Key Points
Question
Is there a difference in patient-reported quality of life among patients with permanent atrial fibrillation (defined as no plan to restore sinus rhythm) and symptoms of heart failure treated with digoxin or bisoprolol (a β-blocker) for heart rate control?
Findings
This randomized clinical trial included 160 adults aged 60 years or older with atrial fibrillation and symptoms of heart failure randomized to digoxin (mean attained dose, 161 μg/d) vs bisoprolol (mean attained dose, 3.2 mg/d). At 6 months, the mean 36-Item Short Form Health Survey physical component summary scores (higher scores are better) were 31.5 for the digoxin group vs 29.3 for the bisoprolol group, a difference that was not statistically significant.
Meaning
There was no statistically significant difference in patient-reported quality of life; the findings support potentially basing decisions about treatment on other end points.
Abstract
Importance
There is little evidence to support selection of heart rate control therapy in patients with permanent atrial fibrillation, in particular those with coexisting heart failure.
Objective
To compare low-dose digoxin with bisoprolol (a β-blocker).
Design, Setting, and Participants
Randomized, open-label, blinded end-point clinical trial including 160 patients aged 60 years or older with permanent atrial fibrillation (defined as no plan to restore sinus rhythm) and dyspnea classified as New York Heart Association class II or higher. Patients were recruited from 3 hospitals and primary care practices in England from 2016 through 2018; last follow-up occurred in October 2019.
Interventions
Digoxin (n = 80; dose range, 62.5-250 μg/d; mean dose, 161 μg/d) or bisoprolol (n = 80; dose range, 1.25-15 mg/d; mean dose, 3.2 mg/d).
Main Outcomes and Measures
The primary end point was patient-reported quality of life using the 36-Item Short Form Health Survey physical component summary score (SF-36 PCS) at 6 months (higher scores are better; range, 0-100), with a minimal clinically important difference of 0.5 SD. There were 17 secondary end points (including resting heart rate, modified European Heart Rhythm Association [EHRA] symptom classification, and N-terminal pro-brain natriuretic peptide [NT-proBNP] level) at 6 months, 20 end points at 12 months, and adverse event (AE) reporting.
Results
Among 160 patients (mean age, 76 [SD, 8] years; 74 [46%] women; mean baseline heart rate, 100/min [SD, 18/min]), 145 (91%) completed the trial and 150 (94%) were included in the analysis for the primary outcome. There was no significant difference in the primary outcome of normalized SF-36 PCS at 6 months (mean, 31.9 [SD, 11.7] for digoxin vs 29.7 [11.4] for bisoprolol; adjusted mean difference, 1.4 [95% CI, −1.1 to 3.8]; P = .28). Of the 17 secondary outcomes at 6 months, there were no significant between-group differences for 16 outcomes, including resting heart rate (a mean of 76.9/min [SD, 12.1/min] with digoxin vs a mean of 74.8/min [SD, 11.6/min] with bisoprolol; difference, 1.5/min [95% CI, −2.0 to 5.1/min]; P = .40). The modified EHRA class was significantly different between groups at 6 months; 53% of patients in the digoxin group reported a 2-class improvement vs 9% of patients in the bisoprolol group (adjusted odds ratio, 10.3 [95% CI, 4.0 to 26.6]; P < .001). At 12 months, 8 of 20 outcomes were significantly different (all favoring digoxin), with a median NT-proBNP level of 960 pg/mL (interquartile range, 626 to 1531 pg/mL) in the digoxin group vs 1250 pg/mL (interquartile range, 847 to 1890 pg/mL) in the bisoprolol group (ratio of geometric means, 0.77 [95% CI, 0.64 to 0.92]; P = .005). Adverse events were less common with digoxin; 20 patients (25%) in the digoxin group had at least 1 AE vs 51 patients (64%) in the bisoprolol group (P < .001). There were 29 treatment-related AEs and 16 serious AEs in the digoxin group vs 142 and 37, respectively, in the bisoprolol group.
Conclusions and Relevance
Among patients with permanent atrial fibrillation and symptoms of heart failure treated with low-dose digoxin or bisoprolol, there was no statistically significant difference in quality of life at 6 months. These findings support potentially basing decisions about treatment on other end points.
Trial Registration
ClinicalTrials.gov Identifier: NCT02391337 and clinicaltrialsregister.eu Identifier: 2015-005043-13
This randomized trial compares the effect of low-dose digoxin vs bisoprolol for heart rate control on quality of life in patients aged 60 years or older with permanent atrial fibrillation and dyspnea.
Introduction
Atrial fibrillation (AF) poses a major challenge to health care delivery, with high cost and rapidly increasing prevalence in an aging population with multiple comorbidities.1 Permanent AF (when patients and physicians jointly decide not to pursue rhythm control) accounted for 50% of patients with AF in a 2010 global registry.2 Yet there is almost no robust evidence to support clinical decision-making.3 Guidance is particularly needed for heart rate control in patients with AF and heart failure because inadequate heart rate control may worsen heart failure,4,5 and the combination of these conditions increases the risk of hospital admission and mortality.6,7
Heart rate control in patients with AF and suspected or diagnosed heart failure is usually limited to a β-blocker, digoxin, or their combination.8 β-Blockers are most widely used due to experience in other cardiovascular conditions9 and in heart failure with reduced ejection fraction in particular because prognosis is improved in patients with sinus rhythm regardless of age or sex.10 However, this finding was not replicated in the subgroup of patients with AF.7 Digoxin is usually a second-line option due to neutral mortality effects in randomized clinical trials (RCTs) of patients with heart failure, reduced left ventricular ejection fraction (LVEF), and sinus rhythm.11 Although there have been safety concerns from observational studies, digoxin is more commonly used in patients who have a greater comorbidity burden, require additional therapy, or are unable to tolerate β-blockers; all factors associated with a higher risk of adverse events.12
The Rate Control Therapy Evaluation in Permanent Atrial Fibrillation (RATE-AF) trial was designed to compare patient-reported quality of life among patients with permanent AF and symptoms of heart failure treated with low-dose digoxin or bisoprolol for heart rate control.
Methods
This study was an open-label, blinded end-point RCT comparing heart rate control using low-dose digoxin or bisoprolol. Without any prior comparative evidence and apparent equipoise for clinical end points,7,12 a 2-sided hypothesis was adopted. The rationale of the study has been described,3 with the design informed by a patient and public involvement team. The trial protocol appears in Supplement 1 and the statistical analysis plan appears in Supplement 2. Approval was obtained from the East Midlands–Derby research ethics committee (16/EM/0178), the Health Research Authority (IRAS 191437), and the Medicines and Healthcare Products Regulatory Agency. All participants provided written informed consent after review of the information leaflet.
Study Participants
Patients were recruited from 3 hospitals and primary care practices in England from 2016 through 2018 and last follow-up occurred in October 2019 (eMethods in Supplement 3). The following inclusion criteria were used: (1) aged 60 years or older, (2) had permanent AF in need of heart rate control from a clinician’s perspective, (3) had breathlessness (equivalent to New York Heart Association [NYHA] ≥class II), and (4) were able to provide written informed consent. Permanent AF was defined as a clinical decision for heart rate control with no plan for cardioversion, treatment with antiarrhythmic drugs, or AF ablation.8
The following exclusion criteria were used: (1) had an established indication for bisoprolol such as myocardial infarction within the last 6 months, (2) had contraindications for bisoprolol or digoxin, (3) had a baseline heart rate less than 60/min, a second- or third-degree heart block, other arrhythmias, pacemaker dependency or planned implantation, obstructive hypertrophic cardiomyopathy, myocarditis, or pericarditis, (4) had received or were planning to undergo heart transplant, (5) had undergone major surgery within prior 3 months, and (6) had any noncardiovascular disease expected to reduce life expectancy (eFigure 1 in Supplement 3).
