Abstract
Welders have an increased risk for cardiovascular disease (CVD) following exposure to welding fumes. The underlying mechanisms are largely unknown; however, oxidative stress, systemic inflammation, and endothelial dysfunction have been suggested as contributing factors to particle-induced CVD. We investigated effects of mild steel welding fume (MSWF) on three target cell types: macrophages, pulmonary epithelial, and vascular endothelial cells. Cells were exposed to MSWF at nontoxic doses for 6 h/day, for five consecutive days. The expression of 40 genes involved in inflammation, fibrosis, and endothelial activation was analyzed. Moreover, changes in the reactive oxygen species production and migration capacity of cells were assessed. The expression of matrix metallopeptidase 1 (MMP1) was induced in both epithelial and endothelial cells following repeated exposure to MSWF. Although MMP1 is important in inflammatory responses in vivo, this effect was not concurrent with changes in the inflammatory status, cell proliferation, and migration capacities, nor did it induce oxidative stress in the cells. Thus, repeated exposure with low doses of MSWF was sufficient neither for inducing inflammatory stress in epithelial cells and macrophages nor for endothelial activation, and higher concentrations of MSWF or the nonparticle fraction of MSWF may be critical in causing the increased risk of CVD observed among welders.
Keywords: Pulmonary inflammation, endothelial activation, oxidative stress, welding fume, cardiovascular disease, welders
Introduction
Welding is an industrial process that employs heat to join metal pieces through melting, leading to generation of complex aerosols of metal fumes, gases, and solid particles. The reaction between air and vaporized metals produces metal oxides, which condense and form complex fume particles of respirable sizes (Antonini et al., 2003). Health effects of exposure to welding fume (WF) are complex, as the WF composition is affected by the type of welding alloy used (Leonard et al., 2010; Zheng et al., 2015). The most common feed wire types used in arc welding are mild steel and stainless steel, and the different WFs generated cause diverse responses correlated with the metal composition, the soluble ion content, and the capability to produce free radicals (Taylor et al., 2003).
An association between occupational exposure to WF and cardiovascular disease (CVD) has been suggested, and some studies report that workers exposed to welding processed particles have an elevated risk of acute myocardial infarction and angina pectoris (Ibfelt et al., 2010; Li et al., 2015; Mocevic et al., 2015). Moreover, metal arc welders have increased risk for ventricular ectopy (Cavallari et al., 2008). WF-exposed animals present pulmonary inflammation and lung injury (Presume et al., 2016; Shoeb et al., 2017), as well as elevated oxidative stress and systemic leukocyte dysfunction (Erdely et al., 2014) leading to increased lesions of atherosclerotic plaques (Erdely et al., 2011). Furthermore, chronic exposure to WF results in welding particle accumulation and deposition of agglomerates within lung cells, particularly inside alveolar macrophages (Antonini et al., 2013). Particles entering the lungs may provoke pulmonary oxidative stress and inflammation, leading to a state of systemic oxidative stress and inflammation. This pro-inflammatory state may in turn promote processes related to CVD, including endothelial dysfunction, atherosclerosis progression, and dyslipidemia (Chin, 2015; Du et al., 2016). Welders show inflammatory cell influx, increased oxidative stress, and significant pulmonary injury (Antonini et al., 2013; Graczyk et al., 2016). WF exposure is also associated with systemic inflammatory responses (Kauppi et al., 2015; Shen et al., 2018), and serum concentrations of cytokines, cell adhesion molecules, and the acute phase proteins have been suggested as biomarkers in WF-induced CVD (Baumann et al., 2018; Fang et al., 2010; Jarvela et al., 2013).
Here, we investigated the involvement of oxidative stress, inflammation, and endothelial dysfunction as principal cellular and molecular mechanisms of CVD development following exposure to mild steel welding fume (MSWF). To do so, the three highly relevant cell types, namely macrophages, epithelial and endothelial cells, were repeatedly exposed to low concentrations of MSWF and patterns of common and differential regulated cellular responses were investigated.
