Key Points
Question
What are the risks of subsequent primary cancers (SPCs) among adult-onset cancer survivors in the United States?
Findings
In this retrospective cohort study that included 1.54 million survivors with a first primary cancer (FPC) between 1992-2011 and who survived at least 5 years, the risk of developing and dying from SPCs was greater than the risk expected in the general population for 18 and 27 of the 30 FPCs among men, respectively, and for 21 and 28 of the 31 FPCs among women, respectively.
Meaning
The findings emphasize the importance of ongoing surveillance and efforts to prevent new cancers among survivors.
Abstract
Importance
The number of cancer survivors who develop new cancers is projected to increase, but comprehensive data on the risk of subsequent primary cancers (SPCs) among survivors of adult-onset cancers are limited.
Objective
To quantify the overall and cancer type-specific risks of SPCs among adult-onset cancer survivors by first primary cancer (FPC) types and sex.
Design, Setting, and Participants
A retrospective cohort study from 12 Surveillance, Epidemiology, and End Results registries in the United States, that included 1 537 101 persons aged 20 to 84 years diagnosed with FPCs from 1992-2011 (followed up until December 31, 2017) and who survived at least 5 years.
Exposures
First primary cancer.
Main Outcomes and Measures
Incidence and mortality of SPCs per 10 000 person-years; standardized incidence ratio (SIR) and standardized mortality ratio (SMR) compared with those expected in the general population.
Results
Among 1 537 101 survivors (mean age, 60.4 years; 48.8% women), 156 442 SPC cases and 88 818 SPC deaths occurred during 11 197 890 person-years of follow-up (mean, 7.3 years). Among men, the overall risk of developing any SPCs was statistically significantly higher for 18 of the 30 FPC types, and risk of dying from any SPCs was statistically significantly higher for 27 of 30 FPC types as compared with risks in the general population. Among women, the overall risk of developing any SPCs was statistically significantly higher for 21 of the 31 FPC types, and risk of dying from any SPCs was statistically significantly higher for 28 of 31 FPC types as compared with risks in the general population. The highest overall SIR and SMR were estimated among survivors of laryngeal cancer (SIR, 1.75 [95% CI, 1.68-1.83]; incidence, 373 per 10 000 person-years) and gallbladder cancer (SMR, 3.82 [95% CI, 3.31-4.39]; mortality, 341 per 10 000 person-years) among men, and among survivors of laryngeal cancer (SIR, 2.48 [95% CI, 2.27-2.72]; incidence, 336 per 10 000 person-years; SMR, 4.56 [95% CI, 4.11-5.06]; mortality, 268 per 10 000 person-years) among women. Substantial variation existed in the associations of specific types of FPCs with specific types of SPC risk; however, only a few smoking- or obesity-associated SPCs, such as lung, urinary bladder, oral cavity/pharynx, colorectal, pancreatic, uterine corpus, and liver cancers constituted considerable proportions of the total incidence and mortality, with lung cancer alone accounting for 31% to 33% of mortality from all SPCs.
Conclusions and Relevance
Among survivors of adult-onset cancers in the United States, several types of primary cancer were significantly associated with greater risk of developing and dying from an SPC, compared with the general population. Cancers associated with smoking or obesity comprised substantial proportions of overall SPC incidence and mortality among all survivors and highlight the importance of ongoing surveillance and efforts to prevent new cancers among survivors.
This cohort study uses SEER registry data to estimate overall and cancer-specific risk for secondary primary cancers among US adults with first primary cancers who lived at least 5 years past their initial diagnosis.
Introduction
There were approximately 16.9 million cancer survivors in 2019 in the United States.1 This number is projected to reach 22.1 million in 2030, due to the aging and growth of the population.2 Long-term survivors face physical, psychosocial, medical, behavioral, and socioeconomic consequences of cancer and its treatment.3 One medical consequence is an increased likelihood of subsequent diagnosis with another cancer.4 In the United States, new malignancies among cancer survivors (ie, subsequent primary cancers [SPCs]) between 2009 and 2013 comprised a substantial proportion of total incident cancers, ranging from 11% in younger adults (aged 20-64 years) to 25% in adults aged 65 years and older.5
The National Cancer Institute monograph published in 2006 described the risk of developing SPCs among cancer survivors diagnosed between 1973 and 2000 in 9 Surveillance, Epidemiology, and End Results Program (SEER) registries.4 Since then, prevalence of cancer risk factors, patterns of treatment(s) and survivorship care, and survival have changed considerably. Except for studies of survivors of childhood or young adult cancers,6,7 few large-scale studies comprehensively examined the risk of developing and dying from SPCs among survivors of adult-onset cancers, with recent studies estimating only incidences for a single primary tumor or for a limited number of cancers.8,9,10 Furthermore, in 1992, the SEER program expanded its geographic coverage by adding registries for greater geographic and racial diversity and greater generalizability to the United States.
The objective of this study was to conduct a comprehensive and systematic analysis of incidence and mortality data (1992-2017) in the United States to describe the risk patterns of SPCs among long-term survivors (≥5 years postdiagnosis) of adult-onset cancers.
Methods
Institutional review board approval and the need for informed consent were exempted by the institutional review board at Morehouse School of Medicine because data were deidentified and publicly available.
Cases of first primary malignant cancers diagnosed among persons aged 20 to 84 years from 1992 until 2011 and recorded in 12 registries and from 1975 until 2011 in 9 registries (eTable 1 in Supplement 1) were classified according to the International Classification of Diseases for Oncology, Third Edition (30 types in men and 31 types in women; eTable 2 in Supplement 1).11,12,13 Race was abstracted from medical records or death certificates and classified as 5 groups (White, Black, American Indian/Alaska Native, Asian/Pacific Islander, unknown) (eMethods in Supplement 1). This information was used to control for differences in the racial distribution between survivors and the general population. The Alaska registry was excluded because of incompatibility of the reference file in the race matching.
SPCs were ascertained following the SEER rules for determining multiple primaries.14 The category of overall SPCs included all new primary cancers except same-type cancer (to minimize bias due to misclassification of recurrence) and nonmelanoma skin cancer. Follow-up of survivors began 5 years after their first primary cancer (FPC) diagnosis and continued until death, loss to follow-up, attaining age 89 (due to concerns regarding underascertainment in the elderly population),4 or December 31, 2017, whichever came first. Multiple SPC diagnoses in a survivor were included to consider tumors that develop after long latency such as those induced by radiation or occurring more commonly at older ages.15
Sensitivity analyses assessed the overall risks of developing SPCs when restricting to only the first SPC (disallowing multiple events in an individual) or by starting follow-up at 1 year after the FPC diagnosis.
Outcomes
The primary outcomes were incidence (per 10 000 person-years) and relative risk of developing SPCs among 5-year survivors of FPCs (standardized incidence ratio [SIR]). The secondary outcomes were mortality and relative risk of dying from an SPC among survivors (standardized mortality ratios [SMR]).
Statistical Analysis
Incidence rates of SPCs among each group of survivors or survivors overall were obtained by dividing the observed number of SPCs by the corresponding total person-years of follow-up and then multiplying by 10 000. The percentage contribution of any given FPC and SPC combination to the total SPC incidence was calculated among all survivors. The SIR (95% CI) was calculated as the ratio of the observed to the expected number of SPCs.4 The expected numbers of SPCs were computed by a weighted sum of stratified incidence rates by sex, age, race, and calendar year from the reference population, which may include multiple primary cancers in an individual. The corresponding excess risks in absolute terms were also calculated and presented in eMethods and eFigure 1 in Supplement 1. All estimates were calculated for overall SPCs and for type-specific (organ-specific) SPCs.4 Due to the substantially lower than expected risk for SPCs after prostate cancer, the estimates for male survivors were also presented after excluding prostate cancer survivors. The mortality and SMR of SPCs were calculated using a similar approach.
Among survivors of the 12 smoking-related cancers, per a report from the US Surgeon General,16 and 12 obesity-related cancers designated by the International Agency for Research on Cancer (eTable 3 in Supplement 1),17 incidences and SIRs were estimated to quantify the risk of developing any types of smoking-related and obesity-related SPCs. Similar analyses were also performed for 7 alcohol-related18 and 10 infection-related19 cancers (eMethods in Supplement 1).
Multicovariate Poisson regression was used to examine SIR trends by diagnosis years (1975-1989, 1990-1999, 2000-2011) for 6 SPCs after the 16 most common FPC types (eMethods in Supplement 1).20
All analyses were stratified by sex and performed using the “MP-SIR” session of SEER*Stat 8.3.6,21 SAS 9.4, or Microsoft Excel Office 365 Proplus. All statistical tests were 2-sided, and P values of less than .05 were considered statistically significant, without multiple comparison corrections as the analyses were exploratory.
Results
Among 1 537 101 survivors (mean age, 60.4 years; 48.8% women; 81.5% White), 41% were aged 65 years or older at FPC diagnosis, and 58.8% lived longer than 10 years after FPC diagnosis, accruing 11 197 890 person-years of follow-up (mean, 7.3 years) (Table 1; eTable 4 in Supplement 1). Among male survivors (excluding those with prostate cancer), there were 49 065 SPC cases (177 per 10 000 person-years) and 28 463 SPC deaths (104 per 10 000 person-years) (Table 2), which corresponded with an 11% higher risk of developing SPCs (SIR, 1.11 [95% CI, 1.10-1.12]) and a 45% higher risk of dying from SPCs (SMR, 1.45 [95% CI, 1.43-1.46]) among survivors compared with the risk in the general population. Among women, there were 62 348 SPC cases (109 per 10 000 person-years) and 34 879 SPC deaths (62 per 10 000 person-years), which corresponded with a 10% higher risk of developing SPCs (SIR, 1.10 [95% CI, 1.09-1.11]) and a 33% higher risk of dying from SPCs (SMR, 1.33 [95% CI, 1.32-1.34]).
