Abstract
The COVID 19 pandemic resulted in a total reduction in the number of hospitalizations for acute coronary syndromes. A consequence of the delay in coronary revascularization has been the resurgence of structural complications of myocardial infarctions. Ventricular septal rupture (VSR) complicating late presenting acute myocardial infarction (AMI) is associated with high mortality despite advances in both surgical repair and perioperative management. Current data suggests a declining mortality with delay in VSR repair; however, these patients may develop cardiogenic shock while waiting for surgery. Available options are limited for patients with VSR who develop right ventricular failure and cardiogenic shock. The survival rate is very low in patients with cardiogenic shock undergoing surgical or percutaneous VSR repair. In this study we present two late presenting ST elevation MI patients who were complicated by rapidly declining hemodynamics and impending organ failure. Both patients were bridged with venoarterial extracorporeal membrane oxygenation (ECMO) to cardiac transplant.
Keywords: STEMI, Ventricular septal rupture, Mechanical circulatory support, VA-ECMO, Heart transplant, COVID-19
Introduction
The incidence of mechanical complications secondary to acute myocardial infarction (AMI) has declined significantly in the past few decades due to advances in early reperfusion with primary percutaneous coronary intervention (PCI) strategy.1 Despite a decline in the number of hospitalizations for acute coronary syndromes in the early months of the COVID-19 pandemic,2 the incidence of mechanical complications has risen.3 This is most likely due to delay in revascularization, which is an established risk factor for development of mechanical complications following AMI.1
Parikh et al. reported two patients with late presenting AMI complicated by VSR.4 One patient expired due to multi-organ failure despite mechanical circulatory support. Second patient underwent surgical repair after a failed percutaneous VSR closure. Similar to the reports from other centers, we have been encountering mechanical complications of late presenting AMI with higher frequency during COVID-19 pandemic compared to the pre-pandemic era. In this report we describe the clinical course of two patients with VSR secondary to late presenting AMI who eventually underwent orthotropic heart transplant at our tertiary medical center.
Patient 1
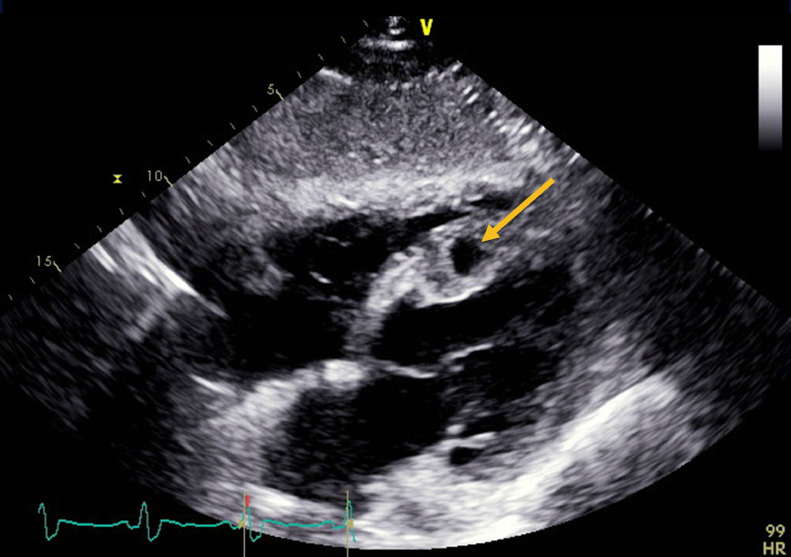
A 56-year-old man presented to the emergency room with 5 days of exertional angina and shortness of breath. A 12-lead ECG demonstrated anterolateral ST-elevation myocardial infarction (STEMI). Emergent left heart catheterization (LHC) showed culprit 100% occlusion of the proximal left anterior descending artery (LAD) and chronic total occlusion of right coronary artery (RCA). Despite undergoing coronary intervention with placement of drug eluting stents (DES), he had no reflow due to organized thrombus and he developed hypotension requiring escalating doses of vasopressors. Emergent right heart catheterization (RHC) demonstrated elevated filling pressures with a pulmonary artery oxygen saturation of 83%. A stat echocardiogram revealed a moderate to large sized ventricular septal rupture (VSR) in the distal third of the interventricular septum with a Qp:Qs ratio of 1.3 and presence of a left ventricular (LV) apical thrombus (Fig. 1 , video 1 and video 2 in supplementary materials). The LV ejection fraction was estimated to be 32% with concomitant right ventricular (RV) dysfunction as evidenced by a tricuspid annular plane systolic excursion (TAPSE) of 1.2 cm.
