Abstract
Background:
The unprecedented situation caused by the coronavirus disease 2019 (COVID-19) pandemic has profoundly affected endoscopic practice in regard to access, volume, and workflow. We aimed to assess the potential changes in the technical outcomes of endoscopic retrograde cholangiopancreatography (ERCP) procedures carried out in patients with confirmed SARS-CoV-2 infection.
Methods:
We conducted an international, multicenter, retrospective, matched case-control study of ERCP procedures carried out in patients with confirmed COVID-19. The main outcome was technical success of the procedure as assessed by the endoscopist, and the secondary outcome was the development of procedure-related adverse events. Each case was matched in a 1:4 ratio with controls extracted from each center’s database in order to identify relevant changes in outcome measures compared with the pre-pandemic era.
Results:
Eighteen procedures performed in 16 COVID-19 patients [14 men, 65 years (9–82)] and 67 controls were included in the final analysis. Technical success was achieved in 14/18 COVID-19 cases, which was significantly lower as compared with the control group (14/18 versus 64/67, p = 0.034), with an endoscopic reintervention required in 9/18 cases. However, the rate of procedure-related adverse events was low in both groups (1/18 versus 10/67, p = 0.44). On multivariable analysis, COVID-19 status remained the only risk factor for technical failure of the procedure [odds ratio of 19.9 (95% confidence interval 1.4–269.0)].
Conclusions:
The COVID-19 pandemic has affected the volume and practice of ERCP, resulting in lower technical success rates without significantly impacting patient safety. Prioritizing cases and following recommendations on safety measures can ensure good outcome with minimal risk in dedicated centers.
Keywords: ERCP, COVID-19, training
Introduction
The coronavirus disease 2019 (COVID-19) pandemic has had a profound impact on medical systems around the globe, placing a tremendous burden on healthcare providers and services. Because of the high infectivity of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and its transmission through airborne droplets of saliva and potential fecal excretion,1 endoscopy staff was deemed particularly vulnerable to infection,2 contributing to the significant reduction in the number of procedures conducted in most endoscopy units. The European Society of Gastrointestinal Endoscopy (ESGE) developed position statements for the practice of gastrointestinal endoscopy during the COVID-19 pandemic, with the aim of minimizing the risk for healthcare workers while prioritizing urgent procedures and enforcing reasonable delays for non-urgent procedures after a case-by-case evaluation.3
It is certain that most units experienced a considerable reduction in the number of procedures performed, as well as a near-complete cessation of all hands-on training programs4–6 for an extended period of time, ranging from several weeks to several months, although the actual impact of these severe restrictions is yet to be properly assessed. A recent Italian survey reported a significant drop in the number of urgent endoscopy procedures carried out during the pandemic,7 despite recommendations that endoscopy for urgent indications (i.e. bleeding, cholangitis, foreign body ingestion) should be carried out in all cases, regardless of SARS-CoV-2 infection status. The decrease was statistically significant for urgent upper gastrointestinal (GI) endoscopy, but not for urgent lower GI and biliopancreatic procedures. Furthermore, the cumbersome nature of the personal protective equipment (PPE) required to safely conduct endoscopy procedures in patients at high risk for SARS-CoV-2 infection can, by itself, impede procedural success and increase the reluctance of the operator to perform complicated and time-consuming interventions such as ERCP.8 While these reports demonstrate significant changes in endoscopic management, there are currently limited data assessing the technical and clinical outcomes of procedures carried out during this period. We aimed to canvass the practice of ERCP in COVID cases and assess whether the technical outcome of ERCP procedures was affected by the changes in practice related to the COVID-19 pandemic.
Methods
Study design
We conducted an international multicenter, retrospective, matched case-control study of ERCP procedures in confirmed cases of SARS-CoV-2 infection.
Case identification and selection
We invited senior endoscopists from 12 referral centers (11 in Europe and 1 in the USA) to identify ERCP procedures conducted in patients with confirmed SARS-CoV-2 infection, with a positive polymerase chain reaction (PCR) test obtained within 14 days prior to the endoscopic procedure. Only 5 out of the 12 centers confirmed having performed ERCP procedures in patients with COVID-19 and provided data for this study. Data regarding the patient (sex, age, indication for the procedure, severity of COVID infection), the procedure (type of sedation used, method of cannulation, type of diagnostic and/or therapeutic intervention, trainee involvement) and its outcome (technical success, procedure-related adverse events, including need for urgent or planned re-intervention) were retrieved from the patient charts and reported by the attending endoscopist using a standard report form. Control cases were identified using each institution’s medical records database, matching the index case with regard to patient age (±5 years), sex, attending endoscopist, indication for procedure, difficulty level (according to the modified Schutz scale9) and papilla anatomy (native papilla versus preexisting sphincterotomy) in a 4:1 ratio. In order to minimize any bias due to changes in personal or institutional practice or experience accumulated by the endoscopist, the most recent procedures which fulfilled the matching criteria, and which were performed prior to the COVID-19 pandemic were included as controls in the final analysis.