There were no exclusion criteria related to known heart failure or according to LVEF. However, patients with decompensated heart failure within the last 14 days were excluded. Kidney dysfunction was not an exclusion criterion because both digoxin and bisoprolol can be safely used with appropriate care and monitoring.13,14 However, patients receiving kidney replacement therapy were excluded due to a lack of safety information.
Participants were asked to self-declare their race based on the code list for the 2011 UK Census. Ethnicity data are collected to monitor for health inequalities in the UK National Health Service. However, participants were able to refuse disclosure of ethnicity.
Randomization and Blinding
After obtaining written informed consent, participants were randomized in a 1:1 ratio to either digoxin or bisoprolol via telephone or a web-based portal using a computer-generated minimization algorithm to ensure balance between the treatment groups for baseline modified European Heart Rhythm Association (EHRA) class and sex (Figure 1). Baseline assessment immediately followed and allocation was concealed until the baseline assessment was complete; thereafter, it was an open-label trial. Alternative β-blocker treatments were acceptable for those with an intolerance to bisoprolol. Patients in both groups were given an appropriate education about AF and its treatments. They were also provided information about the European Society of Cardiology smartphone and tablet application specifically designed for use by patients with AF (https://www.escardio.org/af-apps).15
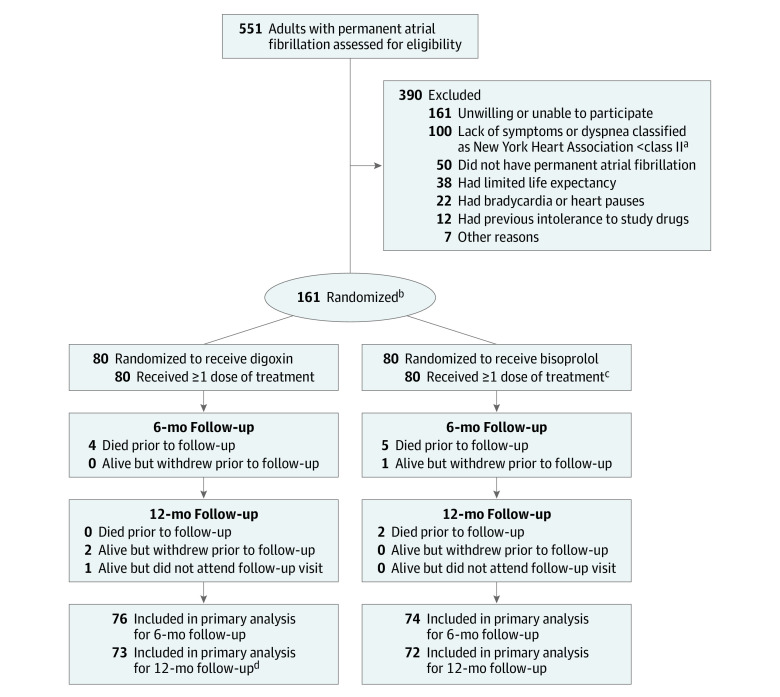
Figure 1. Study Enrollment and Analysis in the RATE-AF Trial of Digoxin vs Bisoprolol for Atrial Fibrillation.
aNew York Heart Association class I indicates no limitation of physical activity, with ordinary physical activity not causing undue fatigue, palpitation, or dyspnea; class II, slight limitation of physical activity, comfortable at rest, but ordinary physical activity resulting in fatigue, palpitation, or dyspnea; class III, marked limitation of physical activity, comfortable at rest, but less than ordinary activity causing fatigue, palpitation, or dyspnea; and class IV, unable to carry out any physical activity without discomfort, symptoms of heart failure at rest, and if any physical activity is undertaken, discomfort increases.
bRandomization included minimization to balance sex and modified European Heart Rhythm Association class at baseline. One person withdrew after randomization before receiving any therapy.
cOr another β-blocker if patient had an intolerance to bisoprolol.
dOne patient completed only 35 of 36 elements of the 36-Item Short Form Health Survey at 12 months.
Outcomes
The primary end point was patient-reported quality of life using the 36-Item Short Form Health Survey physical component summary score (SF-36 PCS) at 6 months after randomization. The SF-36 is a generic quality-of-life questionnaire that was chosen due to concerns about the measurement properties of AF-specific tools.16 Higher scores reflect better quality of life, with a scale range of 0 to 100 for each domain and summary score. Because outcomes for patients with both AF and heart failure resemble those with heart failure,6 the minimal clinically important difference (MCID) for the SF-36 PCS is between 4.1 and 9.2 (anchored to mortality).17 Further detail on outcome derivation and MCIDs for patients with AF are presented in the eMethods in Supplement 3. Investigators were blinded to patient-reported SF-36 PCS responses and scoring was performed after the trial was completed.
The investigator-blinded secondary end points at 6 and 12 months were other SF-36 domains, the 5-level EuroQoL-5D summary index score (range, 0 = death to 1 = complete health; MCID, 0.18), the Atrial Fibrillation Effect on Quality of Life questionnaire (AFEQT; range, 0-100 [a higher score indicates better quality of life]; MCID, 5 points), and N-terminal pro-brain natriuretic peptide (NT-proBNP) level. At 12 months, blinded reevaluation of cardiac function was performed by investigators at a core echocardiography laboratory.18 The unblinded secondary outcomes were the 5-level EuroQoL-5D visual analog score (range, 0-100 [a higher score indicates better quality of life]), symptoms and functional capacity assessed using the modified EHRA classification system and the NYHA classification system, 6-minute walk distance, heart rate, and 24-hour ambulatory electrocardiogram.
The trial also was designed to collect clinical outcomes to assess safety and plan a larger trial. Adverse events were collected at each visit by asking patients if they had experienced any of the common adverse events listed in the summary of product characteristics for each drug and via review of the medical record. All serious adverse events and incident cardiovascular events underwent a process of independent adjudication.
Sample Size
The primary outcome of SF-36 PCS was chosen following review of outcomes relevant to patients by the patient and public involvement team, with the full rationale presented in the design article3 and the population values estimated from previous AF trials. The trial was powered to detect an SD effect size of 0.5 for the SF-36 PCS. This distributional approach was used because MCID varies across different disease populations and because this trial includes patients with both AF and heart failure as well as those with a considerable burden of comorbidity.
In a systematic review, the SD criterion of 0.5 was found to consistently match the MCID regardless of the disease under research,19 and this remains the most common distributional criterion used across different studies.20 With a 2-sided α level of .05, randomizing 144 patients would achieve a power of 85%. Furthermore, assuming that 10% of patients would die or would be lost to follow-up at 6 months, the sample size required was 160 patients. One participant was randomized but did not complete the baseline assessment or start the allocated treatment and the trial steering committee decided to replace this participant to maintain the original sample size.
Statistical Analysis
A statistical analysis plan (Supplement 2) was generated and finalized in advance of the data analysis. The summary results are presented as number and percentage, mean and standard deviation, or median and interquartile range (IQR). The full analysis set consisted of patients who were randomized and received at least 1 dose of therapy. Patients in each group were categorized by the randomized therapy regardless of treatment withdrawal or crossover. The intervention effects were assessed with the group that received bisoprolol or another β-blocker used as the reference category. All model-based analyses were adjusted for the baseline score (when applicable), minimization parameters (sex and modified EHRA symptom classification at baseline), age at randomization, and baseline LVEF (as continuous variables).
