Abstract
Objectives
The purpose of this observational study was to evaluate the effects of radial artery access versus femoral artery access on the risk of 30-day mortality, inhospital bleeding and cardiogenic shock in patients with ST-elevation myocardial infarction undergoing primary percutaneous coronary intervention.
Methods
We used data from the SWEDEHEART registry and included all patients who were treated with primary percutaneous coronary intervention in Sweden between 2005 and 2016. We compared patients who had percutaneous coronary intervention by radial access versus femoral access with regard to the primary endpoint of all-cause death within 30 days, using a multilevel propensity score adjusted logistic regression which included hospital as a random effect.
Results
During the study period, 44,804 patients underwent primary percutaneous coronary intervention of whom 24,299 (54.2%) had radial access and 20,505 (45.8%) femoral access. There were 2487 (5.5%) deaths within 30 days, of which 920 (3.8%) occurred in the radial access and 1567 (7.6%) in the femoral access group. After propensity score adjustment, radial access was associated with a lower risk of death (adjusted odds ratio (OR) 0.70, 95% confidence interval (CI) 0.55–0.88, P = 0.025). We found no interaction between access site and age, gender and cardiogenic shock regarding 30-day mortality. Radial access was also associated with a lower adjusted risk of bleeding (adjusted OR 0.45, 95% CI 0.25–0.79, P = 0.006) and cardiogenic shock (adjusted OR 0.41, 95% CI 0.24–0.73, P = 0.002).
Conclusions
In patients with ST-elevation myocardial infarction, primary percutaneous coronary intervention by radial access rather than femoral access was associated with an adjusted lower risk of death, bleeding and cardiogenic shock. Our findings are consistent with, and add external validity to, recent randomised trials.
Keywords: ST-elevation myocardial infarction, primary PCI, arterial access site
Introduction
Primary percutaneous coronary intervention (PCI) by radial artery access (RA) rather than femoral artery access (FA) has been shown in recent multicentre randomised trials to reduce the risk of death and several other adverse clinical events among patients with ST-elevation myocardial infarction (STEMI).1–3 However, the patients who were included in these trials represent a selected population, and the external validity of the findings in these trials for an unselected cohort of patients who undergo coronary angiography due to STEMI has been questioned.4 Whereas the current European Society of Cardiology (ESC) guideline on the management of patients with STEMI has a strong recommendation for RA,5 the American STEMI guideline does not.6 This difference between the European and American guidelines is reflected in current clinical practice by the fact that RA has become the default strategy in many European countries,7–11 whereas FA remains the default strategy for many American operators. However, there are also large variations in the preferred access site across different operators and hospitals within European countries as well as within the USA.12,13
Our aim was to assess whether the benefits with RA in patients with STEMI undergoing PCI observed in recent randomised clinical trials are reproducible in a nationwide Swedish population of unselected STEMI patients.
Methods
Databases and patient selection
The Swedish Coronary Angiography and Angioplasty Registry (SCAAR) gathers data on all consecutive patients from all hospitals performing coronary angiography and PCI in Sweden. It was established in 1999 and is now part of the national SWEDEHEART (Swedish Web–System for Enhancement and Development of Evidence-Based Care in Heart Disease Evaluated According to Recommended Therapies) registry. The registry is sponsored solely by the Swedish health authorities and receives no commercial funding. The registry’s technology was developed and is administered by the Uppsala Clinical Research Centre. Since 2001, SCAAR has used a web-based case report platform with automatic data surveillance. In total, 30 hospitals in Sweden, including nine university hospitals, have cardiac catheterisation facilities. In SCAAR, a coronary angiographic procedure is described by about 50 variables and a PCI procedure by about 200 variables. After reviewing the clinical information, the PCI physician immediately enters clinical characteristics and procedural details into the registry. SCAAR obtains data on patients’ vital status continuously from the national death registry which, due to the use of mandatory personal identification numbers, has a very high degree of completeness, but it is not reviewed or adjudicated to establish the cause of death. The study population consisted of all patients who were treated by primary PCI for STEMI in Sweden between January 2005 and December 2016. Patients who underwent coronary angiography for presumed STEMI without following PCI (coronary artery bypass grafting (CABG) or no revascularisation) are not included. Patients were stratified according to whether they had RA or FA. Analysis was intention-to-treat (conversion from RA to FA was analysed as RA and vice versa).
Statistics
Adjustments for differences in baseline characteristics were made with the propensity score. The following variables were included in the calculation of the propensity score: age, gender, smoking habits, hypertension, diabetes, hyperlipidaemia, severity of coronary artery disease, previous infarction, previous PCI, previous CABG, anticoagulation therapy with glycoprotein IIb/IIIa receptor antagonists (GP IIb/IIIa), bivalirudin, P2Y12 antagonist, unfractionated heparin (UH)/low-molecular weight heparins (LMWHs), drug-eluting stents (DESs), completeness of revascularisation, number of stents, type of lesion, reperfusion time, pretreatment with P2Y12 antagonist, regular versus off-hours, calendar year, hospital and pharmacological treatment after discharge.