Table 1. Characteristics of Survivors Diagnosed With an Invasive First Primary Cancer in 1992-2011 at Ages 20 to 84 Years and ≥5-Year Survival in 12 SEER Registries.
| Survivorsa | Person-years of follow-up 5 y after first primary cancer | ||
|---|---|---|---|
| No. (%) | Total | Mean | |
| Total | 1 537 101 (100.0) | 11 197 890 | 7.3 |
| Sex | |||
| Men | 786 384 (51.2) | 5 499 426 | 7.0 |
| Women | 750 717 (48.8) | 5 698 464 | 7.6 |
| Age at first primary cancer diagnosis, yb | |||
| 20-39 | 130 296 (8.5) | 1 242 585 | 9.5 |
| 40-49 | 205 737 (13.4) | 1 831 888 | 8.9 |
| 50-64 | 569 091 (37.0) | 4 502 560 | 7.9 |
| 65-84 | 631 977 (41.1) | 3 620 857 | 5.7 |
| Calendar year of first primary cancer diagnosis | |||
| 1992-1999 | 541 867 (35.3) | 5 712 727 | 10.5 |
| 2000-2005 | 471 463 (30.7) | 3 641 246 | 7.7 |
| 2006-2011 | 523 771 (34.1) | 1 843 917 | 3.5 |
| Months of follow-up since first primary cancer | |||
| 60-119 | 1 537 101 (100.0) | 6 137 717 | 4.0 |
| 120-179 | 903 187 (58.8) | 3 255 469 | 3.6 |
| ≥180 | 437 985 (28.5) | 1 804 704 | 4.1 |
| Racec | |||
| White | 1 252 830 (81.5) | 9 224 122 | 7.4 |
| Black | 137 530 (8.9) | 941 916 | 6.8 |
| Asian or Pacific Islander | 124 839 (8.1) | 880 854 | 7.1 |
| American Indian/Alaska Native | 6955 (0.5) | 47 937 | 6.9 |
| Not recorded in SEER registryd | 14 947 (1.0) | 103 061 | 6.9 |
| SEER registry recoded for first primary cancer | |||
| Los Angeles | 311 264 (20.3) | 2 262 141 | 7.3 |
| Seattle (Puget Sound) | 183 429 (11.9) | 1 343 692 | 7.3 |
| Detroit (metropolitan) | 182 366 (11.9) | 1 324 790 | 7.3 |
| San Francisco-Oakland (metropolitan) | 169 744 (11.0) | 1 253 329 | 7.4 |
| Connecticut | 169 628 (11.0) | 1 241 774 | 7.3 |
| Iowa | 133 776 (8.7) | 960 833 | 7.2 |
| Atlanta (metropolitan) | 103 403 (6.7) | 763 923 | 7.4 |
| San Jose-Monterey | 86 822 (5.6) | 645 027 | 7.4 |
| Utah | 73 620 (4.8) | 525 616 | 7.1 |
| New Mexico | 67 306 (4.4) | 474 743 | 7.1 |
| Hawaii | 50 788 (3.3) | 368 945 | 7.3 |
| Rural Georgia | 4955 (0.3) | 33 077 | 6.7 |
| SEER historic stage | |||
| Localized | 673 540 (43.8) | 5 228 418 | 7.8 |
| Localized/regional (prostate cases) | 322 192 (21) | 2 154 564 | 6.7 |
| Regional | 263 132 (17.1) | 1 857 475 | 7.1 |
| Distant | 86 356 (5.6) | 480 412 | 5.6 |
| Unstaged | 131 468 (8.6) | 945 715 | 7.2 |
| Blanke | 60 413 (3.9) | 531 305 | 8.8 |
| Reasons for exit from the current study | |||
| End of study | 925 463 (60.2) | ||
| Death | 550 485 (35.8) | ||
| Lost to follow-up | 61 153 (4.0) | ||
Abbreviation: SEER, Surveillance, Epidemiology, and End Results Program.
Survivor follow-up was until Dec 31, 2017. The current version of the SEER database did not allow determination of variation (standard deviation) for survivor age and person-years of follow-up.
Mean age was 60.4 years.
Race in the SEER registries was based on information abstracted from medical records or death certificates (eMethods in Supplement 1).
Not included in the mortality analysis.
Indicates cases with certain site-year combinations that were not covered by SEER Historic Stage A (see the SEER Variable and Recode Definitions on the SEER Historic Stage A).22
Table 2. The Risk of Developing and Dying From Any Types of Subsequent Primary Cancersa Sorted by Standardized Incidence Ratios of First Primary Cancer Types Among 5-Year Cancer Survivors With First Diagnosis Between 1992 and 2011 in 12 SEER Registries.
| FPC | Survivors, No. (%)b | Observed SPCs, No. (%) | Incidence per 10 000 person-years | Expected SPCs, No.c | SIR (95% CI)d | Observed SPC deaths, No. (%) | Mortality per 10 000 person-years | Expected SPC deaths, No.c | SMR (95% CI)d | Mean age at FPC diagnosise | Mean age at SPC diagnosise | Mean age at SPC deathe | Follow-up since 5 y after FPC, total (mean)b,e |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Men | |||||||||||||
| Larynx | 9107 (1.2) | 2261 (2.4) | 373 | 1291 | 1.75 (1.68-1.83) | 1518 (2.8) | 251 | 571 | 2.66 (2.53-2.80) | 62.5 | 72.7 | 73.7 | 60 693 (6.7) |
| Hodgkin lymphoma | 7278 (0.9) | 659 (0.7) | 101 | 415 | 1.59 (1.47-1.72) | 392 (0.7) | 61 | 145 | 2.71 (2.45-2.99) | 40.1 | 61.2 | 63.2 | 65 167 (9) |
| Kaposi sarcoma | 3962 (0.5) | 424 (0.5) | 119 | 275 | 1.54 (1.40-1.70) | 140 (0.3) | 41 | 98 | 1.43 (1.21-1.69) | 43.4 | 58.3 | 60.8 | 35 753 (9) |
| Pancreas | 1674 (0.2) | 188 (0.2) | 240 | 136 | 1.38 (1.19-1.60) | 110 (0.2) | 141 | 57 | 1.93 (1.59-2.33) | 60.0 | 71.3 | 71.5 | 7846 (4.7) |
| Esophagus | 2746 (0.3) | 416 (0.4) | 273 | 308 | 1.35 (1.23-1.49) | 245 (0.5) | 162 | 132 | 1.86 (1.64-2.11) | 62.8 | 72.2 | 72.6 | 15 226 (5.5) |
| Oral cavity and pharynx | 22 937 (2.9) | 3361 (3.6) | 216 | 2503 | 1.34 (1.30-1.39) | 2704 (5) | 176 | 1028 | 2.63 (2.54-2.73) | 57.5 | 70.1 | 70.6 | 155 247 (6.8) |
| Acute lymphocytic leukemia | 612 (0.1) | 36 (0) | 76 | 27 | 1.31 (0.92-1.82) | 34 (0.1) | 73 | 9 | 3.69 (2.55-5.15) | 38.7 | 60.9 | 64.8 | 4721 (7.7) |
| Gallbladder and other biliary | 1010 (0.1) | 143 (0.2) | 246 | 113 | 1.27 (1.07-1.50) | 197 (0.4) | 341 | 52 | 3.82 (3.31-4.39) | 62.6 | 71.8 | 71.6 | 5812 (5.8) |
| Breast | 1878 (0.2) | 311 (0.3) | 255 | 258 | 1.21 (1.08-1.35) | 157 (0.3) | 131 | 118 | 1.33 (1.13-1.56) | 63.1 | 74.7 | 75.9 | 12 175 (6.5) |
| Lung and bronchus | 20 874 (2.7) | 2447 (2.6) | 223 | 2034 | 1.20 (1.16-1.26) | 1252 (2.3) | 114 | 756 | 1.66 (1.57-1.76) | 64.3 | 73.7 | 74.6 | 109 575 (5.2) |
| Penis and other genital organs | 1592 (0.2) | 252 (0.3) | 230 | 213 | 1.18 (1.05-1.34) | 179 (0.3) | 167 | 97 | 1.84 (1.59-2.14) | 61.5 | 72.8 | 73.4 | 10 939 (6.9) |
| Chronic myeloid leukemia | 2772 (0.4) | 229 (0.2) | 131 | 194 | 1.18 (1.03-1.34) | 150 (0.3) | 87 | 75 | 2.01 (1.70-2.36) | 51.6 | 64.6 | 70.6 | 17 477 (6.3) |
| Anus, anal canal, and anorectum | 1956 (0.2) | 247 (0.3) | 185 | 210 | 1.17 (1.04-1.33) | 210 (0.4) | 158 | 86 | 2.44 (2.13-2.80) | 55.8 | 68.8 | 69.0 | 13 347 (6.8) |
| Non-Hodgkin lymphomaf | 44 576 (5.7) | 5523 (5.9) | 189 | 4751 | 1.16 (1.14-1.20) | 3058 (5.7) | 106 | 2081 | 1.47 (1.42-1.53) | 59.1 | 71.9 | 74.2 | 292 887 (6.6) |
| Thyroid | 12 442 (1.6) | 1268 (1.3) | 129 | 1117 | 1.14 (1.08-1.20) | 456 (0.8) | 47 | 428 | 1.07 (0.97-1.17) | 49.8 | 67.3 | 70.9 | 98 651 (7.9) |
| Acute nonlymphocytic leukemia | 1907 (0.2) | 156 (0.2) | 111 | 137 | 1.14 (0.97-1.33) | 103 (0.2) | 73 | 49 | 2.08 (1.70-2.53) | 47.5 | 66.1 | 65.3 | 14 105 (7.4) |
| Stomach | 6149 (0.8) | 795 (0.8) | 213 | 707 | 1.13 (1.05-1.21) | 637 (1.2) | 171 | 326 | 1.95 (1.81-2.11) | 63.1 | 73.9 | 73.4 | 37 358 (6.1) |
| Eye and orbit | 1645 (0.2) | 230 (0.2) | 192 | 203 | 1.13 (0.99-1.29) | 241 (0.4) | 206 | 87 | 2.76 (2.42-3.13) | 57.9 | 73.3 | 70.7 | 12 010 (7.3) |
| Soft tissue including heart | 5151 (0.7) | 593 (0.6) | 149 | 538 | 1.10 (1.02-1.20) | 402 (0.7) | 103 | 227 | 1.77 (1.61-1.96) | 52.9 | 70.4 | 69.1 | 39 760 (7.7) |
| Urinary bladder | 51 513 (6.6) | 7758 (8.2) | 222 | 7179 | 1.08 (1.06-1.11) | 4732 (8.8) | 136 | 3615 | 1.31 (1.28-1.35) | 65.6 | 75.6 | 77.6 | 349 592 (6.8) |
| Kidney and renal pelvis | 25 977 (3.3) | 3185 (3.4) | 189 | 2957 | 1.08 (1.04-1.12) | 1455 (2.7) | 87 | 1280 | 1.14 (1.08-1.20) | 59.5 | 71.9 | 74.8 | 168 223 (6.5) |
| Testis | 17 913 (2.3) | 834 (0.9) | 47 | 793 | 1.05 (0.99-1.13) | 297 (0.6) | 17 | 243 | 1.23 (1.09-1.38) | 35.8 | 57.4 | 59.6 | 179 310 (10) |