ERCP conditions during COVID-19
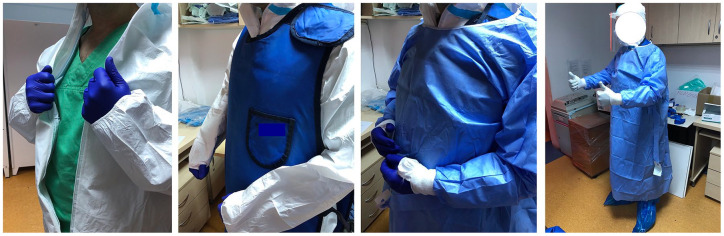
One participating center was designated as a dedicated COVID unit throughout the pandemic; consequently, all positive cases requiring urgent endoscopy from a large catchment area (population >10,000,000) were referred to this center. The other four participating centers had separate COVID circuits and continued to offer endoscopy services to non-COVID-19 patients throughout the pandemic, according to local guidelines and regulations. Briefly, in accordance with World Health Organization recommendations,10 confirmed or probable COVID-19 patients presenting with biliopancreatic conditions were managed in dedicated wards, including adequate facilities for laboratory tests and invasive procedures. In particular, dedicated radiology rooms were available to perform abdominal ultrasound and computed tomography (CT) scan, whereas magnetic resonance imaging (MRI) for COVID-19+ was not available in all centers. Patients were treated in dedicated endoscopy suites, with anesthesiology support available in all cases. Protective measures for the staff, including radiology protection and the use of personal protective equipment (PPE) to prevent SARS-CoV-2 transmission, resulted in a multilayered and cumbersome outfit, including regular scrubs, a first layer of water-resistant PPE overalls (in two of the centers), lead shielding and a second water-resistant layer (surgical gown; Figure 1). Disposable duodenoscopes were employed in one of the centers with the aim of reducing the risk of transmission during the reprocessing process. Finally, negative-pressure rooms were only available in one center.
Figure 1.
Donning of multilayered PPE for ERCP in COVID patients.
COVID, coronavirus disease; ERCP, endoscopic retrograde cholangiopancreatography; PPE, personal protective equipment.
Outcome measures
The main study outcome was the technical success of each procedure [i.e. successful stenting of stricture, complete clearance of all common bile duct (CBD) stones, etc.] as assessed by the attending endoscopist. Procedure-related adverse events as described and graded by Cotton et al.11 constituted the secondary outcome of the study.
Statistical analysis
Data from all participating centers were collected in a central database and analyzed using Statistical Package for Social Sciences v.16 (IBM Corporation, Illinois, USA). Data was analyzed in a two-step fashion; bivariate analysis using the appropriate tests (chi square, Fisher’s exact test, and Student’s t test if the continuous variables had normal distribution, and Mann–Whitney U test if the continuous variables had non-normal distribution) was first carried out for potential risk factors for technical failure of the procedure and procedure-related adverse events. The 95% confidence interval (CI) for proportion comparisons was calculated using an online calculator (https://www.medcalc.org/calc/comparison_of_proportions.php). Multivariable analysis using logistic regression (enter method) was then carried out for the main study outcome, including all variables that had a p level < 0.2 at univariable analysis. A p < 0.05 was considered statistically significant.
The study was conducted in accordance with the Helsinki declaration and approved by local ethics committees.
Results
We identified a total of 18 ERCPs performed in 16 patients with PCR-confirmed SARS-CoV-2 infection by 9 endoscopists from 5 endoscopy units (Colentina Clinical Hospital, Bucharest, Romania; Policlinico Agostino Gemelli, Rome, Italy; University of Leipzig Medical Center, Leipzig Germany; University Hospitals Gasthuisberg, University of Leuven and Erasme Hospital, Brussels, Belgium) between 15 March and 1 July 2020 (Figure 2). Most patients were male (14/16), and the median age was 65 years (range 9–82). PPE was available and employed effectively in all cases, in accordance with ESGE position statements; there were no confirmed cases of infection of medical personnel as a direct result of these procedures. All patients were symptomatic and/or required prioritized interventions according to ESGE recommendations for their biliopancreatic disease;3 however, all of the patients were either asymptomatic or had mild symptoms of COVID-19.12 The most common indication for ERCP was extraction of CBD stones (8/18) and most patients had a native papilla anatomy (13/18). Half of the procedures (9/18) were conducted under general anesthesia, while the remaining procedures were conducted under deep sedation, with anesthesiologist-directed sedation in 8/18 and GE-directed sedation in only one case. Selective cannulation of the desired duct was successful in all cases, with guidewire-assisted cannulation (GW) being the method of choice (11/18), followed by double-guidewire (4/18) and the contrast-guided technique (3/18).
Figure 2.