The estimated propensity score was then used for Kernel-based matching14 (based on Epanechnikov function and bandwidth of 0.06) in multilevel logistic regression which was the primary statistical model. The two groups were compared using multilevel logistic regression, with hospital as a random effect, to account for the hierarchical structure of the database. We imputed missing data with multiple imputation and the chain-equation method,15,16 with five datasets. We included an indicator of missingness, an event indicator and calendar year as regular variables,17 and imputed continuous variables by ordinary least-squares multiple regression, binary variables by logistic regression, and categorical variables by multinomial logistic regression. The imputation procedure and subsequent analyses were done according to Rubin’s protocol18 under the assumption that missing data are missing at random.
Our primary hypothesis was that RA in primary PCI reduces all-cause mortality. The primary endpoint was death at 30 days post PCI. Our secondary hypothesis was that RA in primary PCI reduces inhospital bleeding, stroke and cardiogenic shock (CS). Secondary endpoints were inhospital stroke, inhospital bleeding (defined as bleeding mandating transfusion or operation, cardiac tamponade, fall of haemoglobin more than 20 g/L, haematoma larger than 5 cm, compression time >6 hours, premature cessation of antithrombotic treatment due to bleeding, bleeding mandating other treatment than only local compression, prolongation of hospitalisation, or inctracranial bleeding) or CS (defined as Killip class IV). Patients who had CS at the time of admission to the coronary care unit were excluded from the statistical model in which CS was the outcome variable.
Results
Patient characteristics and treatments
We identified 53,146 patients who underwent primary PCI during the study period (Figure 1). We excluded patients who did not receive acetylsalicylic acid before PCI (N = 6911), patients who were treated with thrombolysis (N = 260) and patients with missing data that were not imputed (N = 1171). The remaining 44,804 patients (28.6% women) were included in the study, of whom 24,299 (26.8% women) had RA. These patients were reported from 30 different hospitals, and the range of reported patients per hospital was 102 to 6471. Between 2005 and 2016, the number of RA increased by 35% per year from 12.3% in 2005 to 85.8% in 2016 (P < 0.001, Figure 2). The characteristics of the patients are presented in Table 1 and procedure related details in Table 2. Patients with a RA access were on average younger, more likely to have heart failure and less likely to have hyperlipidaemia, myocardial infarction, stroke, prior PCI or prior CABG. During PCI, RA patients were more often treated with ticagrelor, prasugrel and bivalirudin but less often with a GP2b/3a receptor antagonist and unfractionated heparin. RA patients were also less likely to have complex coronary artery disease. They were more likely to have complete revascularisation during the index PCI with DESs. Stents were more often placed without prior balloon dilation of the lesion in RA patients. The median time from symptom debut to the first medical contact and from the first medical contact to the start of PCI in the total population was 113 minutes (interquartile range (IQR) 50–293) and 74 minutes (IQR 48–125), respectively, and these times did not differ significantly between the two groups (Table 2). After adjustment for propensity score, the two groups were well balanced.
Figure 1.
Flow chart for patient selection in Swedish Coronary Angiography and Angioplasty Registry (SCAAR).
Figure 2.
The number of primary percutaneous coronary intervention (PCI) procedures performed by radial access per calendar year in Sweden between 2005 and 2016.
Table 1.
Patient characteristics.
| Femoral (N = 20,505) | Missing | Radial (N = 24,299) | Missing | Standardised difference | Standardised difference after adjustment | |||
|---|---|---|---|---|---|---|---|---|
| Age (years) | ||||||||
| Mean age, years | 68 ± 12 | 0 | 67 ± 12 | 0 | 0.036 | 0.097 | ||
| Age >75, n (%) | 5895 (28.6) | 0 | 6232 (25.7) | 0 | 0.065 | 0.087 | ||
| Male sex, n (%) | 14,202 (69.3) | 0 | 17,780 (73.2) | 0 | 0.084 | 0.107 | ||
| Diabetes, n (%) | 3185 (15.5) | 0 | 3608 (14.6) | 0 | 0.014 | 0.028 | ||
| Hypertension, n (%) | 9353 (45.6) | 0 | 11,153 (45.9) | 0 | 0.006 | 0.074 | ||
| Smoking, n/total n (%) | 2350 (11.4) | 1631 (6.7) | ||||||
| Never smoker | 8249 (40.1) | 9652 (39.7) | 0.009 | 0.002 | ||||
| Previous smoker | 5978 (29.5) | 7362 (30.3) | 0.025 | 0.017 | ||||
| Current smoker | 6274 (30.6) | 7281 (30.0) | 0.015 | 0.014 | ||||