| Small intestine | 2771 (0.4) | 313 (0.3) | 183 | 298 | 1.05 (0.94-1.18) | 430 (0.8) | 254 | 127 | 3.39 (3.08-3.73) | 59.0 | 71.0 | 70.8 | 17 059 (6.2) |
| Brain and other nervous system | 4817 (0.6) | 250 (0.3) | 73 | 239 | 1.05 (0.93-1.19) | 146 (0.3) | 43 | 81 | 1.81 (1.53-2.14) | 42.0 | 61.2 | 60.4 | 34 324 (7.1) |
| Bones and joints | 1402 (0.2) | 110 (0.1) | 93 | 108 | 1.02 (0.84-1.24) | 128 (0.2) | 110 | 41 | 3.13 (2.62-3.73) | 44.5 | 64.2 | 63.7 | 11 818 (8.4) |
| Myeloma | 6728 (0.9) | 555 (0.6) | 184 | 550 | 1.01 (0.93-1.10) | 290 (0.5) | 97 | 231 | 1.25 (1.12-1.41) | 61.1 | 70.4 | 72.7 | 30 095 (4.5) |
| Colon and rectum | 74 722 (9.5) | 9720 (10.3) | 190 | 9807 | 0.99 (0.98-1.02) | 5173 (9.6) | 102 | 4650 | 1.11 (1.09-1.15) | 63.3 | 74.9 | 76.8 | 510 732 (6.8) |
| Liver and intrahepatic bile duct | 3629 (0.5) | 215 (0.2) | 132 | 230 | 0.93 (0.82-1.07) | 169 (0.3) | 104 | 85 | 1.99 (1.71-2.32) | 58.9 | 67.8 | 70.0 | 16 276 (4.5) |
| Melanoma of the skin | 46 007 (5.9) | 5098 (5.4) | 138 | 5556 | 0.92 (0.90-0.95) | 2100 (3.9) | 59 | 2412 | 0.87 (0.84-0.91) | 56.3 | 71.6 | 74.6 | 369 019 (8) |
| Prostate | 390 274 (49.6) | 45 029 (47.9) | 165 | 49 246 | 0.91 (0.91-0.93) | 25 476 (47.2) | 94 | 28 456 | 0.9 (0.89-0.91) | 66.4 | 77.4 | 78.7 | 2 732 289 (7) |
| Other and unspecified primary sitesg | 10 363 (1.3) | 1488 (1.6) | 207 | 1160 | 1.28 (1.22-1.35) | 1358 (2.5) | 191 | 479 | 2.84 (2.69-2.99) | 57.6 | 71.1 | 71.3 | 71 938 (6.9) |
| Total | 786 384 | 94 094 | 171 | 93 553 | 1.01 (1.00-1.01) | 53 939 | 99 | 48 121 | 1.12 (1.11-1.13) | 62.4 | 74.4 | 76.0 | 5 499 426 (7) |
| Total excluding prostate cancerh | 396 110 (50.4) | 49 065 (52.1) | 177 | 44 307 | 1.11 (1.10-1.12) | 28 463 (52.8) | 104 | 19 665 | 1.45 (1.43-1.46) | 58.5 | 71.5 | 73.3 | 2 767 137 (7) |
| Women | |||||||||||||
| Larynx | 2096 (0.3) | 463 (0.7) | 336 | 186 | 2.48 (2.27-2.72) | 367 (1.1) | 268 | 80 | 4.56 (4.11-5.06) | 60.9 | 71.1 | 72.6 | 13 767 (6.6) |
| Esophagus | 846 (0.1) | 127 (0.2) | 275 | 67 | 1.89 (1.58-2.25) | 83 (0.2) | 180 | 31 | 2.69 (2.15-3.34) | 65.0 | 72.6 | 73.5 | 4615 (5.5) |
| Hodgkin lymphoma | 6263 (0.8) | 588 (0.9) | 100 | 317 | 1.86 (1.71-2.02) | 259 (0.7) | 44 | 94 | 2.75 (2.42-3.10) | 38.5 | 58.1 | 62.7 | 58 879 (9.4) |
| Acute lymphocytic leukemia | 486 (0.1) | 32 (0.1) | 89 | 21 | 1.53 (1.05-2.17) | 21 (0.1) | 59 | 6 | 3.37 (2.09-5.16) | 41.3 | 62.1 | 59.8 | 3579 (7.4) |
| Anus, anal canal, and anorectum | 3042 (0.4) | 381 (0.6) | 184 | 264 | 1.44 (1.30-1.60) | 313 (0.9) | 152 | 112 | 2.80 (2.51-3.14) | 59.8 | 69.9 | 72.4 | 20 702 (6.8) |
| Oral cavity and pharynx | 10 788 (1.4) | 1220 (2) | 159 | 880 | 1.39 (1.31-1.47) | 1069 (3.1) | 141 | 369 | 2.89 (2.73-3.08) | 58.0 | 70.5 | 72.4 | 76 805 (7.1) |
| Pancreas | 1675 (0.2) | 140 (0.2) | 159 | 102 | 1.37 (1.16-1.62) | 99 (0.3) | 113 | 42 | 2.33 (1.90-2.85) | 59.8 | 70.2 | 70.2 | 8791 (5.2) |
| Kaposi sarcoma | 166 (0) | 17 (0) | 167 | 13 | 1.33 (0.78-2.14) | 8 (0) | 79 | 6 | 1.30 (0.56-2.56) | 63.8 | 73.8 | 78.7 | 1020 (6.1) |
| Urinary bladder | 15 717 (2.1) | 1999 (3.2) | 182 | 1622 | 1.23 (1.18-1.29) | 1159 (3.3) | 106 | 788 | 1.47 (1.39-1.56) | 66.3 | 75.3 | 78.1 | 110 012 (7) |
| Liver and intrahepatic bile duct | 1458 (0.2) | 93 (0.1) | 136 | 76 | 1.22 (0.99-1.50) | 102 (0.3) | 149 | 30 | 3.44 (2.81-4.18) | 60.4 | 69.5 | 71.8 | 6845 (4.7) |
| Non-Hodgkin lymphomaf | 37 467 (5) | 3719 (6) | 148 | 3081 | 1.21 (1.17-1.25) | 2159 (6.2) | 87 | 1373 | 1.57 (1.51-1.64) | 61.5 | 72.7 | 76.0 | 250 718 (6.7) |
| Vulva and other genital organs | 6079 (0.8) | 628 (1) | 143 | 521 | 1.21 (1.12-1.31) | 490 (1.4) | 113 | 225 | 2.18 (1.99-2.38) | 58.9 | 71.1 | 71.7 | 43 858 (7.2) |
| Gallbladder and other biliary | 1314 (0.2) | 138 (0.2) | 169 | 114 | 1.21 (1.02-1.44) | 210 (0.6) | 258 | 54 | 3.90 (3.40-4.47) | 64.4 | 74.3 | 74.3 | 8156 (6.2) |
| Small intestine | 2506 (0.3) | 231 (0.4) | 152 | 195 | 1.19 (1.04-1.35) | 372 (1.1) | 246 | 85 | 4.39 (3.96-4.86) | 60.4 | 72.4 | 71.6 | 15 215 (6.1) |
| Acute nonlymphocytic leukemia | 1861 (0.2) | 136 (0.2) | 93 | 115 | 1.18 (0.99-1.40) | 68 (0.2) | 47 | 38 | 1.77 (1.38-2.25) | 46.9 | 65.5 | 69.2 | 14 649 (7.9) |
| Lung and bronchus | 24 065 (3.2) | 1900 (3) | 144 | 1627 | 1.17 (1.12-1.23) | 1021 (2.9) | 78 | 644 | 1.59 (1.49-1.69) | 64.8 | 73.9 | 75.3 | 131 788 (5.5) |
| Kidney and renal pelvis | 15 980 (2.1) | 1547 (2.5) | 146 | 1337 | 1.16 (1.10-1.22) | 713 (2) | 68 | 582 | 1.22 (1.14-1.32) | 60.5 | 72.2 | 75.0 | 105 906 (6.6) |
| Soft tissue including heart | 4243 (0.6) | 383 (0.6) | 113 | 335 | 1.14 (1.04-1.27) | 260 (0.7) | 78 | 133 | 1.96 (1.73-2.21) | 52.8 | 68.3 | 68.7 | 33 991 (8) |
| Ovary | 18 169 (2.4) | 1399 (2.2) | 107 | 1244 | 1.12 (1.07-1.19) | 709 (2) | 54 | 495 | 1.43 (1.33-1.55) | 55.0 | 68.2 | 71.1 | 131 220 (7.2) |
| Cervix uteri | 20 153 (2.7) | 1632 (2.6) | 86 | 1460 | 1.12 (1.07-1.18) | 1027 (2.9) | 55 | 483 | 2.13 (2.00-2.27) | 46.4 | 63.7 | 67.5 | 189 721 (9.4) |
| Eye and orbit | 1378 (0.2) | 138 (0.2) | 135 | 123 | 1.12 (0.95-1.33) | 222 (0.6) | 223 | 51 | 4.35 (3.80-4.97) | 57.9 | 70.6 | 70.7 | 10 217 (7.4) |
| Thyroid | 43 436 (5.8) | 2861 (4.6) | 81 | 2597 | 1.10 (1.07-1.15) | 890 (2.6) | 26 | 878 | 1.01 (0.95-1.09) | 46.4 | 63.6 | 69.0 | 351 553 (8.1) |
| Breast | 334 273 (44.5) | 25 152 (40.3) | 95 | 23 655 | 1.06 (1.05-1.08) | 13 710 (39.3) | 52 | 12 154 | 1.13 (1.11-1.15) | 58.8 | 72.1 | 74.8 | 2 638 663 (7.9) |
| Colon and rectum | 68 770 (9.2) | 6290 (10.1) | 134 | 6025 | 1.04 (1.02-1.07) | 3415 (9.8) | 73 | 2959 | 1.15 (1.12-1.20) | 65.1 | 75.0 | 77.7 | 470 117 (6.8) |
| Chronic myeloid leukemia | 1969 (0.3) | 123 (0.2) | 97 | 119 | 1.04 (0.86-1.23) | 140 (0.4) | 113 | 44 | 3.21 (2.70-3.79) | 53.4 | 67.4 | 71.5 | 12 633 (6.4) |
| Corpus and uterus, NOS | 62 859 (8.4) | 6145 (9.9) | 124 | 5968 | 1.03 (1.01-1.06) | 2844 (8.2) | 58 | 2611 | 1.09 (1.05-1.13) | 60.3 | 72.4 | 74.8 | 495 025 (7.9) |
| Myeloma | 5578 (0.7) | 324 (0.5) | 134 | 313 | 1.03 (0.93 to 1.16) | 198 (0.6) | 82 | 135 | 1.47 (1.27-1.69) | 63.0 | 71.5 | 74.0 | 24 210 (4.3) |
| Stomach | 4518 (0.6) | 351 (0.6) | 127 | 353 | 1.00 (0.90-1.11) | 270 (0.8) | 98 | 165 | 1.64 (1.45-1.85) | 63.8 | 75.2 | 74.1 | 27 740 (6.1) |
| Melanoma of the skin | 40 414 (5.4) | 3125 (5) | 90 | 3226 | 0.97 (0.94-1.01) | 1229 (3.5) | 37 | 1229 | 1.00 (0.95 to 1.06) | 51.3 | 67.8 | 72.6 | 345 686 (8.6) |
| Brain and other nervous system | 4123 (0.5) | 195 (0.3) | 63 | 201 | 0.97 (0.84-1.12) | 113 (0.3) | 37 | 61 | 1.85 (1.53-2.23) | 42.8 | 60.0 | 62.3 | 31 005 (7.5) |
| Bones and joints | 1252 (0.2) | 76 (0.1) | 69 | 86 | 0.88 (0.70-1.11) | 73 (0.2) | 67 | 29 | 2.48 (1.95-3.12) | 45.6 | 65.6 | 65.6 | 10 972 (8.8) |
| Other and unspecified primary sitesg | 7773 (1) | 795 (1.3) | 158 | 608 | 1.31 (1.22-1.40) | 1266 (22.1) | 254 | 255 | 4.97 (4.70-5.25) | 59.5 | 70.6 | 71.3 | 50 404 (6.5) |
| Total | 750 717 (100) | 62 348 (100) | 109 | 56 853 | 1.10 (1.09-1.11) | 34 879 (100) | 62 | 26243 | 1.33 (1.32-1.34) | 58.2 | 71.2 | 73.9 | 5 698 464 (7.6) |
Abbreviation: FPC, first primary cancer; NOS, not otherwise specified; SEER, Surveillance, Epidemiology, and End Results Program; SIR, standardized incidence ratio; SMR, standardized mortality ratio; SPC, subequent primary cancer.
