Summary
Food represents a limiting resource for the growth and developmental progression of many animal species. As a consequence, competition over food, space, or other resources can trigger territoriality and aggressive behavior. In the monarch butterfly, Danaus plexippus, caterpillars feed predominantly on milkweed, raising the possibility that access to milkweed is critical for growth and survival. Here, we characterize the role of food availability on aggression in monarch caterpillars and find that monarch caterpillars display stereotyped aggressive lunges that increase during development, peaking during the fourth and fifth instar stages. The number of lunges toward a conspecific caterpillar was significantly increased under conditions of low food availability, suggesting resource defense may trigger aggression. These findings establish monarch caterpillars as a model for investigating interactions between resource availability and aggressive behavior under ecologically relevant conditions and set the stage for future investigations into the neuroethology of aggression in this system.
Subject Areas: Biological Sciences, Entomology, Behavioral Neuroscience
Graphical Abstract

Highlights
-
•
Monarch caterpillars display stereotyped aggressive behavior
-
•
Aggression is triggered by limited food availability
-
•
Aggression peaks during the late stages of caterpillar development
Biological Sciences; Entomology; Behavioral Neuroscience
Introduction
Resource competition is a primary driver of behavioral evolution that shapes interactions within conspecifics and between species (Peiman and Robinson, 2010; Brumm and Ritschard, 2011). Animals often compete for diverse resources throughout their lifetimes including territory, mates, and food acquisition. Aggression and territoriality are present from invertebrates through primates, and many of the molecular and neural mechanisms governing aggression are conserved across phyla (Thomas et al., 2015). Food availability represents a critical resource that is particularly important in early life, as insufficient food consumption can potently affect developmental progression and brain development (Ormerod et al., 2017; Brown et al., 2019). Further, even short periods of food restriction can induce mortality during early life when animals often have lower energy stores or are undergoing developmentally specified rapid growth (Dawirs, 1984; Tu and Tatar, 2003). Therefore, while resource competition and aggression are often studied in adult animals, these behaviors are also likely to be adaptive early in life.
Aggressive behavior represents a heterogeneous behavior that consists of offensive aggression, often to acquire resources, and defensive aggression, to protect against predation (Takahashi and Miczek, 2013; Ormerod et al., 2017; Wranghama, 2017). Aggression has been widely documented in eusocial and solitary insects where its function is used to defend territories, establish social hierarchies, and compete for food. For example, multiple species of butterflies have been studied for their territoriality, where males defend sunspots that attract potential mates (Davies, 1977; Wickman and Wiklund, 1983; Stutt and Willmer, 1998; Bergman et al., 2010). Under laboratory conditions, the fruit fly, Drosophila melanogaster, has become a leading model for studying the molecular mechanisms underlying aggression, which can be experimentally induced in the laboratory by pairing male flies in an arena with food (Chen et al., 2002). The standardization of this system, combined with powerful genetics, has allowed for dissection of genes and neural circuits regulating aggressive behavior (Kravitz and Fernandez, 2015; Anderson, 2016; Hoopfer, 2016). Together, intraspecies aggression can be studied in diverse insect species under natural and laboratory conditions, providing the opportunity to investigate how environmental and social factors contribute to this behavior.
Food availability in early life may be particularly important in insect larvae that consume food voraciously throughout most larval stages (Edgar, 2006; Tennessen and Thummel, 2011). Nutritional restriction during larval stages has been shown to delay larval development, as well as reduce adult body size, reproductive performance, and lifespan (Beadle et al., 1938; Dmitriew and Rowe, 2011; Runagall-Mcnaull et al., 2015; Poças et al., 2020). Food consumption in larvae is essential to accommodate the growth necessary to achieve metamorphosis. Larvae from many species are social and often compete for resources (Cody and Diamond, 1975; Kaplan and Denno, 2007) For example, acoustic territoriality in Lepidoptera caterpillars (Yack et al., 2001) and cannibalism in Drosophila larvae, beetles, and other insects (Dickinson, 1992; Vijendravarma et al., 2013; Orrock et al., 2017; Huang et al., 2018) suggest the presence of aggression in early developmental stages. The social interactions and competition for resources raise the possibility that limited food availability triggers aggression during the early stages of insect development.
The monarch butterfly, Danaus plexippus, is a model for investigating the mechanisms underlying migration, navigation, and circadian function (Reppert et al., 2010; Zhan et al., 2011; Tomioka and Matsumoto, 2015). Monarch caterpillars predominantly feed on milkweed and often strip entire plants bare of leaves over a two-week period. In many locations, milkweed is only available for part of the year, placing a significant constraint on monarch development (Yang and Cenzer, 2019). These ethological constraints suggest behavioral repertoires may be present in monarch caterpillars to acquire and defend resources.
To examine whether caterpillars display aggressive behavior, we established a group aggression assay and quantified the presence of aggressive lunges under a number of conditions, as well as the effect of attacks on target conspecifics. Here, we describe aggressive behavior in monarch caterpillars, which occurs during later stages of caterpillar development when resource competition may increase in the wild. Further, limiting food availability promotes aggression, suggesting that this represents a form of resource defense. These findings suggest monarch caterpillars may be used as a model to investigate how resource limitation contributes to regulation of aggressive behavior and subsequent effects on the animals' brain development and function.