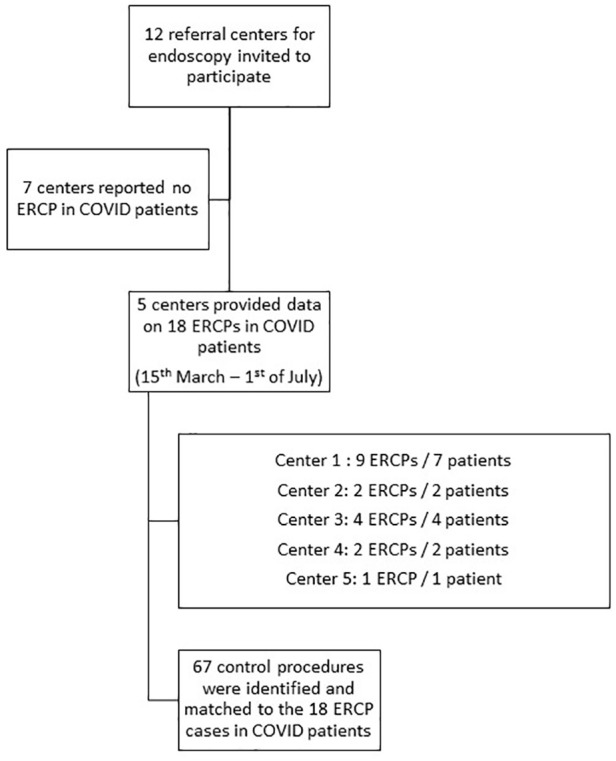
Flowchart of the selection of ERCP procedures included in the final database.
COVID, coronavirus disease; ERCP, endoscopic retrograde cholangiopancreatography.
Technical success was achieved in 14/18 procedures, but endoscopic re-intervention was required in 9/18 cases, including one case where urgent re-intervention for cholangitis occurred within 7 days of the index procedure, and eight more cases where elective re-intervention was undertaken to complete the therapeutic plan. Although all five endoscopy centers have training programs for advanced endoscopy procedures, only one center reported involvement of fellows in two out of their four cases. In both cases, endoscopy fellows with considerable experience in ERCP (>200 procedures) performed the initial part of the intervention which was then completed by the senior attending endoscopist (Table 1). Additional details about the patients and procedures, including comments on particular circumstances due to the COVID-19 pandemic which might have influenced patient care according to the attending endoscopist, are provided in Table 1. Using the electronic medical records available at each institution, we identified four controls for each of the 18 COVID-19 cases, with the exception of cases # 6 and 12 (see Table 1) where only one and, respectively, two controls could be identified using pre-established criteria, resulting in a total of 67 matched controls. There were no significant differences between COVID-19 patients and controls in terms of their baseline clinical characteristics, including the indication for ERCP and the technical difficulty of the planned procedures (Table 2).
Table 1.
Clinical data and technical aspects of procedures in COVID-19 patients.
| ID | Center | Sex | Age | Indication for ERCP | COVID severity | ASA score | Sedation | Cannulation technique | Procedure performed | Technical success | Adverse event | Comments |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Bucharest | F | 61 | Suspected CBD stone in acute pancreatitis | Mild | 2 | AG-deep sedation | GW | Stenting of distal CBD stricture | Yes | No | MRCP/EUS unavailable during COVID lockdown |
| 1 | Bucharest | F | 61 | Stent removal | Mild | 2 | AG-deep sedation | GW | Removal of stent, control balloon sweep of CBD | Yes | No | Early removal of stent to avoid potential delays in recall procedure |
| 2 | Bucharest | M | 71 | CBD stone | Mild | 2 | AG-deep sedation | GW | Balloon extraction of CBD stone | Yes | No | |
| 3 | Bucharest | M | 62 | Distal MBDO (lymphoma) | Mild | 2 | AG-deep sedation | DGW | UCSEMS placement | Yes | No | Same-session EUS/FNA after SEMS placement |
| 4 | Bucharest | M | 77 | CBD stone | Mild | 2 | AG-deep sedation | GW | Sphincterotomy, failed extraction and double-pigtail stent placement | No | No | Difficult stone extraction, prolonged procedure was deemed high-risk and temporary stenting was preferred |
| 5 | Bucharest | M | 65 | CBD stone | Mild | 2 | AG-deep sedation | DGW | Sphincterotomy, failed extraction and double-pigtail stent placement | No | No | Large stone in undilated CBD; failure of mechanical lithotripsy; cholangioscopy not available during COVID pandemic |
| 6 | Bucharest | M | 75 | CBDS and suspected bile leak post-CCY | Mild | 3 | AG-deep sedation | GW | Stent placement | No | No | Cirrhotic patient in a post-surgical setting; temporary stenting was chosen due to comorbidities |
| 7 | Bucharest | M | 65 | Hilar MBDO (cholangiocarcinoma) | Mild | 4 | AG-deep sedation | DGW | Sphincterotomy, bougie dilatation and placement of two 7 Fr PS + pancreatic stent | No | Yes (cholangitis and liver abscess) | Urgent ERCP was performed in a patient with severe cholangitis and acute kidney injury; only a native CT scan was available prior to ERCP |
