donor tissue is distributed to the maximum number of recipients possible. For example, in this study, 4 patients benefited from transplants from a single donor over a time interval of 14 days ( Table 2 ). Despite the relative paucity of donors, the storage protocol using X VIVO-10 with high viability up to 18 days in culture allowed multiple patients to benefit from a single tissue donor. There are limited studies that use prolonged storage methods before performing OAT and no published protocols that ensure high-quality tissue by performing pretransplantation histology and viability assays. One prospective study by McCulloch et al.11 stored grafts for up to 43 days before transplantation in Ringer’s lactate but no pretransplantation viability was performed. A comprehensive analysis of OAT outcomes by Davidson et al.9 also used prolonged storage with a mean graft storage time of 36 days. Despite these prolonged storage times, it has been established that chondrocyte viability and cell density decrease dramatically after 28 days of storage at 4°C, falling below 70%.28 Although the grafts in this study were stored hypothermically, there is evidence that storage of osteochondral allograft tissue in serum-free media at 37°C results in better tissue quality and viability.29 Hypothermic storage of tissue was chosen in this study because of previous experience with hypothermic storage and established tissue harvesting protocols.
In addition to cell viability, the histology of the tissue was analyzed to ensure normal chondrocyte phenotype and distribution throughout the tissue. Tissue was rejected for transplantation if the architecture of the cartilage did not appear normal or there was question about tissue quality or cell morphology, despite the tissue demonstrating high cell viability ( Fig. 3 ). Ensuring high chondrocyte viability has been proven to be essential for long-term graft survival.6 Despite careful donor tissue selection and a standard tissue harvesting protocol, 2 of the 14 tissue donors demonstrated tissue that was unsuitable for transplantation. The effects of transplantation of poor-quality tissue or tissue with low chondrocyte viability is not known, but assessment of tissue quality could be considered as part of allograft storage protocols. This is the first study to our knowledge that has ensured transplantation of high-quality tissue by assessing chondrocyte viability, density, and distribution in the extracellular matrix prior to transplantation of tissue.
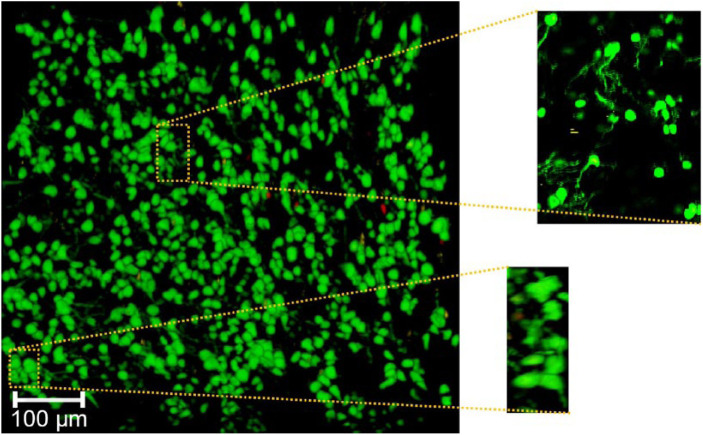
Figure 3.
Syto 13 viability stain of cartilage tissue sample at 100× magnification demonstrating high tissue viability (as demonstrated by the predominance of cells staining green) and associated cell membrane abnormalities. The Syto 13 is leaking out of the cell membranes (as demonstrated in the expanded windows) indicating that although the cells are viable, there are abnormalities associated with their cell membranes since they cannot contain the dye (For interpretation of the references to colours in this figure legend, refer to the online version of this article).
In addition to ensuring transplanted tissue had high pretransplantation cell viability, appropriate cell density and normal tissue architecture, we used multiple clinical outcome scores to compare our results with previously published OAT outcomes. The WOMAC, KOOS, and SF-36 outcomes are evidence that performing OAT using the novel application of X-VIVO 10 produces results similar to previously published outcomes for OAT.9,11 There was an overall improvement in pain, stiffness, and functioning as measured by the WOMAC at both 1 and 2 years. There was statistically significant improvement in pain, but this was not clinically significant.
The KOOS results complement the results from the WOMAC, which demonstrated improvements in pain, quality of life, participation in sports and recreation, activities of daily living, and knee-related symptoms. Previous published data on the KOOS found the minimal significant level of change for patients with knee injuries to be from 5 to 12 points, depending on the specific category.30 Our data demonstrate greater than 20-point improvements in all subcategories analyzed by the KOOS, suggesting drastic clinical improvements in addition to simply gaining statistical significance. McCulloch et al.11 studied the outcomes of 25 patients who underwent osteochondral allograft transplantation with a mean follow-up of 35 months after prolonged storage in Ringer’s lactate. They also demonstrated improvements in KOOS pain, activities of daily livings, quality of life, sports and recreation, and symptoms with similar increases in magnitude and postoperative scores.11
SF-36 outcomes further supported the KOOS and WOMAC data indicating these patients had an improvement in bodily pain, physical functioning, social functioning, and role physical categories.
