Summary
It was posited that functionalities of GPCRs require full-length sequences that are negated by residue deletions. Here we report that significantly truncated nfCCR5QTY and nfCXCR4QTY still bind native ligands. Receptor-ligand interactions were discovered from yeast 2-hybrid screening and confirmed by mating selection. Two nfCCR5QTY (SZ218a, SZ190b) and two nfCXCR4QTY (SZ158a, SZ146a) were expressed in E. coli. Synthesized receptors exhibited α-helical structures and bound respective ligands with reduced affinities. SZ190b and SZ158a were reconverted into non-QTY forms and expressed in HEK293T cells. Reconverted receptors localized on cell membranes and functioned as negative regulators for ligand-induced signaling when co-expressed with full-length receptors. CCR5-SZ190b individually can perform signaling at a reduced level with higher ligand concentration. Our findings provide insight into essential structural components for CCR5 and CXCR4 functionality, while raising the possibility that non-full-length receptors may be resulted from alternative splicing and that pseudo-genes in genomes may be present and functional in living organisms.
Subject Areas: Molecular Biology, Cell Biology, Structural Biology
Graphical Abstract
Highlights
-
•
Y2H screening reveals ligand interaction from truncated CXCR4 and CCR5 in QTY form
-
•
Truncated CCR5QTY and CXCR4QTY can be produced in E. coli and bind native ligands
-
•
Reconverted receptors localize on membranes and regulate cell signaling in HEK293
-
•
Our finding indicates potential presence and function for truncated receptors
Molecular Biology; Cell Biology; Structural Biology
Introduction
Alternative RNA splicing generates a diversity of proteins. Such a process increases the total number of proteins from a limited number of genes (Chaudhary et al., 2019), some of which have a variety of functions. These proteins through alternative RNA splicing are sometimes called truncated proteins; namely, they are no longer full length as encoded by the original gene. During the last few decades, researchers have engineered truncated proteins for both scientific studies and biotechnological applications (Den Dunnen and Van Ommen, 1999; Fersht and Winter, 1992; Fersht, 2008). Most such truncated variants studied are water-soluble proteins (Den Dunnen and Van Ommen, 1999; Fersht, 2008). Few truncated membrane receptor proteins have been systematically studied biochemically and biophysically because they require detergents and notoriously difficult to study.
It is generally believed that truncated membrane receptors are no longer functional. One example is the chemokine receptor CCR5Δ32 mutation of the CCR5 co-receptor for HIV entrance into CD4+ and CD8+ T cells (Deng et al., 1996). The CCR5Δ32 mutation has been shown to prevent HIV infection because it has a deletion of 32 DNA base pairs of the EC2 loop between DNA sequences 553 and 585, resulting in a frameshift translation of a truncated receptor (Samson et al., 1996). There are other examples of truncated receptors that lose function including the V2 vasopressin receptor (Zhu and Wess, 1998), the dopamine D3 receptor (Karpa et al., 2000), the mu opiate receptor (Majumdar et al., 2011), the trkB neural receptor (Middlemas et al., 1991), and others (Wise, 2012).
However, some truncated receptors have been shown to have no obvious functional defects (Wise, 2012). For example, the neurotensin receptor with 5TM loops and a long tail is functionally active to form a heterodimer with NTS2 (Perron et al., 2005). Somatostatin receptors sst5TMD4 and sst5TMD5 are 4TM and 5TM truncated mutants, respectively. They are present in normal and tumor tissues (Cordoba-Chacon et al., 2010; Duran-Prado et al., 2009).
Ling et al. deleted 72 amino acids that corresponded to TM1, IC1, TM2, and EC1 of chemokine receptors CCR5 and CXCR4 to produce slightly truncated receptors (Ling et al., 1999). They showed that such truncated receptors still carried out cell signaling in human embryonic kidney (HEK) 293T cells when exposed to their respective ligands, CCL5 (Rantes) and CXCL12 (SDF1α). Thus, it is possible that some truncated receptors may still bind their ligands and carry out signaling despite significant deletions.
The study of membrane receptor proteins requires detergents to prevent their aggregation in aqueous solutions (Lv et al., 2016; Qing et al., 2019; Vinothkumar and Henderson, 2010). Detergent screening is generally a prerequisite to work on membrane proteins in vitro (Lin and Guidotti, 2009; Skrzypek et al., 2018).
We previously reported a simple QTY code for systematic membrane protein design. The QTY code substitutes hydrophobic amino acids with hydrophilic ones that are structurally similar but with different chemical properties, so as to design the detergent-free, water-soluble, and functional variants of chemokine receptors (Zhang et al., 2018). The QTY code systematically replaces the hydrophobic amino acids Leu, Val, Ile, and Phe with hydrophilic Gln (Q), Thr (T), and Tyr (Y) in the receptors, particularly in the transmembrane domains, based on their structures and electron density map similarity of amino acids. This approach permits flexibility in designing and studying the physiological and functional properties of these chemokine receptors, while providing extra freedom in their utilization by eliminating the necessity of detergents. The QTY variant of chemokine receptors can be readily produced in multiple hosts and purified without any detergents.
During the screening of gene library design of CCR5QTY and CXCR4QTY in the yeast 2-hybrid (Y2H) system (Figure S1), the yeast colonies that bear CCR5QTY and CXCR4QTY in vectors and their respective ligands CCL5 and CXCL12 in vectors underwent stringent screen and complementary mating tests. We obtained the expected full-length detergent-free CCR5QTY and CXCR4QTY variants. Unanticipated non-full-length, truncated variants of CCR5QTY and CXCR4QTY were also found during DNA sequencing of these yeast colony clones.
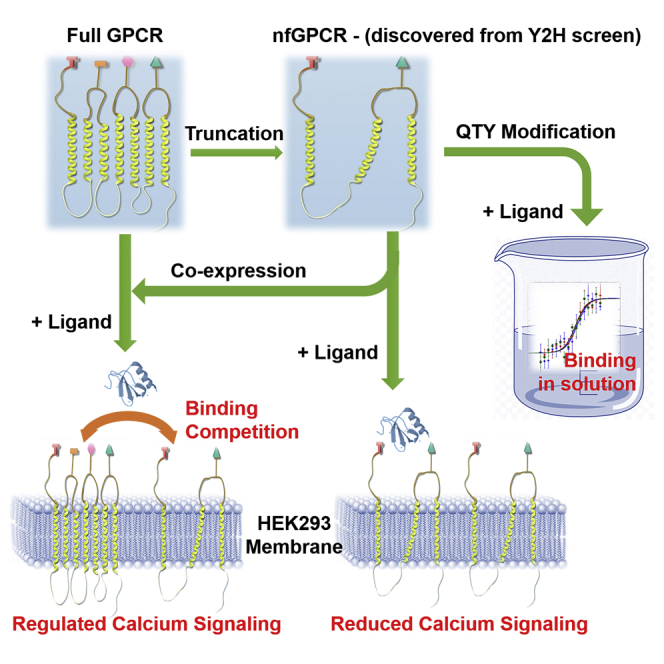
We here report that several non-full-length nfCCR5QTY and nfCXCR4QTY chemokine receptors retain ligand binding in vivo and in vitro. Y2H mating tests revealed many short receptor variants with gene activation via ligand interaction by screening ~3 million gene sequences. We chose two variants of nfCCR5QTY: SZ218a and SZ190b, and two variants of nfCXCR4QTY: SZ158a and SZ146a, to codon-optimize for expression in SF9 insect cells and E. coli. These non-full-length chemokine receptors exhibited binding activity in vitro with their respective ligands, namely, CCL5 for nfCCR5QTY and CXCL12 for nfCXCR4QTY, albeit with reduced affinity. The nfCCR5QTY and nfCXCR4QTY possessed the N terminus and parts of the EC loops, especially the EC3 loop. The truncated receptors also showed the α-helical structure. Two of the truncated receptors, CCR5QTY-SZ190b and CXCR4QTY-SZ158a, were reconverted to non-QTY forms and expressed in HEK293T cells. Confocal microscopy revealed that these receptors preferentially localized on cell membranes. Signaling assays indicated that truncated receptors negatively regulate ligand-induced signaling of full-length receptors when co-expressed. To our great surprise, CCR5-SZ190b, albeit with large deletion of sequence (190aa/352aa), still carried reduced signaling capability to induce intracellular activity at a higher ligand concentration. Our observations raise the plausibility that some so-called pseudogenes may be present and still active in cells. More systematic analyses will be needed to understand the full biological activity for non-full-length genes and their encoded proteins in vivo. Study of these non-full-length receptors can also provide insight into the functionality mechanism for full-length chemokine receptors, which may enable a number of biotechnological, diagnostic, and therapeutic applications.
Results
Y2H Assay for Receptor and Ligand Interactions
The Y2H assay was initially used to study the in vivo interactions between different types of full-length QTY variants and their respective ligands. During this process, numerous short length proteins with QTY modification but not full 7TM structures were discovered to exhibit affinity toward the respective ligands.