Includes second and later primary cancers and all cancer sites except nonmelanoma skin and same-site cancers (see eTable 2 in Supplement 1 for same-site cancers).
See eTable 4 in Supplement 1 for values by FPC types and sex included in the mortality analysis.
Values were extrapolated from the incidence and mortality rates of 12 SEER registries with a comparable distribution of race, sex, age, and calendar year (eMethods in Supplement 1).
SIR indicates the ratio of the observed to the expected number of SPC cases; SMR indicates the ratio of the observed to the expected number of SPC deaths. The SIR and SMR indicate the strength of an association between the FPC and the SPC, which can vary by age at and time since first cancer diagnosis.4
The current version of the SEER database did not allow determination of variation (standard deviation) for survivor age and person-years of follow-up.
Includes chronic lymphocyte leukemia.
Excluded in the further analysis (see eTable 5 in Supplement 1 for specific other sites).
Reported due to the substantially lower than expected risk for SPCs after prostate cancer.
Most survivor groups defined by FPC types and sex had higher than expected risks of developing or dying from SPCs as compared with the risks in the general population. Among men, the risk of developing any SPCs was statistically significantly higher for 18 of the 30 FPC types, and risk of dying from any SPCs was statistically significantly higher for 27 of the 30 FPC types. Among women, the risk of developing any SPCs was statistically significantly higher for 21 of the 31 FPC types, and risk of dying from any SPCs was statistically significantly higher for 28 of the 31 FPC types (Table 2; eTable 5 in Supplement 1). Among FPC types, the greatest SIR in men was observed after laryngeal cancer (SIR, 1.75 [95% CI, 1.68-1.83]; incidence, 373 per 10 000 person-years), followed by survivors of Hodgkin lymphoma (SIR, 1.59 [95% CI, 1.47-1.72]; incidence, 101 per 10 000 person-years). Among FPC types, the greatest SIR in women was observed after laryngeal cancer (SIR, 2.48 [95% CI, 2.27-2.72]; incidence, 336 per 10 000 person-years) followed by survivors of esophageal cancer (SIR, 1.89 [95% CI, 1.58-2.25]; incidence, 275 per 10 000 person-years). The greatest SMR among men was estimated after gallbladder cancer (SMR, 3.82 [95% CI, 3.31-4.39]; mortality, 341 per 10 000 person-years), and among women, the greatest SMR was estimated after laryngeal cancer (SMR, 4.56 [95% CI, 4.11-5.06]; mortality, 268 per 10 000 person-years).
For the 3 most common FPC types, the overall SIRs in men were statistically significantly lower for prostate cancer (SIR, 0.91 [95% CI, 0.91-0.93]; incidence, 165 per 10 000 person-years), were not significantly different for colorectal cancer (SIR, 0.99 [95% CI, 0.98-1.02]; incidence, 190 per 10 000 person-years), and were statistically significantly higher for bladder cancers (SIR, 1.08 [95% CI, 1.06-1.11]; incidence, 222 per 10 000 person-years); whereas the SMR was statistically significantly lower for prostate cancer (SMR, 0.90 [95% CI, 0.89-0.91]; mortality, 94 per 10 000 person-years) and was statistically significantly higher for colorectal cancer (SMR, 1.11 [95% CI, 1.09-1.15]; mortality, 102 per 10 000 person-years) and also for bladder cancer (SMR, 1.31 [95% CI, 1.28-1.35]; mortality, 136 per 10 000 person-years). In women, the SIR and SMR were statistically significantly higher for breast cancer (SIR, 1.06 [95% CI, 1.05-1.08]; incidence, 95 per 10 000 person-years; and SMR, 1.13 [95% CI, 1.11-1.15]; mortality, 52 per 10 000 person-years), colorectal cancer (SIR, 1.04 [95% CI, 1.02-1.07]; incidence, 134 per 10 000 person-years; and SMR, 1.15 [95% CI, 1.12-1.20]; mortality, 73 per 10 000 person-years), and uterine corpus cancer (SIR, 1.03 [95% CI, 1.01-1.06]; incidence, 124 per 10 000 person-years; and SMR, 1.09, [95% CI, 1.05-1.13]; mortality, 58 per 10 000 person-years). Additionally, the risk of developing and dying from SPCs was statistically significantly lower in male survivors of melanoma (of the skin) but for none of the FPC types in women.
Figure 1 and Figure 2 show the percentage contributions of each FPC and SPC combination to the total incidence of SPCs and the SIRs with statistically significantly higher than expected values for 264 associations of the FPC with the risk of specific type of SPCs out of 1007 eligible statistical tests. The corresponding metrics for mortality are presented in Figure 3 and Figure 4 for 274 associations with higher than expected risks out of 760 eligible statistical tests. The patterns in incidence and mortality were generally similar, although the estimates for the corresponding associations were usually greater for SMRs than for SIRs (eTable 6 and eTable 7 in Supplement 1). Results for statistically significantly lower than expected SIRs and SMRs are presented in eFigure 2 in Supplement 1.
Figure 1. Risk of Developing Subsequent Primary Cancers Among 5-Year Male Cancer Survivors.
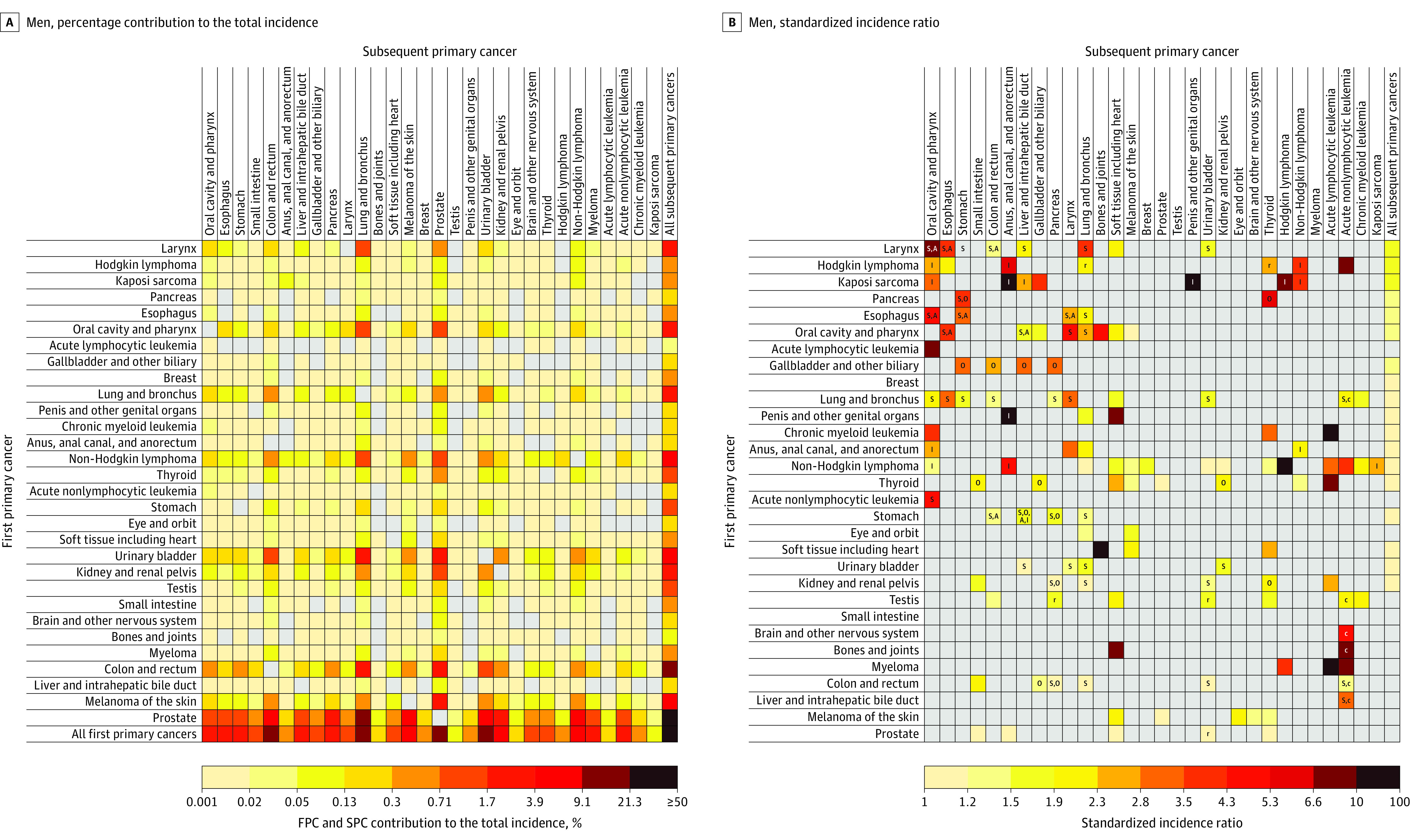
A, Calculated by dividing the observed number of SPC cases of each cell by the total number of observed SPCs. B, Standardized incidence ratios (SIRs) were calculated as the ratio of the observed number of SPCs to the expected number of SPCs in the general population. Letters inside cells indicate major shared risk factors (A, alcohol-associated cancer18; I, infection-associated cancer19; O, obesity-associated cancer17; S, smoking-associated cancer16) or treatment-related SPCs (c, chemotherapy; r, radiotherapy)23,24,25,26 when there was a statistically significant association between the first primary cancer (FPC) and the SPC. Logarithmic color gradients indicate SIRs with statistically significantly higher than expected values based on eligible statistical tests (observed number of SPCs ≥5). Gray cells indicate statistically nonsignificant SIRs or associations not tested due to small number of observed SPCs. The risk of developing SPCs cannot be entirely attributable to risk factors annotated, and additional known and unknown factors may be in play. See Supplement 1 for point estimates and excess absolute incidences.
Figure 2. Risk of Developing Subsequent Primary Cancers Among 5-Year Female Cancer Survivors.