For continuous outcomes, the adjusted mean difference was used. For NT-proBNP level and 6-minute walk distance, the ratio of geometric means was used after log transformation. For binary and categorical outcomes, logistic and ordinal logistic regression models were used. The count data for events were compared using the χ2 test. The change in modified EHRA symptom classification score was compared in an ordinal fashion due to its 5 categories. In addition, the statistical analysis plan prespecified a comparison of patients who had at least a 2-class improvement for the modified EHRA during follow-up. The prespecified subgroup analyses for the primary outcome assessed: (1) sex (male or female); (2) modified EHRA symptom class 1 or 2a vs class 2b, 3, or 4; (3) whether the patient had taken β-blockers within the last month prior to randomization; (4) whether the patient was younger than 75 years vs aged 75 years or older; and (5) whether the patient had LVEF of less than 50% vs LVEF of 50% or greater.
All statistical models were assessed for goodness of fit and interactions as well as to ensure there were no violations of any model assumptions. We checked the normality assumption for continuous outcomes and when this was not met, the data were log transformed prior to the analyses. Due to the limited amount of missing data across all variables and outcomes, the complete case data were used for the analyses and no imputation was performed. The following post hoc tests were performed: (1) estimation of the incidence rate ratio for adverse events (zero-inflated negative binomial model) and count data for primary care visits (negative binomial model) with time used as an offset in all models; (2) AFEQT subscales for symptoms, daily activities, treatment concern, and treatment satisfaction; (3) between-group difference in NYHA class; (4) between-group difference in heart rate deficits; and (5) additional subgroup analysis for the primary outcome relating to baseline heart rate.
Because of the potential for type I error due to multiple comparisons, findings for the analyses of the secondary end points should be interpreted as exploratory. The statistical analyses were performed using Stata version 16 (StataCorp) and SAS version 9.4 (SAS Institute Inc). A 2-tailed P value of .05 was considered a statistically significant difference.
Results
There were 160 patients who completed randomization and received at least 1 dose of allocated treatment. Each group had 80 patients (Figure 1). The mean age was 76 years (SD, 8 years), 46% were women, and 7% self-declared as non-White ethnicity. The majority of patients at baseline had either moderately troubling symptoms without an effect on daily activity (47% with modified EHRA class 2b) or severe symptoms that did impair daily activity (40% with modified EHRA class 3). The mean NYHA class score was 2.4 (SD, 0.6) and 52% had signs of heart failure on clinical examination. The median NT-proBNP level was 1057 pg/mL (IQR, 744-1522 pg/mL) and 19% of patients had LVEF of less than 50% on their echocardiograms. The groups were well balanced at baseline (Table 1). However, there were more signs of heart failure among those randomized to digoxin. The mean heart rate on the baseline 12-lead electrocardiogram was 100/min (SD, 18/min) and was not different between groups. Apart from 1 patient with an absolute contraindication, all the other patients were receiving oral anticoagulants by the end of uptitration.
Table 1. Baseline Characteristics.
| Digoxin (n = 80) | Bisoprolol (n = 80) | |
|---|---|---|
| Demographicsa | ||
| Age, mean (SD), y | 74.5 (8.3) | 76.8 (8.1) |
| Sex, No. (%) | ||
| Female | 36 (45.0) | 38 (47.5) |
| Male | 44 (55.0) | 42 (52.5) |
| Race/heritage/nationality, No. (%)b | ||
| Asian or Asian British | 3 (3.8) | 5 (6.3) |
| Black, African, Caribbean, or Black British | 2 (2.5) | 1 (1.3) |
| White British or Irish | 75 (93.8) | 74 (92.5) |
| Comorbidities, No. (%)c | ||
| Treatment for hypertension | 56 (70.0) | 60 (75.0) |
| Disease of the airways | 24 (30.0) | 18 (22.5) |
| Diabetes | 16 (20.0) | 22 (27.5) |
| Unplanned admission for either AF or heart failure within past 12 mo | 16 (20.0) | 15 (18.8) |
| Previous stroke or transient ischemic attack | 12 (15.0) | 16 (20.0) |
| Atrial fibrillation metrics, No. (%) | ||
| Previous use of antiarrhythmic drugs | 5 (6.3) | 8 (10.0) |
| Previous treatment for AF | ||
| Cardioversion | 6 (7.5) | 9 (11.3) |
| Ablation | 2 (2.5) | 1 (1.3) |
| Modified European Heart Rhythm Association classd | ||
| 1 | 0 | 0 |
| 2a | 3 (3.8) | 3 (3.8) |
| 2b | 34 (42.5) | 40 (50.0) |
| 3 | 38 (47.5) | 27 (33.8) |
| 4 | 5 (6.3) | 10 (12.5) |
| Heart failure metrics | ||
| Previous diagnosis of heart failure, No. (%) | 35 (43.8) | 24 (30.0) |
| Signs of heart failure, No. (%)e | 49 (61.3) | 35 (43.8) |
| N-terminal pro-brain natriuretic peptide level, median (IQR), pg/mL | 1095 (715-1527) | 1041 (753-1480) |
| Echocardiogram result | ||
| LVEF, mean (SD), % | 56.2 (8.8) | 57.6 (10.5) |
| LVEF <50%, No. (%) | 17 (21.3) | 13 (16.3) |
| New York Heart Association class, No. (%)f | ||
| I | 0 | 0 |
| II | 46 (57.5) | 53 (66.3) |
| III | 32 (40.0) | 24 (30.0) |
| IV | 2 (2.5) | 3 (3.8) |
| New York Heart Association class score, mean (SD) | 2.4 (0.5) | 2.4 (0.6) |
| Current use of ACE inhibitor, ARB, or aldosterone antagonist, No. (%) | 49 (61.3) | 45 (56.3) |
| Current use of thiazide or loop diuretics, No. (%) | 23 (28.8) | 26 (32.5) |
| Clinical measurements | ||
| Heart rate, mean (SD), /min | ||
| 12-lead electrocardiogram | 100.1 (16.8) | 99.2 (19.2) |
| Apex beat over 30 s | 98.2 (15.1) | 99.0 (16.8) |
| Radial pulse over 30 sg | 87.8 (12.1) | 86.9 (10.3) |
| Systolic blood pressure level, mean (SD), mm Hg | 134.2 (14.7) | 137.1 (17.5) |
| Creatinine level, median (IQR), mg/dL | 0.96 (0.80-1.10) | 0.98 (0.85-1.19) |
| 6-min walk distance, median (IQR), mh | 321 (120-419) | 330 (90-450) |
Abbreviations: ACE, angiotensin-converting enzyme; AF, atrial fibrillation; ARB, angiotensin receptor blocker; IQR, interquartile range; LVEF, left ventricular ejection fraction.
SI conversion factor: To convert creatinine to μmol/L, multiply by 88.4.
Due to rounding, some categories do not equal 100%.
Self-reported and based on UK Census categories.
Based on patient self-report and clinician review of the medical record.
Class 1 defined as no symptoms from AF; 2a, mild symptoms, normal daily activity not affected and patient not troubled by symptoms; 2b, moderate symptoms, normal daily activity not affected but patient troubled by symptoms; 3, severe symptoms, with normal daily activity affected by symptoms relating to AF; and 4, disabling symptoms, with normal daily activity discontinued.
Consistent with current heart failure as determined by the clinical investigator, including lung crepitations, peripheral edema, raised jugular venous pressure, and abnormal heart sounds.
Class I defined as no limitation of physical activity, with ordinary physical activity not causing undue fatigue, palpitation, or dyspnea; II, slight limitation of physical activity, comfortable at rest, but ordinary physical activity resulting in fatigue, palpitation, or dyspnea; III, marked limitation of physical activity, comfortable at rest, but less than ordinary activity causing fatigue, palpitation, or dyspnea; and IV, unable to carry out any physical activity without discomfort, symptoms of heart failure at rest, and if any physical activity is undertaken, discomfort increases.
Collected immediately before the apex heart rate. The radial pulse deficit demonstrates the degree of discrepancy between the central and peripheral pulse measurements in the context of AF (additional details appear in eTable 2 in Supplement 3).