In Y2H experiments, the ligands and receptors were cloned into custom-made Y2H bait and prey vectors to allow ligand-receptor interactions. The receptor-ligand interaction activates gene transcription, thus enabling yeast cell growth. Only those variants that are folded properly in the intracellular milieu and transported into the yeast nucleus are able to activate gene transcription of the Y2H reporters. The variants were further subjected to control assays to eliminate false-positives. Yeast GAL4 activation and DNA-binding domains are at the C terminus of the fusion proteins, leaving both free receptor and chemokine N terminus. The schematic for Y2H setup is shown in Figure S1.
We screened a library of ~3 million CXCR4QTY receptor variants fused to the C-terminal DNA-binding domain (pGBKC-3C) with CXCR4QTY in bait orientation and CXCL12 in pGADC-2A (C-terminal activation domain) as the prey. Screens were done on stringent medium lacking adenine and histidine and CXCR4QTY library with CXCL12. About 1 in 500 diploid clones activated the HIS3 reporter (0.2%, SD-LTH non-stringent), and about 1 in 25,000 activated HIS3 and ADE2 (0.004%, SD-LTHA stringent). We obtained no selectable clones when the ligand was N-terminally tagged (pGAD-HA).
We picked 22 clones from the CXCR4QTY/CXCL12 screen that grew on high-stringency medium lacking both adenine and histidine and characterized them by colony PCR. Bidirectional sequencing showed 22 selected clones of shortened version of CXCR4 QTY. Figure S2 shows the 15 variants of non-full length CXCR4 clones, all of which contain N terminus and EC3. On the other hand, PCR of random clones after transformation revealed full-length CXCR4 inserts. Hence, the short CXCR4 fragments were specifically selected in the Y2H screen. These characterized CXCR4QTY clones were retested in a 1:1 mating assay with bait CXCL12 with DBD at C terminus (pGADC-2A), and at N terminus (pGAD-HA), and with the two empty vectors as controls. The absence of Y2H reporter activation in the empty vector controls showed that the interactions of these selected CXCR4QTY clones is specific. The interactions were only observed when the ligand was tagged at the C terminus in the Y2H activation domain (AD). The retest results were very reproducible on different selection media, also showing differential interaction strengths among the CXCR4 QTY clones.
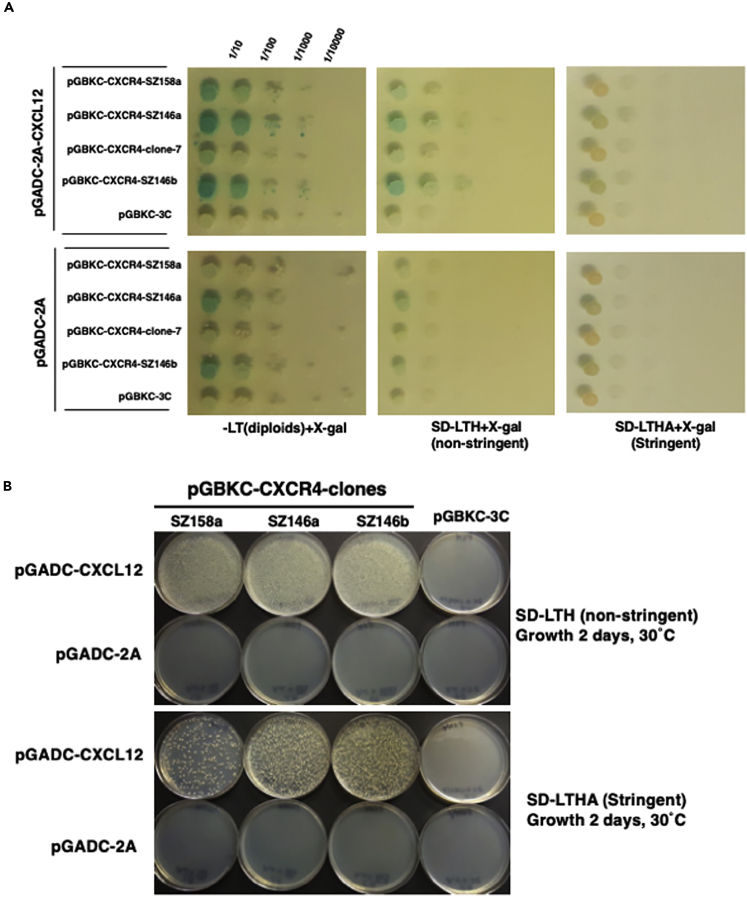
We characterized the interactions between four individual CXCR4QTY clones CXCR4QTY #1 [renamed as SZ158a], #4 [renamed as SZ146a], #7 [not pursued further], and #22 [renamed as 146b] and CXCL12 ligand in semi-quantitative interaction assays (Figures 1A and 1B). Interactions were tested on non-stringent (SD-LTH) and stringent (SD-LTHA) selection media and were found to be fully dependent on the presence of the ligand. Again, it appears that interactions with some of the CXCR4QTY clones SZ146a and 146b are stronger than with numbers SZ158a and #7. As it turned out, the DNA sequences of clones SZ146a and 146b are identical.
Figure 1.
Y2H Mating Assay for nfCXCR4QTY
(A) Yeast colonies were initially picked from plates that allow the activated gene transcription. Cells were spotted in serial 10X dilutions and grown on selective medium with α-Xgal (SD-LT all diploids, SD-LTH non-stringent reporter selection, SD-LTHA stringent reporter selection). The original colonies have been renamed for subsequent studies: #1 (renamed as SZ158a), #4 (renamed as SZ146a), #7 (not pursued further due to weak interaction), and #22 (renamed as SZ146b). SZ146a (#4) and SZ146b (#22) have identical DNA sequence, likely due to PCR amplifications.
(B) The selected clones are further tested on non-stringent (SD-LTH) and stringent (SD-LTHA) selection medium at 30°C for 2 days. If the interactions between the ligand and receptor are strong, these yeast cells will grow, otherwise, cells will not grow. The lower panels are the negative controls without the CXCL12 ligand in the vector, thus no cell growth. Similar screen was carried out for CCR5QTY library with CCL5 ligand.
See also Figures S1–S3.
In addition, we also screened a complex library of ~2 million CCR5QTY variants in the Y2H prey vector with its ligand CCL5 as the bait to find variants that bind with high affinity. Several variants were found and plasmids were then isolated from these clones, transformed into fresh Y187 prey strain, and then were subjected to a stringent 1:1 mating test with original ligand constructs (Figure S3A).
Mating interaction assays showed reporter activation when four selected nfCCR5QTY variants 5CA-12, 5CA-3, 5CA-17 (renamed as SZ190b), and 5NA-43 (renamed as SZ218a) were combined with the native ligand CCL5, but not with the full-length CCR5-22 cloneFigure S3. The growth on non-stringent and stringent media, and alpha-galactosidase color formation by MEL1 marker, shows differences in interaction strength in the Y2H interactions with 5NA-43 being the strongest, followed by 5CA-3 and 5CA-13 with 5CA-12 being the weakest. These results were further confirmed in a more detailed assay with differentially tagged bait ligands CCL5, CX3CL1, and empty vector controls showing preferential binding to CCL5 (Figure S3B). Sequence analysis of 5NA-43 (renamed as SZ218a) is presented in Figure S4. Other clones isolated from the screen (5CA-87) did not show growth under these conditions, similar to empty bait vectors without a ligand insert. The interaction was verified by replicate tests.
Having shown that non-full-length CXCR4QTY and CCR5QTY variants can activate Y2H reporters when combined with their respective ligands, we selected several clones that yeast cells formed into colonies on stringent medium plates. DNA from these colonies was purified and then sequenced. These non-full-length DNA sequences were later cloned into both baculovirus expression vector pOET2 to express in SF9 insect cells and also pET20b+ to express in E. coli. The proteins from both SF9 insect cells and E. coli were affinity purified. Subsequent experiments were carried out to measure the molecular interactions in vitro using microscale thermophoresis (MST).