Fig. 1.
Transthoracic echocardiogram showing the ventricular septal rupture (orange arrow) in subcostal view.
An intra-aortic balloon pump (IABP) was placed for afterload reduction and to temporize his hemodynamic instability. Structural heart and cardiothoracic surgery teams were consulted to determine potential VSR closure options. Due to the apical location of the VSR and presence of LV thrombus he was deemed not to be a suitable candidate for percutaneous closure of the defect. Thus, it was felt that surgical closure with concurrent bypass of the RCA was most appropriate clinically.
While awaiting surgical repair, the patient developed worsening cardiogenic shock with elevation in biventricular filling pressures (Table 1 ). The presence of an LV thrombus precluded the use of Impella. He was referred for veno-arterial extracorporeal membrane oxygenation (VA-ECMO) with placement of an atrial septal drainage cannula for LV venting as a bridge to cardiac replacement therapy given the profound irreversible nature of his hemodynamic compromise.
Table 1.
Hemodynamics pre and post mechanical circulatory support.
| Patient 1 |
Patient 2 |
|||
| Pre-MCS | Post-MCS | Pre-MCS | Post-MCS | |
| RAP (mmHg) | 11 | 11 | 11 | 12 |
| RVP (mmHg) | 55/20 | 55/14 | 43/15 | Not available |
| PAP (mmHg) | 48/24 | 45/19 | 45/22 | 15/12 |
| PAPi | 2.5 | 2.4 | 2.0 | 0.25 |
| PCWP (mmHg) | 22 | 20 | 19 | 13 |
| Cardiac output (L/min) | 2.9 | 6.0 | 6.6 | Not available |
| Cardiac index (L/min/m2) | 1.2 | 2.5 | 3.8 | Not available |
| CPO (Watts) | 0.47 | 0.9 | 3.8 | Not available |
| Lactate (mmol/L) | 2.4 | 0.8 | 1.8 | 0.8 |
| Creatinine (mg/dl) | 1.8 | 0.9 | 1.3 | 0.8 |
| AST/ALT (units/L) | 142/281 | 85/67 | 26/15 | 71/27 |
MCS: mechanical circulatory support; RAP: right atrial pressure; RVP: right ventricle pressure; PAP: pulmonary artery pressure; PAPi: Pulmonary Artery Pulsatility index=(systolic PAP-diastolic PAP)/RAP ; PCWP: pulmonary capillary wedge pressure; CPO: cardiac power output= (mean arterial pressure x cardiac output)/451 ; AST: aspartate transaminase; ALT: alanine transaminase.
The patient was evaluated by the advanced heart failure service and he was listed for a heart transplant. Six days later, he underwent uncomplicated orthotopic heart transplantation, and he was discharged on postoperative day 25 with intact end organ function.
Patient 2
A 53-year-old man presented to an outside hospital with 7 days of progressive exertional chest pain. A 12-lead ECG demonstrated anterolateral STEMI and an emergent LHC revealed 95% stenosis of the mid-LAD and first diagonal artery. Despite revascularization of the LAD with 2 overlapping DES, he developed cardiopulmonary collapse, requiring escalation of vasopressors, placement of an IABP and mechanical ventilation. RHC demonstrated elevated filling pressures and a pulmonary artery oxygen saturation of 87% (Table 1). A stat echocardiogram revealed a large apical VSR (Fig. 2 , Video 3 in supplementary materials) with an LV ejection fraction of 45% and normal RV function with an estimated TAPSE of 1.9 cm.
Fig. 2.
Transthoracic echocardiogram showing the ventricular septal rupture (orange arrow) in subcostal view.
The multidisciplinary cardiogenic shock team was convened, and the patient was transferred to our center for further management. Upon arrival, mechanical circulatory support was escalated to VA-ECMO for cardiopulmonary support with placement of an atrial septal drainage cannula for LV venting, as a bridge to definitive therapy. Structural heart and cardiothoracic surgery teams were consulted to determine potential VSR closure options. Given the large size of the VSR, a surgical approach was favored.
While awaiting surgery, serial echocardiograms demonstrated progressive RV failure. The advanced heart failure service evaluated the patient and he was listed for heart transplant. He remained on VA-ECMO for 16 days prior to undergoing successful, uncomplicated heart transplantation. On post-operative day 4, the patient was noted to have ischemic changes of bilateral toes and an arterial ultrasound demonstrated bilateral digital disease consistent with distal thrombo-embolization, a complication of ECMO necessitating amputation of his toes. On postoperative day 25, patient was discharged home and continues to do well.