Among healthy individuals aged 70 to 80 years, the expected distance is approximately 500 m based on data from 88 persons in a global multicenter study.21
At 6 months, 73 of 76 patients (96%) randomized to digoxin were still taking the drug, the mean dose was 161 µg/d (SD, 55 µg/d), and the mean digoxin level was 0.78 ng/mL (SD, 0.31 ng/mL). At 6 months, 66 of 74 patients (89%) randomized to bisoprolol were still taking the drug, the mean dose was 3.2 mg/d (SD, 1.8 mg/d) in 59 patients (80%), and 7 patients (9%) had switched to an alternative β-blocker due to adverse events (eTable 1 in Supplement 3). Use of the study drugs was similar at 12 months (eTable 1 in Supplement 3).
Over the course of the trial, 5 patients (6.8%) required an additional drug for heart rate control in the digoxin group vs 1 patient (1.4%) in the bisoprolol group. At 12 months, 7 patients (4.8%) were found to be in sinus rhythm (2 in the digoxin group vs 5 in the bisoprolol group), 3 had withdrawn from the study, and 1 could not attend follow-up (Figure 1). The vital status was known for all patients. Heart rate responded similarly in both groups over time (eFigure 2 in Supplement 3). A higher 24-hour heart rate in the digoxin group was noted following uptitration at a mean of 3.1 months (SD, 2.0 months) (adjusted mean difference, 4.3/min [95% CI, 0.7 to 7.9/min]; P = .02). There was no significant difference in resting heart rate at either 6 months (mean of 76.9/min [SD, 12.1/min] in the digoxin group vs mean of 74.8/min [SD, 11.6/min] in the bisoprolol group; adjusted mean difference, 1.5/min [95% CI, −2.0 to 5.1/min]; P = .40) or at 12 months (mean of 75.4/min [SD, 9.9/min] vs mean of 74.3/min [SD, 11.2/min], respectively; adjusted mean difference, 0.3/min [95% CI, −3.0 to 3.5/min]; P = .87). There was no significant difference in exertional heart rate at 6 months or 12 months (eTable 2 in Supplement 3).
Primary End Point
The mean SF-36 PCS normalized for the UK population was 31.9 (SD, 11.7) in the digoxin group at 6 months vs 29.7 (SD, 11.4) in the bisoprolol group (Table 2). There was no significant between-group difference (adjusted mean difference, 1.4 [95% CI, −1.1 to 3.8]; P = .28) and there were no significant findings in the subgroup analyses (eFigure 3 in Supplement 3).
Table 2. Primary Outcome at 6 Months.
| 36-Item Short Form Health Surveya | At baseline | At 6 mo | ||||
|---|---|---|---|---|---|---|
| Digoxin (n = 80) | Bisoprolol (n = 80) | Digoxin (n = 76) | Bisoprolol (n = 74) | Adjusted mean difference (95% CI)b | P value | |
| Physical component summary score (PCS), mean (SD)c | 28.5 (12.0) | 26.7 (10.5) | 31.5 (12.0) | 29.3 (11.7) | 1.3 (−1.2 to 3.9) | .30 |
| PCS normalized for the UK population, mean (SD)c,d | 28.9 (11.6) | 27.2 (10.2) | 31.9 (11.7) | 29.7 (11.4) | 1.4 (−1.1 to 3.8) | .28 |
Patient responds to 36 questions reflecting 8 domains of general physical and emotional health.
Compares digoxin with bisoprolol and was adjusted for baseline values (eg, for PCS, 31.5 vs 29.3, respectively, and not the difference in change from baseline). The bisoprolol group is used as the reference group. Higher values indicate better response with digoxin therapy. All adjusted models also include sex, age at randomization, modified European Heart Rhythm Association class, and left ventricular ejection fraction percentage.
Range is from 0 to 100. Higher values indicate better patient-reported quality of life (additional details regarding the scoring process appear in the eMethods in Supplement 3).
Allows for comparison across studies. A score of 50 is the expected normal score (additional details regarding the component domains appear in eFigure 3 in Supplement 3).
Secondary End Points
Quality of Life
At baseline, quality of life was substantially lower (vs the normal for the UK population) in the SF-36 domains related to physical or functional assessment (eFigure 4 in Supplement 3). There were no significant between-group differences for the SF-36 domains at 6 months (eTable 3 in Supplement 3). At 12 months, compared with patients randomized to bisoprolol, patients randomized to digoxin had significantly better normalized SF-36 scores for vitality (adjusted mean difference, 3.9 [95% CI, 0.8 to 7.0]; P = .01), general health (adjusted mean difference, 2.8 [95% CI, 0 to 5.6]; P = .05), physical functioning (adjusted mean difference, 2.8 [95% CI, 0 to 5.7]; P = .05), and role physical (adjusted mean difference, 3.4 [95% CI, 0 to 6.9]; P = .05) (Table 3). There was no statistically significant difference in the other domains or summaries, including the SF-36 PCS (adjusted mean difference, 1.6 [95% CI, −1.4 to 4.7]; P = .29). The 5-level EuroQoL-5D visual analog scale was significantly better in the digoxin group by 12 months (adjusted mean difference, 5.5 [95% CI, 0.3 to 10.6]; P = .04) compared with the bisoprolol group. The AFEQT overall score was not different at either 6 months or 12 months.
Table 3. Secondary Outcomes at 12 Monthsa.
| At baseline | At 12 mo | |||||
|---|---|---|---|---|---|---|
| Digoxin (n = 80) | Bisoprolol (n = 80) | Digoxin (n = 73) | Bisoprolol (n = 72) | Adjusted mean difference (95% CI)b | P value | |
| Heart rate | ||||||
| 12-lead electrocardiogram, mean (SD), /min | 100.3 (16.8) | 99.2 (19.2) | 75.4 (9.9) | 74.3 (11.2) | 0.3 (−3.0 to 3.5) | .87 |
| Patient-reported quality of life, mean (SD)c | ||||||
| 36-Item Short Form Health Survey | ||||||
| Physical component summary | 28.9 (11.6) | 27.2 (10.2) | 32.5 (13)d | 29.4 (12.4) | 1.6 (−1.4 to 4.7) | .29 |
| Physical functioning | 26.8 (12.6) | 25.9 (12.2) | 31.5 (14.1) | 27.5 (13.0) | 2.8 (0 to 5.7) | .05 |
| Role physical | 31.8 (12.6) | 29.6 (12.1) | 37.0 (12.6) | 32.0 (12.4) | 3.4 (0 to 6.9) | .05 |
| Vitality | 43.4 (9.6) | 40.3 (10.0) | 47.1 (9.9) | 42.0 (10.0) | 3.9 (0.8 to 7.0) | .01 |
| Global health | 40.5 (9.4) | 39 (9.4) | 42.8 (9.9)d | 39.6 (10.0) | 2.8 (0 to 5.6) | .05 |
| 5-level EuroQoL-5D | ||||||
| Summary index score | 0.67 (0.19) | 0.63 (0.22) | 0.66 (0.27) | 0.62 (0.29) | 0.01 (−0.06 to 0.09) | .72 |
| Visual analog scale | 64.0 (16.6) | 61.6 (20.3) | 72.2 (17.0) | 66.2 (17.9) | 5.5 (0.3 to 10.6) | .04 |
| Atrial Fibrillation Effect on Quality of Life questionnaire | ||||||
| Overall score | 62.2 (16.7) | 57.2 (17.6) | 75.6 (17.1) | 68.1 (16.1) | 4.1 (−0.5 to 8.7) | .08 |
| Daily activities subscalee | 44.2 (22.4) | 39.3 (22.4) | 62.0 (25.1) | 48.2 (24.4) | 9.4 (2.9 to 15.9) | .005 |
| Treatment satisfaction subscalee | 55.1 (20.2) | 55.3 (21.2) | 84.1 (14.0) | 75.2 (18.8) | 8.8 (3.3 to 14.3) | .002 |
| Functional outcomes | ||||||
| 2-class improvement from baseline for modified EHRA, No. (%)f | 50 (68.5) | 21 (29.2) | 5.3 (2.5 to 11.3)g | <.001 | ||
| NYHA class score, mean (SD)e,h | 2.4 (0.5) | 2.4 (0.6) | 1.5 (0.6) | 2.0 (0.6) | −0.6 (−0.8 to −0.4) | <.001 |
| 6-min walk distance, median (IQR), mi | 321 (120 to 419) | 330 (90 to 450) | 366 (233 to 435) | 329 (120 to 429) | 1.1 (0.9 to 1.3)j | .25 |
| Cardiac function | ||||||
| NT-proBNP level, median (IQR), pg/mL | 1091 (710 to 1522) | 1041 (753 to 1480) | 960 (626 to 1531) | 1250 (847 to 1890) | 0.77 (0.64 to 0.92)j | .005 |
| Left ventricular ejection fraction, mean (SD), % | 56.2 (8.8) | 57.6 (10.5) | 59.7 (8.7) | 59.8 (7.3) | 0.8 (−1.3 to 3.0) | .45 |
| Ratio of early mitral inflow to annular early diastolic velocity, mean (SD) | 10.7 (4.5) | 10.2 (4.7) | 10.8 (5.1) | 10.8 (5.5) | −0.1 (−1.1 to 0.9) | .81 |
| Diastolic dysfunction composite, No. (%) | 13 (16) | 8 (10) | 8 (11) | 7 (10) | 1.3 (0.3 to 4.8)g | .73 |
Abbreviations: EHRA, European Heart Rhythm Association; IQR, interquartile range; NYHA, New York Heart Association; NT-proBNP, N-terminal pro-brain natriuretic peptide.