Results
To measure aggression in monarch caterpillars, we sought to develop a quantifiable assay for aggression. We first observed caterpillars at the fourth and fifth instar stage, the final stages prior to pupation. In both stages, we noticed a stereotyped aggressive behavior sequence consisting of the aggressive caterpillar orienting toward the conspecific and performing a quick head snap that made physical contact with the target (Figures 1A and 1B and Video S1). This tended to elicit cessation of feeding and a movement away from jointly occupied space by the target caterpillar. We reasoned that these movements constitute aggression.
Figure 1.
Characterization of Aggressive Behavior in fourth and fifth Instar Monarch Caterpillars
Example time lapse of a stereotypical aggressive encounter, characterized by a lateral lunge, between two (A) fourth instar or (B) fifth instar conspecifics. Red arrows indicate a change in caterpillar head movement as it progresses from recognition, initiation, contact, and then cessation of a single attack. The timestamp on each image denotes the duration of the encounter, in milliseconds. Scale bar represents 2cm.
To determine the relationship between aggression and developmental stage, we quantified the number of attacks across multiple instars. Attacks were not observed in third instar caterpillars (data not shown). A comparison between fourth and fifth instar caterpillars revealed significantly more aggressive lunges between fifth instar caterpillars than between fourth instar caterpillars (Figure 2). Together, these findings suggest aggression increases in frequency over the later stages of larval development. We reasoned that the relatively larger size of older caterpillars may increase competition for food resources, thereby promoting aggression.
Figure 2.
Aggressive Behavior Increases in Both fourth and fifth Instar Caterpillars with Decreasing Food Density
(A) Asclepias curassavica of low, intermediate, or high density was provided to fourth and fifth instar caterpillars and aggressive behavior was observed.
(B) There was a significant effect of food quantity on the number of aggressive attacks in fourth instar caterpillars (F2, 45 = 8.84; p < 0.0006). Post hoc analyses revealed a significant increase in the number of aggressive attacks on low food density relative to both intermediate (p < 0.0109) and high food densities (p < 0.0006), while no significant difference between intermediate and high food densities was observed (p < 0.3002).
(C) There was a significant effect of food quantity on the number of aggressive attacks in fifth instar caterpillars (F2, 50 = 15.34; p < 0.0001). Post hoc analyses revealed a significant increase in the number of aggressive attacks on low food density relative to both intermediate (p < 0.0027) and high food densities (p < 0.0001). The number of attacks were also significantly higher on intermediate food density in comparison to high food density (p < 0.0375). Data are represented as mean ± SEM. ∗ = p < 0.05; ∗∗ = p < 0.01; ∗∗∗ = p < 0.001; ∗∗∗∗ = p < 0.0001.
To directly quantify the relationship between food availability and aggression, we generated three conditions that differed in food availability (Figure 2A). Although the total number of attacks was lower among fourth instar caterpillars, there were significantly more attacks among fourth instar caterpillars when food availability was low, with a mean of 18.00 ± 3.17, compared to when food availability was either intermediate or high, with means of 8.33 ± 2.01 and 4.81 ± 1.19, respectively (Figure 2B). The number of attacks among fifth instar caterpillars also correlated with food availability (Figure 2C). We again observed a significantly greater number of aggressive attacks when food availability was low, with a mean of 54.75 ± 7.63, compared to when food availability was either intermediate (28.89 ± 5.55) or high (13.32 ± 1.83). The number of aggressive attacks on intermediate food availability was also greater relative to high food availability. Together, these findings support the notion that low food availability triggers aggression in monarch caterpillars.
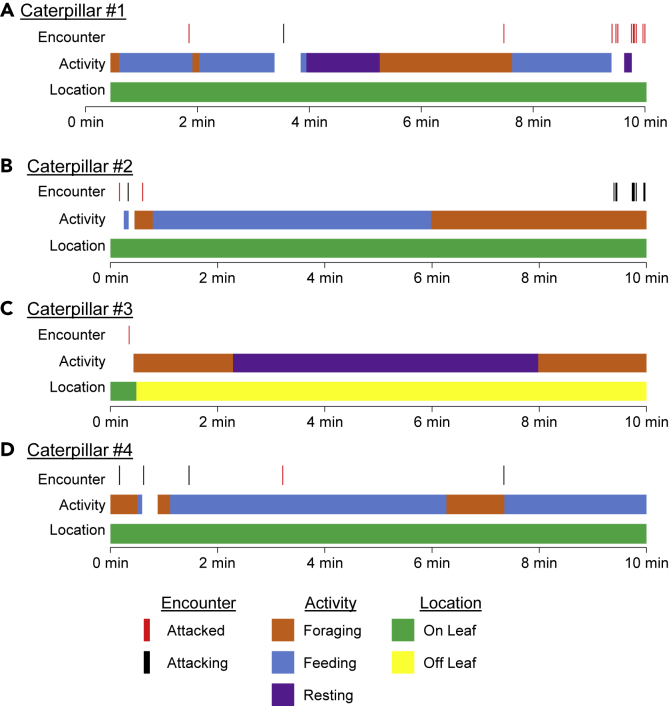
To identify when aggression occurs and its impact on the target conspecific, we generated an ethogram of four caterpillars from a single group, defining behaviors (e.g. foraging, feeding, resting) at the time of each aggressive encounter and their location (e.g. on leaf or off leaf). In nearly all cases, the attacked caterpillar was feeding at the time of the aggressive encounter, suggesting attacks may disrupt feeding and serve to protect a food source (Figure 3). Similarly, the attacking caterpillar tended to be foraging at the time of the attack, suggesting aggression usually occurs when animals are searching for food, rather than actively consuming food. In addition, we cannot rule out the possibility that crowding plays a role in inducing aggression, as this has been shown to contribute to aggressive behavior in many other insect species (Wang and Anderson, 2010; Stevenson and Rillich, 2016). Therefore, this qualitative analysis supports the notion that aggression is associated with food competition, but other factors are likely involved as well.