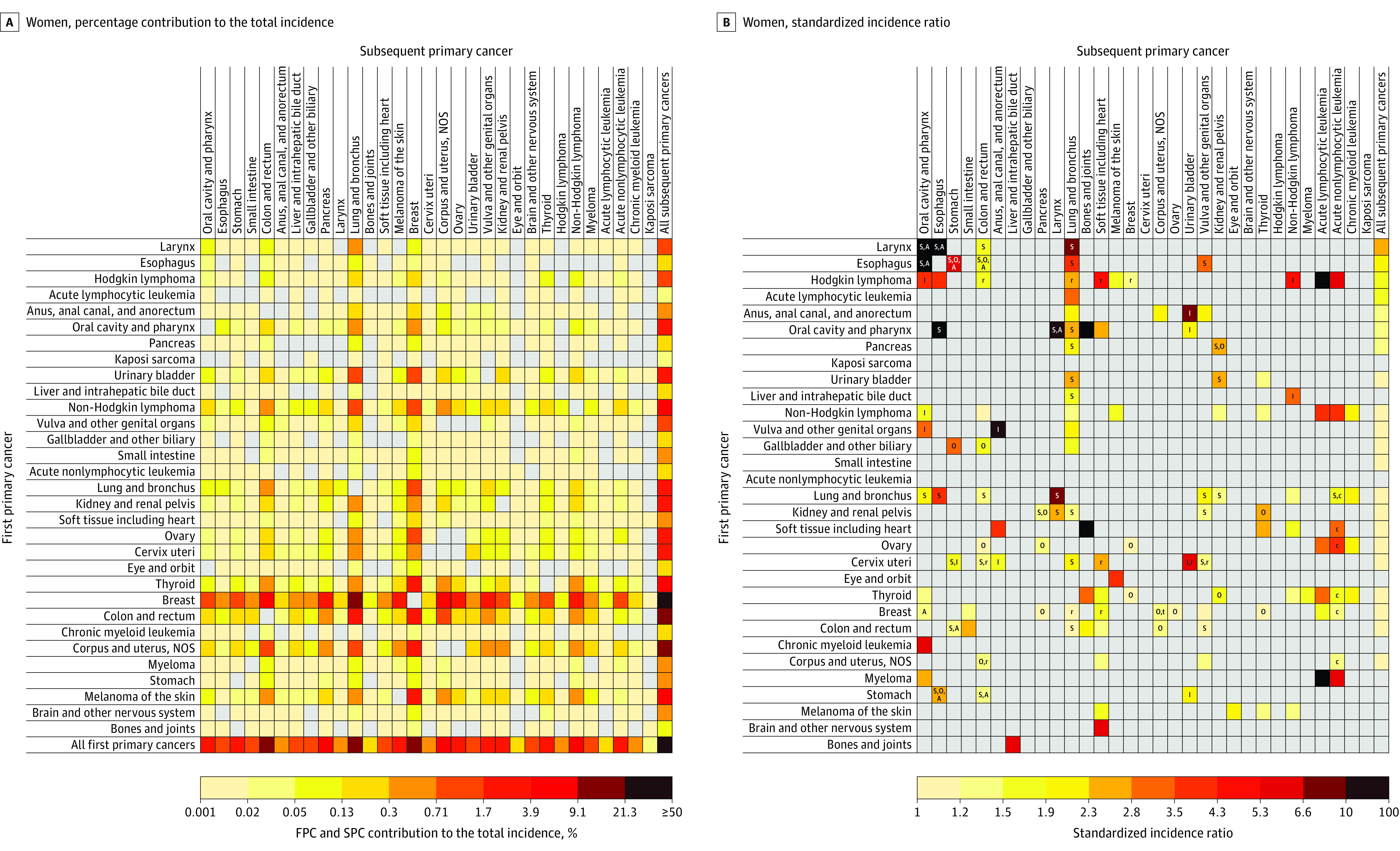
See the legend to Figure 1 for a description of the methods used to calculate the contribution of each cell to total incidence of subsequent primary cancer (A) and the standardized incidence ratios (B) as well as definitions of abbreviations. See Supplement 1 for point estimates and excess absolute incidences. NOS indicates not otherwise specified; t indicates tamoxifen.
Figure 3. Risk of Dying From a Subsequent Primary Cancer Among 5-Year Male Cancer Survivors.
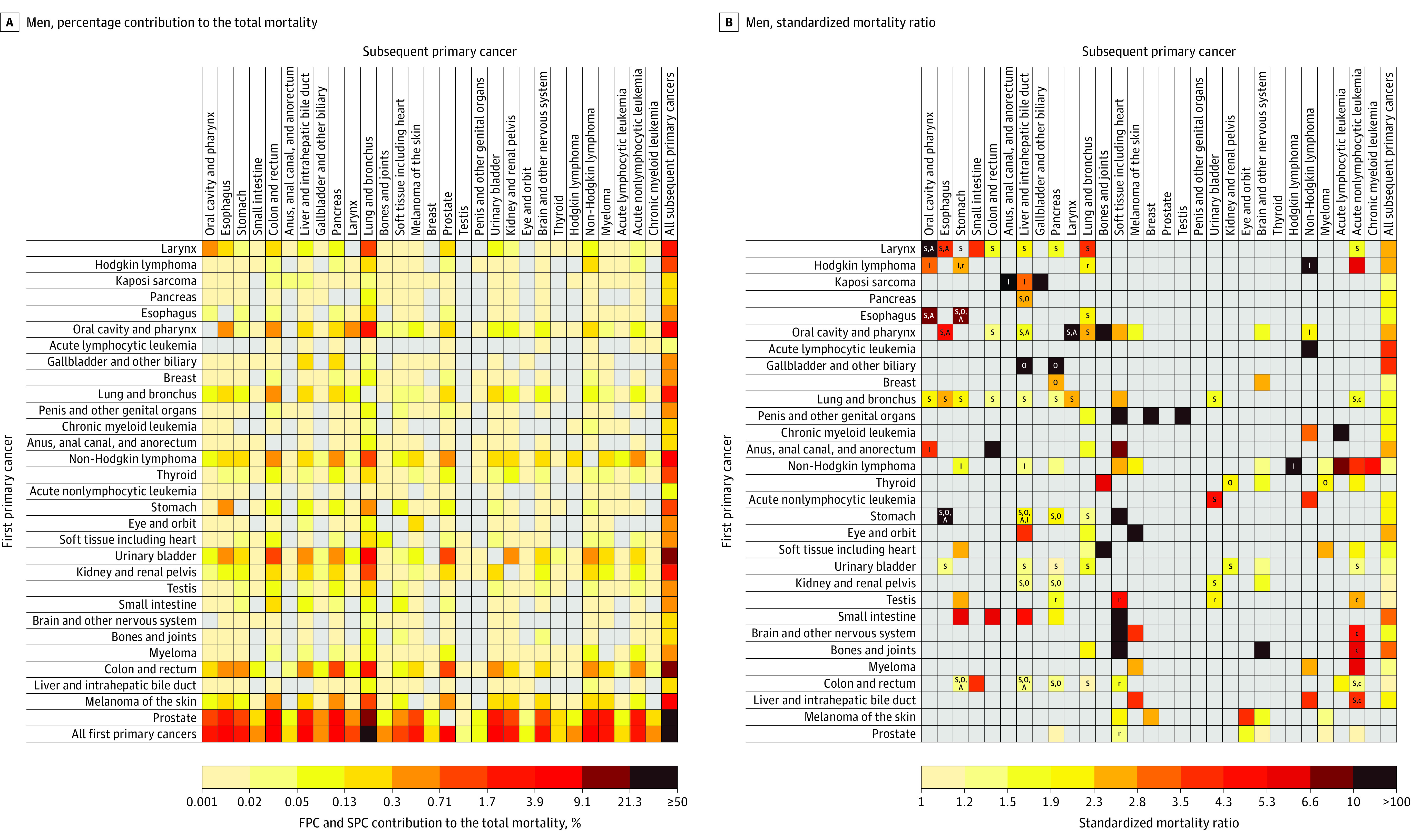
A, Calculated by dividing the observed number of SPC deaths of each cell by the total number of observed SPC deaths. B, Standardized mortality ratios (SMRs) were calculated as the ratio of the observed number of SPC deaths to the expected number of cancer deaths in the general population. Letters inside cells indicate major shared risk factors (A, alcohol-associated cancer18; I, infection-associated cancer19; O, obesity-associated cancer17; S, smoking-associated cancer16) or treatment-related SPCs (c, chemotherapy; r, radiotherapy)23,24,25,26 when there was a statistically significant association between the first primary cancer (FPC) and the SPC. Logarithmic color gradients indicate SIRs with statistically significantly higher than expected values based on eligible statistical tests (observed number of SPCs ≥5). Gray cells indicate statistically nonsignificant SIRs or associations not tested due to small number of observed SPCs. The risk of developing SPCs cannot be entirely attributable to risk factors annotated, and additional known and unknown factors may be in play. See Supplement 1 for point estimates and excess absolute incidences.
Figure 4. Risk of Dying From a Subsequent Primary Cancer Among 5-Year Female Cancer Survivors.