Methods
Characterization of MSWF
MSWF containing 43% (m/m) iron, 22% (m/m) zinc, 1.5% (m/m) manganese, ∼1% (m/m) carbon, and <0.5% (m/m) copper was obtained from the Health and Safety Laboratory (UK) and dispersed essentially as previously described (Jensen et al., 2011). The hydrodynamic diameter was measured by dynamic light scattering and further characterization was performed by field-emission scanning electron microscopy.
Cell exposures and molecular analysis
Human monocytic THP1, bronchial epithelial HBEC-3KT, and microvascular endothelial HMEC-1 cells were exposed to four concentrations (0.035, 0.175, 0.875, or 4.375 µg/ml) of MSWF. Calculations and exposure setup are shown in the Supplementary material, Methods section.
Cellular uptake of MSWF was assessed by confocal microscopy in HBEC-3KT and HMEC-1 cells. Cytotoxicity was assessed by the Cell Counting Kit 8 (CCK-8) assay (Sigma-Aldrich, St. Louis, Missouri). Intracellular reactive oxygen species (ROS) levels were measured using 2′7 ′-dichlorodihydrofluorescein diacetate (Sigma-Aldrich). Cell migration was assessed by live cell imaging using IncuCyte ZOOM (Essen BioScience, Ann Arbor, Michigan). Gene expression was analyzed by a custom RT2 gene array (Qiagen, Hilden, Germany) (Supplementary Table 1). In addition, expression of intracellular adhesion molecule 1 (ICAM1), vascular cell adhesion molecule 1 (VCAM1), and selectin E (SELE) was assessed by real-time polymerase chain reaction. Detailed protocols can be found in the Supplementary material, Methods section. Statistical analyses were performed in STATA v. 16. The values of p < 0.05 were considered significant.
Results
Characteristics of MSWF
Dispersed MSWF had a hydrodynamic diameter of 414.73 ± 44.48 nm and was composed of both single particles of approximately 50 nm in diameter and larger agglomerates/aggregates as shown in Figure 1. The MSWF particles were more dispersed in cell culture media and showed some degree of agglomeration/aggregation after 6 h (Supplementary Table 2).
Figure 1.

Representative SEM image of MSWF. SEM: scanning electron microscopy; MSWF: mild steel welding fume.
Uptake and effects on cell viability
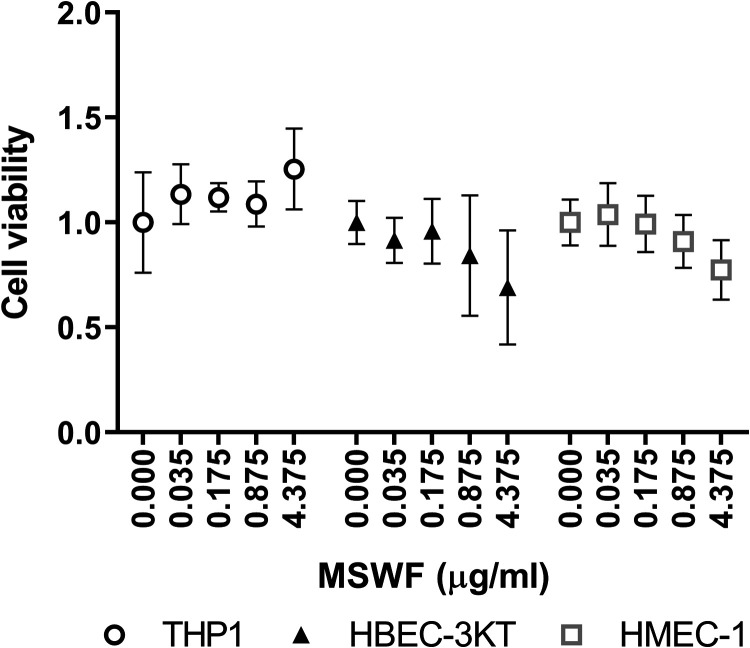
Cellular uptake of MSWF was not visible at day 1 of exposure, but at day 5 of exposure an accumulation of MSWF particles was observed in HBEC-3KT and HMEC-1 cells (Supplementary Figure 1). A trend to a reduction in cell viability was observed in HBEC-3KT and HMEC-1 at day 1 of exposure as shown in Figure 2. However, this trend was not present after 5 days of exposure (data not shown). Based on these data, the lowest occupational relevant concentration and the highest concentration were selected for further analysis.