Discussion
In the absence of surgical repair, the in-hospital mortality rates associated with mechanical complications following AMI exceed 90%.5 Historically, surgical repair is the treatment of choice for VSR complicating AMI. Despite the advances in surgical techniques and perioperative care the mortality rate after surgical repair remains high.6 In a recently published meta-analysis of 41 studies, Matteucci et al. reported an operative mortality of 38.2% (2430 deaths out of 6361 patients).7 Reoperation was performed for residual or recurrent VSR in 7.4% of patients. The post-operative mortality is even higher in patients with RV dysfunction and patients with cardiogenic shock.7 , 8 In recent years percutaneous repair has been proposed as an alternative option in selected patients with high surgical risk and simple rupture amenable to percutaneous repair.1 Schlotter et al. in a meta-analysis of 13 studies showed a 30-day mortality of 32% (14–75%) in patients who underwent percutaneous repair.9 Serious procedural complications were device embolization, arrhythmia and left ventricular rupture. Similar to surgical repair, post percutaneous repair survival rate in the presence of cardiogenic shock is extremely poor.8 , 10 In one study, 30-day mortality rate after percutaneous repair was significantly higher in patients with cardiogenic shock (88%) compared to non-shock patients (38%).10
Given the acuity of illness in these patients, timing of surgery remains controversial.1 There is a significant survival rate improvement with delay in VSR repair11; however the development of hemodynamic compromise requires systemic support to maximize survival while improving potential success of VSR repair. Arnaoutakis et al. in a retrospective study of the Society of Thoracic Surgeons’ database demonstrated the highest mortality in patients who underwent repair in the first 24 h.12 Surgical repair in the first 7 days was associated with higher mortality (54.1%) compared with intervention after 1 week (18.4%).12 Mechanical circulatory support (MCS) has emerged as a potentially suitable temporizing measure for hemodynamic stabilization prior to definitive treatment, as demonstrated in case series.13
VA-ECMO has been used successfully in patients with cardiogenic shock complicating AMI-VSR.5 Timely initiation of VA-ECMO optimizes tissue perfusion and prevents irreversible end organ failure.13 Despite full cardiopulmonary support, VA-ECMO increases LV afterload resulting in enhanced left to right shunting, and progressive RV dysfunction.14 Strategies to vent the LV (i.e. IABP, Impella, atrial septostomy or transatrial drainage cannula) may mitigate these unfavorable hemodynamic effects.14
Both of our patients stabilized hemodynamically with VA-ECMO but showed deteriorating RV function. Since RV dysfunction is associated with higher mortality and poor outcomes post AMI-VSR surgical repair,15 they were listed for heart transplantation.
Conclusion
In the era of COVID-19, heightened public awareness about the potentially fatal consequences of delay in clinical presentation for symptoms suggestive of AMI is imperative. In the case of VSR complicating AMI, VA-ECMO with tailored LV venting may be a viable strategy for hemodynamic stabilization and bridging to surgical repair or cardiac replacement therapy.
Declaration of Competing Interest
Ramesh Singh: Baxter: Speaker; Behnam Tehrani: Medtronic: Consulting, speaker;
Wayne Batchelor: Abbott: Consulting, speaker; Boston Scientific: consulting; vWave: Consulting.
The following authors declare no disclosures or conflict of interest: Hooman Bakhshi, Raghav Gattani, Emmanuel Ekanem, Mehul Desai, Alan M Speir, Shashank Sinha and Matthew W Sherwood.
Funding
No funding was obtained for this study.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.hrtlng.2020.12.013.
Appendix. Supplementary materials
Color Doppler transthoracic echocardiogram showing ventricular septal rupture in subcostal view.
Contrast enhanced transthoracic echocardiogram showing apical thrombus and ventricular septal rupture.
Color Doppler and 2D transthoracic echocardiogram showing ventricular septal rupture in subcostal view.