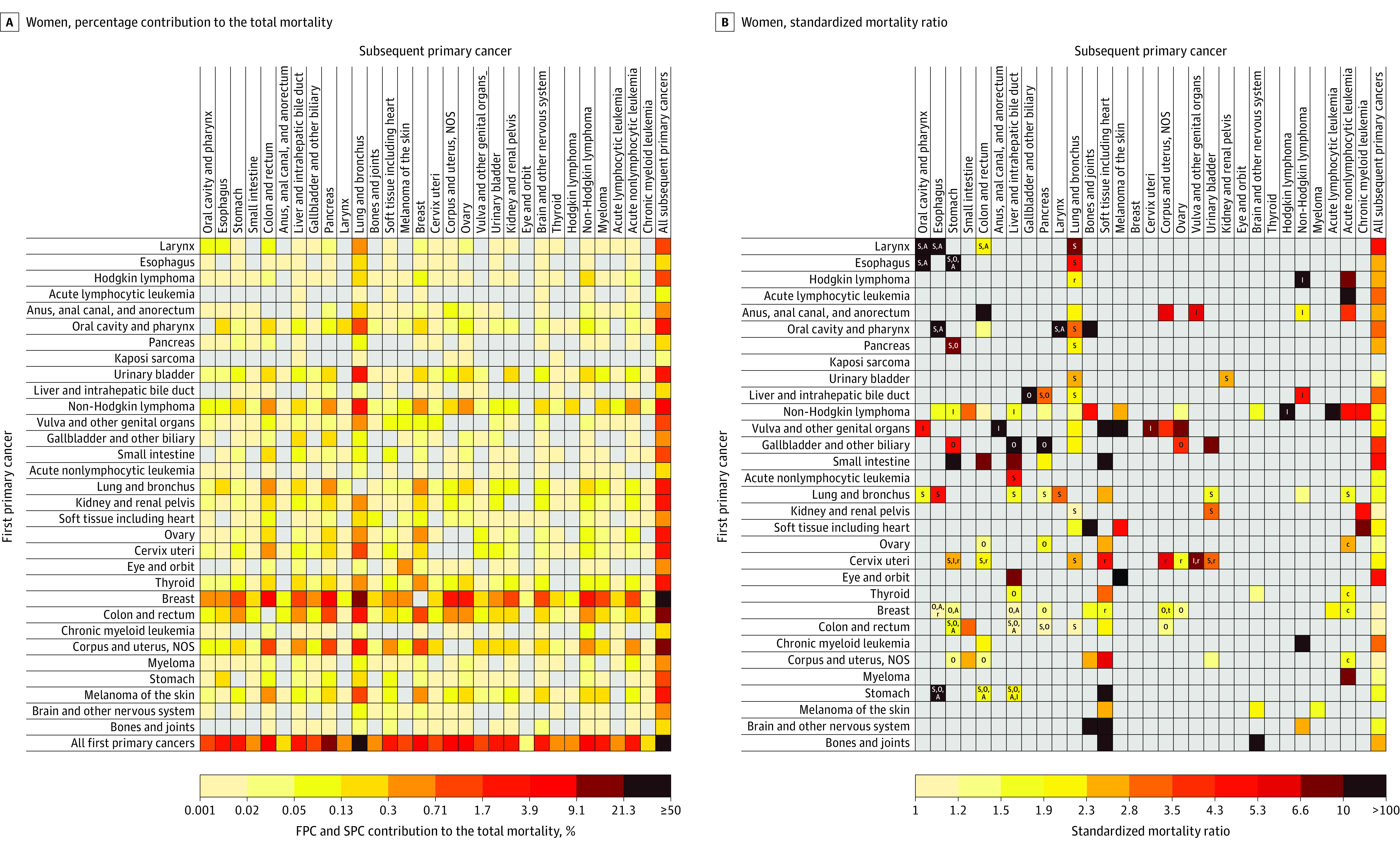
See the legend to Figure 3 for a description of the methods used to calculate the contribution of each cell to the total number of subsequent primary cancer deaths (A) and the standardized mortality ratios (B) as well as definitions of abbreviations. Kaposi sarcoma was not shown as an SPC because mortality data do not specify it as a cause of death. See Supplement 1 for point estimates and excess absolute mortalities. NOS indicates not otherwise specified.
Among all survivors, the 4 SPCs that contributed most to the total incidence of SPCs were lung (19.1%), prostate (13.7%), urinary bladder (11.1%), and colorectal cancers (10.1%) in men; and lung (19.3%), breast (17.3%), colorectal (11.0%), and uterine corpus cancers (7.4%) in women (see the “All first primary cancers” row in Figure 1A and Figure 2A; eTable 8 in Supplement 1). The 4 SPCs that contributed most to the total SPC mortality were lung (33.1% in men; 31.2% in women), colorectal (8.8% in men), pancreatic (8.5% in men; 9.4% in women), followed by non-Hodgkin lymphoma (6.0%) in men, and breast (5.8%) in women (Figure 3A and Figure 4A; eTable 8 in Supplement 1).
The greatest SIR (Figure 1B) and SMR (Figure 3B) in men was observed among survivors of Kaposi sarcoma for anal cancer (SIR, 71.3 [95% CI, 56.8-88.4]; incidence, 23.2 per 10 000 person-years; and SMR, 107.5 [95% CI, 58.8-180.4]; mortality, 4.1 per 10 000 person-years). The greatest SIR in women was among survivors of laryngeal cancer (SIR, 19.3 [95% CI, 14.5-25.1]; incidence, 39.9 per 10 000 person-years) and esophageal cancer (SIR, 17.2 [95% CI, 10.2-27.2]; incidence, 39.0 per 10 000 person-years) for oral cavity/pharyngeal cancer (Figure 2B); whereas the greatest SMR in women was seen among survivors of eye and orbit cancer (SMR, 212.9 [95% CI, 176.2-254.9]; mortality, 118.4 per 10 000 person-years) for melanoma (Figure 4B).
Among survivors of 12 smoking-related cancers, the risks of developing any subsequent smoking-related cancers were statistically significantly higher for all cancers except liver cancer in men and stomach cancer and acute nonlymphocytic leukemia in women, with SIRs ranging from 1.08 (95% CI, 1.04-1.13) after female colorectal cancer to 4.62 (95% CI, 4.17-5.11) after female laryngeal cancer survivors (Figure 5A; eTable 9 in Supplement 1). Among survivors of 12 obesity-related cancers, the risks of developing any subsequent obesity-related cancers were statistically significantly higher among male survivors of pancreatic, gallbladder, thyroid, and colorectal cancers and among female survivors of thyroid, kidney, uterine corpus, and breast cancers (Figure 5B; eTable 9 in Supplement 1). Similarly, the risk for alcohol- and infection-related cancers was significantly higher after most alcohol- and infection-related cancers (eFigure 3 in Supplement 1).
Figure 5. Risk of Developing Any Smoking- or Obesity-Related Subsequent Primary Cancers Among Survivors of Prior Smoking- or Obesity-Related Cancers.
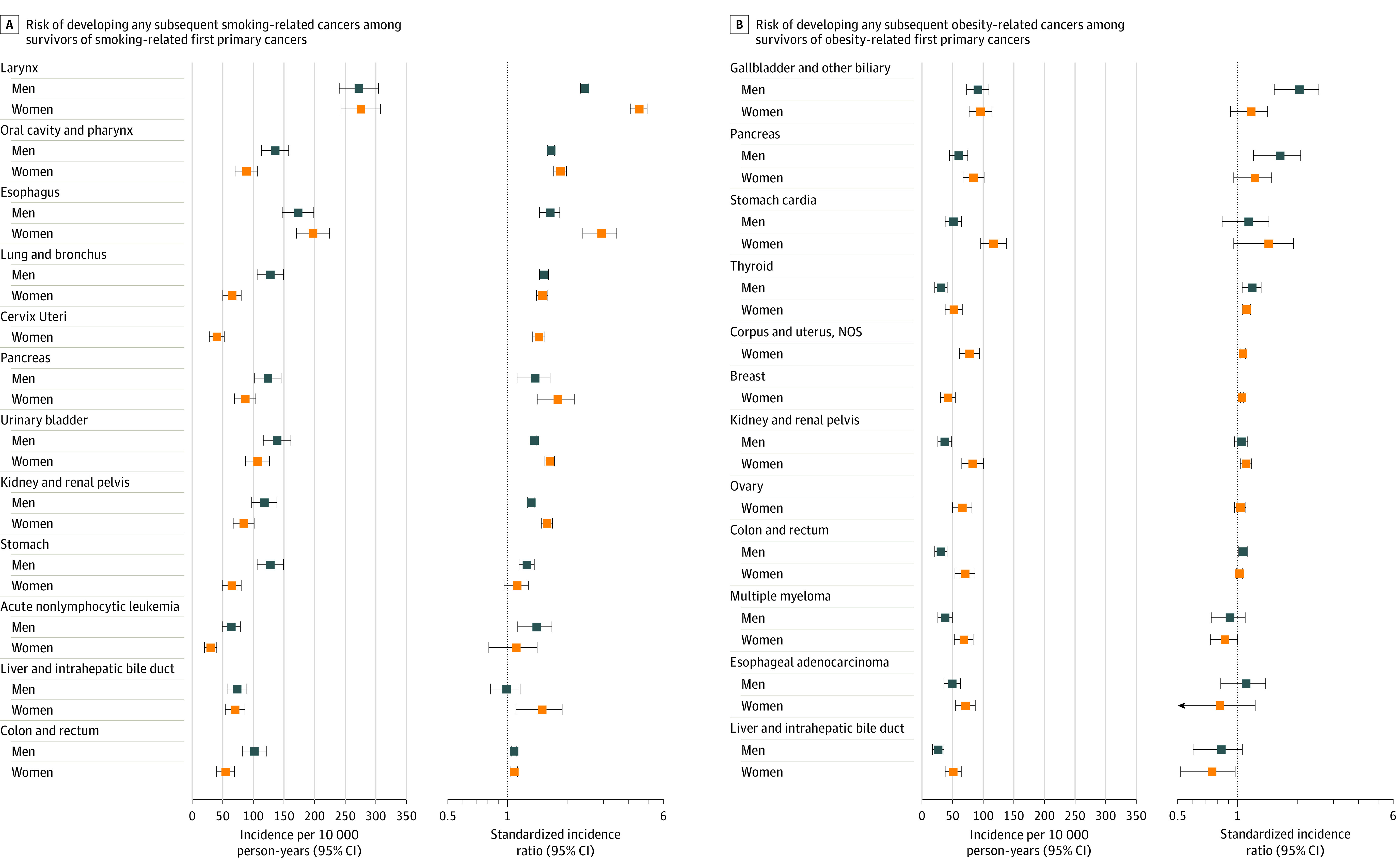
Among each group of survivors of 12 smoking-related cancers,16 incidence per 10 000 person-years and standardized incidence ratios (SIRs) were estimated to quantify the risk of developing any smoking-related subsequent primary cancers (SPCs) (except for SPCs that occurred at the same site as the first primary cancer [FPC]). This was also calculated for 12 obesity-related cancers.17 The risk of developing subsequent smoking- or obesity-related cancer after each FPC cannot be entirely attributable to smoking or obesity because of other risk factors. See Supplement 1 for detailed data.
Temporal trends in SIRs were statistically significant for several cancers (eFigure 4 and eTable 10 in Supplement 1). For example, the ratio of SIRs for lung cancer comparing 2000-2011 with 1975-1989 was 1.52 (95% CI, 1.43-1.61) among laryngeal cancer survivors, and 1.20 (95% CI, 1.16-1.24) among female breast cancer survivors. The corresponding ratio of the SIRs for colorectal cancer was 1.28 (95% CI, 1.20-1.36) among uterine corpus cancer survivors.
In sensitivity analyses, SIRs restricted to the first SPCs among 5-year survivors (eTable 11 in Supplement 1) or expanded to 1-year survivors (eTable 12 in Supplement 1) were generally similar to those from the main analysis regarding the directions of the associations, although the point estimates generally were lower for the former group and higher for the latter group.
Discussion
Based on the most recent population-based incidence data in the US, cancer survivors overall had higher risks of developing or dying from SPCs than the general population. The risk of developing SPCs was significantly higher for 18 of the 30 FPC types in men and for 21 of the 31 FPC types in women, with significantly higher risks of dying from SPCs for the majority of cancer types. Across FPC types, associations with specific types of SPCs varied considerably; however, only a few smoking- or obesity-related SPCs comprised substantial proportions of the total SPC incidence and mortality, highlighting opportunities for primary prevention as central components of survivorship care.