Figure 3.
Ethogram of Four Representative fifth Instar Caterpillars on Intermediate Food Density during a 10-Min Video
For each caterpillar, the time at which an aggressive encounter occurs was recorded, as well as its activity and location, shown as fractions of time. Aggressive encounters were classified as either attacking (black) or being attacked (red). Activities were categorized into foraging (orange), feeding (bule), or resting (purple). The location of each caterpillar was also recorded (on the leaf: green; off the leaf: yellow).
To further investigate the defining features of these aggressive attacks, we quantified multiple parameters during each aggressive encounter and its impact on the targeted caterpillar. In both fourth and fifth instars, nearly all aggressive attacks occurred on the leaf (Figures 4A and 4B). Aggressive attacks were significantly more likely to occur when the target caterpillar was actively feeding and never occurred when a caterpillar was resting (Figures 4C and 4D). The number of aggressive attacks that occurred during foraging was intermediate between those that occurred during feeding and during resting. Immediately following an attack, the attacking caterpillar almost always remained on the leaf and is consistent for both fourth and fifth instars (Figures 4E and 4F). In fourth instars, the target caterpillar left the leaf a significantly greater amount of time than it remained (Figure 4E), while results trended in this direction for fifth instar caterpillars (Figure 4F). Therefore, aggression increases the likelihood that the attacking caterpillar will remain on the food source, while the attacked caterpillar will be displaced.
Figure 4.
Aggressive Behaviors Tend to Occur on the Leaf during Feeding
In fourth (A) and fifth (B) instar caterpillars, there were significantly more aggressive attacks on the leaf than off the leaf (fourth instar: t18 = 5.102; p < 0.0001; fifth instar: t18 = 4.876; p < 0.0001).
(C) There was a significant effect of behavioral state on the number of aggressive attacks in fourth instar caterpillars (F2,27 = 8.047; p < 0.0018). Post hoc analyses revealed that significantly more aggressive attacks occur during feeding relative to resting (p < 0.0012). No significant difference between feeding and foraging (p < 0.1903) or between foraging and resting was observed (p < 0.0876).
(D) There was a significant effect of behavioral state on the number of aggressive attacks in fifth instar caterpillars (F2,27 = 14.98; p < 0.0001). Post hoc analyses revealed that significantly more aggressive attacks occur during feeding relative to both foraging (p < 0.0191) and resting (p < 0.0001). The number of aggressive attacks is also significantly higher during foraging in comparison to during rest (p < 0.0418).
(E) At the fourth instar stage, the aggressive caterpillar is significantly more likely to stay on the leaf (t18 = 4.936; p < 0.0001), while the attacked caterpillar is significantly more likely to leave the leaf (t18 = 2.830; p < 0.0111).
(F) At the fifth instar stage, the aggressive caterpillar is significantly more likely to stay on the leaf (t18 = 7.325; p < 0.0001), but the attacked caterpillar is equally as likely to leave the leaf as they are to stay (t18 = 1.671; p < 0.1120). Data are represented as mean ± SEM. ∗ = p < 0.05; ∗∗ = p < 0.01; ∗∗∗ = p < 0.001; ∗∗∗∗ = p < 0.0001.
Lastly, we sought to investigate whether sensory input contributes to aggressive behavior. Visually-mediated social behavior has been recently identified in Drosophila larvae (Dombrovski et al., 2019), suggesting that simple eyes may convey complex social information. Monarch caterpillars contain six bilateral ocelli allowing for vision (Khan Perveen, 2017). To determine whether visual input is required for aggression, we compared the number of aggressive attacks in fifth instar caterpillars on low food density recorded under standard light conditions or in complete darkness (Figure 5A). No differences in aggression were identified between the groups (Figure 5B), suggesting light input is dispensable for caterpillar aggression. We also investigated the contributions of the tentacles, large mechanosensory appendages that exhibit rapid growth during the fourth and fifth instar stages when aggression increases (Oberhauser and Kuda, 1997). To investigate the contributions of these structures, we compared aggression in caterpillars with bilateral ablation of the tentacles with intact controls (Figure 5C). There was no difference between the groups, suggesting that functioning tentacles are dispensable for the elicitation of aggression (Figure 4D). These findings suggest that alternative sensory modalities such as pheromonal, olfactory, or tactile cues that are independent of the tentacles initiate aggression.