In this study, graft failures were defined by patients who did not subjectively improve compared to their preoperative level of pain and/or functioning and had ongoing symptoms. Using these clinical criteria, 2 patients had additional work-ups that included posttransplant arthroscopy, 1 at 8 months posttransplantation and 1 at 3 years posttransplantation. These arthroscopies revealed graft failures in both patients in the knee. Each of these patients had a lesion that was larger than the cylindrical dowels in the Arthrex OAT system (35 mm) and therefore 2 overlapping dowels were required ( Fig. 2d ). Graft incorporation into the surrounding recipient bone depends on a stable interface between donor and host bone and a robust blood supply from the surrounding cancellous bone so the host bone can replace the graft by creeping substitution.6 When multiple grafts after overlapped, it is possible that the overlapping area is relatively hypovascular and this prevents or slows the subchondral bone from incorporating. Gross et al.6 demonstrated the histological findings of failed OAT included mechanical graft instability resulting in non-union and failure of graft incorporation. The amount of graft overlap in the 2 patients in this series was minimal and these grafts were stable after press fitting. The arthroscopies performed at 8 months and 3 years postoperatively to assess causes for ongoing pain did not demonstrate signs of graft instability, but rather cartilage delamination consistent with a lack of healing between the 2 grafts. An additional possible contributing factor to 1 of the 2 graft failures was postoperative activity. One of the patients participated in high-level mogul skiing and returned to his previous level of activity at 1 year postoperative. It was thought that this high-impact activity in conjunction with the delayed incorporation of the graft resulted in early failure. The time to integration of the graft bone to the surrounding cancellous bone bed has not been determined and it is likely that patients undergoing OAT should be advised to avoid high-impact activity for indefinitely after undergoing surgery to allow for complete incorporation of the underlying subchondral bone and to avoid excessive load transfer through the joint. Despite these 2 failures, there were three successful snowman dowel transplantation. Continued research will focus on the importance of lesion location and relative size in order to recognize optimal treatment strategies.
The perceived limitation of the heterogeneity of the type and location of grafts transplanted is rectified by the significant improvement in clinical outcomes for the majority of patients despite the variety of lesion size and location. The project was initially designed to focus on dowel transplants in medial femoral condyles, but as the list of suitable patients grew, it was realized patients with symptomatic MFC lesions were a small group of patients with OCLs. The indications expanded to include the lateral femoral condyle, tibial plateau, and proximal humerus. The number of transplants reported here is too low to allow for meaningful statistical subgroup analysis at this point in time.
We did not perform viability analysis at set time points to determine the viability of chondrocytes maintained in X-VIVO 10 over time. It should be noted that the tissue stored for the longest amount of time (donor 5) also had the lowest preimplantation viability ( Table 2 ). Further research is necessary to determine how X-VIVO 10 compares with previously established tissue culture media in terms of viability with regard to time. We also acknowledge the exclusion of 2 out of the 14 donors due to either low initial cell viability or abnormal tissue architecture. The effects of these factors on clinical outcomes is unknown and by eliminating the use of this tissue, it further decreased the amount of available tissue for transplantation in a procedure that is already limited in terms of donor supply.
There was no control group to which the treatment group was compared. Besides a case series of primary OAT published by Briggs et al.,31 patients considered candidates for OAT are those who have failed other methods of cartilage restoration and as a result, this “salvage” philosophy is one of the reasons that direct comparison of OAT with these methods.
In conclusion, osteochondral allograft transplantation in the shoulder and knee joints, as performed at our institute, results in good to excellent clinical outcomes with the majority of patients receiving symptomatic relief from traumatic OCLs. The protocol employed here involves performing osteochondral graft architectural analysis to ensure adequate chondrocyte density, viability, and tissue architecture prior to implantation. The careful pre-operative radiographic analysis, including MRI analysis of donor and recipient joints has allowed for custom size and shape matching of donor grafts to recipients. The clinical significance of the protocol described in this article, including ensuring high-quality tissue prior to transplantation and the donor-recipient size matching, has yet to be determined. Careful histological analysis of graft failures is an important future direction that could be employed to help determine the cause of graft failure.