Although some of the higher-risk SPCs among survivors may reflect genetic predisposition and treatment exposures, most of this excess risk is likely due to host factors (eg, aging, immunity) and to lifestyle risk factors (eg, smoking, obesity, alcohol, infection) shared by the first and subsequent cancers.23 The risks of smoking-related SPCs were commonly elevated following many types of smoking-related FPCs, suggesting the role of smoking as a shared risk factor. Among survivors of all FPCs, 4 common smoking-related SPCs—lung, urinary bladder, oral cavity/pharynx, and esophagus—accounted for 26% to 45% of the total SPC incidence and mortality. Furthermore, lung cancer alone comprised 31% to 33% of the total mortality from SPCs. In 2018, 12% of cancer survivors in the US were current smokers,27 with higher prevalence among survivors of smoking-related cancers than survivors of non–smoking-related cancers (20% vs 11%).28 Only 46% of those smokers, however, reported using counseling or medication when trying to quit, and less than 8% reported using both quitting aids,29 suggesting the majority of attempts to quit are inadequately supported. Thus, systematic efforts are needed to increase the provision of counseling and pharmacological interventions for tobacco dependence among survivors.
Similarly, survivors of many obesity-related cancers had an elevated risk of developing obesity-related SPCs. Further, among survivors of all FPCs, 4 common obesity-related cancers—colorectum, pancreas, corpus uteri, and liver—comprised 22% to 26% of total SPC mortality. Prior studies of breast and colorectal cancer survivors demonstrated greater risk of obesity-associated SPCs among those with excess body weight,30,31 suggesting that maintenance of heathy bodyweight may reduce the risk of SPCs. In 2018, 67% of cancer survivors in the US were overweight or obese, and 34% of survivors reported no leisure time physical activity.27 Many cancer survivors, however, report that they have never discussed lifestyle recommendations relevant to bodyweight with their health care providers, nor have they changed these behaviors.32,33 Survivorship care may require greater focus on lifestyle factors, including weight management, physical activity, and healthy eating; survivorship care guidelines recommending health promotion need wider dissemination and implementation in oncology and primary care.34
Infections may also play a role in the risk of SPCs. For example, co-occurrence of human papillomavirus–associated cancers (oral cavity/pharynx, anus, external genital organs, cervix) is likely related to cross-infection at multiple sites.35 The large excess risk of anal cancer among male Kaposi sarcoma survivors and co-occurrence of cancers of the liver, oral cavity/pharynx, and non-Hodgkin lymphoma may reflect underlying immunosuppression, possible role of herpesvirus 8, and behavioral factors that increase chances of viral infection (eg, human immunodeficiency virus, human papillomavirus, hepatitis viruses B and C).8,36 Primary prevention for some of these cancers is possible with vaccination against hepatitis B and treatments of hepatitis C infection and precancerous lesions (eg, advanced anal intraepithelial neoplasia).
The risks of developing SPCs among survivors compared with those in the general population increased for many FPC types between 1975-1989 and 2000-2015. Reasons for these trends are unknown but may include changes in risk factor prevalence and treatment patterns. Consistent with previous studies, the high risks of developing and dying from acute nonlymphocytic leukemia were observed after multiple solid tumor types, likely reflecting late effects of chemotherapy, radiation, or both.24 Higher risks of developing SPCs at sites in close proximity to the FPC (eg, breast cancer after Hodgkin lymphoma; bladder cancer after prostate or testicular cancer) and subsequent soft tissue sarcomas observed after several cancers likely reflect, in part, the effects of radiotherapy.37 Because the use of leukemogenic agents and radiotherapy have increased over time,37,38 quantifying the late effects of prior therapies remains highly relevant for clinicians to inform risk-based long-term follow-up care planning.
In addition to reinforcing the importance of coordinated efforts by primary care clinicians to mitigate the risks of SPCs through survivorship care— counseling to promote adherence to healthy lifestyle recommendations and surveillance and screening for new malignancies—these findings have implications for reducing the economic burden of cancer. With the growing number of long-term survivors, the costs of treating patients with multiple primary cancers will increase, amplifying financial burden for cancer survivors and their families, particularly among elderly persons who may be living on fixed incomes.39 This consideration also has implications for the Medicare program, the primary payer for the population aged 65 years and older, as well as other health care payers.
Limitations
This study has several limitations. First, the overall analysis may conceal important heterogeneity in the risk of SPCs by age, latency, treatment patterns, and other clinical and sociodemographic factors. Although the results were stratified by sex, formal statistical tests were not conducted for these differences. Importantly, this study did not examine risk associated with receipt of radiation and systemic treatments because this information in the registries was classified as “no/unknown” for a considerable proportion of patients (eg, no/unknown, 45% for radiotherapy and 39% for chemotherapy among breast cancer survivors). Second, the accuracy of underlying causes of death on death certificates for SPCs among cancer survivors is unknown. Third, SPCs diagnosed among survivors who migrate out of SEER registry areas are not reportable and, therefore, this risk may be underestimated. However, only 4% of survivors in the analytic samples were lost to follow-up. Fourth, the registry data used in this study cover 9% to 13% of the US population, and results may therefore not be generalizable to the entire population, although SEER is considered as the criterion standard for cancer surveillance and outcomes.40 Fifth, SPCs in the same organ as the FPC were excluded to minimize bias due to misclassification of recurrence; therefore, the incidence rates may underestimate the true risk of SPCs that may include multicentric tumors, new primaries at the other side of paired organs, and those with different histology. Sixth, heightened medical surveillance following the initial cancer diagnosis may increase detection of SPCs, although SPCs occurring within 5 years of first cancer diagnosis were excluded to minimize this bias. Seventh, it is possible that some of the observed statistically significant associations could be due to chance because of multiple testing. Eighth, data were presented for the percentage contributions of smoking-, obesity-, alcohol-, and infections-related cancers to the burden of SPCs among survivors but not for population-attributable fractions of SPCs to these risk factors because of limited data on prevalence of risk factors and relative risks among survivors.
Conclusions
Among survivors of adult-onset cancers in the United States, several types of primary cancers were significantly associated with greater risk of developing and dying from an SPC, compared with the general population. Cancers associated with smoking or obesity comprised substantial proportions of overall SPC incidence and mortality among all survivors and highlight the importance of ongoing surveillance and efforts to prevent new cancers among survivors.
eMethods.
eFigure 1A. Excess Absolute Risk of Developing Subsequent Primary Cancers Among 5-Year Cancer Survivors by Sex
eFigure 1B. Excess Absolute Risk of Dying From Subsequent Primary Caners Among 5-Year Cancer Survivors by Sex
eFigure 2. Standardized Incidence Ratios for the Risk of Subsequent Primary Cancer (A, B) and Standardized Mortality Ratios for the Risk of Dying From a Subsequent Primary Cancer (C, D) With Statistically Significant Lower-Than-Expected Values by Sex
eFigure 3. Risk of Developing Any Alcohol- or Infection-Related Subsequent Primary Cancers Among Survivors of Prior Alcohol- or Infection-Related Cancers
eFigure 4. Ratios of Standardized Incidence Ratios for Select Subsequent Primary Cancer Types Among 5-Year Cancer Survivors by First Primary Cancer Diagnosis Year (1975-1989, 1990-1999, 2000-2011) in 9 SEER Registries
eTable 1. Characteristics Of The Survivors Who Were Diagnosed With An Invasive First Primary Cancer From 1975-2011 At Ages 20-84 And Who Survived For 5 Years Or Longer in 9 SEER registries
eTable 2. Classification Of Cancer Types Based on ICD-O-3 Site and Type Code and Definitions of The Same-Type Cancers
eTable 3. Definitions and Classification of Smoking-, Obesity-, Alcohol-, and Infection-Related Cancers
eTable 4. Number of Survivors and Person-Years of Follow-Up Included in the Mortality Analysis by First Primary Cancer Sites and Sex
eTable 5. Risks of Developing or Dying from Subsequent Primary Cancers Among 5-Year Survivors of Other and Unspecified Primary Cancer Sites by Sex
eTable 6. Risks of Developing Specific Type of Subsequent Primary Cancer Among 5-Year Cancer Survivors in 12 SEER Registries: Incidence, Percent Contribution to the Total Incidence, Standardized Incidence Ratio by First Primary Cancer Types and Sex
eTable 7. Risks of Dying from a Specific Type of Subsequent Primary Cancer Among 5-Year Cancer Survivors in 12 SEER Registries: Mortality, Percent Contribution to the Total Mortality, Standardized Mortality Ratio by First Primary Cancer Types and Sex
eTable 8. Percentage Contribution of Each Subsequent Primary Cancer Types to the Total Incidence of Subsequent Primary Cancer and to the Total Subsequent Primary Cancer Mortality Among All 5-Year Cancer Survivors by Sex in 12 SEER Registries
eTable 9. Risk of Developing Any Smoking- or Any Obesity-Related Subsequent Primary Cancers Among Smoking or Obesity-Related Cancer Survivors
eTable 10. Standardized Incidence Ratios and Ratios of Standardized Ratios by Diagnosis Year (1975-1989, 1990-1999, 2000-2011) for the Risk of Select 6 Subsequent Primary Cancers Among 5-Year Survivors of 16 First Primary Cancer Types in 9 SEER Registries
eTable 11. Results Based on a Single Outcome Analysis for the Risk of Developing Subsequent Primary Cancer Overall Among 5-Year Cancer Survivors in 12 SEER Program Registries
eTable 12. Results Based on the Earlier Start of Follow-Up (Since 1 Year From First Primary Cancer Diagnosis) for the Risk of Developing Subsequent Primary Cancer Overall Among 1-Year Cancer Survivors in the 12 SEER Registries
eReferences.
