Abstract
Background
Chemotherapy usually induces a variety of side-effects in cancer treatment as it cannot tell normal cells apart from cancer cells and kills both. Chinese herbal medicine (CHM) has been regarded as a potential effective intervention for relieving the side-effects of chemotherapy in breast cancer patients.
Objective
This study aims to conduct a comprehensive systematic review and meta-analysis to evaluate the efficacy of CHM as adjuvant therapy for reducing the chemotherapy-induced side-effects in the treatment of breast cancer.
Methods
Main electronic databases were searched up to May 2020 for Randomized Controlled Trials (RCTs) evaluating the effect of CHM on breast cancer patients with chemotherapy. The PRISMA statement was adopted in this study and meta-analyses were performed.
Results
The included studies showed unsatisfied quality. Results based on available literature indicated that the adjunctive use of CHM with chemotherapy may reduce the chemotherapeutic agents-associated adverse events, including nausea and vomiting, diarrhea, alopecia, myelosuppression, and impaired immune function.
Conclusion
A confident conclusion could not be have due to the lack of large scale and high quality trials.
Keywords: herbal medicine, chemotherapy, side effect, breast cancer, meta-analysis
Introduction
Breast cancer is the most common female malignancy, impacting 2.1 million women each year (1). It is the leading cause of cancer-related death in women worldwide, accounting for 23% of all cancers and 14% of cancer-related deaths (2). Chemotherapy plays a crucial role in the treatment of breast cancer (3). It is extensively used in combination with surgery and radiation therapy. Chemotherapy could prolong lifetime and improve survival of cancer patients on one hand, whereas it also causes problematic side-effects, causing a variety of discomfort and burden for the patients (3, 4). The chemical drugs that are selectively destructive to tumor tissues are used in chemotherapy, but these agents cannot tell normal cells apart from cancer cells and kills both, which results in negatively impact compliance with cancer therapy (5). Therefore, developing interventions to alleviate the adverse side-effects induced by chemotherapy and enhance the well-being of patients are urgently needed in clinic.
Notably, the widely use of Chinese Herbal Medicine (CHM) in cancer patients attracts extensive attentions in recent years (6–8). An accumulative number of cancer patients take CHM as complementary and alternative medicine during chemotherapy to reduce side-effects and symptom discomfort as well as to enhance the body’s immune defences (7, 9). As a matter of fact, the use of Traditional Chinese medicine (TCM)-based CHM for breast cancer has been described for more than 2,000 years in China by TCM physicians (10, 11). It is considered that CHM shows the potency to alleviate the toxicities of chemotherapy agents and control the side-effects, such as improves the patient’s quality of life (QoL), prevents recurrence, and prolongs survival (12–15). Therefore, we performed this systematic review and meta-analysis to evaluate the efficacy of CHM as an adjunctive therapy to reduce side-effect of chemotherapy for breast cancer treatment.
Methods
This systematic review was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA). Protocol of this study was registered in International Prospective Register of Systematic Reviews (PROSPERO) with registration number CRD42020186977.
Search Strategy
Main electronic databases including PubMed, ISI Web of Knowledge, EMBASE, CINAHL Plus, AMED, Cochrane Library, China National Knowledge Infrastructure (CNKI), WanFang Data, Chongqing VIP (CQVIP) and SinoMed since the inception of each database up to end of May 2020. The following terms were searched in the databases: (Chinese herbal medicine OR Chinese Medicine OR herbal medicine OR materia medica OR medicinal plants OR herbs OR plant extract OR phytotherapy OR alternative medicine OR complementary medicine) AND (breast cancer or breast tumor or mammary cancer or breast carcinoma) AND (chemotherapy), with slight modifications for individual searches to suit the instructions of different databases.
Study Selection
Types of Studies
The review will include randomized controlled trials (RCTs) using a two-arm or three-arm parallel design for breast cancer patients with chemotherapy-induced side-effects.
Exclusion Criterion
Cross-over trials are not appropriate when an intervention can have a lasting effect that compromises entry to subsequent periods of the trial, or when a disease has a rapid evolution. In our study, CHM always showed a lasting effect on patients, and also, the progression of breast cancer could be fast. Thus, cross-over trials will be excluded. Any study with a sample size of no more than 10 patients will be excluded from this review. Moreover, studies with poor methodological quality (Jadad score = 0/1/2) will also be excluded.
Participants
Trials including participants that meet the diagnostic criteria of breast cancer will be included regardless of the stage of cancer. All eligible participants will be included in this study regardless of their gender age, gender or race. Trials with participants who are not appropriate to receive Chinese medicine therapy, such as bleeding disorder or allergy and other additional severe diseases will be excluded.
Interventions
CHM administered in different dose types, such as decoction, herbal preparation or extract, patented herbal formula, or herbal compounds. CHM may be administrated before or during or after chemotherapy regardless of treatment course via oral or injection. In this review, CHM VS placebo, CHM alone VS conventional western medicine for reducing side-effects of chemotherapy, or CHM combined with conventional western medicine VS conventional western medicine alone will be included.
Outcome Measures
vomiting and nausea: frequency and severity of vomiting/nausea;
diarrhea: frequency and severity of diarrhea
constipation: frequency and severity of constipation
alopecia
myelosuppression: reduction of WBC and blood platelet count
impaired immune function: CD3+, CD4+, CD8+, CD4/CD8 ratio, NK cells population
Data Extraction and Assessment of Methodological Quality
Methodological quality of pooled studies was evaluated by using the risk of bias tools according to Cochrane Handbook version 5.1.0. There are six items of risk of bias for assessing the methodological quality of RCTs: selection bias (random sequence generation and allocation concealment), performance bias (blinding of participants and personnel) and detection bias (blinding of outcome measurement), attrition bias (incomplete outcome data), reporting bias (selective reporting), and other bias. Each item was ranked as low risk, unclear risk, and high risk. For random sequence generation, a low risk of selection bias will be judged if a random component in the sequence generation process such as using a computer random number or a random number table is described while a high risk of selection bias will be considered if a non-random component in the sequence generation process is provided. For allocation concealment, a low risk of selection bias will be judged if the investigators enrolling participants could not foresee assignment, for example, central allocation sequentially numbered, opaque, sealed envelopes, or sequentially numbered drug containers of identical appearance were used, while a high risk of bias will be considered if investigators enrolling participants could possibly foresee assignments, for example, envelopes were unsealed or non-opaque. In terms of performance bias, a low risk was considered when blinding of participants was performed or we judged that the outcome will not be influenced even there was incomplete blinding. Similarly, there is low risk of detection bias when the blinding of the results’ assessment was conducted or we judged that the results will not be influenced by lack of blinding. A low risk of attrition bias will be judged if there were no missing outcome data, and a low risk of reporting bias will be considered when the study protocol is available, or it is clear that the paper include all expected outcomes if the study protocol is not present. Two authors assessed the methodological quality of all trials independently and any disagreements were solved by the third author consensus.
Data Analysis
Cochrane Collaboration Review Manage software (RevMan 5.4) was used for data analysis. Dichotomous data were reported as relative risk (RR) with 95% confidence intervals (95% CI), whereas continuous data were expressed as mean ± standard deviation (SD). In our recruited studies, chemotherapy-induced nausea and vomiting, diarrhea, constipation, alopecia, and myelosuppression were assessed by grading the severity and frequency, presenting as dichotomous data, while the evaluation of the immune function was performed by detecting the population of T cells such as CD3+/CD4+/CD8+ subtypes and Natural Killer (NK) cells, which are presented as mean ± SD. I 2 was used to measure the heterogeneity; when the heterogeneity was significant in the included studies (I 2 > 50%), a random-effect model would be considered; otherwise the fix-effect model was adopted. If I 2 < 0.05, the differences between the CHM treatment groups and control groups were regarded as statistically significant. In the present study, we performed sub-group analysis based on the principles of TCM treatment of breast cancer, which mainly include three aspects: strengthening the body (Fu Zheng, +), eliminating pathogenic factors (Qu Xie, −), or both (Fu Zheng and Qu Xie, ±).
Results
Characteristics of the Included Studies
In initial database searching, total 1,288 studies were retrieved. After duplicated studies removal, there remained 471 records. After further review of the abstracts, 354 records were excluded with reasons of animal studies, retrospective studies, literature reviews or not relevant clinical studies with CHM and chemotherapy. Then only 117 records are eligible for further consideration, and only 80 full-text are available. After full-text review, 20 studies with poor methodological quality as indicated by Jadad score (<3), one study with small sample size (n < 10), one study with crossover trials, four with incomplete data were excluded. So there were a total of 54 studies included for meta-analysis in our study. Among them, three studies were retrieved from English the database and 51 from Chinese database. The flow diagram of the study selection was shown in Figure 1 .
Figure 1.
Study flow diagram.
A total of 4,032 patients were enrolled in these included studies, among which 2,081 patients received CHM interventions while 2,002 patients participated in the control group, and 49 patients dropped out. All included patients in these studies were diagnosed via pathological examination. The baseline characteristics of the included studies were listed in Table 1 . All those studies showed a comparable baseline data. The risk of bias of recruited studies was evaluated by the tool of Cochrane Collaboration. Most of pooled studies performed a random grouping referring to a random number table or using a computer random number, indicating the low risk of selection bias in terms of random sequence generation ( Figures 2 and 3 ). In most of these studies, the allocation concealment and blinding of participants and personnel as well as blinding of outcome assessment were not mentioned, resulting in the unclear/high risk of selection bias, performance bias and detection bias, as shown in Figures 2 and 3 .
Table 1.