| Hyperlipidaemia, n (%) | 5580 (27.2) | 0 | 5859 (24.1) | 0 | 0.071 | 0.061 | ||
| Previous stroke, n (%) | 1386 (6.8) | 1992 (9.7) | 1222 (5.1) | 3496 (14.4) | 0.074 | 0.066 | ||
| History of heart failure, n (%) | 998 (4.9) | 1530 (7.5) | 1576 (6.5) | 3939 (16.2) | 0.07 | 0.071 | ||
| Previous myocardial infarction, n (%) | 4170 (20.3) | 0 | 3545 (14.6) | 0 | 0.152 | 0.079 | ||
| Previous PCI — n (%) | 2989 (14.6) | 0 | 2887 (11.9) | 0 | 0.08 | 0.008 | ||
| Previous CABG, n (%) | 1200 (5.6) | 0 | 396 (1.6) | 0 | 0.224 | 0.028 | ||
| Symptom to first contact, min; median (IQR) | 110 (50–268) | 0 | 115 (50–317) | 0 | 0.016 | 0.001 | ||
| First contact to start of PCI, min; median (IQR) | 73 (45–125) | 0 | 75 (50–126) | 0 | 0.019 | 0.015 | ||
| Prehospital heparin | 4586 (22.6) | 0 | 12,925 (53.2) | 0 | 0.675 | 0.101 | ||
| Days in hospital, mean (±SD) | 5.1 ± 14.7 | 0 | 4.6 ± 5.5 | 0 | 0.120 | 0.131 | ||
| Medications used at time of presentation, n (%) | ||||||||
| Beta-blockers | 6549 (31.9) | 623 (1.6) | 7463 (30.7) | 91 (1.3) | 0.024 | 0.074 | ||
| ACE inhibitor | 3374 (16.6) | 607 (1.6) | 4570 (18.8) | 85 (1.2) | 0.063 | 0.027 | ||
| ARB receptor antagonist | 2220 (10.8) | 602 (1.6) | 3422 (14.8) | 88 (1.3) | 0.097 | 0.015 | ||
| Acetylsalicylic acid | 6604 (32.2) | 414 (1.1) | 7528 (30.9) | 73 (1.1) | 0.023 | 0.073 | ||
| P2Y12 receptor antagonist | 1234 (6.1) | 3971 (10.5) | 1073 (4.4) | 1036 (14.9) | 0.071 | 0.045 | ||
| Statin | 5067 (24.7) | 423 (1.1) | 6826 (28.1) | 75 (1.1) | 0.076 | 0.031 | ||
| OAC or NOAC | 359 (1.6) | 56 (0.2) | 471 (1.9) | 27 (0.4) | 0.017 | 0.018 | ||
PCI: percutaneous coronary intervention; CABG: coronary artery bypass grafting; CPR: cardiopulmonary resurrection; ACE: angiotensin-converting enzyme inhibitor; ARB: angiotensin receptor blocker; OAC: oral anticoagulant; NOAC: novel oral anticoagulant.
Table 2.
Angiography and PCI.
| Femoral (N = 20,505) | Missing | Radial (N = 24,299) | Missing | Standardised difference | Standardised difference after adjustment | |
|---|---|---|---|---|---|---|
| Procedure performed off-hours, n (%) | 12,722 (64.6) | 807 (3.9) | 15,986 (65.8) | 392 (1.6) | 0.046 | 0.006 |
| Infarct-related artery, n/total n (%) | 359 (1.8) | 140 (2.0) | ||||
| RCA | 8002 (39.7) | 8889 (37.2) | 0.050 | 0.126 | ||
| LAD | 8695 (43.2) | 10,873 (45.6) | 0.047 | 0.119 | ||
| LCx | 3183 (15.8) | 3876 (16.2) | 0.001 | 0.035 | ||
| LM | 262 (1.3) | 229 (0.96) | 0.022 | 0.105 | ||
| Arteries with stenosis, n/total n (%) | 130 (0.6) | 31 (0.1) | ||||
| 0 | 158 (0.8) | 183 (0.6) | 0.006 | 0.001 | ||
| 1 | 9186 (44.8) | 12,546 (51.6) | 0.127 | 0.041 | ||
| 2 or 3 no LM | 9815 (47.8) | 10,615 (43.7) | 0.087 | 0.018 | ||
| LM and 1, 2 or 3 | 1212 (5.9) | 920 (3.8) | 0.095 | 0.054 | ||
| Complete revascularization, n/total n (%) | 10,381 (50.6) | 188 (0.9) | 14,779 (60.8) | 256 (1.3) | 0.204 | 0.067 |
| Type of lesion | 126 (0.6) | 38 (0.2) | ||||
| A | 1510 (7.4) | 1694 (7.0) | 0.018 | 0.020 | ||
| B1 | 5617 (27.6) | 7007 (28.9) | 0.027 | 0.030 | ||
| B2 | 6734 (33.3) | 8946 (36.9) | 0.076 | 0.011 | ||
| C | 4666 (23.1) | 4177 (17.3) | 0.144 | 0.080 | ||
| B1 bifurcation | 384 (1.9) | 663 (2.7) | 0.053 | 0.033 | ||
| B2 bifurcation | 754 (3.7) | 1138 (4.7) | 0.047 | 0.028 | ||
| C bifurcation | 569 (2.8) | 593 (2.6) | 0.022 | 0.020 | ||
| Type of stenosis | 8 (0.02) | 3 (0.04) | ||||
| De novo | 19,241 (93.9) | 23,334 (96.0) | 0.097 | 0.065 | ||
| In-stent | 1078 (5.3) | 844 (3.5) | 0.042 | 0.012 | ||
| Other | 172 (0.8) | 116 (0.5) | 0.087 | 0.065 | ||
| PCI with stent, n/total n (%) | 2 (0.001) | 0 (0.01) | ||||
| Drug-eluting stent | 5921 (28.9) | 15,120 (62.2) | 0.648 | 0.017 | ||
| Bare metal stent | 12,722 (62.1) | 7690 (31.7) | 0.707 | 0.045 | ||
| No stent | 1856 (9.0) | 1485 (6.1) | 0.095 | 0.114 | ||
| P2Y12 receptor antagonist* | 0 | 524 (7.5) | ||||
| Clopidogrel | 16,255 (80.8) | 9875 (40.1) | 0.892 | 0.038 | ||
| Ticagrelor | 3148 (15.6) | 12,642 (52.4) | 0.838 | 0.061 | ||
| Prasugrel | 723 (3.6) | 1629 (6.8) | 0.141 | 0.045 | ||
| Thrombus aspiration, n (%) | 4603 (22.5) | 75 (0.36) | 5354 (22.1) | 37 (0.15) | 0.064 | 0.031 |
| Direct stenting, n (%) | 2860 (16.1) | 0 | 3994 (18.1) | 0 | 0.124 | 0.096 |
| Bivalirudin, n (%) | 6869 (34.9) | 817 (3.94) | 12,818 (53.0) | 129 (0.53) | 0.504 | 0.061 |
| GP2b/3a receptor inhibitor, n (%) | 9710 (47.5) | 0 | 5599 (23.1) | 0 | 0.365 | 0.007 |
| Unfractionated heparin, n (%) | 11,890 (58.0) | 11 (0.04) | 15,704 (64.6) | 6 (0.02) | 0.131 | 0.017 |
IRA: infarct-related artery; PCI: percutaneous coronary intervention; RCA: right coronary artery; LAD: left anterior descending artery; LCx: left circumflex artery; LM: left main.