References
- 1.Jones B.M., Kapadia S.R., Smedira N.G., et al. Ventricular septal rupture complicating acute myocardial infarction: a contemporary review. Eur Heart J. 2014;35(31):2060–2068. doi: 10.1093/eurheartj/ehu248. [DOI] [PubMed] [Google Scholar]
- 2.Ahmed T., Nautiyal A., Kapadia S., Nissen S.E. Delayed presentation of STEMI complicated by ventricular septal rupture in the Era of COVID-19 pandemic. JACC Case Rep. 2020;2(10):1599–1602. doi: 10.1016/j.jaccas.2020.05.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pilato E., Pinna G.B., Parisi V., Manzo R., Comentale G. Mechanical complications of myocardial infarction during COVID-19 pandemic: an Italian single-centre experience. Heart Lung. 2020;49(6):779–782. doi: 10.1016/j.hrtlng.2020.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parikh M., Busman M., Dickinson M., Wohns D., Madder R.D. Ventricular septal rupture in 2 patients presenting late after myocardial infarction during the COVID-19 pandemic. JACC Case Rep. 2020;2(12):2013–2015. doi: 10.1016/j.jaccas.2020.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McLaughlin A., McGiffin D., Winearls J., et al. Veno-arterial ECMO in the setting of post-infarct ventricular septal defect: a bridge to surgical repair. Heart Lung Circ. 2016;25(11):1063–1066. doi: 10.1016/j.hlc.2016.02.024. [DOI] [PubMed] [Google Scholar]
- 6.Moreyra A.E., Huang M.S., Wilson A.C., Deng Y., Cosgrove N.M., Kostis J.B. Trends in incidence and mortality rates of ventricular septal rupture during acute myocardial infarction. Am J Cardiol. 2010;106(8):1095–1100. doi: 10.1016/j.amjcard.2010.06.013. [DOI] [PubMed] [Google Scholar]
- 7.Matteucci M., Ronco D., Corazzari C., et al. Surgical repair of post-infarction ventricular septal rupture: systematic review and meta-analysis. Ann Thorac Surg. 2020 doi: 10.1016/j.athoracsur.2020.08.050. [DOI] [PubMed] [Google Scholar]
- 8.Menon V., Webb J.G., Hillis L.D., et al. Outcome and profile of ventricular septal rupture with cardiogenic shock after myocardial infarction: a report from the SHOCK Trial Registry. SHould we emergently revascularize Occluded Coronaries in cardiogenic shocK? J Am Coll Cardiol. 2000;36:1110–1116. doi: 10.1016/s0735-1097(00)00878-0. 3 Suppl A. [DOI] [PubMed] [Google Scholar]
- 9.Schlotter F., de Waha S., Eitel I., Desch S., Fuernau G., Thiele H. Interventional post-myocardial infarction ventricular septal defect closure: a systematic review of current evidence. EuroIntervention. 2016;12(1):94–102. doi: 10.4244/EIJV12I1A17. [DOI] [PubMed] [Google Scholar]
- 10.Thiele H., Kaulfersch C., Daehnert I., et al. Immediate primary transcatheter closure of postinfarction ventricular septal defects. Eur Heart J. 2009;30(1):81–88. doi: 10.1093/eurheartj/ehn524. [DOI] [PubMed] [Google Scholar]
- 11.Jeppsson A., Liden H., Johnsson P., Hartford M., Rådegran K. Surgical repair of post infarction ventricular septal defects: a national experience. Eur J Cardiothorac Surg. 2005;27(2):216–221. doi: 10.1016/j.ejcts.2004.10.037. [DOI] [PubMed] [Google Scholar]
- 12.Arnaoutakis G.J., Zhao Y., George T.J., Sciortino C.M., McCarthy P.M., Conte J.V. Surgical repair of ventricular septal defect after myocardial infarction: outcomes from the Society of Thoracic Surgeons National Database. Ann Thorac Surg. 2012;94(2):443. doi: 10.1016/j.athoracsur.2012.04.020. 436-443discussion-434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rob D., Špunda R., Lindner J., et al. A rationale for early extracorporeal membrane oxygenation in patients with postinfarction ventricular septal rupture complicated by cardiogenic shock. Eur J Heart Failure. 2017;19(S2):97–103. doi: 10.1002/ejhf.852. [DOI] [PubMed] [Google Scholar]
- 14.Pahuja M., Schrage B., Westermann D., Basir M.B., Garan A.R., Burkhoff D. Hemodynamic effects of mechanical circulatory support devices in ventricular septal defect. Circ Heart Fail. 2019;12(7) doi: 10.1161/CIRCHEARTFAILURE.119.005981. [DOI] [PubMed] [Google Scholar]
- 15.Cinq-Mars A., Veilleux S.P., Voisine P., et al. The novel use of heart transplantation for the management of a case with multiple complications after acute myocardial infarction. Can J Cardiol. 2015;31(6):816–818. doi: 10.1016/j.cjca.2015.01.024. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Color Doppler transthoracic echocardiogram showing ventricular septal rupture in subcostal view.
Contrast enhanced transthoracic echocardiogram showing apical thrombus and ventricular septal rupture.
Color Doppler and 2D transthoracic echocardiogram showing ventricular septal rupture in subcostal view.