Sequence Alignments of nfCCR5QTY and nfCXCR4QTY Proteins with Full-Length and Native Counterparts
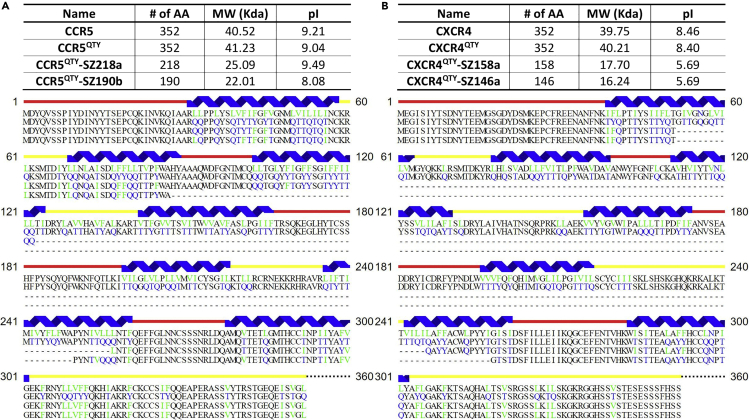
Sequence alignments were performed to show QTY code changes and truncations for nfCCR5QTY and nfCXCR4QTY receptors (Figure 2). Despite the partial inclusion of the gene sequence, all nfCCR5QTY and nfCXCR4QTY proteins strictly follow the rule where only hydrophobic residues in the original transmembrane (TM) regions were replaced by glutamine (Q), threonine (T), and tyrosine (Y). Residues in any other location were untouched, including fragments corresponding to the original N terminus, EC loops, IC loops, and C terminus. The QTY code application diminished the potential existence of hydrophobic TM segments in short receptors, as shown in Figure S5. For full-length CCR5QTY and CXCR4QTY proteins, the latest versions with additional QTY modifications in IC loops and C terminus were presented as described in our previous publications (Qing et al., 2019; Zhang et al., 2018). As an additional note, the sequence of E. coli-synthesized CCR5QTY contains extra modification in IC loops and C terminus when compared with that of SF9-synthesized CCR5QTY as explained in our previous publication (Qing et al., 2019).
Figure 2.
Comparison of Full-Length Native CCR5, CXCR4, Their QTY Variants, and nfCXCR4QTY and nfCCR5QTY Receptors
(A and B) Sequence alignment between (A) native CCR5 (first row), CCR5QTY (second row), nfCCR5QTY-SZ218a (third row), and nfCCR5QTY-SZ190b (fourth row); (B) native CXCR4 (first row), CXCR4QTY (second row), nfCXCR4QTY-SZ158a (third row), and nfCXCR4QTY-SZ146a (fourth row). Substitutions of amino acids are highlighted in different colors. The original hydrophobic L, V, F, and I amino acids are denoted in green; the substitution water-soluble Q, T, and Y amino acids are in blue. The α-helical segments (blue) are shown above the protein sequences, and the external (red) and internal (yellow) loops of the receptors are indicated. Features of native, full-length, and non-full-length QTY chemokine receptors' number of amino acids, pI, and molecular weight are presented.
See also Figures S4–S5.
Figure 2A shows the sequence alignments of different variants of CCR5 receptor proteins. From top to bottom, the sequence in each row corresponds to native CCR5, CCR5QTY, CCR5QTY-SZ218a, and CCR5QTY-SZ190b, respectively. CCR5QTY-SZ218a contains N terminus, TM1, IC1, TM2, EC1, part of TM3, part of TM5, EC3, TM7, and C terminus of the full-length protein with deletion of IC2, TM4, IC3, and TM6. CCR5QTY-SZ190b contains N terminus, TM1, IC1, part of TM2, part of TM6, EC3, TM7, and C terminus of the full-length protein with deletion of EC1, TM3, IC2, TM4, EC2, TM5, and IC3.
Figure 2B shows the sequences alignment of different variants of CXCR4 receptor proteins. From top to bottom, the sequence in each row corresponds to native CXCR4, CXCR4QTY, CXCR4QTY-SZ158a, and CXCR4QTY-SZ146a, respectively. CXCR4QTY-SZ158a contains N terminus, part of TM1, part of TM6, EC3, TM7, and C terminus of the full-length protein with deletion of IC1, TM2, EC1, TM3, IC2, TM4, EC2, TM5, and IC3. CXCR5QTY-SZ146a is similar to CXCR4QTY-SZ158a but with less residues in fused TM1 and TM6.
Full-length CCR5QTY and CXCR4QTY exhibit only slight differences in molecular weight (MW) and isoelectric point (pI) compared with native receptors, whereas non-full-length receptors show a large shift of pI value due to residue deletion, which leads to a severe structural change in folded condition. Additionally, exchanging hydrophobic L, I, V, F with hydrophilic Q, T, and Y induces the formation of inter- and intrahelical hydrogen bonds as well as with surrounding water molecules (Qing et al., 2019).
Ligand-Binding Measurement of nfCCR5QTY and nfCXCR4QTY Receptors Expressed and Purified from SF9 Insect Cells and E. Coli
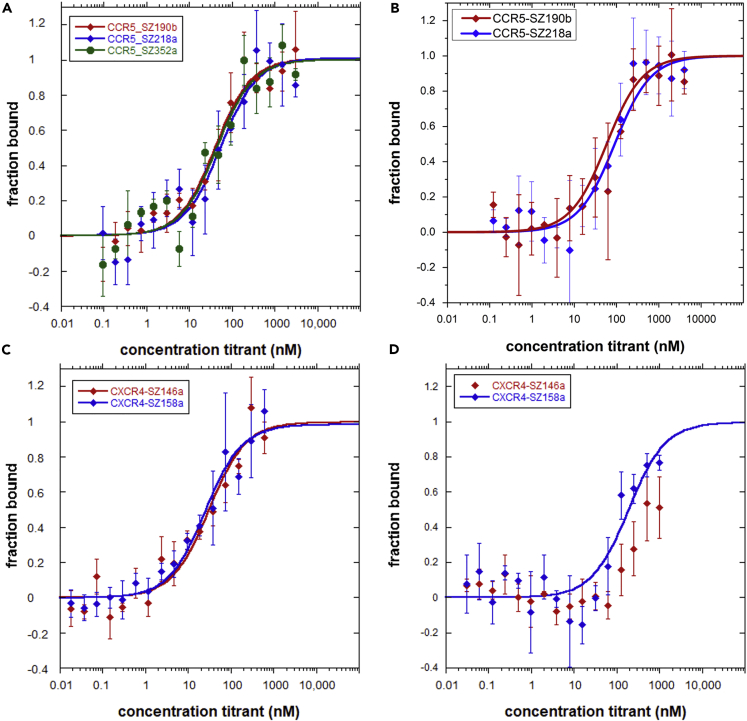
The affinity of nfCCR5QTY and nfCXCR4QTY receptors toward their respective ligands CCL5 and CXCL12 were determined using MST (Figure 3). Purified proteins, as shown in Figure S6, were labeled with fluorescent dye through which the changes in their thermophoretic movement and temperature-related intensity changes upon ligand binding were recorded and plotted against ligand concentration (Seidel et al., 2013). No unspecific adhesion or major aggregation of protein was detected during the measurement for SF9-synthesized proteins. Minor ligand-induced aggregation was observed for E. coli-synthesized proteins, so the corresponding data were analyzed in early MST time trace. For better visualization, all data were replotted as bound fraction versus concentration. Kd values were calculated using the manufacturer-provided Kd model, as presented in the Supplemental Information and Transparent Methods.
Figure 3.
Microscale Thermophoresis (MST) Ligand Binding Measurements
The receptors were labeled with manufacture-provided fluorescent dye. Ligands were obtained commercially and serial diluted in deionized water. Error bars were calculated from three independent repeats of each sample.
(A–D) (A) SF9-synthesized nfCCR5QTY with CCL5, (B) E. coli-synthesized nfCCR5QTY with CCL5, (C) E. coli-synthesized nfCXCR4QTY with CXCL12, (D) E. coli-synthesized nfCXCR4QTY with HIV-1 coat protein gp41-120. The Kd value calculated from the graph can be found in Table 1.
See also Figures S6–S7.
The ligand binding for nfCCR5QTY receptors expressed in both SF9 cells and E. coli were measured (Figures 3A and 3B). Proteins produced from both host systems retain their respective ligand affinity toward CCL5. The ligand-binding measurements were reproducible over several different expressions and purifications. The affinity values obtained for receptors produced in both systems are consistent with each other, with minor variations (Table 1). Full-length CCR5QTY purified from E. coli for CCL5 has a higher affinity than CCR5QTY purified from SF9. This is probably due to the enhanced protein stability in an aqueous environment from additional QTY modification in IC loops and C terminus. On the other hand, nfCCR5QTY receptors purified from SF9 exhibit slightly better affinity compared with counterparts purified from E. coli in spite of having the same sequence. This might be attributed to the refolding process of receptors purified from E. coli where some proteins can misfold into non-functional soluble aggregates and negate the average affinity of the overall system.
Table 1.
Ligand Binding of Non-full-Length Chemokine Receptors CXCR4QTY and CCR5QTY
| CCL5a Kd, nM |
CXCL12a Kd, nM |
gp41-120 Kd, nM |
|
|---|---|---|---|
| CXCR4 native | ~5 | ~200b | |
| CXCR4QTY (E. coli) | 17.3 ± 4.2 | 7.0 ± 1.9 | |
| CXCR4QTY – SZ158a (E. coli) | 246.9 ± 62.2 | ~200 | |
| CXCR4QTY – SZ146a (E. coli) | 301.2 ± 52.2 | Not calculatable | |
| CCR5 native | ~4 | ||
| CCR5QTY (E. coli) | 6.8 ± 2.0 | ||
| CCR5QTY – SZ218a (E. coli) | 87.7 ± 19.5 | ||
| CCR5QTY – SZ190b (E. coli) | 55.8 ± 8.0 | ||
| CCR5QTY (SF9) | 41.1 ± 16.8 | ||
| CCR5QTY – SZ218a (SF9) | 51.7 ± 19.0 | ||
| CCR5QTY – SZ190b (SF9) | 37.8 ± 11.1 |
CCL5 is also called “Rantes,” and CXCL12 is also called “SDF1α” in the literature.