The characteristics of included studies (CHM belongs to Fu Zheng (+), Qu Xie (−), or Fu Zheng and Qu Xie (±)).
| Study | Sample size (T + C)/drop-outs | Diseasestage | Chemotherapy | Experimental CHMs | Control group intervention | Duration | Outcome measures | Jadad scale |
|---|---|---|---|---|---|---|---|---|
| Ansari et al. (16) | 150(57 + 62)/31 | Not mentioned | AC, CAF and TAC | Ginger powder, (−) | Placebo capsules | Twice a day for 3 days | Frequency and grade of CINV | 5 |
| Bai et al. (17) | 64(32 + 32)/0 | I–IV | TEC | Jinlong capsule, (−) | Not mentioned | 12 weeks | Tumor response and chemotoxicity, KPS | 3 |
| Chen et al. (18) | 60(30 + 30)/0 | I–III | CEF : CTX/EPI/5-Fu | Danggui Buxie decoction, (+) | No placebo | 24 weeks | QoL, chemotoxicity, T cells population | 3 |
| Chen et al. (19) | 60(28 + 29)/3 | I–III | GP : GCB + PDD | Sugan Jianpi Sanjie compound, (±) | Not mentioned | 6 weeks | Chemotoxicity, KPS, T cells population | 3 |
| Chen et al. (20) | 56(28 + 28)/0 | Not mentioned | Chemotherapy without mention of specific drugs | Yiqi Jianpi Hewei Therapy. (+) | Ondansetro, and Dexamethasone Sodium Phosphate | 10 days | Gastrointestinal adverse reaction (CTCAE3.0V), QOL, recurrence rate | 3 |
| Cheng (21) | 50(26 + 24)/0 | Not mentioned | TP : TAX/PDD | Fuzheng Kangai compound, (±) | No placebo | 8 weeks | Tumor response and chemotoxicity, KPS | 3 |
| Cheng and Zhang (22) | 40(20 + 20)/0 | IV | XD | Fuzheng Jiedu formula, (±) | Not mentioned | 6 weeks | KPS | 3 |
| Dang and Wang (23) | 48(28 + 20)/0 | I–III | CTF | Aidi injection. (+) | Not mentioned | 9 weeks | Tumor response, KPS, cardiotoxicity, chemotoxicity | 3 |
| Fang and Jia (24) | 30(16 + 14)/0 | II–IV | CE : CTX + EPI | Herbal formula, (±) | No placebo | 12 weeks | Tumor response and chemotoxicity, KPS | 3 |
| Gao et al. (25) | 132(66 + 66)/0 | Not mentioned | AF/AC | Fuzheng Jiandu Xiaoliu Decoction, (±) | No treatment | 6 months | T cells population, gastrointestinal reaction | 3 |
| Guo et al. (26) | 30(10 + 20)/0 | IV | CEF | Modified Sijunzi Decoction. (+) |
No treatment | 3 weeks | T cells population | 3 |
| Guo (27) | 86(43 + 43)/0 | I–III | CEF | Yiqi Yangxie Shengjin formula, (±) | Not mentioned | 9 weeks | Myelosuppression, WHO standard | 3 |
| Hao et al. (28) | 96(49 + 47)/0 | I–III | CEF | Guilu Erxian Decoction, (±) | Not mentioned | 18 weeks | T cells population, KPS | 3 |
| He and Ruan (29) | 80(40 + 40)/0 | II–III | AC, CAF, EC | Xiangsha Liujunzi and Jupi Zhuru decoction. (+) | Not mentioned | 5 days | European society of clinical oncology recommended standards | 3 |
| Hu and He (30) | 68(34 + 34)/0 | I–IV | TA/CAF | Yiqi Jianpi Shugan Decoction, (±) | Placebo | 12 weeks | Changes of cell immune function and quality of life (Karnofsky) | 3 |
| Hu et al. (31) | 52(28 + 24)/0 | II–IV | CAF : CTX/ADM/5-Fu | Yiqi Huoxie decoction. (+) | No placebo | 20 days | Tumor response and chemotoxicity | 3 |
| Huang et al. (32) | 66(37 + 29)/0 | II–IV | CMF : CTX/MTX/5-Fu | Modified Bazhen decoction. (+) | No placebo | 6 weeks | Tumor response, KPS and chemotoxicity | 3 |
| Huang et al. (33) | 60(30 + 30)/0 | IV | CTF | Jianpi Xiaoji decoction, (±) | Not mentioned | More than 4 weeks | quality of life (Karnofsky), T cells population | 4 |
| Huang et al. (34) | 60(30 + 30)/0 | IV | CEF | Huangqi injection. (+) | 18–24 weeks | Tumor response, KPS, chemotoxicity | 3 | |
| Li and Han (35) | 85(44 + 41)/0 | I–IV | CEF | Fuzheng Xiaoliu compound, (±) | No treatment | 9 weeks | Tumor response and chemotoxicity, tumor marker | 3 |
| Li and Gong (36) | 52(32 + 20)/0 | I–III | CEF | Aidi injection. (+) | Not mentioned | 9 weeks | Tumor response, chemotoxicity | 3 |
| Li and Zhong (37) | 120 (60 + 60)/0 | I–IV | FAC | Bushen Shugan Huatan decoction, (±) | Vitamin et al | 18 weeks | WHO standard: nausea and vomiting, myelosuppression, survival rate | 3 |
| Li et al. (38) | 101(61 + 40)/0 | I–III | CMF | Rukang I prescription. (+) | Not mentioned | 6–9 weeks | Tumor response, KPS, chemotoxicity | 3 |
| Li et al. (39) | 75(40 + 35)/0 | I–IV | NE : NDP/EPI | Shenqifuzheng injection. (+) | Not mentioned | 12 weeks | Tumor response and chemotoxicity | 3 |
| Li and Li(40) | 128(64 + 64)/0 | I–II | postoperative chemotherapy of ROBC | Fuzheng Xiaoliu decoction, (±) | Placebo | 18 weeks | T cells, inflammation marker, QoL | 3 |
| Liang et al. (41) | 98(48 + 50)/0 | I–IV | TAC | Huaier Granule, (−) | Not mentioned | 24 weeks | T cells population and survival rate | 3 |
| Liu et al. (42) | 66(31 + 35)/0 | I–II | CAF | Tianzhicao capsule, (±) | Not mentioned | 24 weeks | KPS, chemotoxicity | 3 |
| Liu et al. (43) | 50(25 + 25)/0 | I–IV | TE : TAX/EPI | Renshen Yangrong decoction. (+) | No placebo | 6 weeks | KPS, T cells population | 3 |
| Lu et al. (44) | 90(45 + 45)/0 | I–IV | FEC | Huaier granule, (−) | No treatment | 3 weeks | T cells and NK cells | 4 |
| Lv et al. (45) | 54(27 + 27)/0 | IV | FAC | Yiqi Huoxie Huayu decoction, (±) | 12–16 weeks | Tumor response, KPS, chemotoxicity and immune function | 3 | |
| Ni (46) | 57(30 + 27)/0 | IV | Docetaxel + THP | Gaolisheng injection. (+) | Not mentioned | 13 weeks | Tumor response and chemotoxicity | 3 |
| Semiglazov et al. (47) | 352(169 + 168)/15 | pT1–T3 pN0-N+ (0–10 positive lymph nodes) pM0 |
CMF | 0.5 ml of the study medication PS76A2 (aqueous mistletoe extract). (+) |
0.5 ml placebo | Twice weekly for 16 to 24 consecutive weeks |
FACT-G scale | 3 |
| Sun et al. (48) | 74(37 + 37)/0 | Not mentioned | CEF or TEC | Shugan Jianpi, Fuzheng Xiaoyu decoction, (±) | granisetron hydrochloride | 15 days | QLQ - C30 | 3 |
| Tang et al. (49) | 60(30 + 30)/0 | I–IV | CEF : CTX/EPI/5-Fu | Yiqi Jiedu decoction, (±) | No placebo | 12 weeks | T cells population and KPS | 3 |
| Wang (50) | 60(30 + 30)/0 | II–III | CEF | Taohong Siwu decoction, (±) | Not mentioned | 9 weeks | QoL and chemotoxicity | 3 |
| Wang (51) | 40(20 + 20)/0 | II–IV | TE | Yiqijianpi Huayujiedu decoction, (±) | Not mentioned | 8 weeks | Tumor response, QoL and chemotoxicity | 4 |
| Wen et al. (52) | 60(30 + 30)/0 | IV | TA | Fuzheng Xiaoyan prescription, (±) | Not mentioned | 3 weeks | Tumor response, QoL | 4 |