Figure 2.
Effects of MSWF exposure on cell viability was assessed in THP1, HBEC-3KT, and HMEC-1 cells on day 1 of exposure. The mean viability of control cells was set to 1. Data indicate mean ± SD. MSWF: mild steel welding fume.
Effects on gene expression
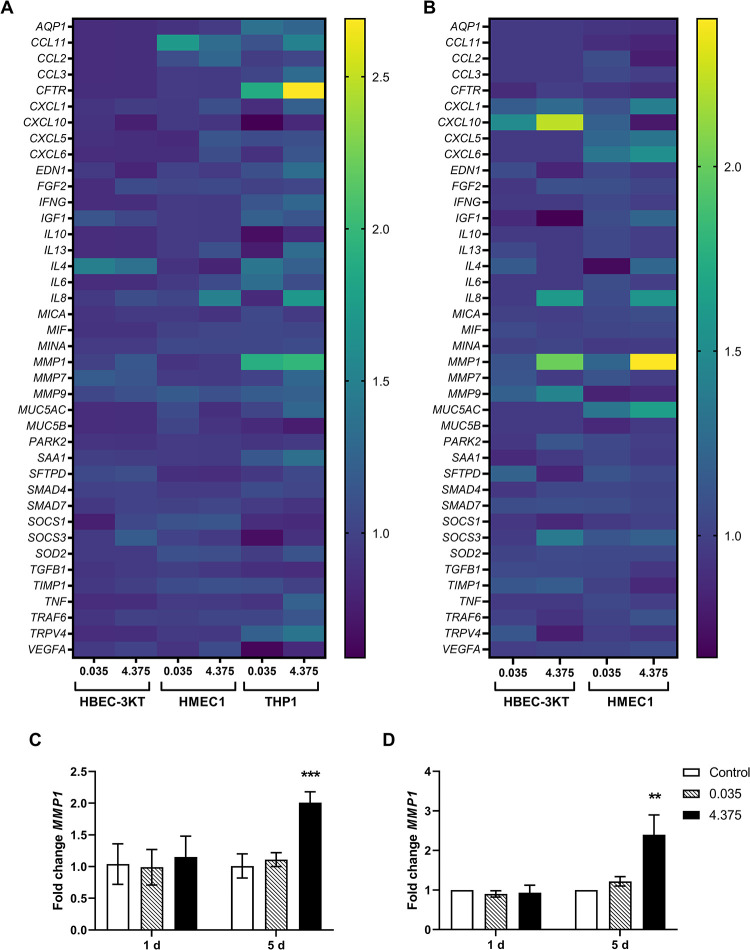
MSWF exposure only induced minor alterations in the expression patterns of inflammation and endothelial activation markers in exposed HBEC-3KT, HMEC-1, and THP1 cells as illustrated in Figure 3(a) and (b). However, matrix metallopeptidase-1 (MMP1) expression showed a significant twofold increase following repeated exposure for 5 days with 4.375 µg/ml MSWF in HBEC-3KT and HMEC-1 cells, in Figure 3(c) and (d). No effects in MMP1 expression were observed after day 1 of exposure or with lower concentrations. Fold changes in gene expression levels can be found in Supplementary Table 3. Furthermore, MSWF exposure did not affect the expression of ICAM, VCAM, and SELE (data not shown).
Figure 3.
Effects of MSWF exposure on the expression of inflammation, fibrosis, and endothelial activation markers. Mean fold changes in expression were illustrated by heat map at (a) 1 day and (b) 5 days of exposure. Changes in MMP1 expression in (c) HBEC-3KT and (d) HMEC-1 cells. Data indicate mean fold changes ± SD. Controls were set to 1. Significance was determined by one-way ANOVA with Dunnett’s test, ***p < 0.001; **p < 0.01. MSWF: mild steel welding fume; MMP1: matrix metallopeptidase 1; ANOVA: analysis of variance.