The Kd ~200 nM was measured by a cell-based assay.
The nfCXCR4QTY receptors SZ158a and SZ146a expressed and purified from E. coli. were evaluated for CXCL12 ligand binding (Figure 3C). Both non-full-length receptors exhibit 15×–18× decrease in ligand affinity when compared with the full-length CXCR4QTY. The affinity value for CXCR4QTY-SZ158a is similar to that of CXCR4QTY-SZ146a but slightly better. Two proteins differ only in TM1 and TM6 in their protein sequence. It is possible that the higher affinity from CXCR4QTY-SZ158a benefits from the longer α-helical chain between the N terminus and EC3, which increases the adaptability of the receptor structure. Ling et al. showed that EC2 and EC3 are very important but EC1 is not crucial in the HEK293 cell signaling. Our results are consistent with their findings.
In addition, nfCXCR4QTY receptors were tested against HIV1 coat glycoprotein gp41-120 (Figure 3D). Both non-full-length receptors show hints of binding with drastically decreased affinity compared with full-length CXCR4QTY. Our results suggest the essential role that the N terminus and EC3 play in HIV entry into cells.
Secondary Structure Analysis of nfCXCR4QTY and nfCCR5QTY Receptors
The secondary structures of nfCXCR4QTY and nfCCR5QTY receptors were analyzed using circular dichroism (CD) and are presented in Figure S7. Both full-length and non-full-length receptors exhibit a predominantly α-helical spectrum, with characteristic minima located at ~208 and ~222 nm. Considering that both native and QTY chemokine receptor variants contain a large portion of α-helices, the results suggest that these proteins are likely to be folded properly. The CD spectra of full-length QTY receptors correspond well with our previous reports. The non-full-length receptors show slightly different spectra, indicating difference in receptors' inter-helical interactions.
Reconverting nfCXCR4QTY and nfCCR5QTY Receptors to Non-QTY Variants for HEK293T Expression
Having verified the ligand activity of nfCXCR4QTY and nfCCR5QTY receptors in solution, we then asked if such truncations might exist and function in an actual human cell line. Truncated QTY receptors with higher ligand affinities, CCR5QTY-SZ190b and CXCR4QTY-SZ158a, were reconverted to the non-QTY form for gene synthesis and HEK293T expression. Sequences for reconverted non-full-length receptors were identified by extracting DNA sequences corresponding to the truncated protein sequences from GeneBank entry (CXCR4: NM_003467.3; CCR5: NM_000579.3). Full-length CXCR4 and CCR5 genes were directly purchased.
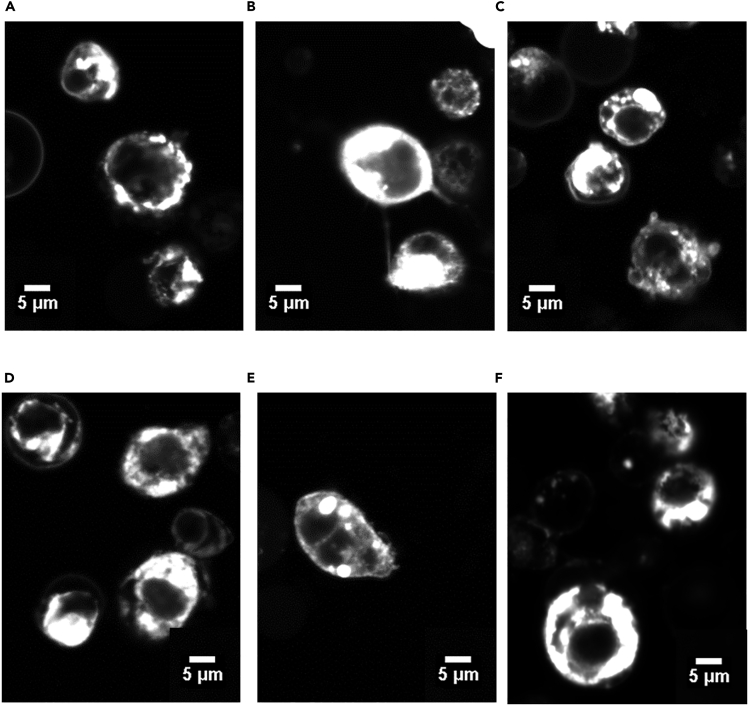
Both full-length and truncated receptors were fused with GFP (green fluorescent protein) on their C terminus and transfected into HEK293T human cells under a human cytomegalovirus promoter (hCMV). The expression and localization of these receptors were visualized using confocal microscopy, as shown in Figures 4A–4F. Cells transfected with full-length and truncated receptors all show enhanced fluorescence at 525 nm on cell membranes using 488-nm laser excitation. Despite a large deletion of sequences, the non-QTY versions of the nfCXCR4 and nfCCR5 still preferentially localize on the cell membranes, due to the existence of residual hydrophobic TM regions. Co-transfection of both non-full-length and full-length receptors shows similar surface distribution. The localization potentially still enables these truncated receptors to function like membrane proteins. Our observation is consistent with previous report that truncated CXCR4 and CCR5 could insert into the cell membrane (Ling et al., 1999).
Figure 4.
Confocal Images of Native Full-Length and Reconverted Non-QTY CXCR4 and CCR5 Truncated Receptors
Tagged with GFP and expressed in HEK293T cell. All receptors exhibited preferential localization on cell membranes.
(A–F) (A) CCR5 full-length, (B) CCR5-SZ190b, (C) CCR5 full-length and CCR5-SZ190b co-transfection, (D) CXCR4 full-length, (E) CXCR4-SZ158a, and (F) CXCR4 full-length and CXCR4-SZ158a co-transfection. Scale bars: 5 μm.
See also Figure S8.
TM segments and topology of non-QTY truncated receptors were predicted via TMHMM Server v. 2.0, as shown in Figure S8. The predicted TM regions correspond well with the sequences within TM in original full-length receptors. CXCR4-SZ158a has a predicted intracellular N terminus, whereas the prediction of CCR5-SZ190b agrees with a schematic we have proposed in Figure 6A. Actual topology of these receptors will need to be verified through detailed structural studies.
Figure 6.
Schematic Illustration of and Data Mining of Non-Full-Length Receptors
(A) Schematic illustration of possible ligand interaction of non-full-length CXCR4 and CCR5 receptors. The ligand-binding motif in N terminus and 3 EC loops are simplified and represented with cartoon blocks. Y2H screen indicated EC3 to be an essential part for CXCL12 binding by nfCXCR4QTY receptors SZ146a and SZ158a. The inter-connect coil between the N terminus and EC3 only slightly reduced the ligand affinity. For nfCCR5QTY, even though SZ218a contains ligand-binding motif EC2 loop, the inter-coil in between EC1 and EC3 may cause undesired spacing between the functional sites and rendered a slightly reduced affinity compared with EC3 containing SZ190b. In these cases, EC3 loop is required for both nfCXCR4QTY and nfCCR5QTY ligand binding as first identified in Y2H in vivo selections.
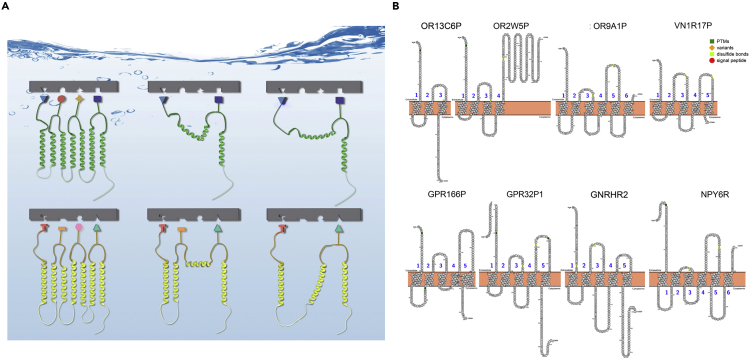
(B) Truncated or mutated GPCRs without full 7TM. Eight truncated GPCRs are mined from the genome database that include three olfactory receptors and one vomeronasal receptor, GNRHR2 (gonadotropin releasing hormone receptor 2), NPY6R (putative neuropeptide Y receptor type 6), and putative GPCRs of unknown function. Common GPCRs have 7TM, but these truncated GPCRs with various deletions have 3TM, 4TM, 5TM, and 6TM. They are presumed to be non-functional. However, no experiments have been carried out to test their biological function. It is plausible that some of them may be still able to bind their respective ligands and carry out signaling in cells.
