Abstract
Extra-adrenal paragangliomas are rare tumors arising from the chromaffin cells of the autonomic nervous system. Retroperitoneal paragangliomas may present as a pancreatic mass. We present a case of a 61-year-old woman with an incidentally found pancreatic mass (7.2 × 6.5 cm) in the CT scan. EUS- guided FNA result was compatible with pancreatic neuroendocrine tumor. Patient underwent pancreaticoduodenectomy and histopathologic assessment revealed the mass was an extra-adrenal paraganglioma. Preoperative diagnosis of pancreatic paragangliomas can be challenging due to imaging and histopathologic similarities with pancreatic neuroendocrine tumors.
Keywords: Pancreatic paraganglioma, pancreatic neuroendocrine tumor, pancreaticoduodenectomy
Introduction
Paragangliomas (PGL) are rare tumors, with annual incidence of 2–8 per 1 million.1 Extra-adrenal PGL originate from chromaffin cells of the autonomic nervous system. Sympathetic PGL arise from the sympathetic chain in the thorax or retroperitoneum; in contrast, parasympathetic PGL are found in the head and neck in association with cranial nerves.2 Retroperitoneal PGL located either in close proximity to the pancreas or primarily arising within the pancreas are even more rare, with fewer than 30 cases reported in the literature.3 Equivocal presenting symptoms and radiologic findings along with its rarity render these tumors a diagnostic challenge. Most of the cases of peripancreatic PGL, as in the present patient, were diagnosed preoperatively as pancreatic neuroendocrine tumors (PNET).4,5
Case description
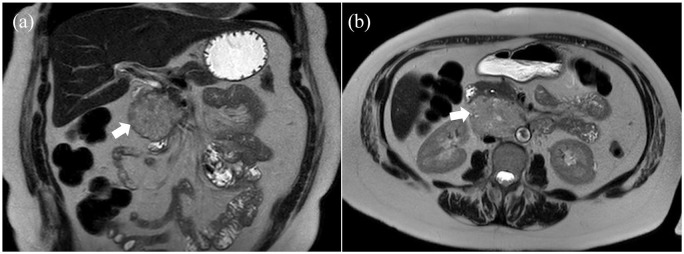
A 61-year old woman was referred for management of a retroperitoneal mass identified on a computed tomography (CT) scan performed as part of the trauma workup after she fell from a ladder. The patient did not have symptoms of abdominal pain or discomfort, fever, nausea, vomiting or change in bowel habit. She did, however, report 3.5 kg of unintentional weight loss over the prior month. Her past medical history was significant for rheumatoid arthritis and chronic obstructive pulmonary disease. She did not have hypertension, palpitation or sweating. On physical examination, her abdomen was soft, nontender and nondistended. No palpable mass was noted. The abdominopelvic MRI showed a mildly T2 hyperintense mass with arterial enhancement measuring 7.2 × 6.5cm, within the pancreatic head and uncinate process (Figure 1).
Figure 1.
Abdominal MRI with contrast shows a mass in pancreatic head and uncinate: (a) coronal and (b) axial views.
The mass abuted the aortocaval region and duodenum. Also, multiple small T2 hyperintense nonenhancing nodules were seen in the spleen. Endoscopic ultrasound (EUS) confirmed a well-defined mass measuring 6.4 × 4.8 cm in the pancreatic head. The pancreatic duct was not dilated (3 mm). EUS-guided fine needle aspiration (FNA) showed neoplastic cells with enlarged nuclei, prominent nucleoli, smooth nuclear contours and moderate amount of cytoplasm. IHC was positive for chromogranin A and synaptophysin. These pathological characteristics were compatible with pancreatic neuroendocrine tumor. Interestingly, during the EUS, the patient experienced an episode of severe hypertension (230/130 mmHg) for 15 minutes that resolved after the patient was intubated and placed under general anesthesia. Laboratory tests including complete blood count, liver function tests, blood glucose, and creatinine were within normal range. Due to the minimal clinical suspicion of pancreatic ductal adenocarcinoma, tumor markers including CA 19-9 were not measured.