Clinical outcomes
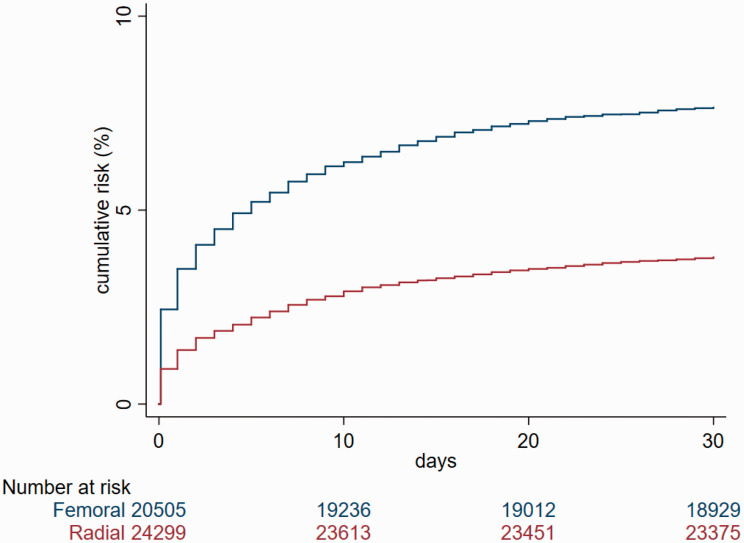
There were 2487 (5.5%) deaths within 30 days, of which 920 (3.8%) occurred in the RA and 1567 (7.6%) in the FA groups (Figure 3). The unadjusted odds ratio (OR) associated with RA versus FA was 0.47 (95% confidence interval (CI) 0.44–0.51) for death within 30 days, 0.56 (95% CI 0.45–0.70) for inhospital bleeding, 0.29 (95% CI 0.23–0.37) for CS and 0.65 (95% CI 0.48–0.87) for stroke. After propensity score adjustment (Table 3), RA was associated with a lower risk of death (adjusted OR 0.70, 95% CI 0.55–0.88, P = 0.025), a lower risk of inhospital bleeding (adjusted OR 0.45, 95% CI 0.25–0.79, P = 0.006) and a lower risk of CS after PCI (adjusted OR 0.41, 95% CI 0.24–0.73, P = 0.002). After adjustment, access site did not modify the risk of stroke (adjusted OR 0.85, 95% CI 0.26–2.84, P = 0.797). We found no interaction between access site and age, gender and CS regarding 30-day mortality and bleeding. Exclusion of patients with CS did not substantially change the estimated risk of death at 30 days (adjusted OR 0.58, 95% CI 0.49–0.76, P < 0.001).
Figure 3.
Cumulative incidence of the primary endpoint in relation to arterial access site.
Table 3.
Primary analysis.
| Femoral (N = 20,505) | Radial (N = 24,299) | Adjusted OR | 95% CI | P value | Missing n (%) | |
|---|---|---|---|---|---|---|
| Primary endpoint | ||||||
| Death at 30 days, n (%) | 1567 (7.6) | 920 (3.8) | 0.70 | 0.55–0.88 | 0.025 | 0 |
| Secondary endpoints | ||||||
| Definite stent thrombosis at 30 days, n (%) | 157 (0.8) | 110 (0.5) | 1.19 | 0.50–2.83 | 0.718 | 0 |
| Cardiogenic shock, n (%) | 1044 (5.1) | 366 (1.5) | 0.41 | 0.24–0.73 | 0.002 | 0 |
| Inhospital bleeding | 718 (3.6) | 486 (2.1) | 0.45 | 0.25–0.79 | 0.006 | 1278 (2.9) |
| Inhospital neurological complications | 67 (0.3) | 51 (0.2) | 0.83 | 0.30–2.28 | 0.721 | 1002 (2.2) |
OR: odds ratio; CI: confidence interval.