Figure 5.
Lighting and Tentacle Laceration Have No Effect on Aggressive Behavior
(A) In the experimental treatment, lights were turned off 1 min prior to testing. The effect of lighting on aggressive behavior was then measured in arenas with low food availability.
(B) Caterpillars were equally as aggressive when the lights are off as they are in lighted conditions (t16 = 1.054. p < 0.3076).
(C) In the experimental treatment, tentacles from each caterpillar were removed 24 hr prior to testing. The effect of tentacle laceration was then measured in arenas with low food availability.
(D) Caterpillars were equally as aggressive without tentacles as they are with intact tentacles (t14 = 0.9964. p < 0.3360). Data are represented as mean ± SEM.
Discussion
Here, we describe aggressive behavior in a caterpillar that consists of physical contact presumably to defend a food source that is critical for development. The physical contact is more likely to occur on food and frequently results in displacement suggesting it may be related to resource acquisition. It is important to note that we observed a high degree of variability between individuals, raising the possibility that interindividual differences in exploration, social behavior, or boldness underlie response differences. Territoriality has been described in other caterpillar species (Kemp, 2000; Yack et al., 2001; Bowen et al., 2008), suggesting aggression may be widespread. Cannibalism is widespread in Lepidoptera larvae (Semlitsch and West, 1988; Dial and Adler, 1990; Zago-Braga and Zucoloto, 2004; Tang et al., 2016; Zhou et al., 2016), raising the possibility that aggression is associated with conspecific competition. This extends beyond Lepidoptera to other insect larvae, as crowded rearing conditions promote cannibalism in Drosophila larvae (Vijendravarma et al., 2013), suggesting a relationship between resource availability and this aggression-linked behavior. In our study, we exclusively examined behavioral lunges, a feature most commonly associated with aggressive behavior (Dankert et al., 2009). Examining acoustic communication and potential cannibalism in monarch caterpillars may inform additional behavioral components associated with resource competition.
Numerous sources of resource competition trigger aggression, including limited availability of territory, mates, and food. Our findings suggest limited food availability triggers aggression in monarch caterpillars. In contrast, in social species like the honeybee, Apis mellifera, rather than an increase in aggression, nutritional stress during larval development promotes cooperation, in which the queen increases colony investment and decreases reproduction (Walton et al., 2018). However, the effect of nutritional state on aggression is likely widespread throughout the animal kingdom as food scarcity induces aggression in amphibians, lizards, and birds (Moore and Marler, 1987; Drummond and Chavelas, 1989; Ducey and Heuer, 1991). In facultatively sublicidal bird species, where aggression commonly results in death of the competitor, the “food amount hypothesis” contends that sibling aggression will vary inversely with food quantity (Mock et al., 1987; Drummond, 2001a). Although less is known about the proximate causes of aggression in nonsiblicidal species (Drummond, 2001b), in the meerkat, Suricata suricatta, the severity of aggressive competition scales with food availability (Hodge et al., 2009). Similarly, in numerous mammalian models, moderate reductions in food availability promote aggression. For example in Siberian hamsters, Phodopus sungorus, food restriction promotes aggression during winter-like photoperiods, a signal of impending limited resources (Hodge et al., 2009; Bailey et al., 2017). Food restriction in utero is also associated with aggression. In baboons, Papio hamadryas, interauterine growth restriction is linked to higher rates of aggressive display behaviors such as aggressive grunts, canine displays, and eyelid flashes (Huber et al., 2015). In Drosophila, which is widely used to study aggression in the laboratory, conspecific aggression is triggered by placing a small amount of high calorie food in the center of an arena (Chen et al., 2002; Lim et al., 2014). Therefore, we hypothesize that aggression induced by limited food availability in monarch caterpillars are likely present in many different species throughout the animal kingdom.
While we systematically tested the relationship between food availability and aggression, we cannot exclude other possibilities. For example, it is possible that low amounts of food brings caterpillars into closer proximity, thereby resulting in aggression. In this case, it is proximity, rather than food availability per se that induces aggression. The conditions, all of which included different amounts of food, were chosen to mimic conditions in the wild. The complete lack of food resulted in roaming behavior, where caterpillars are highly active. This likely represents a foraging state, and few aggressive events were observed (data not shown). Manipulating feeding state prior to testing may provide additional insight into the relationship between feeding state and aggression.