A complete list of the secondary quality-of-life outcomes at both 6 and 12 months appears in eTables 2-4 in Supplement 3.
Compares digoxin with bisoprolol and was adjusted for baseline values (eg, for heart rate, 75.4 vs 74.3, respectively, and not the difference in change from baseline). The bisoprolol group is the reference group. Higher values indicate better response with digoxin therapy. All adjusted models include the baseline score, sex, age at randomization, and baseline modified EHRA class and left ventricular ejection fraction percentage.
For all quality-of-life scales, higher values indicate better patient-reported quality of life. Details on each instrument and the scoring process appear in the eMethods in Supplement 3. The 36-Item Short Form Health Survey (SF-36) and the 5-level EuroQoL-5D instruments are both generic quality-of-life tools; the former has a recall period of 4 weeks and the latter asks about quality of life on that day. The SF-36 values presented are normalized to the UK population (normal value is 50), with low mean values indicative of substantial impairment of quality of life in this patient population. The Atrial Fibrillation Effect on Quality of Life instrument is an atrial fibrillation (AF)–specific quality-of-life tool with a recall period of 4 weeks and with questions tailored to AF symptoms and treatments.
Data are missing for 1 patient for this SF-36 domain.
This is from the post hoc analysis.
Class 1 defined as no symptoms from AF; 2a, mild symptoms, normal daily activity not affected and patient not troubled by symptoms; 2b, moderate symptoms, normal daily activity not affected but patient troubled by symptoms; 3, severe symptoms, with normal daily activity affected by symptoms relating to AF; and 4, disabling symptoms, with normal daily activity discontinued.
Data are expressed as an adjusted odds ratio (95% CI).
Class I defined as no limitation of physical activity, with ordinary physical activity not causing undue fatigue, palpitation, or dyspnea; II, slight limitation of physical activity, comfortable at rest, but ordinary physical activity resulting in fatigue, palpitation, or dyspnea; III, marked limitation of physical activity, comfortable at rest, but less than ordinary activity causing fatigue, palpitation, or dyspnea; and IV, unable to carry out any physical activity without discomfort, symptoms of heart failure at rest, and if any physical activity is undertaken, discomfort increases.
Among healthy individuals aged 70 to 80 years, the expected distance is approximately 500 m based on data from 88 persons in a global multicenter study.21
Due to skewed data, the data are expressed as a ratio of geometric means (95% CI).
Symptoms and Functional Outcomes
The modified EHRA functional classification score was substantially better in the digoxin group during follow-up with 53% of patients reporting a 2-class improvement at 6 months vs 9% of patients in the bisoprolol group (adjusted odds ratio, 10.3 [95% CI, 4.0-26.6]; P < .001) and the significant difference was maintained at 12 months (adjusted odds ratio, 5.3 [95% CI, 2.5-11.3]; P < .001) (Table 3). Only 12 patients (16.4%) remained in class 2b, class 3, or class 4 in the digoxin group vs 32 patients (44.4%) in the bisoprolol group (P < .001; Figure 2). The 6-minute walk distance gradually increased from baseline to 6 months and to 12 months in patients randomized to digoxin, an effect which was not seen in the bisoprolol group; however, there was no significant between-group difference.
Figure 2. Change in Symptom Classification.
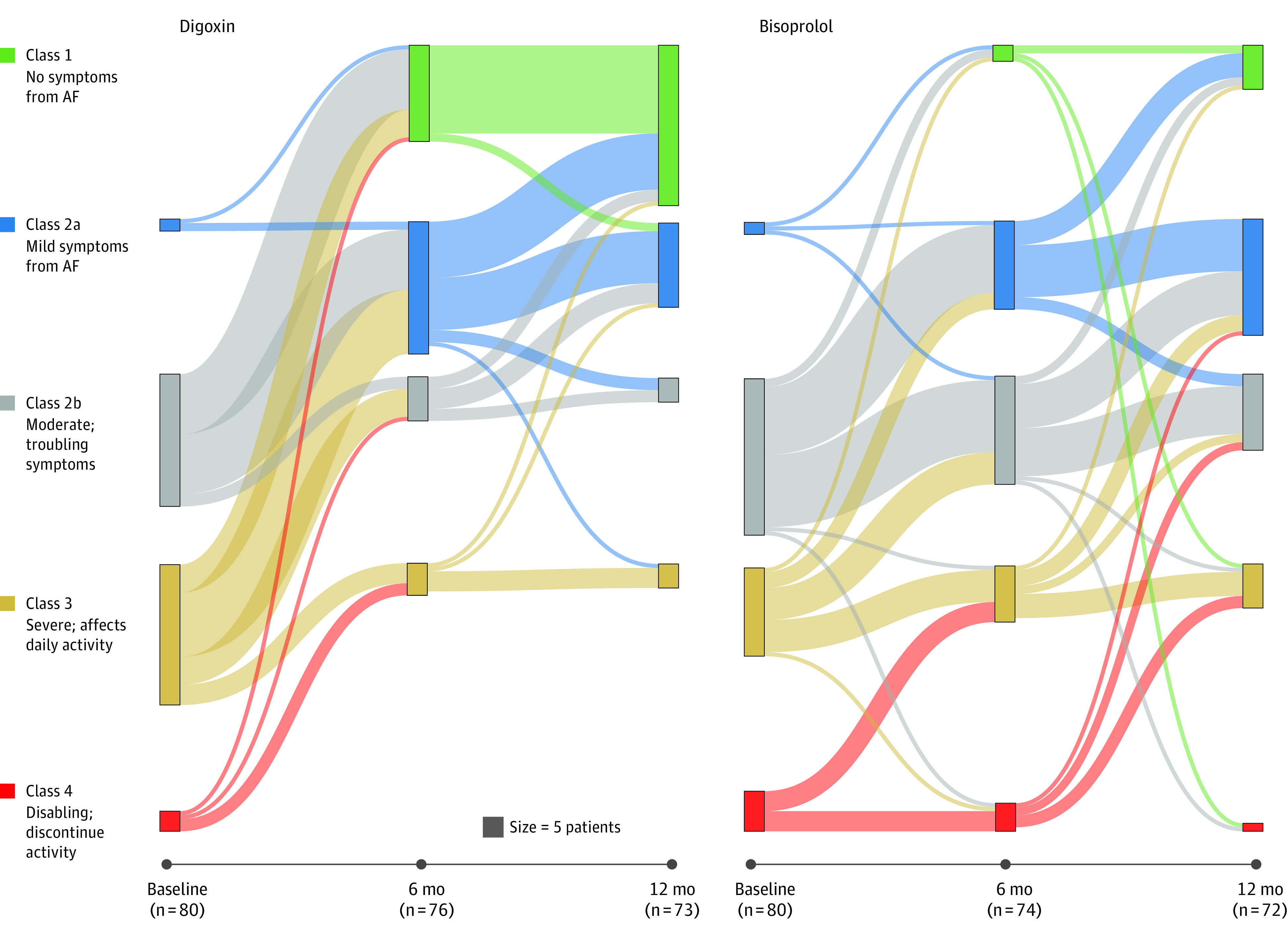
The modified European Heart Rhythm Association (EHRA) score ranks atrial fibrillation (AF)–related symptoms and the effect these have on the patient’s daily life into 5 classes, ranging from asymptomatic (class 1) to disabling (class 4). The modified score subdivides class 2 into “a” (not troubling) and “b” (troubling) to identify patients in need of further intervention. The Sankey plots for participants that attended the 6-month follow-up are displayed with bars proportional to the number of patients in each modified EHRA class at that time point. There were no patients with a modified EHRA class 1 score at baseline in either randomized group. Comparisons of modified EHRA class were made using ordinal logistic regression across all categories for digoxin vs bisoprolol. The adjusted odds ratio at 6 months was 0.12 (95% CI, 0.06-0.25; P < .001) and at 12 months was 0.16 (95% CI, 0.08-0.33; P < .001). An odds ratio of less than 1 indicates superiority of digoxin at both time points. Data on the change in New York Heart Association class during the study appear in eFigure 5 in Supplement 3.