Discussion
The most important finding in our study from the nationwide SWEDEHEART registry is that among 44,804 unselected and consecutive patients with STEMI, coronary angiography and PCI by RA rather than FA access was associated with significantly lower adjusted risks of 30-day mortality, inhospital bleeding and CS. The results of our study are consistent with, and add external validity to, the findings of three recent large randomised trials done on this topic, the RIVAL,3 RIFLE-STEACS1 and MATRIX2 studies. We present one of the largest observational studies on radial versus femoral access in STEMI patients and our findings are congruent with earlier observational studies.19–24
We found that RA, as compared to FA, in patients with STEMI is associated with a considerably reduced risk of dying. This finding is consistent with the findings of the three largest randomised trials that compared RA to FA in patients with acute coronary syndromes undergoing invasive management, the MATRIX, RIVAL-STEACS and RIFLE trials.2 MATRIX, which was the largest of the three trials, randomly allocated 8404 patients with acute coronary syndromes to either RA or FA. MATRIX reported a relative risk reduction for 30-day mortality with RA versus FA of 0.72, which is similar to the adjusted risk reduction observed in our study. In MATRIX, the risk reduction in 30-day mortality was partly responsible for the risk reduction observed with RA versus FA with regard to the primary composite endpoint all-cause death, myocardial infarction, stroke or the Bleeding Academic Research Consortium (BARC)25 level 3 or 5 bleeding. Our results are also consistent with RIFLE-STEACS,1 which randomly allocated 1001 patients with STEMI to RA versus FA in four high-volume centres. RIFLE-ACS reported a significant reduction in the risk of the primary composite endpoint 30-day net adverse cardiac event (cardiac death, stroke, myocardial infarction, target lesion revascularisation and non-CABG bleeding), which was partly driven by a significant reduction from 9.2% to 5.2% in the secondary endpoint 30-day cardiac mortality. Whereas the RIVAL study did not show a significant reduction in the composite primary endpoint (30-day death, myocardial infarction, stroke, non-CABG major bleeding) between RA and FA among 7021 patients with acute coronary syndrome, a prespecified subgroup analysis of the 1958 patients with STEMI showed a reduction in the risk of the primary outcome with RA versus FA.3
There are several possible explanations for the observed reduction in mortality risk with RA versus FA. The observed reduction in mortality with RA versus FA could be related to a reduced risk of bleeding. It is well known that the risk of significant bleeding complications, particularly those directly related to vascular access, is reduced with RA compared to FA. The inhospital bleeding risk was substantially lower with RA than FA in our study, as well as in each of the randomised multicentre trials.1,2 Bleeding complications after PCI, irrespective of whether they are related to the access site,26–29 are associated with an increased risk of dying.27,28,30 In addition to direct life-threatening complications such as haemorrhagic shock, bleeding increases the risk of both myocardial infarction and ischaemic stroke.25,28,30–34 The increased risks of myocardial infarction and stroke for patients who bleed are most likely to be related to cessation of antithrombotic medications, adverse reactions to blood transfusions, activation of the coagulation cascade or by reducing oxygen delivery to already ischaemic tissue. Irrespective of the exact mechanisms through which bleeding after PCI can lead to death, the risk of dying has been shown to be at least as high for patients who have a significant bleed after PCI as for those who have a myocardial infarction after PCI.27,28,30 In light of the strong association between bleeding after PCI and mortality, it is not surprising that strategies to reduce bleeding after PCI have been shown to increase survival,35–37 with the greatest benefit observed in patients with a relatively high bleeding risk, such as STEMI patients.27 Another life-threatening complication that was less likely to occur with RA than FA in our study, and therefore could mediate the reduction in mortality, was CS. The observed RA-associated reduction in the risk of CS is a novel finding because CS was not reported in either MATRIX, RIFLE-ACS or RIVAL, or any of the previously published larger observational studies.1–3,21–24 The exact mechanisms linking RA to a reduced risk of CS in STEMI are not immediately evident from our analysis. However, the observed RA-associated reduction in the risk of CS mirrors the RA-associated risk reduction for bleeding, and several studies have reported a strong association between bleeding risk and the risk of CS.38–42 In the CRUSADE registry,41 patients with major bleeding had a five times higher risk of CS. However, neither CRUSADE nor the other studies were able to establish whether bleeding caused CS,38–40,42 whether CS caused bleeding, or to what extent the association between bleeding and CS is explained by other factors. In our study it is more likely that a bleeding event caused CS than vice versa, because only patients who developed CS after PCI were included in the analysis pertaining to the risk of CS.
Irrespective of the underlying mechanisms, the observed association between access site and the risk of CS is an intriguing hypothesis-generating observation which we believe merits further investigation.43,44 A third possible mediator of the reduction in mortality risk with RA versus FA is the recently described lower risk of acute kidney injury, which is associated with the increased mortality risk45 with RA versus FA. A lower risk of acute kidney injury with RA versus AF has been described for acute coronary syndrome patients46 and may be particularly pronounced in STEMI patients.47,48 A lower risk of acute kidney injury with RA than FA has been suggested to be related to a more extensive mobilisation of endothelial progenitors after radial versus femoral arterial puncture, due to the smaller vessel diameter of the radial artery.49–51 Unfortunately, SWEDHEART does not contain data on the rate of acute kidney injury after PCI.