In most species studied, aggression is dependent on multiple sensory inputs, providing the opportunity to identify specified sensory stimuli that induce aggression (Chen and Hong, 2018). For example, in flies, dedicated pheromone sensing neurons and their cognate receptors are required for aggression (Wang and Anderson, 2010). Further, disruption of the visual system also disrupts aggressive behavior (Hoyer et al., 2008; Ramin et al., 2014). Conversely, we find that aggressive behavior is similar under light and dark conditions, suggesting it occurs independently of visual function. Further, ablation of the tentacles does not impact aggression, suggesting mechanosensory stimuli from these organs are not required. It is possible that other sensory modalities such as acoustic communication, contribute to the regulation of aggression. For example, in the warty birch caterpillar, Drepana bilineata, acoustic cues are used to defend silk leaf mats and shelters from conspecific larvae (Bowen et al., 2008). Further, caterpillars of the silk moth family Bombycoidea display a wide array of defense sounds in response to simulated inter-species aggression (Bura et al., 2016). Future work examining the contributions of olfactory or alternative mechanosensory pathways may help identify neural mechanisms that trigger aggression.
Monarch caterpillars are specialists that feed nearly exclusively on the milkweed (Seiber et al., 1986; Malcolm et al., 1989). It is likely that food resources are scarcer for specialists than generalists or during life stages where animals are dietary specialists, raising the possibility that conspecific aggression is elevated in this species. Food competition may be more prevalent for monarch caterpillars that largely defoliate host plants, generating resource scarcity, particularly in the later stages of larval growth when we observed aggression (Kaplan and Denno, 2007). In addition, it is possible that conspecific aggression originated as a mechanism of inter-species defense. Over 100 different species are known to consume neonate monarch caterpillars (Hermann et al., 2019). Investigating the conditions that trigger aggression in the wild, and in additional Lepidotera caterpillars, will allow for comparative analysis that may provide insight into the ecological factors that promote aggression.
Beyond the study of aggression in caterpillars, monarchs present an emerging model for studying the molecular mechanisms underlying behavior. Monarch caterpillars are widely studied for their migratory behavior that spans generations along the animals range from Southern Mexico through Canada (Brower, 1996; Reppert et al., 2010). Monarch butterflies detect polarized light and use a time-compensated sun compass for migratory navigation (Froy et al., 2003; Reppert et al., 2004). The genome of monarch butterflies has been sequenced, and the recent implementation of gene editing provides the opportunity to investigate the genetic mechanisms underlying behavior (Zhan et al., 2011; Merlin et al., 2013; Markert et al., 2016). Our findings that monarch caterpillars exhibited aggressive behavior under conditions of limited food availability provide the opportunity to examine the role of genes identified in other leading models of aggression in an ecologically relevant system.
Limitations of the Study
While our work identifies aggressive behavior in monarch caterpillars, a number of limitations to this work will guide future investigation. For example, our studies are limited to a laboratory setting and it will be critical to quantify the behavior in nature. To this point, it is possible that the behavior is regulated by the circadian clock and by seasonality. These aspects can be investigated by manipulating the circadian cycle under laboratory conditions or measuring the behavior in nature. Finally, our findings reveal that limiting food availability increases aggressive behavior, but this limitation may be a consequence of the reduction in food brining animals into closer proximity. This can be directly addressed by systematically manipulating the nutrients within the available food or testing animals in different sized arenas.
Resource Availability
Lead Contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Alex Keene (Keenea@fau.edu).
Material Availability
This study did not generate new unique reagents.
Data and Code Availability
This study did not generate any new code. All original and processed data will be made available upon request.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
This work was supported by NSF awards DEB 174231 and IOS 165674 to A.C.K.. We are grateful for technical support from Christine Merlin (Texas A&M) and for guidance on establishing aggression assays from Kenta Asahina (Salk Institute).
Author Contributions
Conceptualization, J.C., O.G., E.B.B., and A.C.K.; Methodology, J.C., O.G., E.B.B., and A.C.K.; Investigation, J.C., O.G., and E.B.B.; Writing –Original Draft, J.C., E.B.B., and A.C.K.; Writing –Review & Editing, E.B.B. and A.C.K.; Funding Acquisition, A.C.K.; Resources, A.C.K.; Supervision, E.B.B. and A.C.K.
Declaration of Interests
The authors declare no competing interests.
Published: November 19, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2020.101791.
Contributor Information
Elizabeth B. Brown, Email: elizabethbrown@fau.edu.
Alex C. Keene, Email: keenea@fau.edu.