| Xiong et al. (53) | 92(50 + 42)/0 | I–III | TAC | Huaier granule, (−) | Placebo | 18 weeks | T cells population and NK cells | 3 |
| Xu (54) | 60(30 + 30)/0 | Not mentioned | Doxorubicin, Cyclophosphamide, 5-Fu | Sini Decoction. (+) | Not mentioned | 2 weeks | Heart toxicity, WHO standard | 3 |
| Yang (55) | 59(31 + 28)/0 | IV | NVB + THP | Aidi injection. (+) | Not mentioned | 6 weeks | Tumor response, KPS and immune function | 3 |
| Yang and Sun (56) | 51(27 + 24)/0 | Not mentioned | CMF : CTX/MTX/5-Fu | Modified Liujunzi decoction. (+) | No placebo | 9 weeks | Tumor response and chemotoxicity | 3 |
| Yang et al. (57) | 38(20 + 18)/0 | I–IV | CEF : CTX/EPI/5-Fu | Taohongsiwu decoction, (±) | No placebo | 9 weeks | Tumor response and chemotoxicity | 3 |
| Yang (58) | 52(26 + 26)/0 | Not mentioned | CEF or TEC | Yiqi Jianpi Hewei Therapy. (+) | Ondansetro, and Dexamethasone Sodium Phosphate | 10 days | Gastrointestinal adverse reaction (CTCAE3.0V) | 3 |
| Yu and Pang (59) | 164(82 + 82)/0 | III–IV | CAF | Fuzheng Xiaozheng Qudu formula therapy, (±) | Not mentioned | 24 weeks | KPS | 3 |
| Yu et al. (60) | 93(47 + 46)/0 | I–IV | Docetaxel and adriamycin | Xiaoaiping tablet, (−) | Placebo | 2 weeks during each chemotherapy cycle | QoL; BWB, EWB, FWB, PWB, and SWB scores |
5 |
| Zhang (61) | 50(26 + 24)/0 | I–IV | NP : NDP/PDD | Herbal formula, (±) | No placebo | 4 weeks | Tumor response and chemotoxicity, KPS | 3 |
| Zhang (62) | 70(40 + 30)/0 | Not mentioned | CTX+EPI | Fuzheng Jiandu Xiaoliusan formula, (±) | Not mentioned | 5 months | Adverse effect, CD4, CD8 | 3 |
| Zhang and Dang (63) | 60(30 + 30)/0 | I–IV | NP: NDP+PDD | Fuzheng Guben compound. (+) | Not mentioned | 4 weeks | RECIST, KPS, chemotoxicity | 3 |
| Zhang and Wang (64) | 110(55 + 55)/0 | I–III | FEC | Shenmai injection. (+) | No treatment | 19 weeks | TCM symptom score and T cells population | 4 |
| Zhang et al. (65) | 80(40 + 40)/0 | I–III | CEF | Huangqi Taohong decoction, (±) | Not mentioned | 18 weeks | Immune system | 3 |
| Zhang et al. (66) | 45(23 + 22)/0 | III–IV | CTF | Fuzheng Quyu Jiedu prescription, (±) | 6 weeks | QoL and immune system | 3 | |
| Zhang (67) | 50(25 + 25)/0 | Not mentioned | GT | Shugan Jianpi Yishen decoction, (±) | Not mentioned | 15 weeks | Tumor response (RECIST), Chemotoxicity (CTCAE v3.0) | 3 |
| Zhong (68) | 40(20 + 20)/0 | I–IV | TE | Shugan Tiaoli Chongren decoction, (±) | Not mentioned | 6 weeks | QoL, KPS, and chemotoxicity | 3 |
| Zhou (69) | 108(54 + 54)/0 | II–IV | Not shown | Guipi & Guilu Erxian Decoction, (±) | Placebo | 2 months | WBC count | 3 |
AC, Adriamycin + Cyclophosphamide; CAF, Cytoxan + Adriamycin + Fluorouracil; TAC, Taxotere + Adriamycin + Cyclophosphamide; TEC, Taxotere + Epirubicin + Cyclophosphamide; CEF, CTX/EPI/5-Fu, Cyclophosphamide + Epirubicin + 5-Fluorouracil; GP, GCB + PDD: Gemcitabine + Cisplatin; TP, TAX/PDD: Taxotere + Cisplatin; XD, Docetaxel + Capecitabine; CTF, Cyclophosphamide + Pirarubicin + 5-Fluorouracil; CE, CTX + EPI: Cyclophosphamide + Epirubicin; EC, Epirubicin + Cyclophosphamide; TA, Taxotere + Adriamycin; CAF, CTX/ADM/5-Fu: Cytoxan + Adriamycin + Fluorouracil; CMF, CTX/MTX/5-Fu: Cytoxan + Methotrexate + Fluorouracil; FAC, Fluorouracil + Adriamycin + Cytoxan; NE, NDP/EPI: Nedaplatin + Epirubicin; TE, TAX/EPI: Taxotere + Epirubicin; FEC, 5-Fluorouracil (5FU) + Epirubicin + Cyclophosphamide; THP, Taxotere + Herceptin + Perjeta; CMF, Cyclophosphamide + Methotrexate + 5-Fluorouracil; NVB + THP, Vinorelbine + Taxotere + Herceptin + Perjeta; NP, NDP/PDD: Nedaplatin + cisplatin; CTX + EPI, Cyclophosphamide + Epirubicin; GT, Gemcitabine + Paclitaxel.
Figure 2.
Risk of bias graph.
Figure 3.

Risk of bias summary.
Chemotherapy-Induced Nausea and Vomiting
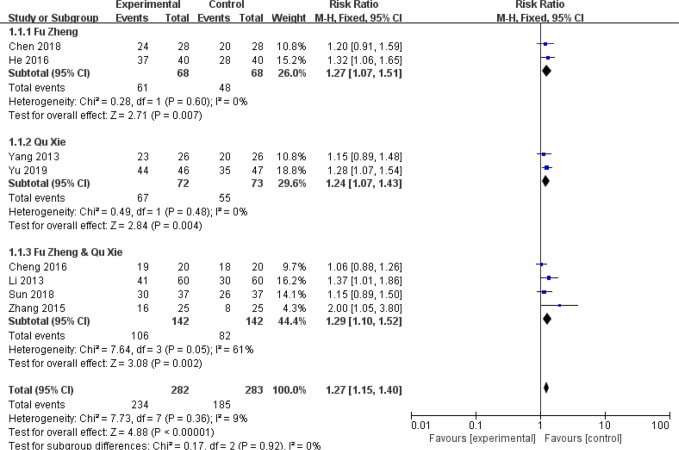
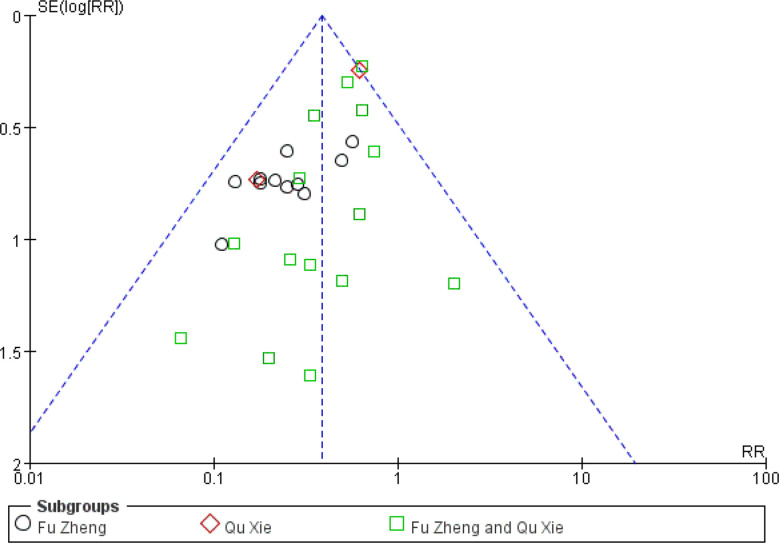
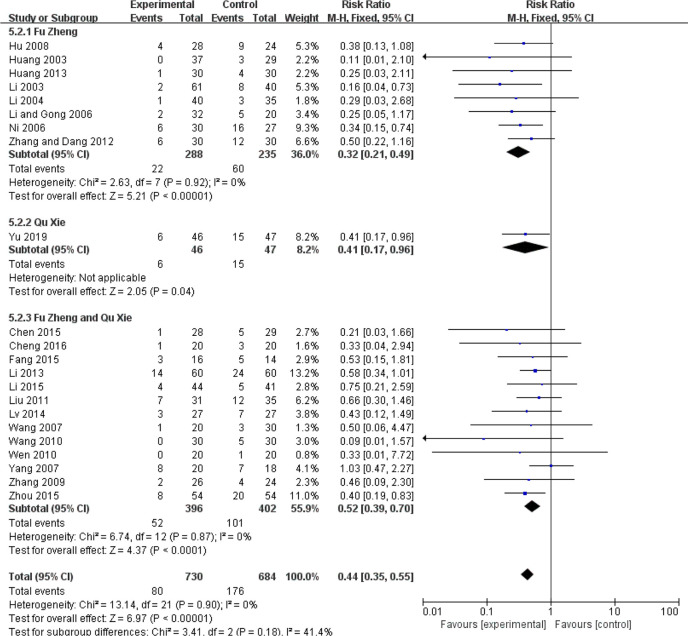
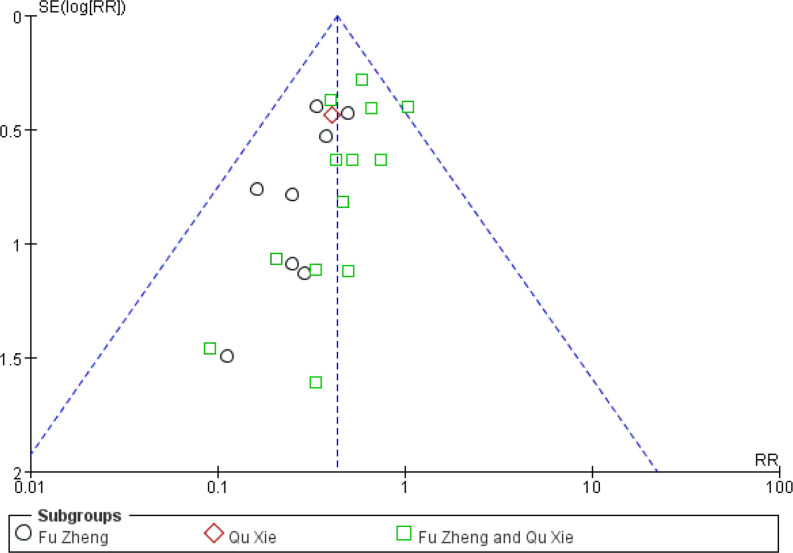
Among the side-effects induced by chemotherapy, chemotherapy-induced nausea and vomiting (CINV) is the most common symptom (70, 71). According to the chemotherapy-induced toxicity grading criteria, for example CTCAE version 4, none/one to two episodes/three to five episodes in 24 h was graded as Grade 0/1/2, which could be considered as mild to moderate toxicity, while others such as ≥6 episodes in 24 h are severe toxicity. In certain grade of our pooled studies, there are only zero or one or two events in either the experimental or control groups. To avoid the imprecision of the pooled results due to few events of some grades, combination of these data from several grades is adopted. The frequency of CINV with grades 0–II was significantly higher in the CHM group than in the control group (RR = 1.27, 95% CI = 1.15–1.4, I2 = 9%, eight studies (18, 22, 29, 37, 48, 58, 60, 67), 665 patients Figures 4 and 5 ). For each sub-group analysis, the frequency of CINV with grades 0–II was significantly higher in the CHM group than in the control group. Moreover, there is no statistical significance between these sub-groups (p = 0.92). Regarding the frequency of grades III–IV, which indicated severe nausea and vomiting (RR = 0.39, 95% CI = 0.32–0.48, I2 = 0%, 28 studies (17, 19, 20, 22–24, 29, 31, 32, 34–39, 42, 45, 48, 50–52, 56–58, 60, 61, 63, 67, 68), 1,774 patients, Figures 6 and 7 ), was lower significantly in patients receiving CHM treatment, suggesting the beneficial effects of CHM in alleviating CINV. Our sub-group analysis showed that the frequency of grades III–IV was significantly lower in the CHM group than in the control group. Notably, there is statistical significance between these sub-groups (p = 0.04), suggesting CHM belonging to different TCM theory showed different magnitude of therapeutic effects. In the study of Ansari et al., the criteria used are the one defined by the authors (grades 0–III); thus we did not include this study to perform the meta-analysis. This study found that ginger reduced vomiting severity from 1.4 ( ± 1.04) to 0.71 ( ± 0.86) in all sessions, but the results showed no statistically significance. Only in patients of the AC sub-group, ginger treatment significantly decreased the severity of vomiting (0.64 ± 0.87) compared to those from the placebo group (1.13 ± 1.12).