Study limitations
This was an observational study and provides only evidence of association, not cause. We cannot exclude residual confounding or selection bias. A proportion of patients had missing data. We do not have data on cause-specific mortality. We have no data on blood pressure or heart rate on admission, that is, factors independently associated with the development of CS52 and patients in Killip classes II–III were not excluded.
In conclusion, in patients with STEMI, primary PCI via RA rather than FA was associated with an adjusted lower risk of death, bleeding and CS. Our findings are consistent with, and add external validity to, recent randomised trials, and support the ESC guideline class Ia recommendation for the use of radial access for primary PCI in STEMI.
Conflict of interest
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Swedish Heart–Lung Foundation, the Swedish Research Council and ALF Göteborg.
References
- 1.Romagnoli E, Biondi-Zoccai G, Sciahbasi A, et al. Radial versus femoral randomized investigation in ST-segment elevation acute coronary syndrome: the RIFLE-STEACS (Radial Versus Femoral Randomized Investigation in ST-Elevation Acute Coronary Syndrome) study. J Am Coll Cardiol 2012; 60: 2481–2489. [DOI] [PubMed] [Google Scholar]
- 2.Valgimigli M, Gagnor A, Calabró P, et al. Radial versus femoral access in patients with acute coronary syndromes undergoing invasive management: a randomised multicentre trial. Lancet 2015; 385: 2465–2476. [DOI] [PubMed] [Google Scholar]
- 3.Jolly SS, Yusuf S, Cairns J, et al. Radial versus femoral access for coronary angiography and intervention in patients with acute coronary syndromes (RIVAL): a randomised, parallel group, multicentre trial. Lancet 2011; 377: 1409–1420. [DOI] [PubMed] [Google Scholar]
- 4.Shah R, Ahmed AJ. Validity of randomized trials comparing radial versus femoral access in acute coronary syndrome. JACC Cardiovasc Interv 2016; 9: 1517–1518. [DOI] [PubMed] [Google Scholar]
- 5.Ibanez B, James S, Agewall S, et al. ; and Group ESCSD. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J 2018; 39: 119–177. [DOI] [PubMed] [Google Scholar]
- 6.O’Gara PT, Kushner FG, Ascheim DD, et al. ; and American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2013; 127: e362–e425. [DOI] [PubMed] [Google Scholar]
- 7.Barbato E, Dudek D, Baumbach A, et al. Current trends in coronary interventions: an overview from the EAPCI registries. EuroIntervention 2017; 13: Z8–Z10. [DOI] [PubMed] [Google Scholar]
- 8.Serrador Frutos AM, Jimenez-Quevedo P, Perez de Prado A, et al. Spanish Cardiac Catheterization and Coronary Intervention Registry. 26th Official Report of the Spanish Society of Cardiology Working Group on Cardiac Catheterization and Interventional Cardiology (1990–2016). Rev Esp Cardiol (Engl Ed) 2017; 70: 1110–1120. [DOI] [PubMed] [Google Scholar]
- 9.Biswas S, Duffy SJ, Lefkovits J, et al. Australian trends in procedural characteristics and outcomes in patients undergoing percutaneous coronary intervention for ST-elevation myocardial infarction. Am J Cardiol 2018; 121: 279–288. [DOI] [PubMed] [Google Scholar]
- 10.Rigattieri S, Valsecchi O, Sciahbasi A, et al. Current practice of transradial approach for coronary procedures: a survey by the Italian Society of Interventional Cardiology (SICI-GISE) and the Italian Radial Club. Cardiovasc Revasc Med 2017; 18: 154–159. [DOI] [PubMed] [Google Scholar]
- 11.Johnman C, Pell JP, Mackay DF, et al. Clinical outcomes following radial versus femoral artery access in primary or rescue percutaneous coronary intervention in Scotland: retrospective cohort study of 4534 patients. Heart 2012; 98: 552–557. [DOI] [PubMed] [Google Scholar]
- 12.Hamon M, Pristipino C, Di Mario C, et al. ; European Association of Percutaneous Cardiovascular I, Working Group on Acute Cardiac Care of the European Society of Cardiology and Working Group on Thrombosis on the European Society of Cardiology. Consensus document on the radial approach in percutaneous cardiovascular interventions: position paper by the European Association of Percutaneous Cardiovascular Interventions and Working Groups on Acute Cardiac Care** and Thrombosis of the European Society of Cardiology. EuroIntervention 2013; 8: 1242–1251. [DOI] [PubMed] [Google Scholar]
- 13.Mamas MA, Nolan J, de Belder MA, et al. ; British Cardiovascular Intervention Society and the National Institute for Clinical Outcomes Registry. Changes in arterial access site and association with mortality in the United Kingdom: observations from a national percutaneous coronary intervention database. Circulation 2016; 133: 1655–1667. [DOI] [PubMed] [Google Scholar]
- 14.Heckman JJ, Ichimura H, Todd P. Matching as an econometric evaluation estimator. Rev Econ Studies 1998; 65: 261–294. [Google Scholar]