Supplemental Information
References
- Anderson D.J. Circuit modules linking internal states and social behaviour in flies and mice. Nat. Rev. Neurosci. 2016;17:692–704. doi: 10.1038/nrn.2016.125. [DOI] [PubMed] [Google Scholar]
- Bailey A.M., Rendon N.M., O’Malley K.J., Demas G.E. Food as a supplementary cue triggers seasonal changes in aggression, but not reproduction, in Siberian hamsters. Physiol. Behav. 2017;167:298–308. doi: 10.1016/j.physbeh.2016.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beadle G.W., Tatum E.L., Clancy C.W. Food level in relation to rate of development and eye pigmentation in Drosophila melanogaster. Biol. Bull. 1938;75:447–462. [Google Scholar]
- Bergman M., Olofsson M., Wiklund C. Contest outcome in a territorial butterfly: the role of motivation. Proc. R. Soc. B. 2010;277:3027–3033. doi: 10.1098/rspb.2010.0646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen J.L., Mahony S.J., Mason A.C., Yack J.E. Vibration-mediated territoriality in the warty birch caterpillar Drepana bilineata. Physiol. Entomol. 2008;33:238–250. [Google Scholar]
- Brower L.P. Monarch butterfly orientation: missing pieces of a magnificent puzzle. J. Exp. Biol. 1996;199:93–103. doi: 10.1242/jeb.199.1.93. [DOI] [PubMed] [Google Scholar]
- Brown E.B., Slocumb M.E., Szuperak M., Kerbs A., Gibbs A.G., Kayser M.S., Keene A.C. Starvation resistance is associated with developmentally specified changes in sleep, feeding and metabolic rate. J. Exp. Biol. 2019;222:jeb191049. doi: 10.1242/jeb.191049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brumm H., Ritschard M. Song amplitude affects territorial aggression of male receivers in chaffinches. Behav. Ecol. 2011;22:310–316. [Google Scholar]
- Bura V.L., Kawahara A.Y., Yack J.E. A comparative analysis of sonic defences in Bombycoidea caterpillars. Sci. Rep. 2016;6:31469. doi: 10.1038/srep31469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P., Hong W. Neural circuit mechanisms of social behavior. Neuron. 2018;98:16–30. doi: 10.1016/j.neuron.2018.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S., Lee A.Y., Bowens N.M., Huber R., Kravitz E.A. Fighting fruit flies: a model system for the study of aggression. Proc. Natl. Acad. Sci. U S A. 2002;99:5664–5668. doi: 10.1073/pnas.082102599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cody M., Diamond J. Belknap Press; 1975. Ecology and Evolution of Communities. [Google Scholar]
- Dankert H., Wang L., Hoopfer E.D., Anderson D.J., Perona P. Automated monitoring and analysis of social behavior in Drosophila. Nat. Methods. 2009;6:297–303. doi: 10.1038/nmeth.1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies N.B. Prey selection and the search strategy of the spotted flycatcher (Muscicapa striata): a field study on optimal foraging. Anim. Behav. 1977;25:1016–1033. [Google Scholar]
- Dawirs R.R. Influence of starvation on larval development of Carcinus maenas L. (Decapoda : portunidae) J. Exp. Mar. Biol. Ecol. 1984;80:47–66. [Google Scholar]
- Dial C.I., Adler P.H. Larval behavior and cannibalism in Heliothis zea (Lepidoptera: noctuidae) Ann. Entomol. Soc. Am. 1990;83:258–263. [Google Scholar]
- Dickinson J.L. Egg cannibalism by larvae and adults of the milkweed leaf beetle (Labidomera clivicollis, Coleoptera: chrysomelidae) Ecol. Entomol. 1992;17:209–218. [Google Scholar]
- Dmitriew C., Rowe L. The effects of larval nutrition on reproductive performance in a food-limited adult environment. PLoS One. 2011;6:e17399. doi: 10.1371/journal.pone.0017399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dombrovski M., Kim A., Poussard L., Vaccari A., Acton D., Spillman E., Condron B., Yuan Q. A plastic visual pathway regulates cooperative behavior in Drosophila larvae. Curr. Biol. 2019;29:1–11. doi: 10.1016/j.cub.2019.04.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond H. A revaluation of the role of food in broodmate aggression. Anim. Behav. 2001;61:517–526. [Google Scholar]
- Drummond H. The control and function of agonism in avian broodmates. Adv. Study Behav. 2001;30:261–301. [Google Scholar]
- Drummond H., Chavelas C.G. Food shortage influences sibling aggression in the blue-footed booby. Anim. Behav. 1989;37:806–819. [Google Scholar]
- Ducey P.K., Heuer J. Effects of food availability on intraspecific aggression in salamanders of the genus Ambystoma. Can. J. Zool. 1991;69:288–290. [Google Scholar]
- Edgar B.A. How flies get their size: genetics meets physiology. Nat. Rev. Genet. 2006;7:907–916. doi: 10.1038/nrg1989. [DOI] [PubMed] [Google Scholar]
- Froy O., Gotter A.L., Casselman A.L., Reppert S.M. Illuminating the circadian clock in monarch butterfly migration. Science. 2003;300:1303–1305. doi: 10.1126/science.1084874. [DOI] [PubMed] [Google Scholar]