Figure 4.
Forest plot of CINV in patients of breast cancer (toxicity grades 0–II).
Figure 5.
Funnel plot of CINV in patients of breast cancer (toxicity grades 0–II).
Figure 6.
Forest plot of CINV in patients of breast cancer (toxicity grades III–IV).
Figure 7.
Funnel plot of CINV in patients of breast cancer (toxicity grades III–IV).
Diarrhea
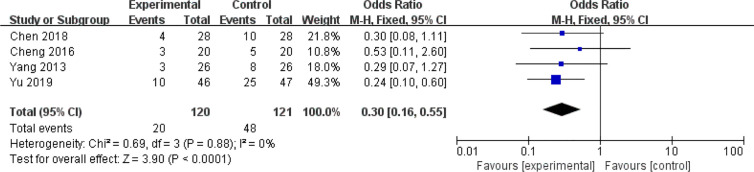
Another common gastrointestinal adverse effects of chemotherapy is diarrhea, which occurs in up to 60% of cancer patients treated with chemotherapy, and 10% have severe diarrhea (72, 73). As shown in Figures 8 and 9 , the events of severe diarrhea in the CHM treated group were significantly lowered than that of the control group (RR = 0.3, 95% CI = 0.16–0.55, I2 = 0%, four studies (20, 22, 58, 60), 241 patients, Figures 8 and 9 ). Among those four studies, two studies (22, 60) defined the severity of diarrhea by using the World Health Organization (WHO) criteria, while the other two evaluated it according to CTCAE3.0V. Although the criteria used is not the same, they are comparable because both of them defined the severity into five level grades (the higher number, the more severe it is). Of note, the study by Yu et al. (60) showed high quality, which was assessed as low risk of selection bias, performance bias, detection bias, attrition bias, and reporting bias. In this study, Xiaoaiping, a drug composed of the Chinese herb Marsdeniae tenacissimae (Z20063919), showed remarkable effects in the reduction of diarrhea.
Figure 8.
Forest plot of diarrhea in patients of breast cancer (toxicity grades III–IV).
Figure 9.
Funnel plot of diarrhea in patients of breast cancer (toxicity grades III–IV).
Constipation
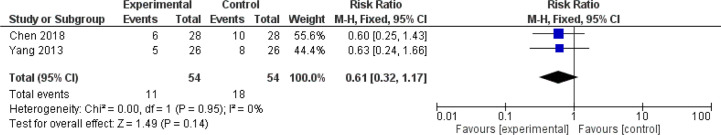
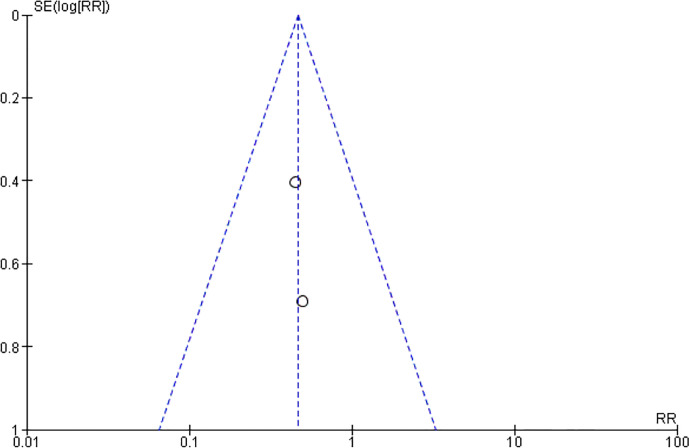
In some cases, chemotherapy induced the disordered lining of the intestine, resulting in constipation (73). The altered eating habits or reduced activity level due to fatigue and decreased appetite may also cause bowel irregularity and constipation (74, 75). Two of our recruited studies (20, 58) have assessed the efficacy of a traditional CHM named Yiqi Jianpi Hewei on chemotherapy induced constipation in breast cancer patients. Figures 10 and 11 showed Yiqi Jianpi Hewei therapy alleviated the occurrence rate of severe constipation, while without statistical significant difference (grades II–IV, RR = 0.61, 95% CI = 0.32–1.17, I2 = 0%, two studies (20, 58), 108 patients).
Figure 10.
Forest plot of constipation in patients of breast cancer (toxicity grades III–IV).
Figure 11.
Funnel plot of constipation in patients of breast cancer (toxicity grades III–V).
Alopecia
As chemotherapeutic agents generally interfere with cells of rapidly dividing, not only tumor cells but also hair follicles, it may cause alopecia (76, 77). After the chemotherapy is finished, hair commonly grows back but in some cases hair cannot regrow (73, 74). In our recruited studies, two studies (59, 60) enrolled 257 patients evaluated the effect of two CHM on alleviating alopecia. As shown in Figures 12 and 13 , Xiaoaiping and another herbal formula Fuzheng Xiaozheng Qudu could significantly reduce the events of alopecia caused by chemotherapy in patients with breast cancer (RR = 0.39, 95% CI = 0.17–0.88, I2 = 0%). There is another randomized, double-blind, multi-center clinical trial to study the effect and mechanism of YH0618 granule on chemotherapy-induced hair loss in breast cancer patients (78). Because this clinical trial is still on-going, and no completed data is available, this study has to be excluded in this meta-analysis.
Figure 12.
Forest plot of alopecia in patients of breast cancer (toxicity grades III–IV).
Figure 13.
Funnel plot of alopecia in patients of breast cancer (toxicity grades III–IV).
Reduced White Blood Cell and Blood Platelet Count
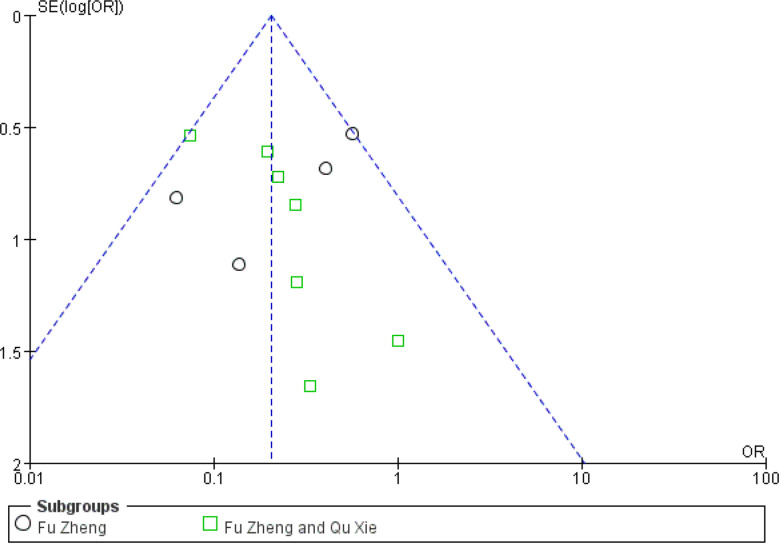
The reduced white blood cell (WBC) and blood platelet count are used to assess myelosuppression, which is another common side-effect of chemotherapy, referring to impaired bone marrow activity (79, 80). For WBC reduction, the occurrence rate of grades III–IV was significantly reduced in the CHM groups (RR = 0.44, 95% CI = 0.35–0.55, I2 = 0%, 22 studies (19, 22, 24, 31, 32, 34–39, 42, 45, 46, 50–52, 57, 60, 61, 63, 69), 1,414 patients, Figures 14 and 15 ). Sub-group analysis showed that the occurrence rate of grades III–IV was significantly reduced in each sub-group. There is no statistical significance between these sub-groups (p = 0.18). Regarding blood platelet count, the reduction of blood platelet in the CHM groups is much less than that of the control groups (RR = 0.2, 95% CI=0.13–0.31, I2 = 17%, 11 studies (19, 22, 34, 35, 37, 39, 42, 45, 50–52, 61, 63), 827 patients, Figures 16 and 17 ).