Cardiac Function
In the digoxin group, the median NT-proBNP level decreased from 1095 pg/mL (IQR, 715-1527 pg/mL) at baseline to 1058 pg/mL (IQR, 626-1531 pg/mL) at 6 months, and then to 960 pg/mL (IQR, 626-1531 pg/mL) at 12 months. In contrast, the median NT-proBNP level increased from 1041 pg/mL (IQR, 753-1480 pg/mL) at baseline in the bisoprolol group to 1209 pg/mL (IQR, 837-1531 pg/mL) at 6 months, and then to 1250 pg/mL (IQR, 847-1890 pg/mL) at 12 months. There was no significant between-group difference at 6 months (ratio of geometric means, 0.85 [95% CI, 0.70-1.03]; P = .09), but statistical significance was reached at 12 months (ratio of geometric means, 0.77 [95% CI, 0.64-0.92]; P = .005; Table 3). The mean LVEF increased in both groups, with no statistically significant between-group difference for systolic or diastolic function at 12 months (Table 3).
Post Hoc End Points
The daily activities and treatment satisfaction subscales of the AFEQT were significantly better in the digoxin group, compared with the bisoprolol group, at both time points (Table 3 and eTable 4 in Supplement 3). Treatment with digoxin was associated with significantly lower NYHA class, compared with the bisoprolol group, at both 6 months (mean of 1.5 [SD, 0.6] vs mean of 2.0 [SD, 0.6], respectively; adjusted mean difference, −0.6 [95% CI, −0.7 to −0.4]; P < .001) and at 12 months (mean of 1.5 [SD, 0.6] vs mean of 2.0 [0.6]; adjusted mean difference, −0.6 [95% CI, −0.8 to −0.4]; P < .001) (eFigure 5 in Supplement 3).
Adverse Events
There were significantly fewer adverse events in patients randomized to digoxin (20 patients [25%] had ≥1 adverse event) compared with patients randomized to bisoprolol (51 patients [64%] had ≥1 adverse event) (χ2 = 24.91; P < .001) (Table 4 and eTable 5 in Supplement 3). The total number of treatment-related adverse events was 29 in the digoxin group vs 142 in the group that received bisoprolol or another β-blocker and the post hoc incidence rate ratio was 0.30 (95% CI, 0.15-0.59; P < .001).
Table 4. Clinical Events Through 12 Months.
| Outcome | Digoxin (n = 80) | Bisoprolol (n = 80) | ||
|---|---|---|---|---|
| No. of events | No. of patients | No. of events | No. of patients | |
| Death | 4a | 4 | 7b | 7 |
| Adjudicated cardiovascular eventsc | 3d | 2 | 15e | 12 |
| Unplanned hospitalizations | 12 | 11 | 28 | 19 |
| ≥2 hospital admissions | 1 | 1 | 9 | 9 |
| Serious adverse eventsf | 16 | 13 | 37 | 21 |
| Treatment-related adverse eventsg | 29 | 20 | 142 | 51 |
| Primary care visits in addition to study visitsh | 192 | 64 | 228 | 68 |
| Due to atrial fibrillation | 6 | 4 | 30 | 21 |
| Due to other cardiovascular cause | 16 | 9 | 34 | 23 |
| Due to noncardiovascular or other cause | 170 | 61 | 164 | 58 |
The causes of death were ischemic heart disease, bladder cancer, aspiration pneumonia (in the context of colon cancer), and liver cirrhosis (in the context of alcoholic liver disease).
The causes of death were congestive cardiac failure, decompensated heart failure (in the context of severe valve disease), non-Hodgkin lymphoma, cardio-renal syndrome, myocardial infarction, pancreatic cancer, and perforated bowel secondary to diverticular disease.
An independent clinician reviewed medical records, blood results, and imaging and completed a prespecified structured case report form that was sent directly to the trials unit.
The primary causes were myocardial infarction, peripheral edema (after diuretics were inadvertently paused), and palpitations (with no change to management).
The primary causes were pacemaker implantation in 2 patients (bradycardia, pauses, or both), decompensated heart failure in 3 patients, myocardial infarction in 2 patients, troponin-negative chest pain in 2 patients, acute stroke in 2 patients, and collapse and bradycardia, heart failure and bradycardia, rapid atrial fibrillation and dyspnea, and endocarditis in 1 patient.
Defined as any adverse event, adverse reaction, or unexpected adverse reaction that results in death, is life-threatening, requires hospitalization or prolongation of existing hospitalization, results in persistent or significant disability or incapacity, or consists of a congenital anomaly or birth defect. All events underwent appraisal by a principal investigator within 1 working day, followed by confirmatory processes by the chief investigator.
At each study visit, patients were asked to report any adverse events since the last visit from a list taken from the summary of product characteristics for each drug.
On average, there were 3.2 primary care visits per patient in addition to trial visits. In a national survey in Scotland, the average number of visits per patient (with newly diagnosed atrial fibrillation) was between 4.2 and 7.8.22
The total number of adjudicated serious adverse events was 16 in the digoxin group (in 13 patients) vs 37 in the group that received bisoprolol or another β-blocker (in 21 patients). Three adjudicated cardiovascular events occurred in 2 patients in the digoxin group compared with 15 events in 12 patients in the group that received bisoprolol or another β-blocker. Among those randomized to digoxin, 4 patients (5.0%) died compared with 7 patients (8.8%) randomized to bisoprolol. Of these deaths, 1 (1.3%) was related to cardiovascular causes in the digoxin group compared with 4 (5.0%) in the group that received bisoprolol or another β-blocker.