Effects on oxidative stress and cell migration
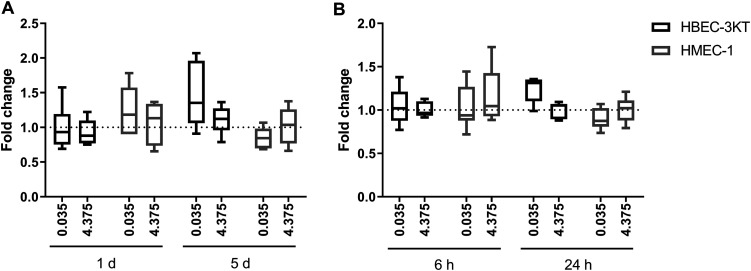
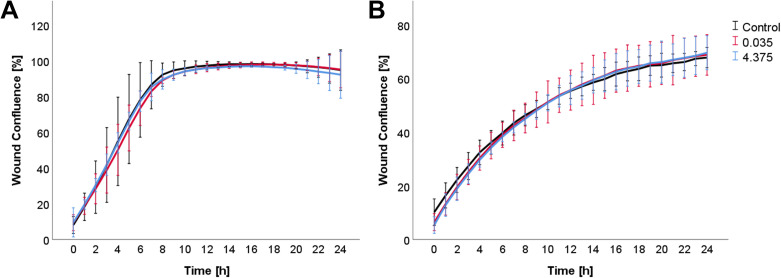
Neither direct exposure with MSWF nor exposure to conditioned media from THP1 cells affected ROS production in HBEC-3KT and HMEC-1 cells as shown in Figure 4. Similarly, exposure to MSWF-conditioned media did not affect the migration of epithelial and endothelial cells as shown in Figure 5.
Figure 4.
Analysis of oxidative cell stress after MSWF exposure. Intracellular ROS levels were measured in HBEC-3KT and HMEC-1 cells exposed to (a) MSWF for 1 and 5 days and (b) conditioned media for 6 and 24 h. Controls were set to 1. Boxes represent median and 5 and 95 percentiles. MSWF: mild steel welding fume.
Figure 5.
Analysis of cell migration. Migration of (a) HBEC-3KT and (b) HMEC-1 cells following exposure to conditioned media was assessed by live cell imaging. Data represent mean ± SD.
Discussion
The toxicity of WF is dependent on particle size, distribution, morphology, and chemical composition, as well as on concentration and exposure time. Moreover, metal release, rather than total metal content, is a critical determinant of toxicity (McCarrick et al., 2019). In this study, MSWF exposure did not induce significant cytotoxic effects on macrophages and epithelial and endothelial cells; however, the utilized concentrations were low and of occupational relevance. Generally, toxic effects of WF exposure have been observed at higher concentrations than those included in the current study (Lai et al., 2016; Leonard et al., 2010; McCarrick et al., 2019).
WF may induce pulmonary inflammation, and stainless steel welding fume (SSWF) appears to be more pneumotoxic and has greater inflammatory potential than MSWF, plausibly through differences in the metal constituents (Badding et al., 2014; Taylor et al., 2003). In this study, the expression of several inflammatory markers was not affected by MSWF exposure. However, an increased expression of MMP1 was observed in epithelial cells after repeated exposure with MSWF. MMPs play important roles in pulmonary inflammation, fibrosis, and COPD and are involved in the recovery from lung damage (Gueders et al., 2006). MMPs are activated by several pro-inflammatory cytokines and growth factors, and MMP1 expression is increased in alveolar epithelial cells during pulmonary fibrosis, and they represses mitochondrial respiration and oxidative stress, while promoting cell proliferation and migration (Herrera et al., 2013). Enhanced MMP1 expression was, however, not concurrent with increased ROS production or alterations in the expression of superoxide dismutase 2, nor were changes in expression of TIMP metallopeptidase inhibitor 1 (TIMP1) observed. TIMP1 is a known regulator of MMP activity as it binds to and inhibits MMPs (Brew and Nagase, 2010). The expression of MMPs and TIMPs is coordinately regulated and dependent on the inflammatory status, and dysregulation may potentially lead to tissue injury (Shapiro, 2009). Furthermore, epithelial cell proliferation and migration were not affected by direct MSFW exposure or exposure to conditioned media. Size and age of the particles may affect the generation of free radicals and ROS (Leonard et al., 2010) and, thus, effects of exposure to newly produced WF could possibly induce a more prominent effect on ROS production.