See also Figure S9.
Cell Signaling of Full-Length and Truncated CXCR4 and CCR5 Receptors
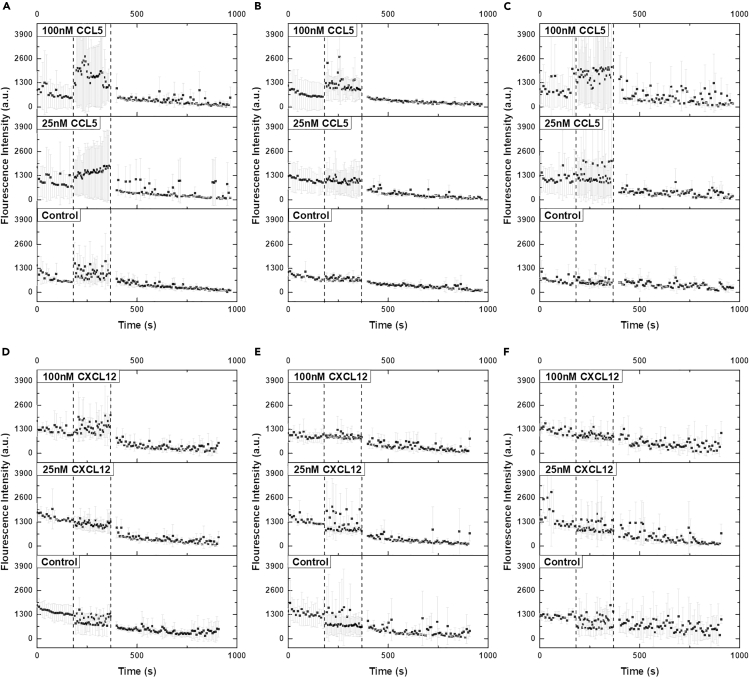
The biological functionality of CXCR4-SZ158a and CCR5-SZ190b in HEK293T cell were determined by carrying out ligand-induced signaling assays using a calcium indicator and comparing with full-length receptors. Plasmids encoding (1) full-length, (2) truncated, or (3) both of the genes were co-transfected with Gαq into HEK293T cells. The cells were then stained using calcium-sensitive dye Rhod-4 with excitation/emission wavelength at 540/590 nm and monitored by a plate reader upon application of respective ligands. Free calcium interacts with the fluorophore to cause intensity increase in the well when signaling is triggered by the full-length or truncated receptors upon the addition of the ligands. Amplitude of the fluorescent changes correlates to the ligand-receptor interactions and depends on Gαq-coupled signaling pathway (Kufareva et al., 2014; Lorenzen et al., 2018). Two concentrations of ligands, 25nM and 100nM, were applied to the wells to determine concentration dependence. The calcium fluorescence change was recorded as a function of time (Figures 5A–5F). Three independent biological repeats of each group were conducted to eliminate error and obtain statistical significance.
Figure 5.
Ligand-Induced Calcium Signaling Assays of Native Full-Length and Reconverted Non-QTY CXCR4 and CCR5 Truncated Receptors Co-transfected with Gαq in HEK293T Cells
(A–F) (A) CCR5 full-length, (B) CCR5-SZ190b, (C) CCR5 full-length and CCR5-SZ190b co-transfection, (D) CXCR4 full-length, (E) CXCR4-SZ158a, and (F) CXCR4 full-length and CXCR4-SZ158a co-transfection. Signaling was monitored by a calcium-sensitive dye Rhod-4. Fluorescence readings were recorded as a function of time. Two concentrations of respective ligand, 100nM (upper panel) and 25nM (middle panel), are added to the stained cells and compared with a negative control sample (lower panel). Two events were marked with dashed lines in the graph, representing the addition of ligand (first dashed line) and resting of plate (second dashed line), respectively. The fluorescence intensities were averaged from three independent experiments and normalized to baseline for illustration purposes. Error bars were shown for individual data points.
Figures 5A–5C show the fluorescence response from CCR5, CCR5-SZ190b, and mix of the two receptors when CCL5 is added. Ligand at two concentrations was added at the time point corresponding to the first dashed line. After 180 s of data acquisition, at the second dashed line, the plate was allowed to rest for an additional 180 s and resume data collection for another 10 min until the fluorescence intensity fully recovered to baseline level. Full-length CCR5 exhibits the most significant fluorescence change at both of the ligand concentrations (Figure 5A). In ideal conditions, the time-dependent fluorescent change should present a bell-like shape (Caers et al., 2014). The different trends in Figure 5A (100 nM) and Figure 5A (25 nM) resembles the different stages in an ideal response model and are likely due to the ligand diffusion at different concentrations, which can be the rate-limiting factor.
Despite a large deletion of sequences, CCR5-SZ190b also processes a discernable ligand-induced calcium signaling at 100 nM CCL5 concentration, albeit with much lower calcium response intensity. The signaling is not triggered at 25 nM ligand concentration, as shown in Figure 5B, where no fluorescence change was observed. When CCR5 and CCR5-SZ190b are co-expressed, the fluorescence changing profile at 100 nM CCL5 strongly resembles that of CCR5 at 25 nM CCL5, showing an increasing trend on fluorescence, but with an intensity similar to that of the full-length CCR5. The hysteresis effect might suggest that, when truncated CCR5 co-exists on the cell membrane with CCR5, it can act like a ligand sink to negatively regulate the binding event between CCR5 and CCL5, prolonging the reaction without diminishing it.
Figures 5D–5F show the calcium fluorescence response from CXCR4, CXCR4-SZ158a, and a mix of the two receptors when CXCL12 is added. No discernable signaling response is observed except for CXCR4 with 100 nM CXCL12 added. Such signaling is again negated when CXCR4 is co-expressed with CXCR4-SZ158a. There are dubious fluorescence increases for CXCR4-SZ158a and co-expressed cultures when 25 nM CXCL12 is added, but data is not significant enough to draw a conclusion.
Taken together, this set of data shows that certain truncated receptors, such as CCR5-SZ190b, can carry out limited signaling function at high ligand concentrations when individually expressed. However, both the truncated receptors preferentially behave like a ligand sink and negatively regulate binding between ligand and full-length receptors when co-expressed. Such signal-regulating effects were commonly observed on soluble single transmembrane cytokine receptors (Heaney and Golde, 1998) and human-engineered “decoy receptors” (Kariolis et al., 2014; Wise, 2012). The signaling assays infer that, if these non-full-length receptors are indeed present in living organisms, they can provide an additional level of regulatory functions by affecting the binding and signaling of the full-length receptors.
Schematic Representations for nfCXCR4QTY and nfCCR5QTY Receptors
A schematic representation of how nfCXCR4QTY and nfCCR5QTY receptors interact with their respective ligands is shown in Figure 6A. Tamamis et al. simulated the ligand interaction of native CXCR4 and CCR5 proteins (Tamamis and Floudas, 2014a, b). The primary interaction between receptors' EC components with ligands were suggested with key residues referenced. We use graphical illustrations to represent the motifs on N terminus and EC loops that are responsible for ligand binding. In spite of a large deletion of a primary sequence, many of the key motifs are still present in the non-full-length variant chemokine receptors, rendering ligand affinity. Both non-full-length CXCR4QTY-SZ158a and SZ146a are similar in retention of their EC components, thus they have close affinity values. Interestingly, CCR5QTY-SZ190b shows slightly higher ligand affinity compared with CCR5QTY-SZ218a, with one less EC loop. One possible explanation could be the orientation of TM3+TM5. The rigidity of the non-parallel α-helix may prevent EC3 from forming a proper binding pocket with N terminus and EC1 against its ligand.
Truncation of the receptors also affects the signaling capability for the non-QTY version of the receptors. For CXCR4-SZ158a, it is likely that there is only one TM segment, and the lack of any intracellular loop would be insufficient to carry out signaling activity. For CCR5-SZ190b, there may likely be three TM α-helices and one intracellular loop (IC1) that might still be capable of performing reduced signaling albeit with significant deletion. Such a hypothesis will be verified in our further research.
Data Mining for Native Non-Full-length GPCR
In light of our finding on non-full-length chemokine receptors with significant deletions of residues, we carried out a data mining search for native GPCRs with alternative splicing or frameshift mutations that resulted in truncations (Figure 6B). Eight truncated GPCRs are mined from the genome databases that include three olfactory receptors, one vomeronasal receptor, GNRHR2 (gonadotropin-releasing hormone receptor 2), NPY6R (putative neuropeptide Y receptor type 6), and two putative GPCRs of unknown function. Common GPCRs have 7TM domains, but these truncated GPCRs with various deletions have 3TM, 4TM, 5TM, and 6TM domains. They are presumed to be non-functional and thus neglected directly. No systematic efforts have been made to study their biological function. It is plausible that some of them may be still able to bind their respective ligands and perform certain functions in cells. Systematic experiments will be needed to test and verify if some may be still functional and retain biological relevance.