| 7 | Bucharest | M | 65 | Reintervention for Hilar MBDO | Mild | 4 | General anesthesia | GW | Stent removal, Bougie dilatation, brush cytology + biopsies, placement of one 8.5 Fr and one 7 Fr PS to the left and right lobes | Yes | No | Ascites development precluded percutaneous drainage, so a second ERCP was attempted in this case |
| 8 | Rome | M | 59 | Acute biliary pancreatitis with CBD stone and cholangitis | Mild | 3 | General anesthesia | CG | Sphincterotomy and extraction of CBD stones | Yes | No | SARS-CoV-2 infection was diagnosed at triage for endoscopy |
| 9 | Rome | M | 66 | CBD stones | Mild | 3 | General anesthesia | CG | Sphincterotomy and extraction of CBD stones | Yes | No | SARS-CoV-2 infection was diagnosed at triage for endoscopy |
| 10 | Leuven | M | 56 | Benign CBD stricture | Mild | 3 | General anesthesia | DGW | Stent placement | Yes | No | ERCP unnecessarily delayed because of COVID positivity and restrictions |
| 11 | Leuven | M | 69 | Disconnected pancreatic-duct syndrome | Mild | 4 | General anesthesia | GW | Stent placement in MPD | Yes | No | Reevaluation after LAMS placement for acute necrotic pancreatitis |
| 12 | Leuven | M | 9 | Post-LT bile leak | Mild | 3 | General anesthesia | GW | Stenting of the CBD | Yes | No | Liver transplant for PFIC with postoperative ischemic cholangiopathy and anastomotic leak |
| 13 | Leuven | M | 65 | Benign bile-duct stricture | Mild | 2 | General anesthesia | GW | Balloon dilatation and FCSEMS placement | Yes | No | Referral after recurrent benign stricture and contracting COVID-19 |
| 14 | Leipzig | M | 64 | CBD stones | Mild | 2 | General anesthesia | GW | Sphincterotomy and extraction of CBD stones | Yes | No | |
| 15 | Leipzig | M | 58 | Hilar MBDO (cholangiocarcinoma) | Mild | 3 | GE-guided sedation | GW | Sphincterotomy, balloon dilatation and stenting of stricture | Yes | No | Scheduled for plastic stent exchange |
| 16 | Brussels | F | 82 | Distal MBDO (cholangiocarcinoma) | Mild | 3 | General anesthesia | GW | Sphincterotomy, brushing at the level of the stricture and placement of SEMS | Yes | No |
AG, anesthesiologist guided; AKI, acute kidney injury; ASA score, American Society of Anesthesiologists physical status score; CBD, common bile duct; CBDS, common bile duct stones; CCY, cholecystectomy; CG, contrast guided; COVID-19, coronavirus disease 2019; CT, computed tomography; DGW, double-guidewire cannulation; EUS, endoscopic ultrasound; FCSEMS, fully covered SEMS; FNA, fine-needle aspiration; GE, gastroenterologist; GW, guidewire-led cannulation; LAMS, lumen-apposing metal stent; LT, liver transplant; MBDO, malignant bile-duct obstruction; MPD, main pancreatic duct; MRCP, magnetic resonance cholangiopancreatography; PFIC, progressive familial intrahepatic cholestasis; PS, plastic stent; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SEMS, self-expandable metal stent; UCSEMS, uncovered SEMS.
Table 2.
Comparison of baseline characteristics of COVID-19 cases and controls.
| COVID-19 cases | Control cases | p value | |
|---|---|---|---|
| Sex (male/female) | 14/4 | 49/18 | 0.77 |
| Median age (range) | 65 years (9–82) | 64 (19–86) | 0.98 |
| Indication for ERCP | 0.99 | ||
| CBD stones | 8/18 | 30/67 | |
| Distal malignant stricture | 2/18 | 8/67 | |
| Hilar malignant stricture | 3/18 | 12/67 | |
| Bile leak | 0/18 | 0/67 | |
| Benign CBD stricture | 2/18 | 8/67 | |
| Other | 3/18 | 9/67 | |
| Median bilirubin levels (range) | 3.7 mg/dl (0.3–25) | 1.6 mg/dl (0.1–28.7) | 0.28 |
| Native papilla anatomy | 13/18 | 44/67 | 0.77 |
| Type of sedation used | |||
| GE-directed sedation | 1/18 | 11/67 | 0.44 |
| A-directed sedation | 8/18 | 30/67 | |
| General anesthesia | 9/18 | 26/67 | |
| Median ASA score * | III | III | 0.75 |
| Technical difficulty of the procedure | |||
| Grade I | 9/18 | 38/67 | 0.87 |
| Grade II | 7/18 | 23/67 | |
| Grade III | 2/18 | 6/67 | |
ASA score was available in all COVID-19 cases but only 25/67 control cases (data not captured in all electronic medical records).