There were fewer primary care visits in the digoxin group related to either AF or another cardiovascular cause compared with the group that received bisoprolol or another β-blocker. Among patients randomized to digoxin, pacing devices were required for 0 patients compared with 3 patients (4.2%) randomized to bisoprolol. Two patients (2.7%) in the group that received bisoprolol or another β-blocker required pacing devices for bradycardia indications. Pauses on the 24-hour recording occurred in 33% of patients randomized to digoxin (mean duration of the longest pause, 2.8 [SD, 0.4] seconds) and in 39% of patients randomized to the bisoprolol group (mean duration of the longest pause, 3.2 [SD, 1.9] seconds).
Discussion
Among patients aged 60 years or older with permanent AF and symptoms of heart failure treated with low-dose digoxin or bisoprolol, there was no statistically significant difference in quality of life at 6 months. These findings support basing decisions about treatment on other end points.
This trial was designed to address a major evidence gap in the management of patients with AF and included outcomes of concern for patients in this increasing population.23 Heart rate control is often the sole treatment for impaired quality of life in the context of permanent AF (when there has been a joint decision by the patient and physician not to pursue attempts at restoring normal sinus rhythm). Without adequate RCTs, clinicians have relied on anecdotal experience to guide prescription of heart rate control therapy, often defaulting to β-blockers in routine practice. Despite the long history of digoxin,24 nonacute RCTs are only available in the context of heart failure with sinus rhythm.12
The mechanism of action for digoxin is proposed to include a neurohormonal component (antiadrenergic or provagal), an electrophysiological component (increased atrioventricular node refractory period), a cellular component (inhibition of the sodium-potassium adenosine triphosphatase pump), and resultant hemodynamic changes.13 In contrast, β-blockers are used to target β1-adrenergic receptors. Even though β-blockers have been widely studied across different cardiovascular indications, there is a lack of data specifically for treatment of AF.9 In an individual patient-level meta-analysis7 of the landmark double-blind RCTs including patients with heart failure with reduced ejection fraction, β-blockers substantially reduced all-cause mortality in sinus rhythm (n = 13 942; hazard ratio, 0.73 [95% CI, 0.67-0.80]; P < .001), but not in the subgroup with AF at baseline (n = 3063; 0.97 [95% CI, 0.83-1.14]; P = .73). The distinct relationship in AF between heart rate and prognosis may contribute to this difference in efficacy.25 In the only major RCT comparing heart rate targets in AF,26 strict heart rate control (predominantly using β-blockers) did not reduce a composite of clinical events compared with lenient control.
This trial was designed with a 2-sided hypothesis for the primary outcome to detect a difference of 0.5 SD in SF-36 PCS. This approach was chosen because an SD of 0.5 is consistently reflective of the MCID across a range of diseases.19 The MCIDs for SF-36 vary according to the methods used (criterion, anchor-based, or distributional methods) as well as the disease. In a study of 31 325 Medicare patients with heart failure published by the instrument developers,17 the MCIDs for SF-36 PCS were 4.1 (corresponding to a 20% increased mortality risk) and 9.2 (corresponding to a 50% increased mortality risk). In independent studies that used the SF-36 PCS, an MCID of 5.5 has been suggested for cervical myelopathy,27 10 for knee arthritis,28 7.2 for rheumatoid arthritis,29 5.0 for pulmonary fibrosis,30 and 8.2 for carotid artery disease.31 Although the MCID approaches have been criticized,32 these ranges are consistent with clinical correlates seen in rhythm control trials of patients with AF (eTable 6 in Supplement 3), including a recent study in which a score difference of 8.9 in SF-36 general health had clinical relevance.33 The upper 95% confidence limit for the primary outcome comparing digoxin with bisoprolol in this trial was 3.9, suggesting that the difference in the effect of these drugs on SF-36 PCS at 6 months (adjusted for baseline score) is not a clinically important difference.
The secondary end points should be considered exploratory and hypothesis generating. By 12 months, 8 of 20 outcomes were significantly different (all favoring digoxin) and 12 outcomes were null. There was better symptom control with digoxin for both AF and heart failure–related symptoms, which is consistent with a significantly lower NT-proBNP level and number of adverse events. There was no requirement for pacemakers, no increase in pauses, and no deterioration in LVEF with digoxin therapy. In contrast to short-term RCTs, there was no statistically significant difference for longer-term heart rate control with digoxin compared with bisoprolol or an alternate β-blocker. Concerns regarding the use of digoxin, such as the narrow therapeutic window and drug interactions, were not an issue with the low-dose approach used in the current study.
Entry criteria relating to heart failure were avoided due to the difficulties in ascertaining this diagnosis in AF both for heart failure with reduced ejection fraction (for which there are no data on the validity of measuring systolic function in AF34) and also heart failure with preserved LVEF (for which symptomatic improvement using diuretics may be required to separate overlapping diagnostic features5). The majority of patients in the trial also had other comorbidities, and discussions from the patient focus groups suggested that the benefit to AF-related symptoms was often offset by enhanced appreciation of these comorbidities (particularly large-joint arthritis), leading to a neutral effect on overall quality of life.23 This may explain why no significant between-group difference was identified for the summary quality-of-life domains and the 6-minute walk distance, which highlights the importance of broad and inclusive management of patients with AF8 and an integrated management approach.35
Limitations
This study has several limitations. First, the trial used an open-label design because a blinded approach was determined to be impractical in the context of the embedded health care design and unethical due to the lack of prior trial data and the potential need for additional therapy with intercurrent illness or hospitalization (extremely common in this older comorbid patient group). The trial design maintained the benefits associated with a strict randomization procedure, whereas the blinded end-point assessment helped to reduce bias (especially because the primary end point was subjective).
Second, although there was a considerable and statistically significant between-group difference for the prespecified comparison of the adverse events, this end point was secondary and the trial lacked power for comparison of major adverse cardiovascular events, which deserves further study.
Third, the findings do not apply to patients with severe reduction in LVEF (because data in the trial were limited) or to those admitted with uncontrolled AF or decompensated heart failure because acute heart rate control in these scenarios is often more challenging. With broad inclusion criteria and minimal exclusion criteria, the patients in this trial reflect usual clinical practice of those requiring outpatient heart rate control with permanent AF and symptoms of heart failure.
Conclusions
Among patients with permanent atrial fibrillation and symptoms of heart failure treated with low-dose digoxin or bisoprolol, there was no statistically significant difference in quality of life at 6 months. These findings support potentially basing decisions about treatment on other end points.