- 15.van Buuren S. Multiple imputation of discrete and continuous data by fully conditional specification. Stat Meth Med Res 2007; 16: 219–242. [DOI] [PubMed] [Google Scholar]
- 16.Angeras O, Albertsson P, Karason K, et al. Evidence for obesity paradox in patients with acute coronary syndromes: a report from the Swedish Coronary Angiography and Angioplasty Registry. Eur Heart J 2013; 34: 345–353. [DOI] [PubMed] [Google Scholar]
- 17.White IR, Royston P. Imputing missing covariate values for the Cox model. Stat Med 2009; 28: 1982–1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rubin DB. Inference and missing data. Biometrika 1976; 63: 581–590. [Google Scholar]
- 19.De Luca G, Schaffer A, Wirianta J, et al. Comprehensive meta-analysis of radial vs femoral approach in primary angioplasty for STEMI. Int J Cardiol 2013; 168: 2070–2081. [DOI] [PubMed] [Google Scholar]
- 20.Bertrand OF, Belisle P, Joyal D, et al. Comparison of transradial and femoral approaches for percutaneous coronary interventions: a systematic review and hierarchical Bayesian meta-analysis. Am Heart J 2012; 163: 632–648. [DOI] [PubMed] [Google Scholar]
- 21.Bauer T, Hochadel M, Brachmann J, et al. Use and outcome of radial versus femoral approach for primary PCI in patients with acute ST elevation myocardial infarction without cardiogenic shock: results from the ALKK PCI registry. Cathet Cardiovasc Interv: official journal of the Society for Cardiac Angiography & Interventions 2015; 86 (Suppl. 1): S8–S14. [DOI] [PubMed] [Google Scholar]
- 22.Baklanov DV, Kaltenbach LA, Marso SP, et al. The prevalence and outcomes of transradial percutaneous coronary intervention for ST-segment elevation myocardial infarction: analysis from the National Cardiovascular Data Registry (2007 to 2011). J Am Coll Cardiol 2013; 61: 420–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mamas MA, Ratib K, Routledge H, et al. Influence of arterial access site selection on outcomes in primary percutaneous coronary intervention: are the results of randomized trials achievable in clinical practice? JACC Cardiovasc Interv 2013; 6: 698–706. [DOI] [PubMed] [Google Scholar]
- 24.Valgimigli M, Saia F, Guastaroba P, et al. Transradial versus transfemoral intervention for acute myocardial infarction: a propensity score-adjusted and -matched analysis from the REAL (REgistro regionale AngiopLastiche dell’Emilia-Romagna) multicenter registry. JACC Cardiovasc Interv 2012; 5: 23–35. [DOI] [PubMed] [Google Scholar]
- 25.Mehran R, Rao SV, Bhatt DL, et al. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the Bleeding Academic Research Consortium. Circulation 2011; 123: 2736–2747. [DOI] [PubMed] [Google Scholar]
- 26.Yatskar L, Selzer F, Feit F, et al. Access site hematoma requiring blood transfusion predicts mortality in patients undergoing percutaneous coronary intervention: data from the National Heart, Lung, and Blood Institute Dynamic Registry. Cathet Cardiovasc Interv: official journal of the Society for Cardiac Angiography & Interventions 2007; 69: 961–966. [DOI] [PubMed] [Google Scholar]
- 27.Chhatriwalla AK, Amin AP, Kennedy KF, et al. Association between bleeding events and in-hospital mortality after percutaneous coronary intervention. JAMA 2013; 309: 1022–1029. [DOI] [PubMed] [Google Scholar]
- 28.Ndrepepa G, Berger PB, Mehilli J, et al. Periprocedural bleeding and 1-year outcome after percutaneous coronary interventions: appropriateness of including bleeding as a component of a quadruple end point. J Am Coll Cardiol 2008; 51: 690–697. [DOI] [PubMed] [Google Scholar]
- 29.Verheugt FW, Steinhubl SR, Hamon M, et al. Incidence, prognostic impact, and influence of antithrombotic therapy on access and nonaccess site bleeding in percutaneous coronary intervention. JACC Cardiovasc Interv 2011; 4: 191–197. [DOI] [PubMed] [Google Scholar]
- 30.Manoukian SV, Feit F, Mehran R, et al. Impact of major bleeding on 30-day mortality and clinical outcomes in patients with acute coronary syndromes: an analysis from the ACUITY Trial. J Am Coll Cardiol 2007; 49: 1362–1368. [DOI] [PubMed] [Google Scholar]
- 31.Ben-Yehuda O, Redfors B. Validation of the Bleeding Academic Research Consortium bleeding definition: towards a standardized bleeding score. J Am Coll Cardiol 2016; 67: 2145–2147. [DOI] [PubMed] [Google Scholar]
- 32.Eikelboom JW, Mehta SR, Anand SS, et al. Adverse impact of bleeding on prognosis in patients with acute coronary syndromes. Circulation 2006; 114: 774–782. [DOI] [PubMed] [Google Scholar]
- 33.Mehran R, Pocock SJ, Nikolsky E, et al. A risk score to predict bleeding in patients with acute coronary syndromes. J Am Coll Cardiol 2010; 55: 2556–2566. [DOI] [PubMed] [Google Scholar]