A, anesthesiologist; ASA score, American Society of Anesthesiologists physical status score; CBD, common bile duct; COVID-19, coronavirus disease 2019; ERCP, endoscopic retrograde cholangiopancreatography; GE, gastroenterologist.
Cannulation was successful in all cases, and there was no statistically significant difference in terms of cannulation methods used in COVID-19 cases as compared with controls (p = 0.22), although, notably, there was no precut access in the COVID-19 group, while we identified 7/67 cases of precut in the control group (Table 3).
Table 3.
Comparison of technical characteristics and procedure-related outcomes between COVID-19 cases and the control group.
| COVID-19 cases | Control cases | % difference (controls–cases) and 95% CI | p value | |
|---|---|---|---|---|
| Cannulation method | N/A | |||
| GW | 11/18 | 47/67 | ||
| CG | 3/18 | 7/67 | 0.22 | |
| DGW | 4/18 | 5/67 | ||
| Precut | 0/18 | 7/67 | ||
| Other | 0/18 | 1/67 | ||
| Failed | 0/18 | 0/67 | ||
| Technical success | 14/18 (77.7%, 95% CI 55.3–93.5%) | 64/67 (95.5%, 95% CI 87.4–99.0%) | 17.8% (2.3–40.9%) | 0.034** |
| Need for re-intervention | 9/18 (50%) | 20/67 (29.8%) | −20.2% (−43.2% to +3.8%) | 0.16 |
| Procedure-related AE | 1/18 (5.5%) | 10/67 (14.9%) | 9.4% (−11.8% to +20.7%) | 0.44 |
| Trainee involvement | 2/18 (11.1%, 95% CI 1.3–34.7%) | 25/67 (37.3%, 95% CI 25.8–49.9%) | 26.2% (2.0–40.6%) | 0.045** |
Statistically significant using Fisher’s exact test.
AE, adverse event; CI, confidence interval; COVID-19, coronavirus disease 2019; N/A, not applicable.
Technical success of the procedure was lower in the COVID-19 cases as compared with the control group (14/18 versus 64/67, p = 0.034, Fisher’s exact); however, there was no significant difference in the need for urgent or planned endoscopic re-intervention to complete the treatment plan [9/18 cases versus 20/67 cases in the control group (p = 0.16, Fisher’s exact)]. There was no significant difference in the occurrence of procedure-related adverse events (1/18 versus 10/67, p = 0.44, Fisher’s exact). On multivariable analysis, after adjusting for sex, age, Schutz difficulty grade, endoscopy center and attending endoscopist, COVID-19 status remained the only risk factor for technical failure of the procedure, with an odds ratio of 19.9 (95% CI 1.4–269).
Discussion
At the time of writing this paper, there were over 28,000,000 confirmed cases of SARS-CoV-2 infection, resulting in more than 900,000 deaths worldwide,13 with numbers expected to increase significantly as the pandemic evolves. Severe lockdown measures across Europe and the USA, including a drastic limitation of endoscopy procedures, aimed to protect patients and medical staff alike, have clearly affected patient care.
To our knowledge, this is the first study analyzing the impact of COVID-19-related restrictions on the clinical outcome and procedure-related aspects of ERCP, one of the most technically demanding endoscopic procedures. Our study showed a significant decrease in the proportion of technically successful procedures in COVID-19 patients as compared with a historical group of cases performed by the same endoscopists, for similar indications (including technical difficulty of the procedure according to the Schutz scale), and in a similar group of patients. Furthermore, the rate of planned or urgent re-interventions to complete endoscopic treatment was higher in the COVID-19 group, although it did not reach the threshold for statistical significance. By reviewing the individual cases and the attending endoscopists’ comments, it seems possible that this change in performance was mainly a result of adopting a ‘stent-and-run’ policy, aimed at ensuring adequate drainage and resolving the acute episode, with re-intervention planned for a later point at which the patient had presumably cleared the infection. Current guidelines on quality in ERCP14 endorse a ‘one-stop-shop’ approach to ERCP, recommending CBD clearance in >95% cases and successful stenting of distal strictures in >95% cases. However, in the setting of the COVID-19 pandemic, several factors should weigh in on the decision algorithm and acceptable technical outcome of a procedure. The new situation could significantly increase procedure-related morbidity and mortality,15 the costs of repeating an initially incomplete ERCP procedure and the personal risk to medical staff attending to highly contagious patients in a difficult working environment16 (cumbersome PPE, limited access to high-end imaging techniques, etc).