Discussion
The discovery of truncated chemokine receptors nfCCR5QTY and nfCXCR4QTY stimulated us to ask new questions about the deterministic factors for native GPCR functionality: if similar non-full-length receptors exist in humans and how they interact with the full-length receptors and if they have some regulatory activities. It has been suggested that certain truncated receptors can form dimers or oligomers to hinder the transport of full-length receptors to the cell surface (Wise, 2012).
Elucidating the Essential Components for Binding Events
Numerous efforts were devoted to identifying the key residues responsible for ligand binding and HIV infection for CXCR4 and CCR5 receptors. Researchers used either mutation-based methods (Abrol et al., 2014; Brelot et al., 2000; Choi et al., 2005; Howard et al., 1999; Lopalco, 2010; Wescott et al., 2016) or computer simulations (Abrol et al., 2014; Tamamis and Floudas, 2014a, b) to reveal specific amino acids without which the activity of the proteins will be severely hindered. Our approach is complementary with a mutation-based analysis and cross-referenced with computer simulations. Proteins are analyzed by fractions through which the essential components can be identified. For instance, EC3 of CXCR4 is identified as a key component for CXCL12 binding as only receptors with EC3 are observed with gene activation in Y2H assays. A simple analogy is shown in Figure S9 where not all five fingers are necessary to hold a teacup.
Implications and Future Studies of Truncated Membrane Receptors
Our observation of non-full-length functional CCR5 and CXCR4 variants raises more questions than it provides answers. Some questions are as follows. (1) Are there DNA sequences specifically coding for non-full-length receptors in all genomes? (2) Are they capable of performing regulatory functions in vivo at another level? (3) What are the smallest functional receptors that can exist in vivo? (4) Are they synthesized and subsequently cleared?
It is plausible that there are a few means of generating non-full-length receptors and proteins in general through (1) alternative RNA splicing (Ambros, 2004; Chaudhary et al., 2019), (2) SINE and LINE transposon insertions and deletions (Adams et al., 1980; Cordaux and Batzer, 2009; Deininger et al., 1981; Ewing and Kazazian, 2011; Singer, 1982; Vassetzky and Kramerov, 2013; Wicker et al., 2007), (3) frameshift mutations resulting in premature translational termination, and (4) non-AUG translation initiation (Ghosh et al., 1967; Kearse and Wilusz, 2017). Many gene identification bioinformatics search for receptors and proteins with AUG as the translational initiation, and most experiments probe for RNA, rather than proteins. Therefore, it is plausible that such non-full-length proteins may have been overlooked.
Suggested Systematic Experimental Studies
To identify and study non-full-length receptors, or more generally, non-full-length proteins, alternative methods are required to find, experimentally characterize, and finally understand their possible biological functions. These methods include (1) performing RNA sequencing using long-read sequencing technology to seek corresponding transcripts and identify non-AUG initial codons, (2) isolating proteins from 1D and 2D membrane protein-specific gels (Carrette et al., 2006; Santucci et al., 2015; Westermeier, 2014) combined with mass spectroscopy identifications, and (3) generating specific monoclonal antibodies (mAbs) for particular regions of known proteins as probes to find non-full-length proteins in various cellular regions and all tissues of every cell cycle as function of time, for example, generation of mAbs (Hashimoto et al., 2018; Huang et al., 2016) for membrane receptors of every intracellular and extracellular loop and N and C termini. The final method is (4) isolating proteins from 1D and 2D protein gels to carry out single protein molecule sequencing using the latest aerolysin nanopore method (Ouldali et al., 2020).
Think Differently and Ask Unusual Questions
Before microRNAs were unexpectedly discovered (Lee et al., 1993; Wightman et al., 1993), such small RNA species and other noncoding RNAs were also overlooked. Since then, microRNAs have been found to be indispensable in every aspect for biological regulations, especially for highly evolved biological systems. Recently, a number of mini-proteins or micro-proteins, previously identified as peptides or small open reading frames, have been found to play a very important role in all aspects of biological regulation (Anderson et al., 2015; Bhati et al., 2018; Camarero, 2017; Carvunis et al., 2012; D'Lima et al., 2017; Delcourt et al., 2018; Graeff et al., 2016; Ingolia et al., 2011; Kageyama et al., 2011; Orr et al., 2019; Saghatelianr and Couso, 2015; Singh et al., 2019; Staudt and Wenkel, 2011). Our unexpected discovery of truncated membrane receptor variants in this study may thus alert us again to venture beyond current paradigms to discover, characterize, and design proteins.
Limitation of the Study
Here we discovered non-full-length CCR5 and CXCR4 chemokine receptors that still retain ligand affinity and partial signal transduction capability. As this study is based on the convenient QTY design code and Y2H screening system in vitro, the truncation of the full-length receptors in vivo resulted from alternative splicing or pseudo-genes might differ in exact sequence. Herein, as suggested in the Discussion section, a systematic study of the human genome is required to identify actual truncated genes that serve the function. Details of experiments are proposed in the Discussion section. Nevertheless, our work provides valuable insights on the structure-function relation of non-full-length receptors and their possible regulatory functions in living organism.
On the other hand, due to the ongoing pandemic of COVID-19 and institution closedown, we were only able to access a regular Tecan Spark microplate reader for the calcium signaling experiments. The unit lacks an auto-injection module, which is essential to continuously monitor ligand-induced fluorescence change. A hardware-enforced 5-s delay is estimated between the addition of the ligand and the start of measurement. As the ligand-induced calcium signaling is a transient process, such delay negates our ability to evaluate the whole period of ligand-receptor interaction. We expect that a plate reader with auto-injection and shaking module would be able to fully elucidate how non-full-length receptor responds to ligand under different condition. In the current study, we introduced ligand diffusion factor by not pipetting or shaking the plate to prolong the response time. We also conducted three independent biological repeats to average out the intensity fluctuations to establish statistical significance of experiments. Albeit not in ideal conditions, we consider our current approach illustrative enough to reveal the potential functions of these non-full-length receptors in vivo.
Resource Availability
Lead Contact
Further information and requests for materials should be directed to lead contact, Rui Qing, Ruiqing@mit.edu.
Materials Availability
All new unique genes generated in this study are available from the lead contact with a completed materials transfer agreement. Genes for native and QTY version of non-full-length CXCR4 and CCR5 chemokine receptors will also be deposited on Addgene for research use.
Data and Code Availability
This published article includes all datasets generated or analyzed during this study.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
This work was primarily funded by OH2 Laboratories and the MIT Center for Bits and Atoms Consortium that includes Bay Valley Innovation Center (Shanghai). We also thank Dorrie Langsley for carefully helping English editing.
Author Contributions
R.Q., F.T., P.C., and S.Z. designed the experiments. R.Q., P.C., G.Y., Q.H., H.C., J.N., B.S., J.K., B.M., T.S., C.B. performed the experiments, R.Q., F.T., P.C., G.Y., B.S., and S.Z. wrote the paper.
Declaration of Interests
This research was in part funded by an MIT startup OH2 Laboratories. S.Z. fully discloses that he has a minority stake in this startup for the invention.
Published: October 28, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2020.101670.
Contributor Information
Rui Qing, Email: ruiqing@mit.edu.
Shuguang Zhang, Email: shuguang@mit.edu.