Figure 14.
Forest plot of WBC reduction in patients of breast cancer (toxicity grades III–IV).
Figure 15.
Funnel plot of WBC reduction in patients of breast cancer (toxicity grades III–IV).
Figure 16.
Forest plot of blood platelet reduction in patients of breast cancer (toxicity grades III–IV).
Figure 17.
Funnel plot of blood platelet reduction in patients of breast cancer (toxicity grades III–IV).
Impaired Immune Functions
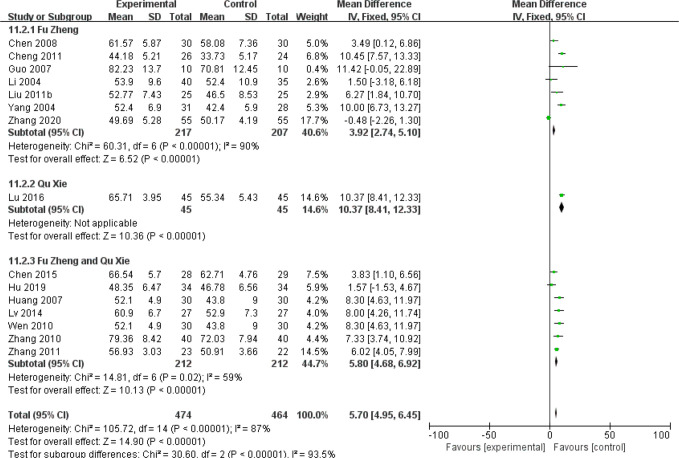
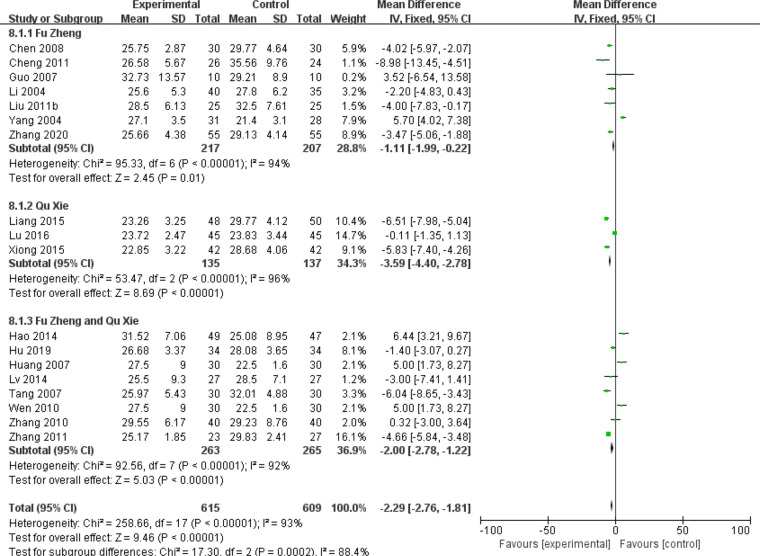
Chemotherapy would weaken the immune system (81). The impaired immune functions include the neutropenia, reduced T cells population and natural killer (NK) cells as well as serum IgG level (81, 82). Data of those cell population are continuous and mean difference analysis is adopted to decrease the influence of mean value difference. In terms of T cells, the population of CD3+ cells were significantly increased in patients with CHM intervention (MD = 5.7, 95% CI = 4.95–6.45, I2 = 87%, 15 studies (18, 19, 21, 26, 30, 33, 39, 43–45, 52, 55, 64–66), 938 patients, Figure 18 ). Regarding CD4+ cells (MD = 5.18, 95% CI = 4.68–5.69, I2 = 96%, 19 studies (18, 19, 21, 26, 28, 30, 33, 39, 41, 43–45, 52, 55, 64–66), 1,231 patients, Figure 19 ) and CD8+ cells (MD = −0.47, 95% CI = -0.59–0.34, I2 = 92%, 18 studies (18, 19, 21, 26, 28, 30, 33, 39, 41, 43–45, 52, 53, 55, 63, 65, 66), 1,124 patients, Figure 20 ) also increased in the CHM groups compared to that of the control group. Sub-group analysis showed that for each Fu Zheng, Qu Xie or Fu Zheng and Qu Xie sub-groups, the immune functions were significantly improved via CHM intervention. Interestingly, there is still high heterogeneity within each sub-group, which suggests there is another factor causing the heterogeneity of impaired immune functions. The ratio of CD4+/CD8+ also increased by CHM treatment (MD = 0.34, 95% CI = 0.29–0.38, I2 = 79%, 18 studies (18, 19, 25, 27, 28, 30, 33, 39, 41, 43–45, 49, 52, 55, 64–66), 1,274 patients, Figure 21 ). Figure 22 indicated the population of NK cells are raised in breast cancer patients from the CHM groups (MD = 0.71, 95% CI = 0.50–0.92, I2 = 84%, 5 studies (30, 44, 45, 53, 65), 384 patients). However, of note, all of those studies showed high heterogeneity, which might result from the measure approaches, different cancer stages and age of patients, as well as sample size.
Figure 18.
Forest plot of CD3+ in patients of breast cancer after chemotherapy.
Figure 19.
Forest plot of CD4+ in patients of breast cancer after chemotherapy.
Figure 20.
Forest plot of CD8+ in patients of breast cancer after chemotherapy.
Figure 21.
Forest plot of CD4+/CD8+ ratio in patients of breast cancer after chemotherapy.
Figure 22.
Forest plot of NK cells in patients of breast cancer after chemotherapy.
Discussion
Currently, the treatment of breast cancer includes surgery, chemotherapy, radiation therapy, hormonal therapy, targeted therapy, and immunotherapy (3, 5), among which, chemotherapy is extensively used in not only early-stage invasive but also advanced-stage breast cancer, as well as before surgery to shrink the tumor in some cases (83). Generally, a combination of two or more chemotherapeutic agents is adopted as chemotherapy regimens for breast cancer (84). It shows remarkable efficacy in destroying cancer cells but also causes non-negligible adverse effects on patients, including nausea and vomiting, diarrhea, constipation, alopecia, myelosuppression, impaired immune function, etc. The severity of side-effects depends on the regimen type, dose used, treatment length, and general health of patients. Although several western medicines such as dexamethasone, 5-HT3 receptor antagonist, glutathione, magnesium sulfate and calcium gluconate have been used to reduce chemotherapy-induced side effects, they still hardly satisfy the requirements of patients (85). Thus, an adjuvant treatment to manage these side-effects is expected.
Increasing CHM is clinically used in breast cancer patients receiving chemotherapy for its beneficial effects in alleviating the side-effects. As a matter of fact, there are several systematic reviews and meta-analyses evaluating the efficacy of CHM combined with conventional therapy such as surgery, chemotherapy, or endocrine therapy for breast cancer (86–88). The tumor response, quality of life, hot flashes were particularly focused. In 2016, there are two review articles (86, 87) performed meta-analysis to assess CHM as adjunctive therapy to chemotherapy for breast cancer. Both of them did not get a confident conclusion due to the limited data. They did not identify as many studies as it could have. In this study, we have collected as many studies as we can and performed a meta-analysis to assess the efficacy of CHM in alleviating side-effects induced by chemotherapy in breast cancer patients. Plentiful studies have evaluated the tumor response and quality of life after adjunctive treatment of CHM and chemotherapy, but we focused on the adverse effects, and only studies with evaluation of chemotoxicity are included for meta-analysis in our study. All available data of those recruited studies were used without intentional selection. Regarding CINV, diarrhea, constipation, alopecia, and myelosuppression, dichotomy data is used to describe the frequency of adverse events. Although the criteria for classification are slightly different, those data from different studies showed low heterogeneity. Results showed that patients treated with different CHMs could significantly alleviate symptoms such as CINV, diarrhea, alopecia, and myelosuppression. In terms of the evaluation of immune function, the level of CD3+ T cells, CD4+ population, CD8+ population, CD4+/CD8+ ratio and the NK cell population are presented as continuous data. Although results after meta-analysis showed CHM intervention significantly improved T cells and NK cell population, significant heterogeneity was observed among these pooled studies. The different regimens, treatment duration, therapeutic dose, as well as age and cancer stage of patients might contribute to the high heterogeneity. To obtain more reliable conclusion, sub-group analysis according to chemotherapeutic regiments or cancer stage should be performed. In the present study, we performed sub-group analysis based on the principles of TCM treatment of breast cancer, which mainly include three aspects: strengthening the body (Fu Zheng, +), eliminating pathogenic factors (Qu Xie, −), or both (Fu Zheng and Qu Xie, ±). Sub-group analysis showed that for each Fu Zheng, Qu Xie or Fu Zheng and Qu Xie sub-groups, the immune functions were significantly improved via CHM intervention. Interestingly, there is still high heterogeneity within each sub-group, which suggests there is another factor causing the heterogeneity of impaired immune functions.
In this meta-analysis study, we have paid special attention to CINV, diarrhea, constipation, alopecia, myelosuppression, and reduced immune function, which are common symptoms of chemotherapy-associated adverse effect. Beside them, there are still many other symptoms which have been reported in breast cancer patients receiving chemotherapy, such as flash hot, kidney injury, liver injury, and cardiotoxicity from time to time (89, 90). However, the limited studies we obtained have clearly evaluated the efficacy of CHM on those adverse symptoms of chemotherapy-treated breast cancer patients due to the relatively low occurrence rate. In the study of Cheng and Zhang (22), they recruited 40 chemotherapy-treated breast cancer patients and assessed the relative level of aminotransferase in groups receiving Fuzheng Jiedu decoction or not. The results showed this decoction could slightly reduce the incidence rate of increased aminotransferase, two patients from the CHM group and four patients from the control group. In another study (59), the events of abnormal liver and kidney function in chemotherapy-treated breast cancer patients were recorded. There are two patients who reported abnormal liver and kidney function in the CHM group (Modified Fuzheng Xiaozheng Qudu decoction) while eight patients in the control group. Xu (54) has reported the effect of an herbal formula Sini Decoction on alleviating the anthracycline associated cardiotoxicity in breast cancer patients. This study claimed that Sini Decoction effectively slowed down cardiac toxicity and reduced the process of myocardial damage. More trials are needed to further confirm the positive role of CHM in ameliorating those chemotherapy-associated toxicities.