Another interesting finding in our study was the change in cannulation technique, with no precut being performed in the COVID-19 group as compared with the control group (7/67), although this difference was not statistically significant. This finding could be related to the decrease in hands-on trainee involvement in the COVID-19 cases compared with historical controls (p = 0.045), as some data suggests trainee involvement could be a potential risk factor for failure of conventional cannulation methods requiring salvage precut by the supervising endoscopist.17 In fact, all but one of the attending centers had suspended their training programs for endoscopy during the pandemic.18
Sedation practices varied across the participating centers; while general anesthesia was employed in 50% of the procedures, gastroenterologist-directed and anesthesiologist-directed deep sedation were also employed and there was no statistically significant difference in the type of sedation used in COVID-19 cases compared with historical controls. While evaluation of sedation practices was beyond the scope of this paper, it is important to consider the choice of sedation in relationship to the risk of viral transmission for the attending staff; furthermore, a recent case report has suggested that propofol-based sedation could aggravate the clinical condition of COVID-19 patients.19
Finally, the rate of procedure-related adverse events was low in both study groups (1/18 versus 10/67, p = 0.76), with a single reported case of cholangitis in a complex hilar stricture in a COVID-19 case which was successfully managed by endoscopic re-intervention. The low rate of procedure-related adverse events was in line with previous data from our group,20 reflecting the fact that the procedures were performed by experienced endoscopists working in referral centers for endoscopy. These favorable results underline the importance of following high-quality standards and guidelines for special situations. For the time being, perhaps it would be advisable to approach COVID-19 patients requiring urgent ERCP in a similar fashion to other special patient groups such as pregnant patients21 in the sense that a strong indication for intervention is required and that only highly experienced endoscopists should be involved in order to reduce exposure time and associated risks.
Our study has several limitations which should be acknowledged. First, we could only identify 18 ERCP cases performed in patients with active SARS-CoV-2 infection, despite the fact that we conducted an international multicenter study. A recent single-center study from a tertiary referral center in Milan, one of the most affected regions in Europe, also showed a dramatic drop in biliopancreatic procedures performed at the height of the pandemic, with only one ERCP and no endoscopic ultrasound procedures performed during their study timeframe.22 In light of these findings, we believe our data are probably an accurate reflection of endoscopy practice during the pandemic, confirmed through a well-established network of investigators in 12 referral centers across Europe and the USA (Figure 2), but we acknowledge the potential for selection bias and the limitations inherent to our particular study design. Furthermore, the case-control design of the study could also limit the generalizability of our results. However, we strived to identify adequate control cases for each COVID-19 cases, taking into account clinically relevant characteristics which are usually included in the analysis of the technical performance of ERCP (i.e. patient sex, age, indication of the procedure, Schutz scale of difficulty, attending endoscopist).20 Another limitation of the study is the inclusion of only PCR-confirmed cases in the analysis, excluding other high-risk procedures performed during the study interval in patients who were either untested or ultimately proven negative on PCR testing. We opted to include only cases where the increased risk for the attending staff was certain and acknowledged by all the team members, as a way of accurately reflecting the high-stress working conditions which would likely impact clinical practice. The influence of stress on the performance of tasks requiring a high-degree of concentrated effort is shown to be detrimental to outcomes in various scenarios.23 Finally, the presence of an active respiratory infection constitutes a potential bias since it could influence the clinically relevant outcome variables such as intensive-care-unit admission and post-procedure mortality rates. However, since all patients in our study were classified as asymptomatic or mildly symptomatic with regard to their COVID-19 disease, we consider the risk for bias to be low.
Another aspect which needs to be underscored is the fact that the extraordinary situation created by the COVID pandemic has led to very different responses across different countries and endoscopy units, both in terms of infrastructure and medical practice. Our case cohort is heterogeneous both in respect to underlying disease and endoscopic management, but this should be interpreted not as a limitation of the study but rather a reflection of the particular circumstances surrounding the practice of ERCP in COVID patients, and a starting point for reassessing how to provide optimal care for our patients at this time.
As the COVID-19 pandemic continues to unfold, and with an increased pressure of re-opening endoscopy wards likely to result in an increased number of procedures in COVID-19 patients,24 it is paramount to continue providing safe and effective treatment, with the aim of minimizing risks to patients and healthcare workers alike. Prospective study cohorts, including a wide variety of procedures performed during the pandemic (including COVID-19 confirmed, suspected and negative patients) could help assess the impact of individual factors such as the perceived risk of contamination and limitations of cumbersome PPE on the technical outcome of the procedures, helping to further adapt our practice in response to these new challenges facing the endoscopist.
In conclusion, our findings suggest that the extraordinary situation created by the COVID-19 pandemic has significantly influenced the practice of ERCP, resulting in a highly variable practice of endoscopic therapy across different centers. However, these changes do not seem to have significantly impacted patient safety despite resulting in a significant increase in the rate of re-interventions required. Further, larger prospective studies should assess if a ‘stent-and-run’ paradigm could be adopted in selected cases, with the aim of shortening procedure time and staff exposure, as well as how, and when, trainee involvement could be envisioned for these procedures.
Acknowledgments
The first two authors have contributed equally to study design, data collection, and drafting of the paper.
Footnotes
Authors’ contributions: Theodor Voiosu, Andrei Voiosu, Ivo Boškoski, Marianna Arvanitakis, Michiel Bronswijk, Andreea Benguș, Beatrice Orlandini, and Marcus Hollenbach were involved in study design, data gathering, analysis of the data, and drafting of the paper. Paul Bălănescu was involved in study design, statistical analysis of the data, and drafting of the paper. Daniel Blero, Schalk Van der Merwe, Radu Bogdan Mateescu, Jacques Devière, and Guido Costamagna were involved in study design and revising the of the paper for important intellectual content. All authors reviewed and approved the final version of the paper.