Supplemental Information
References
- Abrol R., Trzaskowski B., Goddard W.A., Nesterov A., Olave I., Irons C. Ligand- and mutation-induced conformational selection in the CCR5 chemokine G protein-coupled receptor. Proc. Natl. Acad. Sci. U S A. 2014;111:13040–13045. doi: 10.1073/pnas.1413216111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams J.W., Kaufman R.E., Kretschmer P.J., Harrison M., Nienhuis A.W. A family of long reiterated DNA-sequences, one copy of which is next to the human beta-globin gene. Nucleic Acids Res. 1980;8:6113–6128. doi: 10.1093/nar/8.24.6113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambros V. The functions of animal microRNAs. Nature. 2004;431:350–355. doi: 10.1038/nature02871. [DOI] [PubMed] [Google Scholar]
- Anderson D.M., Anderson K.M., Chang C.L., Makarewich C.A., Nelson B.R., McAnally J.R., Kasaragod P., Shelton J.M., Liou J., Bassel-Duby R. A micropeptide encoded by a putative long noncoding RNA regulates muscle performance. Cell. 2015;160:595–606. doi: 10.1016/j.cell.2015.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhati K.K., Blaakmeer A., Paredes E.B., Dolde U., Eguen T., Hong S.Y., Rodrigues V., Straub D., Sun B., Wenkel S. Approaches to identify and characterize microProteins and their potential uses in biotechnology. Cell. Mol. Life Sci. 2018;75:2529–2536. doi: 10.1007/s00018-018-2818-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brelot A., Heveker N., Montes M., Alizon M. Identification of residues of CXCR4 critical for human immunodeficiency virus coreceptor and chemokine receptor activities. J. Biol. Chem. 2000;275:23736–23744. doi: 10.1074/jbc.M000776200. [DOI] [PubMed] [Google Scholar]
- Caers J., Peymen K., Suetens N., Temmerman L., Janssen T., Schoofs L., Beets I. Characterization of G Protein-coupled receptors by a fluorescence-based calcium mobilization assay. J. Vis. Exp. 2014;28:e51516. doi: 10.3791/51516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camarero J.A. Cyclotides, a versatile ultrastable micro-protein scaffold for biotechnological applications. Bioorg. Med. Chem. Lett. 2017;27:5089–5099. doi: 10.1016/j.bmcl.2017.10.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrette O., Burkhard P.R., Sanchez J.C., Hochstrasser D.F. State-of-the-art two-dimensional gel electrophoresis: a key tool of proteomics research. Nat. Protoc. 2006;1:812–823. doi: 10.1038/nprot.2006.104. [DOI] [PubMed] [Google Scholar]
- Carvunis A.R., Rolland T., Wapinski I., Calderwood M.A., Yildirim M.A., Simonis N., Charloteaux B., Hidalgo C.A., Barbette J., Santhanam B. Proto-genes and de novo gene birth. Nature. 2012;487:370–374. doi: 10.1038/nature11184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhary S., Khokhar W., Jabre I., Reddy A.S.N., Byrne L.J., Wilson C.M., Syed N.H. Alternative splicing and protein diversity: plants versus animals. Front. Plant Sci. 2019;10:708. doi: 10.3389/fpls.2019.00708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi W.T., Tian S.M., Dong C.Z., Kumar S., Liu D.X., Madani N., An J., Sodroski J.G., Huang Z.W. Unique ligand binding sites on CXCR4 probed by a chemical biology approach: implications for the design of selective human immunodeficiency virus type 1 inhibitors. J. Virol. 2005;79:15398–15404. doi: 10.1128/JVI.79.24.15398-15404.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordaux R., Batzer M.A. The impact of retrotransposons on human genome evolution. Nat. Rev. Genet. 2009;10:691–703. doi: 10.1038/nrg2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordoba-Chacon J., Gahete M.D., Duran-Prado M., Pozo-Salas A.I., Malagon M.M., Gracia-Navarro F., Kineman R.D., Luque R.M., Castano J.P. Identification and characterization of new functional truncated variants of somatostatin receptor subtype 5 in rodents. Cell. Mol. Life Sci. 2010;67:1147–1163. doi: 10.1007/s00018-009-0240-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Lima N.G., Ma J., Winkler L., Chu Q., Loh K.H., Corpuz E.O., Budnik B.A., Lykke-Andersen J., Saghatelian A., Slavoff S.A. A human microprotein that interacts with the mRNA decapping complex. Nat. Chem. Biol. 2017;13:174–180. doi: 10.1038/nchembio.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deininger P.L., Jolly D.J., Rubin C.M., Friedmann T., Schmid C.W. Base sequence studies of 300 nucleotide renatured repeated human DNA clones. J. Mol. Biol. 1981;151:17–33. doi: 10.1016/0022-2836(81)90219-9. [DOI] [PubMed] [Google Scholar]
- Delcourt V., Staskevicius A., Salzet M., Fournier I., Roucou X. Small proteins encoded by unannotated ORFs are rising stars of the proteome, confirming shortcomings in genome annotations and current vision of an mRNA. Proteomics. 2018;18:e1700058. doi: 10.1002/pmic.201700058. [DOI] [PubMed] [Google Scholar]
- Den Dunnen J.T., Van Ommen G.J.B. The protein truncation test: a review. Hum. Mutat. 1999;14:95–102. doi: 10.1002/(SICI)1098-1004(1999)14:2<95::AID-HUMU1>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Deng H.K., Liu R., Ellmeier W., Choe S., Unutmaz D., Burkhart M., DiMarzio P., Marmon S., Sutton R.E., Hill C.M. Identification of a major co-receptor for primary isolates of HIV-1. Nature. 1996;381:661–666. doi: 10.1038/381661a0. [DOI] [PubMed] [Google Scholar]
- Duran-Prado M., Gahete M.D., Martinez-Fuentes A.J., Luque R.M., Quintero A., Webb S.M., Benito-Lopez P., Leal A., Schulz S., Gracia-Navarro F. Identification and characterization of two novel truncated but functional isoforms of the somatostatin receptor subtype 5 differentially present in pituitary tumors. J. Clin. Endocr. Metab. 2009;94:2634–2643. doi: 10.1210/jc.2008-2564. [DOI] [PubMed] [Google Scholar]
- Ewing A.D., Kazazian H.H. Whole-genome resequencing allows detection of many rare LINE-1 insertion alleles in humans. Genome Res. 2011;21:985–990. doi: 10.1101/gr.114777.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fersht A., Winter G. Protein engineering. Trends Biochem. Sci. 1992;17:292–294. doi: 10.1016/0968-0004(92)90438-f. [DOI] [PubMed] [Google Scholar]
- Fersht A.R. From the first protein structures to our current knowledge of protein folding: delights and scepticisms. Nat. Rev. Mol. Cell Biol. 2008;9:650–654. doi: 10.1038/nrm2446. [DOI] [PubMed] [Google Scholar]
- Ghosh H.P., Soll D., Khorana H.G. Studies on polynucleotides .67. Initiation of protein synthesis in vitro as studied by using ribopolynucleotides with repeating nucleotide sequences as messengers. J. Mol. Biol. 1967;25:275–&. doi: 10.1016/0022-2836(67)90142-8. [DOI] [PubMed] [Google Scholar]
- Graeff M., Straub D., Eguen T., Dolde U., Rodrigues V., Brandt R., Wenkel S. MicroProtein-mediated recruitment of CONSTANS into a TOPLESS trimeric complex represses flowering in arabidopsis. PLoS Genet. 2016;12:e1005959. doi: 10.1371/journal.pgen.1005959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto Y., Zhou W., Hamauchi K., Shirakura K., Doi T., Yagi K., Sawasaki T., Okada Y., Kondoh M., Takeda H. Engineered membrane protein antigens successfully induce antibodies against extracellular regions of claudin-5. Sci. Rep. 2018;8:8383. doi: 10.1038/s41598-018-26560-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaney M.L., Golde D.W. Soluble receptors in human disease. J. Leukoc. Biol. 1998;64:135–146. doi: 10.1002/jlb.64.2.135. [DOI] [PubMed] [Google Scholar]
- Howard O.M.Z., Shirakawa A.K., Turpin J.A., Maynard A., Tobin G.J., Carrington M., Oppenheim J.J., Dean M. Naturally occurring CCR5 extracellular and transmembrane domain variants affect HIV-1 co-receptor and ligand binding function. J. Biol. Chem. 1999;274:16228–16234. doi: 10.1074/jbc.274.23.16228. [DOI] [PubMed] [Google Scholar]
- Huang R., Kiss M.M., Batonick M., Weiner M.P., Kay B.K. Generating recombinant antibodies to membrane proteins through phage display. Antibodies. 2016;5:11. doi: 10.3390/antib5020011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingolia N.T., Lareau L.F., Weissman J.S. Ribosome profiling of mouse embryonic stem cells reveals the complexity and dynamics of mammalian proteomes. Cell. 2011;147:789–802. doi: 10.1016/j.cell.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kageyama Y., Kondo T., Hashimoto Y. Coding vs non-coding: translatability of short ORFs found in putative non-coding transcripts. Biochimie. 2011;93:1981–1986. doi: 10.1016/j.biochi.2011.06.024. [DOI] [PubMed] [Google Scholar]
- Kariolis M.S., Miao Y.R., Ii D.S.J., Kapur S., Mathews I.I., Giaccia A.J., Cochran J.R. An engineered Axl 'decoy receptor' effectively silences the Gas6-Axl signaling axis. Nat. Chem. Biol. 2014;10:977–983. doi: 10.1038/nchembio.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpa K.D., Lin R.W., Kabbani N., Levenson R. The dopamine D3 receptor interacts with itself and the truncated D3 splice variant D3nf: D3-D3nf interaction causes mislocalization of D3 receptors. Mol. Pharmacol. 2000;58:677–683. doi: 10.1124/mol.58.4.677. [DOI] [PubMed] [Google Scholar]