Though we strictly performed this meta-analysis according to review procedure claimed by the Cochrane Collaboration, this study has several limitations. First, the quality of pooled studies is generally poor. Most of these studies did not mention the allocation concealment and blinding of participants and personnel as well as blinding of outcome assessment, resulting in the unclear/high risk of selection bias, performance bias, and detection bias, which may overestimate the efficacy of intervention. Moreover, the detailed approaches of random grouping, follow-up and drop-out rate are not clear in most studies, particularly for Chinese papers. The details of sample size calculation are also missing in our pooled studies. Additionally, most of the studies haven’t provided placebo to patients of the control group, which may also lead to performance bias. Due to the nature of CHM, it is difficult to have placebo that could well mimic herbs (91). This is still a limitation and challenge of many trials of Chinese medicine. Secondly, there might exist publication bias in our enrolled studies. Almost all studies presented the positive data, but negative results might be unreported. Thirdly, the CHM intervention approaches are different from each other. In addition to the CHM composition (herbal formula, single herb, pure compound), the way to take the medicine (oral intake or intravenous injection) and dose used may contribute to high heterogeneity. Lastly, due to language barrier, only papers in English and Chinese are included in our study. Since CHM is also widely used in other Asian countries such as South Korea and Japan, additional studies published in their local language should also be considered.
At present, although the usage of CHM in the treatment of breast cancer has been described for thousands of years in China, accepting CHM remains a challenge nowadays in western countries. One of the reasons is due to language barrier. Most clinical trials regarding the use of CHM on breast cancer are reported in Chinese. Thus, more clinical trials reported in English are warranted to facilitate the globalization of Chinese Medicine. Another concern raised from scientists and physicians is whether CHM would have an influence on the pharmacokinetics or anti-cancer effect of chemotherapeutic regimens when used in combination (92). In some animal studies, it is reported that some of CHM such as berberine showed no interaction with chemotherapeutic agents and did not reduce the anti-tumor efficacy (93–95). In clinic trials, however, few studies performed pharmacokinetics evaluation of chemotherapeutic agents in patients receiving CHM treatment. Last but not least, scientific evidence of molecular mechanism underlying its beneficial effects on chemotherapy induced adverse effects is required to comprehensively understand and more appropriately use CHM in clinical for breast cancer.
Conclusion
Because of the limitations of low quality of the enrolled trials, it is hard to reach a firm conclusion. Nevertheless, our work suggested the potencies of CHM to facilitate the management of chemotherapy toxicity, and more efforts are needed to promote the application of CHM in the clinic. Larger sample size, double-blinded, randomized, placebo-controlled clinical trials with modern and rigorous methodology are expected to offer more convincing evidence to show the efficacy and safety of CHM in the management of chemotherapy-associated side-effects. Moreover, it is of great importance to further observe whether CHM has an influence on the metabolism of chemotherapeutic drugs and anti-tumor effects in clinical trial. In addition, more efforts should be made in identifying molecular signaling pathways involved in chemotoxicity in breast cancer patients, which could facilitate the understanding of the role of CHM in the management of cancer treatment.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Author Contributions
YF and EY conceived and designed the study. SL and T-hS developed the search terms and drafted the manuscript. GT, H-YT, and NW reviewed the protocol and revised the manuscript. BN and CC initiated the idea and revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
The study was financially supported by Innovation and Technology Commission: The 2nd Phase of Integrative Joint Organizational Platform (IJOP) Disease Collaborative Panel (Project Code: 200009062), Wong’s Donation (Project Code: 200006276), the Gaia Family Trust for Modern Oncology of Chinese Medicine (Project Code: 200007008), Research Grant Council, HKSAR (Project Code RGC GRF 17152116), and Health and Medical Research Fund (Project Codes 15162961, 16172751 and 17181101).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- 1. Momenimovahed Z, Salehiniya H. Epidemiological characteristics of and risk factors for breast cancer in the world. Breast Cancer (Dove Med Press) (2019) 11:151–64. 10.2147/BCTT.S176070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Torre LA, Islami F, Siegel RL, Ward EM, Jemal A. Global Cancer in Women: Burden and Trends. Cancer Epidemiol Biomarkers Prev (2017) 26:444–57. 10.1158/1055-9965.EPI-16-0858 [DOI] [PubMed] [Google Scholar]
- 3. Waks AG, Winer EP. Breast Cancer Treatment: A Review. JAMA (2019) 321(3):288–300. 10.1001/jama.2018.19323 [DOI] [PubMed] [Google Scholar]
- 4. Nadia H, Frédérique PL, Javier C, Gnant M, Houssami N, Poortmans P, et al. Breast cancer. Nat Rev Dis Primers (2019) 5(1):66. 10.1038/s41572-019-0111-2 [DOI] [PubMed] [Google Scholar]
- 5. Anampa J, Makower D, Sparano JA. Progress in adjuvant chemotherapy for breast cancer: an overview. BMC Med (2015) 13:195. 10.1186/s12916-015-0439-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Xiang Y, Guo Z, Zhu P, Chen J, Huang Y. Traditional Chinese medicine as a cancer treatment: Modern perspectives of ancient but advanced science. Cancer Med (2019) 8(5):1958–75. 10.1002/cam4.2108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. So TH, Chan SK, Lee VH, Chen BZ, Kong FM, Lao LX. Chinese Medicine in Cancer Treatment - How is it Practised in the East and the West? Clin Oncol (2019) 31(8):578–88. 10.1016/j.clon.2019.05.016 [DOI] [PubMed] [Google Scholar]
- 8. Lu CL, Li X, Zhou HM, Zhang C, Yang YY, Feng LR, et al. Randomised controlled trials of traditional Chinese medicine in cancer care published in Chinese: an overview. Lancet (2019) 394:s26. 10.1016/S0140-6736(19)32362-1 [DOI] [Google Scholar]
- 9. Chui PL. Cancer- and chemotherapy-related symptoms and the use of complementary and alternative medicine. Asia Pac J Oncol Nurs (2019) 6(1):4–6. 10.4103/apjon.apjon_51_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Liu C, Chen Y, Huang Y, Chen SY, Tsai MY. Chemotherapy in conjunction with traditional Chinese medicine for survival of patients with early female breast cancer: protocol for a non-randomized, single center prospective cohort study. Trials (2019) 20:741. 10.1186/s13063-019-3848-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Meng H, Peng N, Yu M, Sun X, Ma Y, Yang GW, et al. Treatment of triple-negative breast cancer with Chinese herbal medicine. Medicine (2017) 96(44):e8408. 10.1097/MD.0000000000008408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jiao L, Dong C, Liu J, Chen ZW, Zhang L, Xu JF, et al. Effects of Chinese Medicine as adjunct medication for adjuvant chemotherapy treatments of non-small cell lung cancer patients. Sci Rep (2017) 7:46524. 10.1038/srep46524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nie J, Zhao CL, Li D, Chen J, Yu B, Wu XL, et al. Efficacy of traditional Chinese medicine in treating cancer (Review). Biomed Rep (2016) 4(1):3–14. 10.3892/br.2015.537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wu JM, Liu Y, Fang CT, Lin LZ, Lu LM. Traditional Chinese Medicine preparation combined therapy may improve chemotherapy efficacy: A systematic review and meta-analysis. Evidence-Based Complement Altern Med (2019) 1:1–18. 10.1155/2019/5015824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wu YH, Chen HY, Lai CH, Yeh CS, Pang JHS, Qiu JT, et al. Use of Chinese herbal medicine improves chemotherapy-induced thrombocytopenia among gynecological cancer patients: An observational study. Evidence-Based Complement Altern Med (2018). 10.1155/2018/4201325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ansari M, Porouhan P, Mohammadianpanah M, Omidvari S, Mosalaei A, Ahmadloo N, et al. Efficacy of ginger in control of chemotherapy induced nausea and vomiting in breast cancer patients receiving doxorubicin-based chemotherapy. Asian Pac J Cancer Prev (2016) 17:3877–80. [PubMed] [Google Scholar]
- 17. Bai JW, Wu WM. Efficacy analysis of Jin Long Capsule (JLC) in neoadjuvant chemotherapy of breast cancer. Chin J Clin Oncol (2014) 4:246–9. [Google Scholar]
- 18. Chen QT, Zhu HX, Liu ZQ, Wang HB, Xu Y, Li Y. Clinical observation on the effect-enhancing and toxicity-reducing efficacy of Dangguibuxue decoction for postoperative breast cancer patients with chemotoxicity. J Hebei Med Coll Contin Educ (2008) 6:50–1. [Google Scholar]