Footnotes
Acknowledgments and Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by the Calgary Health Trust.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval: This research was reviewed and approved by the Conjoint Health Research Ethics Board (University of Calgary) REB15-0132.
Informed Consent: Written consent was obtained from each patient prior to enrollment in the study.
Trial Registration: Not applicable.
References
- 1. Bedi A, Feeley BT, Williams RJ., 3rd Management of articular cartilage defects of the knee. J Bone Joint Surg Am. 2010;92(4):994-1009. [DOI] [PubMed] [Google Scholar]
- 2. Messner K, Maletius W. The long-term prognosis for severe damage to weight-bearing cartilage in the knee: a 14-year clinical and radiographic follow-up in 28 young athletes. Acta Orthop Scand. 1996;67(2):165-8. [DOI] [PubMed] [Google Scholar]
- 3. Widuchowski W, Widuchowski J, Trzaska T. Articular cartilage defects: study of 25 124 knee arthroscopies. Knee. 2007;14(3):177-82. [DOI] [PubMed] [Google Scholar]
- 4. Buckwalter JA, Mankin HJ. Articular cartilage: degeneration and osteoarthritis, repair, regeneration, and transplantation. Instr Course Lect. 1998;47:487-504. [PubMed] [Google Scholar]
- 5. Farr J, Cole B, Dhawan A, Kercher J, Sherman S. Clinical cartilage restoration: evolution and overview. Clin Orthop Relat Res. 2011;469(10):2696-705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gross AE, Kim W, Las Heras F, Backstein D, Safir O, Pritzker KP. Fresh osteochondral allografts for posttraumatic knee defects: long-term followup. Clin Orthop Relat Res. 2008;466(8):1863-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Beaver RJ, Mahomed M, Backstein D, Davis A, Zukor DJ, Gross AE. Fresh osteochondral allografts for post-traumatic defects in the knee. A survivorship analysis. J Bone Joint Surg Br. 1992;74(1):105-10. [DOI] [PubMed] [Google Scholar]
- 8. Chahal J, Gross AE, Gross C, et al. Outcomes of osteochondral allograft transplantation in the knee. Arthroscopy. 2013;29(3):575-88. [DOI] [PubMed] [Google Scholar]
- 9. Davidson PA, Rivenburgh DW, Dawson PE, Rozin R. Clinical, histologic, and radiographic outcomes of distal femoral resurfacing with hypothermically stored osteoarticular allografts. Am J Sports Med. 2007;35(7):1082-90. [DOI] [PubMed] [Google Scholar]
- 10. LaPrade RF, Botker J, Herzog M, Agel J. Refrigerated osteoarticular allografts to treat articular cartilage defects of the femoral condyles. A prospective outcomes study. J Bone Joint Surg Am. 2009;91(4):805-11. [DOI] [PubMed] [Google Scholar]
- 11. McCulloch PC, Kang RW, Sobhy MH, Hayden JK, Cole BJ. Prospective evaluation of prolonged fresh osteochondral allograft transplantation of the femoral condyle: minimum 2-year follow-up. Am J Sports Med. 2007;35(3):411-20. [DOI] [PubMed] [Google Scholar]
- 12. Hunter S, Timmermann S, Schachar N, Muldrew K. The effects of hypothermic storage on chondrocyte survival and apoptosis in human articular cartilage. Cell Preservation Technol. 2006;4(2):82-90. [Google Scholar]
- 13. Ware JE, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30(6):473-83. [PubMed] [Google Scholar]
- 14. Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988;15(12):1833-40. [PubMed] [Google Scholar]
- 15. Kirkley A, Griffin S, McLintock H, Ng L. The development and evaluation of a disease-specific quality of life measurement tool for shoulder instability. The Western Ontario Shoulder Instability Index (WOSI). Am J Sports Med. 1998;26(6):764-72. [DOI] [PubMed] [Google Scholar]
- 16. Downie WW, Leatham PA, Rhind VM, Wright V, Branco JA, Anderson JA. Studies with pain rating scales. Ann Rheum Dis. 1978;37(4):378-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Roos EM, Roos HP, Lohmander LS, Ekdahl C, Beynnon BD. Knee Injury and Osteoarthritis Outcome Score (KOOS)—development of a self-administered outcome measure. J Orthop Sports Phys Ther. 1998;28(2):88-96. [DOI] [PubMed] [Google Scholar]