Endothelial dysfunction is a key event in the development of CVD, as it actively participates in the process of lesion formation, predisposing to vasoconstriction, platelet activation, leukocyte adhesion, oxidative stress, thrombosis, coagulation and inflammation (Verma et al., 2003). However, endothelial cells may be activated without being dysfunctional, and intact activated endothelium can contribute to disease initiation and progression (Galley and Webster, 2004). Particle exposure can induce endothelial activation by increasing intracellular levels of pro-inflammatory cytokines and chemokines and inducing oxidative stress (Hu et al., 2016). Here, no alterations in the expression of adhesion molecules and endothelial makers were detected following MSWF exposure in HMEC-1 cells. Endothelial cells exposed to conditioned media also did not show any changes in migration capacity. Moreover, no notable changes in inflammatory markers and ROS production were observed, despite an increase in MMP1 expression following repeated exposure of endothelial cells to MSWF. These data suggest that MSWF exposure is not sufficient in inducing endothelial activation at the concentrations investigated in this study. Interestingly, welders show no change in endothelial function following exposure to MSWF (Kauppi et al., 2015; Li et al., 2015). Higher reactivity and stronger inflammatory responses have been observed for SSWF than for MSWF (Leonard et al., 2010; Shoeb et al., 2017; Taylor et al., 2003), and thus, an involvement of endothelial activation in SSWF-induced CVD cannot be ruled out.
Although the expression of MMP1 was induced in both epithelial and endothelial cells following repeated exposure to MSWF, this effect was not concurrent with changes in the inflammatory status of cells, nor with changes in the proliferation and migration capacities and oxidative stress responses of the cells. Moreover, repeated exposure with low concentrations of MSWF was not sufficient in inducing epithelial inflammation and endothelial activation. Notably, this study only focused on the particle fraction of the WF exposure and did not consider systemic effects in workers following exposure. Thus, plausibly the soluble fractions of WF may be crucial for health outcome, and WF-induced cardiovascular effects cannot be allotted to the particle fraction only.
Supplemental material
Supplementary_Figures for Effects of mild steel welding fume particles on pulmonary epithelial inflammation and endothelial activation by Johanna Samulin Erdem, Yke Jildouw Arnoldussen, Sepideh Tajik, Dag G Ellingsen and Shanbeh Zienolddiny in Toxicology and Industrial Health
Suppl_methods for Effects of mild steel welding fume particles on pulmonary epithelial inflammation and endothelial activation by Johanna Samulin Erdem, Yke Jildouw Arnoldussen, Sepideh Tajik, Dag G Ellingsen and Shanbeh Zienolddiny in Toxicology and Industrial Health
Acknowledgements
The authors are grateful to Kristine Haugen Anmarkrud and Heidi Ødegaard Notø for providing excellent technical assistance.
Footnotes
Author contributions: JSE and YJA contributed equally to this work.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported through intramural funds from National Institute of Occupational Health and received no specific grant from any other funding agency in public, commercial or not-for-profit sector.
ORCID iD: Johanna Samulin Erdem  https://orcid.org/0000-0002-2102-3012
https://orcid.org/0000-0002-2102-3012
Supplemental material: Supplemental material for this article is available online.