Conflict of interest statement: Michiel Bronswijk received travel grants from Taewoong, Takeda, and Prion Medical. Schalk van der Merwe holds the Cook chair in interventional endoscopy and holds consultancy agreements with Cook, Pentax and Olympus. Professor Guido Costamagna is a consultant for Cook Medical, Boston Scientific, and Olympus. Ivo Boškoski is a consultant for Apollo Endosurgery, Cook Medical, and Boston Scientific; board member for Endo Tools; research grant recipient from Apollo Endosurgery. Also, he has received food and beverage compensation from Apollo Endosurgery, Cook Medical, Boston Scientific, and Endo Tools. The other authors report no conflict of interest.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Ethics: The study was conducted in accordance with the Helsinki declaration and approved by local ethics committees.
ORCID iDs: Theodor Voiosu  https://orcid.org/0000-0003-3250-4900
https://orcid.org/0000-0003-3250-4900
Ivo Boškoski  https://orcid.org/0000-0001-8194-2670
https://orcid.org/0000-0001-8194-2670
Contributor Information
Theodor Voiosu, Gastroenterology Department, Colentina Clinical Hospital, 19–21 Stefan Cel Mare Bvd, Spitalul Clinic Colentina, Pavilion B, Gastroenterologie, Bucharest, Romania; Gastroenterology Department, Colentina Clinical Hospital, Bucharest, Romania; Internal Medicine Department, Carol Davila University of Medicine and Pharmacy, Bucharest, Romania.
Andrei Voiosu, Gastroenterology Department, Colentina Clinical Hospital, Bucharest, Romania.
Ivo Boškoski, Digestive Endoscopy Unit, Fondazione Policlinico Universitario Agostino Gemelli IRCCS, Rome, Italy; Center for Endoscopic Research Therapeutics and Training (CERTT), Catholic University of Rome, Italy.
Marianna Arvanitakis, Department of Gastroenterology, Hepatopancreatology, and Digestive Oncology, CUB Hôpital Erasme, Université Libre de Bruxelles, Brussels, Belgium.
Michiel Bronswijk, Department of Gastroenterology and Hepatology, University Hospitals Gasthuisberg, University of Leuven, Leuven, Belgium.
Marcus Hollenbach, Division of Gastroenterology; Medical Department II (Oncology, Gastroenterology, Hepatology, Pulmonology, Infectious Diseases), University of Leipzig Medical Center, Leipzig, Germany.
Andreea Benguş, Gastroenterology Department, Colentina Clinical Hospital, Bucharest, Romania.
Paul Bălănescu, Internal Medicine Department, Carol Davila University of Medicine and Pharmacy, Bucharest, Romania.
Beatrice Orlandini, Digestive Endoscopy Unit, Fondazione Policlinico Universitario Agostino Gemelli IRCCS, Rome, Italy; Center for Endoscopic Research Therapeutics and Training (CERTT), Catholic University of Rome, Italy.
Daniel Blero, Department of Gastroenterology, Hepatopancreatology, and Digestive Oncology, CUB Hôpital Erasme, Université Libre de Bruxelles, Brussels, Belgium.
Schalk Van der Merwe, Department of Gastroenterology and Hepatology, University Hospitals Gasthuisberg, University of Leuven, Leuven, Belgium.
Radu Bogdan Mateescu, Gastroenterology Department, Colentina Clinical Hospital Bucharest, Romania; Internal Medicine Department, Carol Davila University of Medicine and Pharmacy, Bucharest, Romania.
Jacques Devière, Department of Gastroenterology, Hepatopancreatology, and Digestive Oncology, CUB Hôpital Erasme, Université Libre de Bruxelles, Brussels, Belgium.
Guido Costamagna, Digestive Endoscopy Unit, Fondazione Policlinico Universitario Agostino Gemelli IRCCS, Rome, Italy; Center for Endoscopic Research Therapeutics and Training (CERTT), Catholic University of Rome, Italy.