Trial protocol
Statistical analysis plan
eMethods
eFigure 1. RATE-AF trial flowchart and selection criteria
eFigure 2. Change in heart rate
eFigure 3. Subgroup analyses for the primary outcome
eFigure 4. Change in quality of life
eFigure 5. Change in NYHA classification
eTable 1. Medication usage over time
eTable 2. Resting and exertional heart rate
eTable 3. Generic quality of life data
eTable 4. AF-specific quality of life data
eTable 5. Adverse event reporting over 12-months
eTable 6. Studies utilizing the SF36 survey in patients with AF
eReferences
Data sharing statement
References
- 1.Lane DA, Skjøth F, Lip GYH, Larsen TB, Kotecha D. Temporal trends in incidence, prevalence, and mortality of atrial fibrillation in primary care. J Am Heart Assoc. 2017;6(5):e005155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chiang CE, Naditch-Brûlé L, Murin J, et al. . Distribution and risk profile of paroxysmal, persistent, and permanent atrial fibrillation in routine clinical practice. Circ Arrhythm Electrophysiol. 2012;5(4):632-639. [DOI] [PubMed] [Google Scholar]
- 3.Kotecha D, Calvert M, Deeks JJ, et al. . A review of rate control in atrial fibrillation, and the rationale and protocol for the RATE-AF trial. BMJ Open. 2017;7(7):e015099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kotecha D, Piccini JP. Atrial fibrillation in heart failure: what should we do? Eur Heart J. 2015;36(46):3250-3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kotecha D, Lam CS, Van Veldhuisen DJ, et al. . Heart failure with preserved ejection fraction and atrial fibrillation: vicious twins. J Am Coll Cardiol. 2016;68(20):2217-2228. [DOI] [PubMed] [Google Scholar]
- 6.Kotecha D, Chudasama R, Lane DA, et al. . Atrial fibrillation and heart failure due to reduced versus preserved ejection fraction. Int J Cardiol. 2016;203:660-666. [DOI] [PubMed] [Google Scholar]
- 7.Kotecha D, Holmes J, Krum H, et al. . Efficacy of β blockers in patients with heart failure plus atrial fibrillation: an individual-patient data meta-analysis. Lancet. 2014;384(9961):2235-2243. [DOI] [PubMed] [Google Scholar]
- 8.Kirchhof P, Benussi S, Kotecha D, et al. . 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37(38):2893-2962. [DOI] [PubMed] [Google Scholar]
- 9.Ziff OJ, Samra M, Howard JP, et al. . Beta-blocker efficacy across different cardiovascular indications: an umbrella review and meta-analytic assessment. BMC Med. 2020;18(1):103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kotecha D, Manzano L, Krum H, et al. . Effect of age and sex on efficacy and tolerability of β blockers in patients with heart failure with reduced ejection fraction: individual patient data meta-analysis. BMJ. 2016;353:i1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Digitalis Investigation Group The effect of digoxin on mortality and morbidity in patients with heart failure. N Engl J Med. 1997;336(8):525-533. [DOI] [PubMed] [Google Scholar]
- 12.Ziff OJ, Lane DA, Samra M, et al. . Safety and efficacy of digoxin: systematic review and meta-analysis of observational and controlled trial data. BMJ. 2015;351:h4451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ziff OJ, Kotecha D. Digoxin: the good and the bad. Trends Cardiovasc Med. 2016;26(7):585-595. [DOI] [PubMed] [Google Scholar]
- 14.Kotecha D, Gill SK, Flather MD, et al. . Impact of renal impairment on beta-blocker efficacy in patients with heart failure. J Am Coll Cardiol. 2019;74(23):2893-2904. [DOI] [PubMed] [Google Scholar]
- 15.Kotecha D, Chua WWL, Fabritz L, et al. . European Society of Cardiology smartphone and tablet applications for patients with atrial fibrillation and their health care providers. Europace. 2018;20(2):225-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kotecha D, Ahmed A, Calvert M, et al. . Patient-reported outcomes for quality of life assessment in atrial fibrillation: a systematic review of measurement properties. PLoS One. 2016;11(11):e0165790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ware JE, Gandek B, Sinclair SJ, Kosinski M. Measuring and Improving Health Outcomes: An SF-36 Primer for the Medicare Health Outcomes Survey. Health Assessment Lab and QualityMetric Inc; 2004. [Google Scholar]
- 18.Bunting KV, Steeds RP, Slater LT, et al. . A practical guide to assess the reproducibility of echocardiographic measurements. J Am Soc Echocardiogr. 2019;32(12):1505-1515. [DOI] [PubMed] [Google Scholar]
- 19.Norman GR, Sloan JA, Wyrwich KW. Interpretation of changes in health-related quality of life: the remarkable universality of half a standard deviation. Med Care. 2003;41(5):582-592. [DOI] [PubMed] [Google Scholar]
- 20.Mouelhi Y, Jouve E, Castelli C, Gentile S. How is the minimal clinically important difference established in health-related quality of life instruments? review of anchors and methods. Health Qual Life Outcomes. 2020;18(1):136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Casanova C, Celli BR, Barria P, et al. . The 6-min walk distance in healthy subjects: reference standards from seven countries. Eur Respir J. 2011;37(1):150-156. [DOI] [PubMed] [Google Scholar]
- 22.Murphy NF, Simpson CR, Jhund PS, et al. . A national survey of the prevalence, incidence, primary care burden and treatment of atrial fibrillation in Scotland. Heart. 2007;93(5):606-612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jones J, Stanbury M, Haynes S, et al. . Importance and assessment of quality of life in symptomatic permanent atrial fibrillation: patient focus groups from the RATE-AF trial. Cardiology. 2020;145(10):666-675. [DOI] [PubMed] [Google Scholar]
- 24.Withering W. An Account of the Foxglove and Some of Its Medical Uses: Practical Remarks on Dropsy and Other Diseases. Swinney; 1785. [Google Scholar]
- 25.Kotecha D, Flather MD, Altman DG, et al. . Heart rate and rhythm and the benefit of beta-blockers in patients with heart failure. J Am Coll Cardiol. 2017;69(24):2885-2896. [DOI] [PubMed] [Google Scholar]
- 26.Van Gelder IC, Groenveld HF, Crijns HJGM, et al. . Lenient versus strict rate control in patients with atrial fibrillation. N Engl J Med. 2010;362(15):1363-1373. [DOI] [PubMed] [Google Scholar]
- 27.Zhang Y, Zhou F, Sun Y. Assessment of health-related quality of life using the SF-36 in Chinese cervical spondylotic myelopathy patients after surgery and its consistency with neurological function assessment: a cohort study. Health Qual Life Outcomes. 2015;13:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Teo BJX, Koh JSB, Jiang L, et al. . Association of the 36-Item Short Form Health Survey physical component summary score with patient satisfaction and improvement 2 years after total knee arthroplasty. JAMA Netw Open. 2019;2(2):e190062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ward MM, Guthrie LC, Alba MI. Clinically important changes in Short Form 36 Health Survey scales for use in rheumatoid arthritis clinical trials: the impact of low responsiveness. Arthritis Care Res (Hoboken). 2014;66(12):1783-1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Witt S, Krauss E, Barbero MAN, et al. . Psychometric properties and minimal important differences of SF-36 in idiopathic pulmonary fibrosis. Respir Res. 2019;20(1):47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jiang Q, Lin T, Qu L. Predictors of health-related quality of life for mental health status in patients after carotid endarterectomy. World Neurosurg. 2019;126:e379-e384. [DOI] [PubMed] [Google Scholar]
- 32.King MT. A point of minimal important difference (MID): a critique of terminology and methods. Expert Rev Pharmacoecon Outcomes Res. 2011;11(2):171-184. [DOI] [PubMed] [Google Scholar]
- 33.Blomström-Lundqvist C, Gizurarson S, Schwieler J, et al. . Effect of catheter ablation vs antiarrhythmic medication on quality of life in patients with atrial fibrillation: the CAPTAF randomized clinical trial. JAMA. 2019;321(11):1059-1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kotecha D, Mohamed M, Shantsila E, et al. . Is echocardiography valid and reproducible in patients with atrial fibrillation? a systematic review. Europace. 2017;19(9):1427-1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kotecha D, Breithardt G, Camm AJ, et al. . Integrating new approaches to atrial fibrillation management: the 6th AFNET/EHRA Consensus Conference. Europace. 2018;20(3):395-407. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial protocol
Statistical analysis plan
eMethods
eFigure 1. RATE-AF trial flowchart and selection criteria
eFigure 2. Change in heart rate
eFigure 3. Subgroup analyses for the primary outcome
eFigure 4. Change in quality of life
eFigure 5. Change in NYHA classification
eTable 1. Medication usage over time
eTable 2. Resting and exertional heart rate
eTable 3. Generic quality of life data
eTable 4. AF-specific quality of life data
eTable 5. Adverse event reporting over 12-months
eTable 6. Studies utilizing the SF36 survey in patients with AF
eReferences
Data sharing statement