References
- Antonini JM, Lewis AB, Roberts JR, et al. (2003) Pulmonary effects of welding fumes: Review of worker and experimental animal studies. American Journal of Industrial Medicine 43(4): 350–360. [DOI] [PubMed] [Google Scholar]
- Antonini JM, Roberts JR, Schwegler-Berry D, et al. (2013) Comparative microscopic study of human and rat lungs after overexposure to welding fume. Annals of Occupational Hygiene 57(9): 1167–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badding MA, Fix NR, Antonini JM, et al. (2014) A comparison of cytotoxicity and oxidative stress from welding fumes generated with a new nickel-, copper-based consumable versus mild and stainless steel-based welding in RAW 264.7 mouse macrophages. PLoS One 9(6): e101310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann R, Gube M, Markert A, et al. (2018) Systemic serum amyloid A as a biomarker for exposure to zinc and/or copper-containing metal fumes. Journal of Exposure Science and Environmental Epidemiology 28(1): 84–91. [DOI] [PubMed] [Google Scholar]
- Brew K, Nagase H. (2010) The tissue inhibitors of metalloproteinases (TIMPs): An ancient family with structural and functional diversity. Biochimica et Biophysica Acta (BBA)—Molecular Cell Research 1803(1): 55–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavallari JM, Eisen EA, Fang SC, et al. (2008) PM2.5 metal exposures and nocturnal heart rate variability: A panel study of boilermaker construction workers. Environmental Health 7: 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin MT. (2015) Basic mechanisms for adverse cardiovascular events associated with air pollution. Heart 101(4): 253–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Y, Xu X, Chu M, et al. (2016) Air particulate matter and cardiovascular disease: The epidemiological, biomedical and clinical evidence. Journal of Thoracic Disease 8(1): E8–E19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdely A, Antonini JM, Young SH, et al. (2014) Oxidative stress and reduced responsiveness of challenged circulating leukocytes following pulmonary instillation of metal-rich particulate matter in rats. Particle and Fibre Toxicology 11: 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdely A, Hulderman T, Salmen-Muniz R, et al. (2011) Inhalation exposure of gas-metal arc stainless steel welding fume increased atherosclerotic lesions in apolipoprotein E knockout mice. Toxicology Letters 204(1): 12–16. [DOI] [PubMed] [Google Scholar]
- Fang SC, Eisen EA, Cavallari JM, et al. (2010) Circulating adhesion molecules after short-term exposure to particulate matter among welders. Occupational and Environmental Medicine 67(1): 11–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galley HF, Webster NR. (2004) Physiology of the endothelium. British Journal of Anaesthesia 93(1): 105–113. [DOI] [PubMed] [Google Scholar]
- Graczyk H, Lewinski N, Zhao J, et al. (2016) Increase in oxidative stress levels following welding fume inhalation: A controlled human exposure study. Particle and Fibre Toxicology 13(1): 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gueders MM, Foidart JM, Noel A, et al. (2006) Matrix metalloproteinases (MMPs) and tissue inhibitors of MMPs in the respiratory tract: Potential implications in asthma and other lung diseases. European Journal of Pharmacology 533(1): 133–144. [DOI] [PubMed] [Google Scholar]
- Herrera I, Cisneros J, Maldonado M, et al. (2013) Matrix metalloproteinase (MMP)-1 induces lung alveolar epithelial cell migration and proliferation, protects from apoptosis, and represses mitochondrial oxygen consumption. The Journal of biological chemistry 288(36): 25964–25975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H, Wu J, Li Q, et al. (2016) Fine particulate matter induces vascular endothelial activation via IL-6 dependent JAK1/STAT3 signaling pathway. Toxicology Research 5(3): 946–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibfelt E, Bonde JP, Hansen J. (2010) Exposure to metal welding fume particles and risk for cardiovascular disease in Denmark: A prospective cohort study. Occupational and Environmental Medicine 67(11): 772–777. [DOI] [PubMed] [Google Scholar]
- Jarvela M, Kauppi P, Tuomi T, et al. (2013) Inflammatory response to acute exposure to welding fumes during the working day. International Journal of Occupational Medicine and Environmental Health 26(2): 220–229. [DOI] [PubMed] [Google Scholar]
- Jensen KA, Kembouche Y, Christiansen E, et al. (2011) Final protocol for producing suitable manufactured nanomaterial exposure media [Web-report] The generic NANOGENOTOX dispersion protocol—Standard operation procedure (SOP) Available at: http://www.nanogenotox.eu/index.php?option=com_content&view=article&id=136&Itemid=158.