References
- 1. Wilson NM, Norton A, Young FP, et al. Airborne transmission of severe acute respiratory syndrome coronavirus-2 to healthcare workers: a narrative review. Anaesthesia 2020; 75: 1086–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gu J, Han B, Wang J. COVID-19: gastrointestinal manifestations and potential fecal-oral transmission. Gastroenterology 2020; 158: 1518–1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gralnek IM, Hassan C, Beilenhoff U, et al. ESGE and ESGENA position statement on gastrointestinal endoscopy and the COVID-19 pandemic. Endoscopy 2020; 52: 483–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Belle A, Barret M, Bernardini D, et al. Impact of the COVID-19 pandemic on the gastrointestinal endoscopic activity in France. Endoscopy 2020; 52: 1111–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Boškoski I, Pecere S, Bove V, et al. Impact of SARS-CoV-2 on a high volume endoscopy center in Italy. Dig Liver Dis 2020; 52: 819–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pawlak KM, Kral J, Khan R, et al. Impact of COVID-19 on endoscopy trainees: an international survey. Gastrointest Endosc. Epub ahead of print 11 June 2020. DOI: 10.1016/j.gie.2020.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Salerno R, Conti CB, De Silvestri A, et al. The impact of covid-19 pandemic on urgent endoscopy in Italy: a nation-wide multicenter study. Scand J Gastroenterol 2020; 55: 870–876. [DOI] [PubMed] [Google Scholar]
- 8. An P, Huang X, Wan X, et al. ERCP during the pandemic of COVID-19 in Wuhan, China. Gastrointest Endosc. Epub ahead of print 16 April 2020. DOI: 10.1016/j.gie.2020.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Schutz SM, Abbott RM. Grading ERCPs by degree of difficulty: a new concept to produce more meaningful outcome data. Gastrointest Endosc 2000; 51: 535–539. [DOI] [PubMed] [Google Scholar]
- 10. World Health Organization. Global surveillance for COVID-19 caused by human infection with COVID-19 virus: interim guidance, 20 March 2020, https://www.who.int/docs/default-source/coronaviruse/global-surveillance-for-covid-v-19-final200321-rev.pdf (2020, accessed 30 June 2020).
- 11. Cotton PB, Eisen GM, Aabakken L, et al. A lexicon for endoscopic adverse events: report of an ASGE workshop. Gastrointest Endosc 2010; 71: 446–454. [DOI] [PubMed] [Google Scholar]
- 12. Epidemiology Working Group for NCIP Epidemic Response and Chinese Center for Disease Control and Prevention. [The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China]. Zhonghua Liu Xing Bing Xue Za Zhi 2020; 41: 145–151. [DOI] [PubMed] [Google Scholar]
- 13. Worldometer. COVID-19 coronavirus pandemic, https://www.worldometers.info/coronavirus/ (accessed 11 September 2020).
- 14. Domagk D, Oppong KW, Aabakken L, et al. Performance measures for ERCP and endoscopic ultrasound: a European Society of Gastrointestinal Endoscopy (ESGE) quality improvement initiative. Endoscopy 2018; 50: 1116–1127. [DOI] [PubMed] [Google Scholar]
- 15. Dumonceau JM, Kapral C, Aabakken L, et al. ERCP-related adverse events: European Society of Gastrointestinal Endoscopy (ESGE) guideline. Endoscopy 2020; 52: 127–149. [DOI] [PubMed] [Google Scholar]
- 16. Bove V, Schepis T, Boškoski I, et al. Bilio-pancreatic endoscopy during COVID-19 pandemic. Therap Adv Gastroenterol 2020; 13: 1756284820935187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Voiosu T, Voiosu A, Benguş A, et al. Trainee involvement increases precut rates and delays access to the common bile duct without an increase in procedure-related adverse events: a brave new world of ERCP training? Rom J Intern Med 2018; 56: 55–61. [DOI] [PubMed] [Google Scholar]
- 18. Marasco G, Nardone OM, Maida M, et al. Impact of COVID-19 outbreak on clinical practice and training of young gastroenterologists: a European survey. Dig Liver Dis. Epub ahead of print 23 May 2020. DOI: 10.1016/j.dld.2020.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Soh M, Hifumi T, Isokawa S, et al. The authors’ response: propofol and sedation in patients with coronavirus disease 2019. Am J Emerg Med. Epub ahead of print 18 June 2020. DOI: 10.1016/j.ajem.2020.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Voiosu T, Boskoski I, Voiosu AM, et al. Impact of trainee involvement on the outcome of ERCP procedures: results of a prospective multicenter observational trial. Endoscopy 2020; 52: 115–122. [DOI] [PubMed] [Google Scholar]
- 21. ASGE Standard of Practice Committee, Shergill AK, Ben-Menachem T, et al. Guidelines for endoscopy in pregnant and lactating women. Gastrointest Endosc 2012; 76: 18–24. [DOI] [PubMed] [Google Scholar]
- 22. Elli L, Tontini GE, Filippi E, et al. Efficacy of endoscopic triage during the Covid-19 outbreak and infective risk. Eur J Gastroenterol Hepatol 2020; 32: 1301–1304. [DOI] [PubMed] [Google Scholar]
- 23. Hasher L, Zacks RT. Automatic and effortful processes in memory. J Exp Psychol Gen 1979; 108: 356–388. [Google Scholar]
- 24. Gralnek I, Hassan C, Beilenhoff U, et al. ESGE and ESGENA position statement on gastrointestinal endoscopy and COVID-19: an update on guidance during the post-lockdown phase and selected results from a membership survey. Endoscopy. Epub ahead of print 8 July 2020. DOI: 10.1055/a-1213-5761. [DOI] [PMC free article] [PubMed] [Google Scholar]