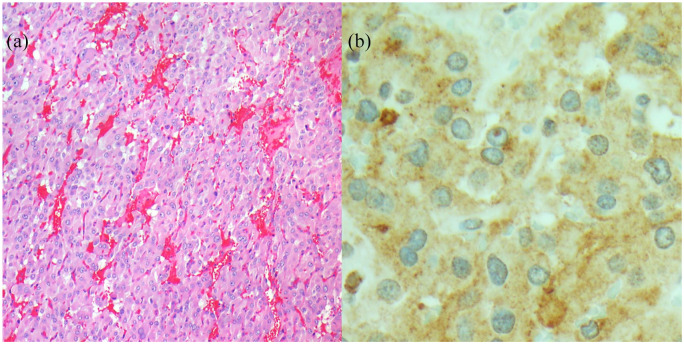
The patient underwent pancreaticoduodenectomy (Whipple procedure). Intraoperatively, the large retroperitoneal mass with dense adhesions to the aorta and pancreatic head was noted and required extensive dissection. Unexpected severe hypertension with systolic pressure up to 250 mmHg with tumor manipulation was followed by hypotension after tumor resection. The mass was located in the para-aortic region with adhesions to the aorta and pancreatic head. Although radiologically the tumor seemed to be intra-pancreatic, during the operation, we found that it was primarily a retroperitoneal mass with extensive adhesions to the pancreatic head, but not definitively involving the pancreatic parenchyma. This finding, along with the initial hypertensive and later hypotensive episodes made PGL the likely diagnosis. Pathologic assessment of the specimen, postoperatively, revealed a 6.5 cm mass adherent to the pancreatic surface but not grossly invading the pancreatic parenchyma (Figure 2).
Figure 2.
Microscopic images of the mass: (a) 100X, H&E section; Nested architecture of enlarged epithelioid cells surrounded by a delicate capillary network and (b) 400X; Immunohistochemistry staining for chromogranin A showing granular cytoplasmic staining.
Immunohistochemical staining was positive for S100, chromogranin A, synaptophysin and GATA3. These findings were consistent with an extra-adrenal paraganglioma.
Following surgery, the patient was managed according to our enhanced recovery after surgery (ERAS) pathway6 and was discharged on postoperative day 5 without any complications. Serum metanephrine level was measured postoperatively as baseline for follow up. In one-year follow-up, the patient is in good overall health, CT scan showed no recurrence and serum metanephrine level was in the normal range.
Discussion
Paraganglionic tissue is composed of neuroepithelial cells, derived from the neural crest.7 Instead of transforming into neurons, these cells secret epinephrine and norepinephrine in to the blood. Pheochromocytomas arise from the paraganglionic tissue within the adrenal medulla. PGL are tumors of the autonomic nervous system.
Sympathetic PGL mostly present as retroperitoneal masses or because of symptoms. If functional, patients may experience symptoms of catecholamine excess, including headache, sweating, and palpitation. Nonfunctional tumors may either manifest with mass effect symptoms such as abdominal pain or discomfort due to abdominal mass, or they may be incidental findings during work up for other indications such as trauma, as in our patient. It is unclear, whether intrapancreatic PGL are primary tumors originating from ectopic paraganglionic tissue within the pancreas or represent infiltration of retroperitoneal tumors in to the pancreatic parenchyma.8,9 Rarity of this type of tumor further hampers delineating its pathophysiological origins. As PGL have a tendency to adhere to adjacent organs, in peripancreatic cases, it may invade the pancreatic parenchyma; hence, typical pancreatic masses are often the first differential diagnosis based on the preoperative imaging. Although our patient had only one episode of hypertensive crisis during EUS, in the presence of CT finding of pancreatic head mass and FNA proving pancreatic neuroendocrine tumor, our clinical suspicion was guided away from PGL until intraoperative blood pressure fluctuations were noted and it became clear after resection that the tumor was not arising from the pancreas. Therefore, we did not measure 24-h urinary norepinephrine excretion prior to the operation. Previously, in a case series, it was shown that one in six FNAs misdiagnosed the PGL as a PNET and discussed that the similar histopathologic characteristics of these tumors make pathologic diagnosis challenging.10
Although prior case reports have suggested that the lack of biliary and pancreatic duct obstruction benefits the diagnosis of PGL, this is also true in most PNETs.9 Regarding the specific radiographic features of the PGL, one study found that four of eight evaluable cases in the literature demonstrated ample draining vessels around the tumor in dynamic CT images.4 Another study proposed that signal changes manifesting between arterial and venous phases in contrast-enhanced MRI (washout pattern) is helpful in distinguishing PGL.3 However, due to the rarity of this disease, specificity and sensitivity of these findings cannot be determined.