- Kauppi P, Järvelä M, Tuomi T, et al. (2015) Systemic inflammatory responses following welding inhalation challenge test. Toxicology Reports 2: 357–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai CY, Lai CH, Chuang HC, et al. (2016) Physicochemistry and cardiovascular toxicity of metal fume PM2.5: A study of human coronary artery endothelial cells and welding workers. Scientific Reports 6: 33515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard SS, Chen BT, Stone SG, et al. (2010) Comparison of stainless and mild steel welding fumes in generation of reactive oxygen species. Particle and Fibre Toxicology 7: 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li HQ, Hedmer M, Karedal M, et al. (2015) A cross-sectional study of the cardiovascular effects of welding fumes. Plos One 10(7): e0131648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarrick S, Wei Z, Moelijker N, et al. (2019) High variability in toxicity of welding fume nanoparticles from stainless steel in lung cells and reporter cell lines: The role of particle reactivity and solubility. Nanotoxicology 13(10): 1293–1309. [DOI] [PubMed] [Google Scholar]
- Mocevic E, Kristiansen P, Bonde JP. (2015) Risk of ischemic heart disease following occupational exposure to welding fumes: A systematic review with meta-analysis. International Archives of Occupational and Environmental Health 88(3): 259–272. [DOI] [PubMed] [Google Scholar]
- Presume M, Simon-Deckers A, Tomkiewicz-Raulet C, et al. (2016) Exposure to metal oxide nanoparticles administered at occupationally relevant doses induces pulmonary effects in mice. Nanotoxicology 10(10): 1535–1544. [DOI] [PubMed] [Google Scholar]
- Shapiro SD. (2009) Chapter 28—Matrix degrading proteinases in COPD and asthma In: Barnes PJ, Drazen JM, Rennard SI, et al. (eds) Asthma and COPD, 2nd edn. Oxford: Academic Press, pp. 343–352. [Google Scholar]
- Shen S, Zhang R, Zhang J, et al. (2018) Welding fume exposure is associated with inflammation: A global metabolomics profiling study. Environmental Health 17(1): 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoeb M, Kodali V, Farris B, et al. (2017) Evaluation of the molecular mechanisms associated with cytotoxicity and inflammation after pulmonary exposure to different metal-rich welding particles. Nanotoxicology 11(6): 725–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor MD, Roberts JR, Leonard SS, et al. (2003) Effects of welding fumes of differing composition and solubility on free radical production and acute lung injury and inflammation in rats. Toxicological Sciences 75(1): 181–191. [DOI] [PubMed] [Google Scholar]
- Verma S, Buchanan Michael R, Anderson Todd J. (2003) Endothelial function testing as a biomarker of vascular disease. Circulation 108(17): 2054–2059. [DOI] [PubMed] [Google Scholar]
- Zheng W, Antonini JM, Lin YC, et al. (2015) Cardiovascular effects in rats after intratracheal instillation of metal welding particles. Inhalation Toxicology 27(1): 45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary_Figures for Effects of mild steel welding fume particles on pulmonary epithelial inflammation and endothelial activation by Johanna Samulin Erdem, Yke Jildouw Arnoldussen, Sepideh Tajik, Dag G Ellingsen and Shanbeh Zienolddiny in Toxicology and Industrial Health
Suppl_methods for Effects of mild steel welding fume particles on pulmonary epithelial inflammation and endothelial activation by Johanna Samulin Erdem, Yke Jildouw Arnoldussen, Sepideh Tajik, Dag G Ellingsen and Shanbeh Zienolddiny in Toxicology and Industrial Health