- 34.Lindsey JB, Marso SP, Pencina M, et al. Prognostic impact of periprocedural bleeding and myocardial infarction after percutaneous coronary intervention in unselected patients: results from the EVENT (evaluation of drug-eluting stents and ischemic events) registry. JACC Cardiovasc Interv 2009; 2: 1074–1082. [DOI] [PubMed] [Google Scholar]
- 35.Yusuf S, Mehta SR, Chrolavicius S, et al. Comparison of fondaparinux and enoxaparin in acute coronary syndromes. N Engl J Med 2006; 354: 1464–1476. [DOI] [PubMed] [Google Scholar]
- 36.Mehran R, Lansky AJ, Witzenbichler B, et al. Bivalirudin in patients undergoing primary angioplasty for acute myocardial infarction (HORIZONS-AMI): 1-year results of a randomised controlled trial. Lancet (London, England ) 2009; 374: 1149–1159. [DOI] [PubMed] [Google Scholar]
- 37.De Servi S, Mariani G, Mariani M, et al. How to reduce mortality in ST-elevation myocardial infarction patients treated with primary percutaneous coronary interventions: cut the bleeding. Curr Med Res Opin 2013; 29: 189–194. [DOI] [PubMed] [Google Scholar]
- 38.Numasawa Y, Kohsaka S, Ueda I, et al. Incidence and predictors of bleeding complications after percutaneous coronary intervention. J Cardiol 2017; 69: 272–279. [DOI] [PubMed] [Google Scholar]
- 39.Young K, Earl T, Selzer F, et al. Trends in major entry site complications from percutaneous coronary intervention (from the Dynamic Registry). Am J Cardiol 2014; 113: 626–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mehta SK, Frutkin AD, Lindsey JB, et al. Bleeding in patients undergoing percutaneous coronary intervention: the development of a clinical risk algorithm from the National Cardiovascular Data Registry. Circulation Cardiovasc Interv 2009; 2: 222–229. [DOI] [PubMed] [Google Scholar]
- 41.Subherwal S, Bach RG, Chen AY, et al. Baseline risk of major bleeding in non-ST-segment-elevation myocardial infarction: the CRUSADE (Can Rapid risk stratification of Unstable angina patients Suppress ADverse outcomes with Early implementation of the ACC/AHA Guidelines) Bleeding Score. Circulation 2009; 119: 1873–1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Boden H, Velders MA, van der Hoeven BL, et al. In-hospital major bleeding and its clinical relevance in patients with ST elevation myocardial infarction treated with primary percutaneous coronary intervention. Am J Cardiol 2013; 112: 1533–1539. [DOI] [PubMed] [Google Scholar]
- 43.Thiele H, Allam B, Chatellier G, et al. Shock in acute myocardial infarction: the Cape Horn for trials? Eur Heart J 2010; 31: 1828–1835. [DOI] [PubMed] [Google Scholar]
- 44.van Diepen S, Katz JN, Albert NM, et al. Contemporary management of cardiogenic shock: a scientific statement from the American Heart Association. Circulation 2017; 136: e232–e268. [DOI] [PubMed] [Google Scholar]
- 45.Kooiman J, Seth M, Nallamothu BK, et al. Association between acute kidney injury and in-hospital mortality in patients undergoing percutaneous coronary interventions. Circulation Cardiovasc Interv 2015; 8: e002212. [DOI] [PubMed] [Google Scholar]
- 46.Ando G, Cortese B, Russo F, et al. ; for the MATRIX Investigators. Acute kidney injury after radial or femoral access for invasive acute coronary syndrome management: AKI-MATRIX. J Am Coll Cardiol 2017; 69: 2592–2603. [DOI] [PubMed] [Google Scholar]
- 47.Ando G, Costa F, Trio O, et al. Impact of vascular access on acute kidney injury after percutaneous coronary intervention. Cardiovasc Revasc Med 2016; 17: 333–338. [DOI] [PubMed] [Google Scholar]
- 48.Cortese B, Sciahbasi A, Sebik R, et al. Comparison of risk of acute kidney injury after primary percutaneous coronary interventions with the transradial approach versus the transfemoral approach (from the PRIPITENA urban registry). Am J Cardiol 2014; 114: 820–825. [DOI] [PubMed] [Google Scholar]
- 49.Mitchell A, Fujisawa T, Mills NL, et al. Endothelial progenitor cell biology and vascular recovery following transradial cardiac catheterization. J Am Heart Assoc 2017; 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Leone AM, Valgimigli M, Giannico MB, et al. From bone marrow to the arterial wall: the ongoing tale of endothelial progenitor cells. Eur Heart J 2009; 30: 890–899. [DOI] [PubMed] [Google Scholar]
- 51.Gao M, Yao Q, Liu Y, et al. Association between mobilization of circulating endothelial progenitor cells and time or degree of injury from angioplasty in patients with exertional angina: a prospective study. Exp Ther Med 2015; 10: 809–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Auffret V, Cottin Y, Leurent G, et al. Predicting the development of in-hospital cardiogenic shock in patients with ST-segment elevation myocardial infarction treated by primary percutaneous coronary intervention: the ORBI risk score. Eur Heart J 2018; 39: 2090–2102. [DOI] [PubMed] [Google Scholar]