- 18. Gudas R, Gudaite A, Pocius A, Gudiene A, Cekanauskas E, Monastyreckiene E, et al. Ten-year follow-up of a prospective, randomized clinical study of mosaic osteochondral autologous transplantation versus microfracture for the treatment of osteochondral defects in the knee joint of athletes. Am J Sports Med. 2012;40(11):2499-508. [DOI] [PubMed] [Google Scholar]
- 19. Bentley G, Biant LC, Vijayan S, Macmull S, Skinner JA, Carrington RW. Minimum ten-year results of a prospective randomised study of autologous chondrocyte implantation versus mosaicplasty for symptomatic articular cartilage lesions of the knee. J Bone Joint Surg Br. 2012;94(4):504-9. [DOI] [PubMed] [Google Scholar]
- 20. Knutsen G, Drogset JO, Engebretsen L, Grøntvedt T, Isaksen V, Ludvigsen TC, et al. A randomized trial comparing autologous chondrocyte implantation with microfracture. Findings at five years. J Bone Joint Surg Am. 2007;89(10):2105-12. [DOI] [PubMed] [Google Scholar]
- 21. Knutsen G, Engebretsen L, Ludvigsen TC, Drogset JO, Grøntvedt T, Solheim E, et al. Autologous chondrocyte implantation compared with microfracture in the knee. A randomized trial. J Bone Joint Surg Am. 2004;86-A(3):455-64. [DOI] [PubMed] [Google Scholar]
- 22. Saris DB, Vanlauwe J, Victor J, Almqvist KF, Verdonk R, Bellemans J, et al. ; TIG/ACT/01/2000EXT Study Group. Treatment of symptomatic cartilage defects of the knee: characterized chondrocyte implantation results in better clinical outcome at 36 months in a randomized trial compared to microfracture. Am J Sports Med. 2009;37(Suppl 1):10S-19S. [DOI] [PubMed] [Google Scholar]
- 23. Basad E, Ishaque B, Bachmann G, Sturz H, Steinmeyer J. Matrix-induced autologous chondrocyte implantation versus microfracture in the treatment of cartilage defects of the knee: a 2-year randomised study. Knee Surg Sports Traumatol Arthrosc. 2010;18(4):519-27. [DOI] [PubMed] [Google Scholar]
- 24. Dozin B, Malpeli M, Cancedda R, Bruzzi P, Calcagno S, Molfetta L, et al. Comparative evaluation of autologous chondrocyte implantation and mosaicplasty: a multicentered randomized clinical trial. Clin J Sport Med. 2005;15(4):220-6. [DOI] [PubMed] [Google Scholar]
- 25. Ghazavi MT, Pritzker KP, Davis AM, Gross AE. Fresh osteochondral allografts for post-traumatic osteochondral defects of the knee. J Bone Joint Surgery Br. 1997;79(6):1008-13. [DOI] [PubMed] [Google Scholar]
- 26. Levy YD, Gortz S, Pulido PA, McCauley JC, Bugbee WD. Do fresh osteochondral allografts successfully treat femoral condyle lesions? Clin Orthop Relat Res. 2013;471(1):231-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mahomed MN, Beaver RJ, Gross AE. The long-term success of fresh, small fragment osteochondral allografts used for intraarticular post-traumatic defects in the knee joint. Orthopedics. 1992;15(10):1191-9. [DOI] [PubMed] [Google Scholar]
- 28. Williams SK, Amiel D, Ball ST, Allen RT, Wong VW, Chen AC, et al. Prolonged storage effects on the articular cartilage of fresh human osteochondral allografts. J Bone Joint Surg Am. 2003;85-A(11):2111-20. [DOI] [PubMed] [Google Scholar]
- 29. Garrity JT, Stoker AM, Sims HJ, Cook JL. Improved osteochondral allograft preservation using serum-free media at body temperature. Am J Sports Med. 2012;40(11):2542-8. [DOI] [PubMed] [Google Scholar]
- 30. Collins NJ, Misra D, Felson DT, Crossley KM, Roos EM. Measures of knee function: International Knee Documentation Committee (IKDC) Subjective Knee Evaluation Form, Knee Injury and Osteoarthritis Outcome Score (KOOS), Knee Injury and Osteoarthritis Outcome Score Physical Function Short Form (KOOS-PS), Knee Outcome Survey Activities of Daily Living Scale (KOS-ADL), Lysholm Knee Scoring Scale, Oxford Knee Score (OKS), Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC), Activity Rating Scale (ARS), and Tegner Activity Score (TAS). Arthritis Care Res (Hoboken). 2011;63(suppl 11):S208-S228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Briggs DT, Sadr KN, Pulido PA, Bugbee WD. The use of osteochondral allograft transplantation for primary treatment of cartilage lesions in the knee. Cartilage. 2015;6(4):203-7. [DOI] [PMC free article] [PubMed] [Google Scholar]