- Hermann S.L., Blackledge C., Haan N.L., Myers A.T., Landia D.A. Predators of monarch butterfly eggs and neonate larvae are more diverse than previously recognised. Sci. Rep. 2019;9:14304. doi: 10.1038/s41598-019-50737-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodge S.J., Thornton A., Flower T.P., Clutton-Brock T.H. Food limitation increases aggression in juvenile meerkats. Behav. Ecol. 2009;20:930–935. [Google Scholar]
- Hoopfer E.D. Neural control of aggression in Drosophila. Curr. Opin. Neurobiol. 2016;38:109–118. doi: 10.1016/j.conb.2016.04.007. [DOI] [PubMed] [Google Scholar]
- Hoyer S.C., Eckart A., Herrel A., Zars T., Fischer S.A., Hardie S.L., Heisenberg M. Octopamine in male aggression of Drosophila. Curr. Biol. 2008;18:159–167. doi: 10.1016/j.cub.2007.12.052. [DOI] [PubMed] [Google Scholar]
- Huang J., Miller J.R., Walker E.D. Cannibalism of egg and neonate larvae by late stage conspecifics of Anopheles gambiae (Diptera: Culicidae): implications for ovipositional studies. J. Med. Entomol. 2018;55:801–807. doi: 10.1093/jme/tjy059. [DOI] [PubMed] [Google Scholar]
- Huber H.F., Ford S.M., Bartlett T.Q., Nathanielsz P.W. Increased aggressive and affiliative display behavior in intrauterine growth restricted baboons. J. Med. Primatol. 2015;44:143–157. doi: 10.1111/jmp.12172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan I., Denno R.F. Interspecific interactions in phytophagous insects revisited: a quantitative assessment of competition theory. Ecol. Lett. 2007;10:977–994. doi: 10.1111/j.1461-0248.2007.01093.x. [DOI] [PubMed] [Google Scholar]
- Kemp D.J. Contest behavior in territorial male butterflies: does size matter? Behav. Ecol. 2000;11:591–596. [Google Scholar]
- Khan Perveen F. BoD - Books on Demand; 2017. Lepidoptera. [Google Scholar]
- Kravitz E.A., Fernandez M.D.I.P. Aggression in Drosophila. Behav. Neurosci. 2015;129:549–563. doi: 10.1037/bne0000089. [DOI] [PubMed] [Google Scholar]
- Lim R.S., Eyjólfsdóttir E., Shin E., Perona P., Anderson D.J. How food controls aggression in Drosophila. PLoS One. 2014;9:e105626. doi: 10.1371/journal.pone.0105626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malcolm S.B., Cockrell B.J., Brower L.P. Cardenolide fingerprint of monarch butterflies reared on common milkweed, Asclepias syriaca L. J. Chem. Ecol. 1989;15:819–853. doi: 10.1007/BF01015180. [DOI] [PubMed] [Google Scholar]
- Markert M.J., Zhang Y., Enuameh M.S., Reppert S.M., Wolfe S.A., Merlin C. Genomic access to monarch migration using TALEN and CRISPR/Cas9-mediated targeted mutagenesis. G3 (Bethesda) 2016;6:905–915. doi: 10.1534/g3.116.027029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlin C., Beaver L.E., Taylor O.R., Wolfe S.A., Reppert S.M. Efficient targeted mutagenesis in the monarch butterfly using zinc-finger nucleases. Genome Res. 2013;23:159–168. doi: 10.1101/gr.145599.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mock D.W., Lamey T.C., Ploger B.J. Proximate and ultimate roles of food amount in regulating egret sibling aggression. Ecology. 1987;68:1760–1772. doi: 10.2307/1939867. [DOI] [PubMed] [Google Scholar]
- Moore M.C., Marler C.A. Effects of testosterone manipulations on nonbreeding season territorial aggression in free-living male lizards. Sceloporus Jarrovi. Gen. Comp. Endocrinol. 1987;65:225–232. doi: 10.1016/0016-6480(87)90170-5. [DOI] [PubMed] [Google Scholar]
- Oberhauser K., Kuda K. University of Minnesota. Department of Ecology, Evolution and Behavior; 1997. A Field Guide to Monarch Caterpillars (Danaus plexippus) pp. 1–14. [Google Scholar]
- Ormerod K.G., LePine O.K., Abbineni P.S., Bridgeman J.M., Coorssen J.R., Mercier A.J., Tattersall G.J. Drosophila development, physiology, behavior, and lifespan are influenced by altered dietary composition. Fly. 2017;11:153–170. doi: 10.1080/19336934.2017.1304331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orrock J., Connolly B., Kitchen A. Induced defences in plants reduce herbivory by increasing cannibalism. Nat. Ecol. Evol. 2017;1:1205–1207. doi: 10.1038/s41559-017-0231-6. [DOI] [PubMed] [Google Scholar]
- Peiman K.S., Robinson B.W. Ecology and evolution of resource-related heterospecific aggression. Q. Rev. Biol. 2010;85:133–158. doi: 10.1086/652374. [DOI] [PubMed] [Google Scholar]
- Poças G.M., Crosbie A.E., Mirth C.K. When does diet matter? The roles of larval and adult nutrition in regulating adult size traits in Drosophila melanogaster. J. Insect Physiol. 2020:104051. doi: 10.1016/j.jinsphys.2020.104051. [DOI] [PubMed] [Google Scholar]
- Ramin M., Domocos C., Slawaska-Eng D., Rao Y. Aggression and social experience: genetic analysis of visual circuit activity in the control of aggressiveness in Drosophila. Mol. Brain. 2014;7:55. doi: 10.1186/s13041-014-0055-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reppert S.M., Zhu H., White R.H. Polarized light helps monarch butterflies cavigate. Curr. Biol. 2004;14:155–158. doi: 10.1016/j.cub.2003.12.034. [DOI] [PubMed] [Google Scholar]