- 19. Chen HH, Wang W, Shi GX, Li JW. Effect of Chinese herbal compound combining chemotherapy on recurrence breast cancer. Liaoning J Tradit Chin Med (2015) 5:1003–5. [Google Scholar]
- 20. Chen QY, Fu XY, Chen FL. Effect of Chinese herbal medicine Yiqi Jianpi Hewei Therapy on postoperative gastrointestinal reaction of patients with breast cancer after chemotherapy. Clin J Chin Med (2018) 10(24):98–100. [Google Scholar]
- 21. Cheng Y. Clinical observation on Fuzheng Kanai compound combined TP for advanced breast cancer. Med Technol (2011) 12:92–3. [Google Scholar]
- 22. Cheng Z, Zhang YH. Clinical study of Fuzheng Jiedu combined with chemotherapy in the treatment of 20 cases of advanced breast cancer. Jiangsu Chin Med (2016) 48(8):40–2. [Google Scholar]
- 23. Dang XG, Wang L. Evaluation of efficacy on breast cancer treated by Aidi injection plus CTF program of neoadjuvant chemotherapy and the impacts on serum sFas. World J Integr Tradit Western Med (2010) 5(1):54–6. [Google Scholar]
- 24. Fang LL, Jia Y. Method of promoting blood circulation to remove blood stasis and detoxication combined with chemotherapy on breast cancer of intermediate stage and advanced stage. J Shanxi Coll Tradit Chin Med (2015) 2:52–3. [Google Scholar]
- 25. Gao NN, Zou ML, Ma X, He XA, Xu J. The effect of strengthening vital qi and reducing toxicity herbs on patients with chemotherapy after breast cancer surgery. Modern Chin Clin Med (2018) 25(2):22–5. [Google Scholar]
- 26. Guo Y, Yao QH, Yang WH, Luo T. Clinica l research on the method of replenishing Qi to invigorate spleen interfering in the spleen deficiency syndrome that caused by chemotherapy to mastocarcinoma patients. Chin Arch Tradit Chin Med (2007) 25(12):2443–5. [Google Scholar]
- 27. Guo XG. Improvement effect of Yiqiyangxueshengjin prescription on bone marrow suppression for breast cancer patients undergoing chemotherapy. Guangxi Chin Med (2018) 41(6):13–5. [Google Scholar]
- 28. Hao SZ, Chen J, Pan YR, Zou YP, Deng SH, Liu LH. Effect of Guilu-Erxian decoction on bone marrow suppression and immune function of breast cancer patients treated with chemotherapy. Int J Tradit Chin Med (2014) 36(1):17–9. [Google Scholar]
- 29. He JF, Ruan XQ. Clinical observation of gastrointestinal reaction caused by chemotherapy of breast Cancer with Qi deficiency and phlegm blocking treated by Chinese herbal compound. Med Inf (2016) 29(15):61–2. [Google Scholar]
- 30. Hu YT, He F. Effect of Yiqi Jianpi Shugan Decoction on patients undergoing chemotherapy after breast cancer surgery. Guangming Chin Med (2019) 34(15):2336–8. [Google Scholar]
- 31. Hu JH, Yuan YF, He JQ. Yiqihuoxue decoction combined with new adjuvant chemotherapy for 28 cases breast cancer. Hunan J Tradit Chin Med (2008) (2) 38–9. [Google Scholar]
- 32. Huang ZF, Wei JS, Shi ZY, Zhong K. Study of Bazhen decoction and chemotherapy on mammary cancer in metaphase or later period. Modern J Integr Tradit Chin Western Med (2003) 11:1123–4. [Google Scholar]
- 33. Huang ZF, Liu JB, Chen QS, Li HZ, Zhang ZJ, Huang CJ. Effect of Jianpi Xiaoji Decoction combined with chemotherapy on quality of life and immune function in patients with advanced breast cancer. J N Chin Med (2007) 39(5):88–90. [Google Scholar]
- 34. Huang CJ, Liu JB, Liao TH. Effect of Astragalus injection combined chemotherapy on life quality and immune function in advanced breast cancer. Nei Mongol J Tradit Chin Med (2013) 32(32):66–7. [Google Scholar]
- 35. Li LF, Han M. The effect of YIqihuoxue method as an adjuvant therapy for postoperative breast cancer and its impact on patients’ quality of life. Sichuan Jouranl Tradit Chin Med (2015) 3:119–21. [Google Scholar]
- 36. Li XQ, Gong SB. Effect of Aidi injection combined with CEF regimn chemotherapy in the treatment of breast cancer. Pharmacol Clinics Chin Mater Med (2006) 3(22):176–7. [Google Scholar]
- 37. Li QQ, Zhong XY. Clinical observation of Traditional Chinese Medicine reducing toxic side effects of chemotherapy after breast cancer operation. J Med Theor Prac (2013) 26(13):1743–4. [Google Scholar]
- 38. Li XQ, Wang Y, Zhao ST, Ni XH, Ma SQ. Clinical observation on no. 1 decoction of Rukang affecting breast cancer matastasis of 61 cases. Chin J Tradit Med Sci Technol (2003) 10(5):305–6. [Google Scholar]
- 39. Li XL, Tian QY, Ma WJ. Clinical observation on Senqifuzheng injection combined with chemotherpay for advanced breast cancer. Modern Oncol (2004) 12(6):574–5. [Google Scholar]
- 40. Li Z, Li ZY. Fuzheng Xiaoliu decoction in the treatment of patients undergoing chemotherapy after radical operation of breast cancer. Modern Med Health (2017) 33(10):1460–2. [Google Scholar]
- 41. Liang YQ, Yin WJ, Qian W, Wang HL, Ma C, Du HY. Effects of Huaier Granule combined with systemic chemotherapy on immunologic function and prognosis for advanced breast cancer patients. Chin J Bases Clinics Gen Surg (2015) 12:1482–6. [Google Scholar]
- 42. Liu J, Wei SX, Lu M. The observation of attenuation and synergy of postoperative chemotherapy matching with traditional Chinese drug Tianzhicao capsule in breast cancer. Med J West China (2011) 5:896–7. [Google Scholar]
- 43. Liu C, Wu LY, Zhao HJ, Yang N. Clinical observation on Rensenyangrong decoction for the Qi-blood-deficiency type of breast cancer which caused by new adjuvant chemotherapy. Western J Tradit Chin Med (2011) 11:8–11. [Google Scholar]
- 44. Lu MQ, Kong QZ, Lu HD, Li W, Xu BQ, Zhang R, et al. Effect of Huaier granule on T lymphocyte subsets in patients after breast cancer surgery. Maternal Child Health Care China (2016) 31(20):4125–7. [Google Scholar]
- 45. Lv Y, Huang LZ, Mao D, Dai XJ, Yang J, Zhang SQ. Clinical research on treatment of advanced breast cancer based on Yiqi Huoxue method combined with chemotherapy. Acta Chin Med Pharmacol (2014) 42(3):93–6. [Google Scholar]
- 46. Ni XL. Clinical study of gaolishen injection combined with chemotherapy for the advanced breast cancer. China Pract Med (2006) 1(4):64–5. [Google Scholar]
- 47. Semiglazov VF, Stepula VV, Dudov A, Schnitker J, Mengs U. Quality of life is improved in breast cancer patients by standardised mistletoe extract PS76A2 during chemotherapy and follow-up: A Randomised, Placebo-controlled, Double-blind, Multicentre Clinical Trial. Anticancer Res (2006) 26:1519–30. [PubMed] [Google Scholar]
- 48. Sun CX, Yan CH, Yang DH, An ZW, Chen JJ, Fang J, et al. Effect of Chinese Medicine of Shugan Jianpi, Fuzheng Xiaoyu on gastrointestinal adverse reaction and quality of life of breast cancer patients in chemotherapy after operation. J Hebei TCM Pharmacol (2018) 33(1):9–11. [Google Scholar]
- 49. Tang JW, Li L, Tang XY. Yiqijiedu decoction combined with chemotherapy for 30 cases breast cancer (type Qi-deficiency while toxin flourishing. Tradit Chin Med Res (2007) 7:28–9. [Google Scholar]
- 50. Wang YH. Effect on quality of life about paients with breast cancer received neoadjuvant chemotherapy and Taohong Siwu Tang. Guangzhou, China: Guangzhou University of Chinese Medicine; (2007). [Google Scholar]
- 51. Wang LF. Impact on QOL of patients with advanced breast cancer treated by YiQiJianPi and HuaYuJieDu method combined with TE regiment chemotherapy. Nanjing, China: The First Clinical Medical College, Nanjing University of Chinese Medicine; (2010). [Google Scholar]
- 52. Wen HY, Ma YL, Wang CX. Effect on QOL and immunity of patients with advanced breast cancer treated by fuzheng xiaoyan decoction combined with chemotherapy. Jiang Su Chin Med (2010) 42(8):23–4. [Google Scholar]
- 53. Xiong Y, Zhu YL, Xu XD. Effects of Huaier Granule combined with TAC chemotherapy on immunologic function andprognosis in triple negative breast cancer patients after operation. Int J Surg (2015) 42(9):608–11. [Google Scholar]
- 54. Xu XY. The effect of Chinese medicine Sini decoction on preventing and treating acute cardiotoxicity of anthracycline chemotherapy drugs in breast cancer. Guide China Med (2017) 15(19):198–9. [Google Scholar]
- 55. Yang L. Clinical study of Aidi injection combined with chemotherapy on advanced breast cancer. Chin J Integr Tradit Western Med (2004) 24(8):755–6. [Google Scholar]
- 56. Yang L, Sun S. Clinical research into the treatment of mammary cancer by chemotherapy combined with TCM drugs before operation. J Hennan Univ Chin Med (2004) 4:40–1. [Google Scholar]
- 57. Yang HY, Tong CL, Huang M. Clinical observation on Taohongsiwu decoction combined with new adjuvant chemotherapy for breast cancer with stagnation of blood stasis. Modern J Integr Tradit Chin Western Med (2007) 10:1327–8. [Google Scholar]
- 58. Yang HX. Clinical observation on the treatment of gastrointestinal adverse reactions after chemotherapy for breast cancer with Traditional Chinese Medicine Yiqi Jianpi. Auhui Med Pharm J (2013) 17(11):1967–8. [Google Scholar]