Ultimately, taking into consideration the difficulty of preoperative diagnosis with no pathognomonic features, a definite diagnosis is only achievable with a histopathologic examination of the resected tumor. Chromaffin (chief) cells form small nests that are surrounded by sustentacular cells, giving rise to the characteristic Zellballen pattern of PGL.11 By immunohistochemistry, the sustentacular cells are positive for S100 protein, while the chromaffin cells show reactivity to synaptophysin and chromogranin A.10
PGL has the potential of becoming malignant through metastasis or recurrence.1 Hence, surgical resection is considered the only definitive treatment.
Conclusion
Since the pancreatic paraganglioma can present as a pancreatic neuroendocrine tumor, this diagnosis should be considered as one of the differential diagnosis of a pancreatic mass.
Lessons learned
Extra-adrenal paragangliomas can rarely present as a pancreatic mass.
The diagnosis may be challenging since it can mimic a neuroendocrine tumor.Pathology including immunohistochemistry of the resected mass provides the definitive diagnosis.
Footnotes
Contributorship: Arezou Abbasi wrote the first draft of the manuscript. Kristina M Wakeman provided the pathological information and figures. Venu G Pillarisetty conceived of the manuscript, assisted with writing, and edited it. All authors reviewed and edited the manuscript and approved the final version of the manuscript.
Declaration of conflicting interest: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethical approval (include full name of committee approving the research and if available mention reference number of that approval): University of Washington Medical Centre does not require ethical approval for reporting individual cases.
Informed Consent: Written informed consent was obtained from the patient for anonymised information to be published in this article.
ORCID iD: Arezou Abbasi  https://orcid.org/0000-0001-8337-9332
https://orcid.org/0000-0001-8337-9332
References
- 1. Zhang L, Liao Q, Hu Y, et al. Paraganglioma of the pancreas: a potentially functional and malignant tumor. World J Surg Oncol 2014; 12(1): 218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Turchini J, Cheung VK, Tischler AS, et al. Pathology and genetics of phaeochromocytoma and paraganglioma. Histopathology 2018; 72(1): 97–105. [DOI] [PubMed] [Google Scholar]
- 3. Liang W, Xu S. CT and MR Imaging findings of pancreatic paragangliomas: a case report. Medicine 2016; 95(9): e2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Misumi Y, Fujisawa T, Hashimoto H, et al. Pancreatic paraganglioma with draining vessels. World J Gastroenterol 2015; 21(31): 9442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nguyen E, Nakasaki M, Lee TK, et al. Diagnosis of paraganglioma as a pancreatic mass: a case report. Diagn Cytopathol 2018; 46(9): 804–806. [DOI] [PubMed] [Google Scholar]
- 6. Daniel SK, Thornblade LW, Mann GN, et al. Standardization of perioperative care facilitates safe discharge by postoperative day five after pancreaticoduodenectomy. PLoS One 2018; 13(12): e0209608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lightfoot N, Santos P, Nikfarjam M. Paraganglioma mimicking a pancreatic neoplasm. JOP 2011; 12(3): 259–261. [PubMed] [Google Scholar]
- 8. Lin S, Peng L, Huang S, et al. Primary pancreatic paraganglioma: a case report and literature review. World J Surg Oncol 2015; 14(1): 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Meng L, Wang J, Fang S-H. Primary pancreatic paraganglioma: a report of two cases and literature review. World J Gastroenterol 2015; 21(3): 1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Singhi AD, Hruban RH, Fabre M, et al. Peripancreatic paraganglioma: a potential diagnostic challenge in cytopathology and surgical pathology. Am J Surg Pathol 2011; 35(10): 1498–1504. [DOI] [PubMed] [Google Scholar]
- 11. Zeng J, Simsir A, Oweity T, et al. Peripancreatic paraganglioma mimics pancreatic/gastrointestinal neuroendocrine tumor on fine needle aspiration: report of two cases and review of the literature. Diagn Cytopathol 2017; 45(10): 947–952. [DOI] [PubMed] [Google Scholar]