- Kearse M.G., Wilusz J.E. Non-AUG translation: a new start for protein synthesis in eukaryotes. Gene Dev. 2017;31:1717–1731. doi: 10.1101/gad.305250.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kufareva I., Stephens B.S., Holden L.G., Qin L., Zhao C.X., Kawamura T., Abagyan R., Handel T.M. Stoichiometry and geometry of the CXC chemokine receptor 4 complex with CXC ligand 12: molecular modeling and experimental validation. Proc. Natl. Acad. Sci. U S A. 2014;111:E5363–E5372. doi: 10.1073/pnas.1417037111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee R.C., Feinbaum R.L., Ambros V. The C. elegans heterochronic gene lin-4 encodes small rnas with antisense complementarity to lin-14. Cell. 1993;75:843–854. doi: 10.1016/0092-8674(93)90529-y. [DOI] [PubMed] [Google Scholar]
- Lin S.H., Guidotti G. Purification of membrane proteins. Method Enzymol. 2009;463:619–629. doi: 10.1016/S0076-6879(09)63035-4. [DOI] [PubMed] [Google Scholar]
- Ling K., Wang P., Zhao J., Wu Y.L., Cheng Z.J., Wu G.X., Hu W., Ma L., Pei G. Five-transmembrane domains appear sufficient for a G protein-coupled receptor: functional five-transmembrane domain chemokine receptors. Proc. Natl. Acad. Sci. U S A. 1999;96:7922–7927. doi: 10.1073/pnas.96.14.7922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopalco L. CCR5: from natural resistance to a new anti-HIV strategy. Viruses. 2010;2:574–600. doi: 10.3390/v2020574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzen E., Ceraudo E., Berchiche Y.A., Rico C.A., Fürstenberg A., Sakmar T.P., Huber T. G protein subtype–specific signaling bias in a series of CCR5 chemokine analogs. Sci. Signal. 2018;11:eaao6152. doi: 10.1126/scisignal.aao6152. [DOI] [PubMed] [Google Scholar]
- Lv X.C., Liu J.L., Shi Q.Y., Tan Q.W., Wu D., Skinner J.J., Walker A.L., Zhao L.X., Gu X.X., Chen N. In vitro expression and analysis of the 826 human G protein-coupled receptors. Protein Cell. 2016;7:325–337. doi: 10.1007/s13238-016-0263-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumdar S., Grinnell S., Le Rouzic V., Burgman M., Polikar L., Ansonoff M., Pintar J., Pan Y.X., Pasternak G.W. Truncated G protein-coupled mu opioid receptor MOR-1 splice variants are targets for highly potent opioid analgesics lacking side effects. Proc. Natl. Acad. Sci. U S A. 2011;108:19778–19783. doi: 10.1073/pnas.1115231108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middlemas D.S., Lindberg R.A., Hunter T. Trkb, a neural receptor protein-tyrosine kinase - evidence for a full-length and 2 truncated receptors. Mol. Cell Biol. 1991;11:143–153. doi: 10.1128/mcb.11.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr M.W., Mao Y., Storz G., Qian S.B. Alternative ORFs and small ORFs: shedding light on the dark proteome. Nucleic Acids Res. 2019;48:1029–1042. doi: 10.1093/nar/gkz734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouldali H., Sarthak K., Ensslen T., Piguet F., Manivet P., Pelta J., Behrends J.C., Aksimentiev A., Oukhaled A. Electrical recognition of the twenty proteinogenic amino acids using an aerolysin nanopore. Nat. Biotechnol. 2020;38:176–181. doi: 10.1038/s41587-019-0345-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perron A.L., Sarret P., Gendron L., Stroh T., Beaudet A. Identification and functional characterization of a 5-transmembrane domain variant isoform of the NTS2 neurotensin receptor in rat central nervous system. J. Biol. Chem. 2005;280:10219–10227. doi: 10.1074/jbc.M410557200. [DOI] [PubMed] [Google Scholar]
- Qing R., Han Q., Skuhersky M., Chung H., Badr M., Schubert T., Zhang S. QTY code designed thermostable and water-soluble chimeric chemokine receptors with tunable ligand affinity. Proc. Natl. Acad. Sci. U S A. 2019;116:25668–25676. doi: 10.1073/pnas.1909026116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saghatelianr A., Couso J.P. Discovery and characterization of smORF-encoded bioactive polypeptides. Nat. Chem. Biol. 2015;11:909–916. doi: 10.1038/nchembio.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samson M., Libert F., Doranz B.J., Rucker J., Liesnard C., Farber C.M., Saragosti S., Lapoumeroulie C., Cognaux J., Forceille C. Resistance to HIV-1 infection in Caucasian individuals bearing mutant alleles of the CCR-5 chemokine receptor gene. Nature. 1996;382:722–725. doi: 10.1038/382722a0. [DOI] [PubMed] [Google Scholar]
- Santucci L., Bruschi M., Ghiggeri G.M., Candiano G. The latest advancements in proteomic two-dimensional gel electrophoresis analysis applied to biological samples. Methods Mol. Biol. 2015;1243:103–125. doi: 10.1007/978-1-4939-1872-0_6. [DOI] [PubMed] [Google Scholar]
- Seidel S.A.I., Dijkman P.M., Lea W.A., van den Bogaart G., Jerabek-Willemsen M., Lazic A., Joseph J.S., Srinivasan P., Baaske P., Simeonov A. Microscale thermophoresis quantifies biomolecular interactions under previously challenging conditions. Methods. 2013;59:301–315. doi: 10.1016/j.ymeth.2012.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer M.F. Sines and lines - highly repeated short and long interspersed sequences in mammalian genomes. Cell. 1982;28:433–434. doi: 10.1016/0092-8674(82)90194-5. [DOI] [PubMed] [Google Scholar]
- Singh D.R., Dalton M.P., Cho E.E., Pribadi M.P., Zak T.J., Seflova J., Makarewich C.A., Olson E.N., Robia S.L. Newly discovered micropeptide regulators of SERCA form oligomers but bind to the pump as monomers. J. Mol. Biol. 2019;431:4429–4443. doi: 10.1016/j.jmb.2019.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skrzypek R., Iqbal S., Callaghan R. Methods of reconstitution to investigate membrane protein function. Methods. 2018;147:126–141. doi: 10.1016/j.ymeth.2018.02.012. [DOI] [PubMed] [Google Scholar]
- Staudt A.C., Wenkel S. Regulation of protein function by 'microProteins. EMBO Rep. 2011;12:35–42. doi: 10.1038/embor.2010.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamamis P., Floudas C.A. Elucidating a key anti-HIV-1 and cancer-associated Axis: the structure of CCL5 (rantes) in complex with CCR5. Sci. Rep. 2014;4:5447. doi: 10.1038/srep05447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamamis P., Floudas C.A. Elucidating a key component of cancer metastasis: CXCL12 (SDF-1 alpha) binding to CXCR4. J. Chem. Inf. Model. 2014;54:1174–1188. doi: 10.1021/ci500069y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassetzky N.S., Kramerov D.A. SINEBase: a database and tool for SINE analysis. Nucleic Acids Res. 2013;41:D83–D89. doi: 10.1093/nar/gks1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinothkumar K.R., Henderson R. Structures of membrane proteins. Q. Rev. Biophys. 2010;43:65–158. doi: 10.1017/S0033583510000041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wescott M.P., Kufareva I., Paes C., Goodman J.R., Thaker Y., Puffer B.A., Berdougo E., Rucker J.B., Handel T.M., Doranz B.J. Signal transmission through the CXC chemokine receptor 4 (CXCR4) transmembrane helices. Proc. Natl. Acad. Sci. U S A. 2016;113:9928–9933. doi: 10.1073/pnas.1601278113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westermeier R. Looking at proteins from two dimensions: a review on five decades of 2D electrophoresis. Arch. Physiol. Biochem. 2014;120:168–172. doi: 10.3109/13813455.2014.945188. [DOI] [PubMed] [Google Scholar]
- Wicker T., Sabot F., Hua-Van A., Bennetzen J.L., Capy P., Chalhoub B., Flavell A., Leroy P., Morgante M., Panaud O. A unified classification system for eukaryotic transposable elements. Nat. Rev. Genet. 2007;8:973–982. doi: 10.1038/nrg2165. [DOI] [PubMed] [Google Scholar]
- Wightman B., Ha I., Ruvkun G. Posttranscriptional regulation of the heterochronic gene lin-14 by lin-4 mediates temporal pattern-formation in C. elegans. Cell. 1993;75:855–862. doi: 10.1016/0092-8674(93)90530-4. [DOI] [PubMed] [Google Scholar]
- Wise H. The roles played by highly truncated splice variants of G protein-coupled receptors. J. Mol. Signal. 2012;7:13. doi: 10.1186/1750-2187-7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S.G., Tao F., Qing R., Tang H.Z., Skuhersky M., Corin K., Tegler L., Wassie A., Wassie B., Kwon Y. QTY code enables design of detergent-free chemokine receptors that retain ligand-binding activities. Proc. Natl. Acad. Sci. U S A. 2018;115:E8652–E8659. doi: 10.1073/pnas.1811031115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X.Y., Wess J. Truncated V2 vasopressin receptors as negative regulators of wild-type V2 receptor function. Biochemistry. 1998;37:15773–15784. doi: 10.1021/bi981162z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This published article includes all datasets generated or analyzed during this study.