- Reppert S.M., Gegear R.J., Merlin C. Navigational mechanisms of migrating monarch butterflies. Trends Neurosci. 2010;33:399–406. doi: 10.1016/j.tins.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runagall-Mcnaull A., Bonduriansky R., Crean A.J. Dietary protein and lifespan across the metamorphic boundary: protein-restricted larvae develop into short-lived adults. Sci. Rep. 2015;5:11783. doi: 10.1038/srep11783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiber J.N., Brower L.P., Lee S.M., McChesney M.M., Cheung H.T.A., Nelson C.J., Watson T.R. Cardenolide connection between overwintering monarch butterflies from Mexico and their larval food plant, Asclepias syriaca. J. Chem. Ecol. 1986;12:1157–1170. doi: 10.1007/BF01639002. [DOI] [PubMed] [Google Scholar]
- Semlitsch R.D., West C.A. Size-dependent cannibalism in noctuid caterpillars. Oecologia. 1988;77:286–288. doi: 10.1007/BF00379200. [DOI] [PubMed] [Google Scholar]
- Stevenson P.A., Rillich J. Controlling the decision to fight or flee: the roles of biogenic amines and nitric oxide in the cricket. Curr. Zool. 2016;62:265–275. doi: 10.1093/cz/zow028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stutt A.D., Willmer P. Territorial defence in speckled wood butterflies: do the hottest males always win? Anim. Behav. 1998;55:1341–1347. doi: 10.1006/anbe.1998.0728. [DOI] [PubMed] [Google Scholar]
- Takahashi A., Miczek K. Neurogenetics of aggressive behavior: studies in rodents. Curr. Top. Behav. neurosci. 2013;17:3–44. doi: 10.1007/7854_2013_263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang T., Zhao C., Xu L., Qiu L. Factors affecting larval cannibalism in the cotton bollworm, Helicoverpa armigera (Hübner) (Lepidoptera: noctuidae) Oriental Insects. 2016;50:L23–L33. [Google Scholar]
- Tennessen J.M., Thummel C.S. Coordinating growth and maturation - insights from Drosophila. Curr. Biol. 2011;21:R750–R757. doi: 10.1016/j.cub.2011.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas A.L., Davis S.M., Dierick H.A. Of fighting flies, mice, and men: are some of the molecular and neuronal mechanisms of aggression universal in the animal kingdom? PLoS Genet. 2015;11:e1005416. doi: 10.1371/journal.pgen.1005416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomioka K., Matsumoto A. Circadian molecular clockworks in non-model insects. Curr. Opin. Insect Sci. 2015;7:58–64. doi: 10.1016/j.cois.2014.12.006. [DOI] [PubMed] [Google Scholar]
- Tu M.P., Tatar M. Juvenile diet restriction and the aging and reproduction of adult Drosophila melanogaster. Aging cell. 2003;2:327–333. doi: 10.1046/j.1474-9728.2003.00064.x. [DOI] [PubMed] [Google Scholar]
- Vijendravarma R.K., Narasimha S., Kawecki T.J. Predatory cannibalism in Drosophila melanogaster larvae. Nat. Commun. 2013;4:1789. doi: 10.1038/ncomms2744. [DOI] [PubMed] [Google Scholar]
- Walton A., Dolezal A.G., Bakken M.A., Toth A.L. Hungry for the queen: honeybee nutritional environment affects worker pheromone response in a life stage-dependent manner. Funct. Ecol. 2018;32:2699–2706. [Google Scholar]
- Wang L., Anderson D.J. Identification of an aggression-promoting pheromone and its receptor neurons in Drosophila. Nature. 2010;463:227–231. doi: 10.1038/nature08678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickman P.O., Wiklund C. Territorial defence and its seasonal decline in the speckled wood butterfly (Pararge aegeria) Anim. Behav. 1983;31:1206–1216. [Google Scholar]
- Wranghama R.W. Two types of aggression in human evolution. Proc. Natl. Acad. Sci. U S A. 2017;115:245–253. doi: 10.1073/pnas.1713611115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yack J.E., Smith M.L., Weatherhead P.J. Caterpillar talk: acoustically mediated territoriality in larval Lepidoptera. Proc. Natl. Acad. Sci. U S A. 2001;98:11371–11375. doi: 10.1073/pnas.191378898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L.H., Cenzer M.L. Seasonal windows of opportunity in milkweed–monarch interactions. Ecology. 2019;101:e02880. doi: 10.1002/ecy.2880. [DOI] [PubMed] [Google Scholar]
- Zago-Braga R.C., Zucoloto F.S. Cannibalism studies on eggs and newly hatched caterpillars in a wild population of Ascia monuste (Godart) (Lepidoptera, Pieridae) Rev. Bras. de Entomol. 2004;48:415–420. [Google Scholar]
- Zhan S., Merlin C., Boore J.L., Reppert S.M. The monarch butterfly genome yields insights into long-distance migration. Cell. 2011;147:P1171–P1185. doi: 10.1016/j.cell.2011.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J., Dudash M.R., Fenster C.B. Cannibalism during early larval development of Hadena ectypa Morrison (Lepidoptera: noctuidae) Proc. Entomol. Soc. Wash. 2016;118:450–455. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This study did not generate any new code. All original and processed data will be made available upon request.