- 59. Yu Q, Pang SJ. Chinese medicine combined with chemotherapy for advanced breast cancer and its serum CEA, Effects of CA125, CA153 and CA19 -9 levels. Modern J Integr Tradit Chin Western Med (2019) 28(17):1878–80. [Google Scholar]
- 60. Yu FJ, Li YM, Zou JQ, Jiang LS, Wang C, Tang Y, et al. The Chinese herb Xiaoaiping protects against breast cancer chemotherapy-induced alopecia and other side effects: a randomized controlled trial. J Int Med Res (2019) 47(6):2607–14. 10.1177/0300060519842781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Zhang PY. Chinese herbal compound combined with chemotherapy for 50 cases advanced breast cancer. Liaoning J Tradit Chin Med (2009) (4) 130–1. [Google Scholar]
- 62. Zhang LB. Clinical study on the effect of Fuzheng attenuated Traditional Chinese Medicine on breast cancer postoperative chemotherapy. Diet Health (2018) 5(27):28–9. [Google Scholar]
- 63. Zhang SX, Dang H. The method of Fuzheng Guben combined with NP chemotherapy for 30 cases advanced breast cancer. Guangming J Chin Med (2012) 5:980–2. [Google Scholar]
- 64. Zhang SP, Wang YC. Effects of Shenmai injection on myocardial function and immune function in patients with postoperative chemotherapy of breast cancer. Eval Anal Drug-use Hospitals China (2020) 20(2):214–6. [Google Scholar]
- 65. Zhang WH, Zhao CY, Zhang XF, et al. Effect of radix astragali taohong decoction on dellular immune function in breast cancer patients with postoperative chemotherapy. Chin J Breast Dis (2010) 2(4):137–43. [Google Scholar]
- 66. Zhang YC, Li XJ, Jia YJ, Chen J, Sun YY. Clinical observation of “Fuzheng Quyu Jiedu Prescription” combining with chemotherapy in treating postoperative breast cancer. Shanghai J Tradit Chin Med (2011) 45(11):64–6. [Google Scholar]
- 67. Zhang XL. Treatment of metastatic negative type of liver stagnation with combined chemotherapy of soothing liver, spleen and kidney analysis of the effect of breast cancer. China Prac Med (2015) 10(33):177–8. [Google Scholar]
- 68. Zhong R. Clinical Rearch of Shu Gan Tiao Li Chong Ren. Decoction with the Chemotherapy in the Treatment of Breast Cancers QOL. Nanjing, China: The First Clinical Medical College, Nanjing University of Chinese Medicine; (2009). [Google Scholar]
- 69. Zhou LY. Observation of guipi decoction and guilu erxian decoction in treating 54 cases of myelosuppression after chemotherapy of breast cancer. J Pract Tradit Chin Med (2015) 31(7):615. [Google Scholar]
- 70. Rao KV, Faso A. Chemotherapy-induced nausea and vomiting: optimizing prevention and management. Am Health Drug Benefits (2012) 5(4):232–40. [PMC free article] [PubMed] [Google Scholar]
- 71. Janelsins MC, Tejani MA, Kamen C, Peoples AR, Mustian KM, Morrow GR. Current pharmacotherapy for chemotherapy-induced nausea and vomiting in cancer patients. Expert Opin Pharmacother (2013) 14(6):757–66. 10.1517/14656566.2013.776541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Stein A, Voigt W, Jordan K. Chemotherapy-induced diarrhea: pathophysiology, frequency and guideline-based management. Ther Adv Med Oncol (2010) 2(1):51–63. 10.1177/1758834009355164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. McQuade RM, Stojanovska V, Abalo R, Bornstein JC, Nurgali K. Chemotherapy-Induced Constipation and Diarrhea: Pathophysiology, Current and Emerging Treatments. Front Pharmacol (2016) 7:414. 10.3389/fphar.2016.00414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Boussios S, Pentheroudakis G, Katsanos K, Pavlidis N. Systemic treatment-induced gastrointestinal toxicity: incidence, clinical presentation and management. Ann Gastroenterol (2012) 25(2):106–18. [PMC free article] [PubMed] [Google Scholar]
- 75. Gibson RJ, Keefe DMK. Cancer chemotherapy-induced diarrhoea and constipation: mechanisms of damage and prevention strategies. Support Care Cancer (2006) 14:890. 10.1007/s00520-006-0040-y [DOI] [PubMed] [Google Scholar]
- 76. Rossi A, Fortuna MC, Caro G, Pranteda G, Garelli V, Pompili U, et al. Chemotherapy-induced alopecia management: Clinical experience and practical advice. J Cosmet Dermatol (2017) 16(4):537–41. 10.1111/jocd.12308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Balagula Y, Rosen ST, Lacouture ME. The emergence of supportive oncodermatology: the Study of dermatologic adverse events to cancer therapies. J Am Acad Dermatol (2011) 65(3):624–35. 10.1016/j.jaad.2010.06.051 [DOI] [PubMed] [Google Scholar]
- 78. You JS, Guo L, Huang M, Shi XL, Lin MD, Guo Z, et al. The effect and mechanism of YH0618 granule on chemotherapy- induced hair loss in patients with breast cancer: study protocol for a randomized, double-blind, multi-center clinical trial. Trials (2019) 20:719. 10.1186/s13063-019-3893-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Wang Y, Probin V, Zhou D. Cancer therapy-induced residual bone marrow injury-Mechanisms of induction and implication for therapy. Curr Cancer Ther Rev (2006) 2(3):271–9. 10.2174/157339406777934717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Barreto JN, McCullough KB, Ice LL, Smith JA. Antineoplastic Agents and the Associated Myelosuppressive Effects: A Review. J Pharm Pract (2014) 27(5):440–6. 10.1177/0897190014546108 [DOI] [PubMed] [Google Scholar]
- 81. Kang DH, Weaver MT, Park NJ, Smith B, McArdle T, Carpenter J. Significant impairment in immune recovery after cancer treatment. Nurs Res (2009), 105–14, 58(2). 10.1097/NNR.0b013e31818fcecd [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Hu W, Wang G, Huang D, Sui M, Xu Y. Cancer immunotherapy based on natural killer cells: current progress and new opportunities. Front Immunol (2019) 10:1205. 10.3389/fimmu.2019.01205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Moo TA, Sanford R, Dang C, Morrow M. Overview of Breast Cancer Therapy. PET Clinics (2018) 13(3):339–54. 10.1016/j.cpet.2018.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Huang CY, Ju DT, Chang CF, Muralidhar Reddy P, Velmurugan BK. A review on the effects of current chemotherapy drugs and natural agents in treating non-small cell lung cancer. BioMedicine (2007) 7(4):23. 10.1051/bmdcn/2017070423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Chen D, Zhao J, Cong W. Chinese Herbal Medicines Facilitate the Control of Chemotherapy-Induced Side Effects in Colorectal Cancer: Progress and Perspective. Front Pharmacol (2018) 9:1442. 10.3389/fphar.2018.01442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Zhu L, Li L, Li Y, Wang J, Wang Q. Chinese Herbal Medicine as an Adjunctive Therapy for Breast Cancer: A Systematic Review and Meta-Analysis. Evid Based Complement Alternat Med (2016), 9469276. 10.1155/2016/9469276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Sun X, Zhang X, Nian JY, Guo J, Yin Y, Zhang GL, et al. Chinese Herbal Medicine as Adjunctive Therapy to Chemotherapy for Breast Cancer: A Systematic Review and Meta-Analysis. Evidence-Based Complement Altern Med: eCAM (2016) 2016:3281968. 10.1155/2016/3281968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Chung V, Wu X, Hui E, Ziea TC, Ng FL, Ho ST, et al. Effectiveness of Chinese herbal medicine for cancer palliative care: overview of systematic reviews with meta-analyses. Sci Rep (2015) 5:18111. 10.1038/srep18111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Perez IE, Taveras Alam S, Hernandez GA, Sancassani R. Cancer Therapy-Related Cardiac Dysfunction: An Overview for the Clinician. Clin Med Insights Cardiol (2019) 13:1179546819866445. 10.1177/1179546819866445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Horie S, Oya M, Nangaku M, Yasuda Y, Komastsu Y, Yauagita M, et al. Guidelines for treatment of renal injury during cancer chemotherapy 2016. Clin Exp Nephrol (2018) 22(1):210–44. 10.1007/s10157-017-1448-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Zick SM, Schwabl H, Flower A, Chakraborty B, Hirschkorn K. Unique aspects of herbal whole system research. Explore (N York N Y) (2009) 5(2):97–103. 10.1016/j.explore.2008.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Bayat Mokhtari R, Homayouni TS, Baluch N, Morgatskaya E, Kumar S, Das B, et al. Combination therapy in combating cancer. Oncotarget (2017) 8(23):38022–43. 10.18632/oncotarget.16723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Li X, Yang G, Li X, Zhang Y, Yang J, Chang J, et al. Traditional Chinese Medicine in Cancer Care: A Review of Controlled Clinical Studies Published in Chinese. PLoS One (2013) 8(6). 10.1371/annotation/b53a0b8b-3eb6-44a2-9c37-bc9bb66bfe7e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Pan Y, Zhang F, Zhao Y, Shao D, Zheng X, Chen YJ, et al. Berberine enhances chemosensitivity and induces apoptosis through dose-orchestrated AMPK signaling in breast cancer. J Cancer (2017) 8(9):1679–89. 10.7150/jca.19106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Luo H, Vong CT, Chen H, Gao Y, Lyu P, Qiu L, et al. Naturally occurring anti-cancer compounds: shining from Chinese herbal medicine. Chin Med (2019) 14:48. 10.1186/s13020-019-0270-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.