References
- 1.Miller KD, Nogueira L, Mariotto AB, et al. Cancer treatment and survivorship statistics, 2019. CA Cancer J Clin. 2019;69(5):363-385. doi: 10.3322/caac.21565 [DOI] [PubMed] [Google Scholar]
- 2.Bluethmann SM, Mariotto AB, Rowland JH. Anticipating the “Silver Tsunami”: prevalence trajectories and comorbidity burden among older cancer survivors in the United States. Cancer Epidemiol Biomarkers Prev. 2016;25(7):1029-1036. doi: 10.1158/1055-9965.EPI-16-0133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.El-Shami K, Oeffinger KC, Erb NL, et al. American Cancer Society Colorectal Cancer Survivorship Care Guidelines. CA Cancer J Clin. 2015;65(6):428-455. doi: 10.3322/caac.21286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Curtis RE, Freedman DM, Ron E, et al. , eds. New Malignancies Among Cancer Survivors: SEER Cancer Registries, 1973-2000. National Cancer Institute; 2006. NIH publication 05-5302. [Google Scholar]
- 5.Murphy CC, Gerber DE, Pruitt SL. Prevalence of prior cancer among persons newly diagnosed with cancer: an initial report from the Surveillance, Epidemiology, and End Results Program. JAMA Oncol. 2018;4(6):832-836. doi: 10.1001/jamaoncol.2017.3605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bright CJ, Reulen RC, Winter DL, et al. Risk of subsequent primary neoplasms in survivors of adolescent and young adult cancer (Teenage and Young Adult Cancer Survivor Study): a population-based, cohort study. Lancet Oncol. 2019;20(4):531-545. doi: 10.1016/S1470-2045(18)30903-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee JS, DuBois SG, Coccia PF, Bleyer A, Olin RL, Goldsby RE. Increased risk of second malignant neoplasms in adolescents and young adults with cancer. Cancer. 2016;122(1):116-123. doi: 10.1002/cncr.29685 [DOI] [PubMed] [Google Scholar]
- 8.Hessol NA, Whittemore H, Vittinghoff E, et al. Incidence of first and second primary cancers diagnosed among people with HIV, 1985-2013: a population-based, registry linkage study. Lancet HIV. 2018;5(11):e647-e655. [DOI] [PubMed] [Google Scholar]
- 9.Adjei Boakye E, Buchanan P, Hinyard L, et al. Trends in the risk and burden of second primary malignancy among survivors of smoking-related cancers in the United States. Int J Cancer. 2019;145(1):143-153. doi: 10.1002/ijc.32101 [DOI] [PubMed] [Google Scholar]
- 10.Gilbert DC, Wakeham K, Langley RE, Vale CL. Increased risk of second cancers at sites associated with HPV after a prior HPV-associated malignancy, a systematic review and meta-analysis. Br J Cancer. 2019;120(2):256-268. doi: 10.1038/s41416-018-0273-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.National Cancer Institute Surveillance, Epidemiology, and End Results Program SEER*Stat Database: Incidence—SEER Research Plus Data. 9 Registries, November 2019 submission (1975-2017)—linked to county attributes—total US, 1969-2018 counties. Accessed August 25, 2020. http://www.seer.cancer.gov
- 12.National Cancer Institute Surveillance, Epidemiology, and End Results Program SEER*Stat Database: Incidence—SEER Research Plus Data. 13 Registries (excluding Arkansas), November 2019 submission (1992-2017)—linked to county attributes—total US, 1969-2018 counties. Accessed October 16, 2020. http://www.seer.cancer.gov
- 13.National Cancer Institute Surveillance, Epidemiology, and End Results Program SEER*Stat Database: Incidence—SEER Research Plus Data. 13 Registries (excluding Arkansas), November 2019 submission (1992-2017) for SMRs—linked to county attributes—total US, 1969-2018 counties. Accessed August 25, 2020. http://www.seer.cancer.gov
- 14.Johnson C, Peace S, Adamo P, Fritz A, Percy-Laurry A, Edwards BK. Multiple Primary and Histology Coding Rules: January 1, 2007. National Cancer Institute, Surveillance, Epidemiology and End Results Program. Accessed October 10, 2020. https://seer.cancer.gov/tools/mphrules/2007_mphrules_manual_08242012.pdf
- 15.United Nations Scientific Committee on the Effects of Atomic Radiation Effects of Ionizing Radiation. Annex A: Epidemiological Studies of Radiation and Cancer, Volume 1. United Nations; 2006. Accessed October 10, 2020. https://www.unscear.org/docs/publications/2006/UNSCEAR_2006_Annex-A-CORR.pdf
- 16.US Department of Health and Human Services Smoking Cessation: A Report of the Surgeon General. US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2020. [Google Scholar]
- 17.Lauby-Secretan B, Scoccianti C, Loomis D, Grosse Y, Bianchini F, Straif K; International Agency for Research on Cancer Handbook Working Group . Body fatness and cancer—viewpoint of the IARC Working Group. N Engl J Med. 2016;375(8):794-798. doi: 10.1056/NEJMsr1606602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.World Cancer Research Fund/American Institute for Cancer Research Continuous Update Project Expert Report 2018. Alcoholic drinks and the risk of cancer. Accessed October 10, 2020. https://www.wcrf.org/dietandcancer/recommendations/limit-alcohol-consumption
- 19.Bouvard V, Baan R, Straif K, et al. ; WHO International Agency for Research on Cancer Monograph Working Group . A review of human carcinogens—part B: biological agents. Lancet Oncol. 2009;10(4):321-322. doi: 10.1016/S1470-2045(09)70096-8 [DOI] [PubMed] [Google Scholar]
- 20.Yasui Y, Liu Y, Neglia JP, et al. A methodological issue in the analysis of second-primary cancer incidence in long-term survivors of childhood cancers. Am J Epidemiol. 2003;158(11):1108-1113. doi: 10.1093/aje/kwg278 [DOI] [PubMed] [Google Scholar]
- 21.National Cancer Institute Surveillance, Epidemiology, and End Results Program SEER*Stat software version 8.3.6. Accessed October 10, 2020. https://www.seer.cancer.gov/seerstat
- 22.National Cancer Institute Surveillance, Epidemiology, and End Results Program Variable and Recode Definitions on the SEER Historic Stage A. Accessed October 10, 2020. https://seer.cancer.gov/seerstat/variables/seer/yr1973_2009/lrd_stage/index.html
- 23.Travis LB, Demark Wahnefried W, Allan JM, Wood ME, Ng AK. Aetiology, genetics and prevention of secondary neoplasms in adult cancer survivors. Nat Rev Clin Oncol. 2013;10(5):289-301. doi: 10.1038/nrclinonc.2013.41 [DOI] [PubMed] [Google Scholar]
- 24.Morton LM, Dores GM, Schonfeld SJ, et al. Association of chemotherapy for solid tumors with development of therapy-related myelodysplastic syndrome or acute myeloid leukemia in the modern era. JAMA Oncol. 2019;5(3):318-325. doi: 10.1001/jamaoncol.2018.5625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Berrington de Gonzalez A, Gilbert E, Curtis R, et al. Second solid cancers after radiation therapy: a systematic review of the epidemiologic studies of the radiation dose-response relationship. Int J Radiat Oncol Biol Phys. 2013;86(2):224-233. doi: 10.1016/j.ijrobp.2012.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dracham CB, Shankar A, Madan R. Radiation induced secondary malignancies: a review article. Radiat Oncol J. 2018;36(2):85-94. doi: 10.3857/roj.2018.00290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.National Cancer Institute Cancer Trends Progress Report: Life After Cancer. Accessed August 15, 2020. https://www.progressreport.cancer.gov/after
- 28.Gritz ER, Talluri R, Fokom Domgue J, Tami-Maury I, Shete S. Smoking behaviors in survivors of smoking-related and non–smoking-related cancers. JAMA Netw Open. 2020;3(7):e209072. doi: 10.1001/jamanetworkopen.2020.9072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gallaway MS, Glover-Kudon R, Momin B, et al. Smoking cessation attitudes and practices among cancer survivors—United States, 2015. J Cancer Surviv. 2019;13(1):66-74. doi: 10.1007/s11764-018-0728-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Druesne-Pecollo N, Touvier M, Barrandon E, et al. Excess body weight and second primary cancer risk after breast cancer: a systematic review and meta-analysis of prospective studies. Breast Cancer Res Treat. 2012;135(3):647-654. doi: 10.1007/s10549-012-2187-1 [DOI] [PubMed] [Google Scholar]
- 31.Gibson TM, Park Y, Robien K, et al. Body mass index and risk of second obesity-associated cancers after colorectal cancer: a pooled analysis of prospective cohort studies. J Clin Oncol. 2014;32(35):4004-4011. doi: 10.1200/JCO.2014.56.8444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Anderson C, Sandler DP, Weinberg CR, et al. Age- and treatment-related associations with health behavior change among breast cancer survivors. Breast. 2017;33:1-7. doi: 10.1016/j.breast.2017.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rai A, Chawla N, Han X, et al. Has the quality of patient-provider communication about survivorship care improved? J Oncol Pract. 2019;15(11):e916-e924. doi: 10.1200/JOP.19.00157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Runowicz CD, Leach CR, Henry NL, et al. American Cancer Society/American Society of Clinical Oncology Breast Cancer Survivorship Care Guideline. CA Cancer J Clin. 2016;66(1):43-73. doi: 10.3322/caac.21319 [DOI] [PubMed] [Google Scholar]
- 35.Neumann F, Jégu J, Mougin C, et al. Risk of second primary cancer after a first potentially-human papillomavirus-related cancer: a population-based study. Prev Med. 2016;90:52-58. doi: 10.1016/j.ypmed.2016.06.041 [DOI] [PubMed] [Google Scholar]
- 36.Mukhtar F, Ilozumba M, Utuama O, Cimenler O. Change in pattern of secondary cancers after Kaposi sarcoma in the era of antiretroviral therapy. JAMA Oncol. 2018;4(1):48-53. doi: 10.1001/jamaoncol.2017.2395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Berrington de Gonzalez A, Kutsenko A, Rajaraman P. Sarcoma risk after radiation exposure. Clin Sarcoma Res. 2012;2(1):18. doi: 10.1186/2045-3329-2-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bryant AK, Banegas MP, Martinez ME, Mell LK, Murphy JD. Trends in radiation therapy among cancer survivors in the United States, 2000-2030. Cancer Epidemiol Biomarkers Prev. 2017;26(6):963-970. doi: 10.1158/1055-9965.EPI-16-1023 [DOI] [PubMed] [Google Scholar]
- 39.Yabroff KR, Lund J, Kepka D, Mariotto A. Economic burden of cancer in the United States: estimates, projections, and future research. Cancer Epidemiol Biomarkers Prev. 2011;20(10):2006-2014. doi: 10.1158/1055-9965.EPI-11-0650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Harlan LC, Hankey BF. The surveillance, epidemiology, and end-results program database as a resource for conducting descriptive epidemiologic and clinical studies. J Clin Oncol. 2003;21(12):2232-2233. doi: 10.1200/JCO.2003.94.023 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods.
eFigure 1A. Excess Absolute Risk of Developing Subsequent Primary Cancers Among 5-Year Cancer Survivors by Sex
eFigure 1B. Excess Absolute Risk of Dying From Subsequent Primary Caners Among 5-Year Cancer Survivors by Sex
eFigure 2. Standardized Incidence Ratios for the Risk of Subsequent Primary Cancer (A, B) and Standardized Mortality Ratios for the Risk of Dying From a Subsequent Primary Cancer (C, D) With Statistically Significant Lower-Than-Expected Values by Sex
eFigure 3. Risk of Developing Any Alcohol- or Infection-Related Subsequent Primary Cancers Among Survivors of Prior Alcohol- or Infection-Related Cancers
eFigure 4. Ratios of Standardized Incidence Ratios for Select Subsequent Primary Cancer Types Among 5-Year Cancer Survivors by First Primary Cancer Diagnosis Year (1975-1989, 1990-1999, 2000-2011) in 9 SEER Registries
eTable 1. Characteristics Of The Survivors Who Were Diagnosed With An Invasive First Primary Cancer From 1975-2011 At Ages 20-84 And Who Survived For 5 Years Or Longer in 9 SEER registries
eTable 2. Classification Of Cancer Types Based on ICD-O-3 Site and Type Code and Definitions of The Same-Type Cancers
eTable 3. Definitions and Classification of Smoking-, Obesity-, Alcohol-, and Infection-Related Cancers
eTable 4. Number of Survivors and Person-Years of Follow-Up Included in the Mortality Analysis by First Primary Cancer Sites and Sex
eTable 5. Risks of Developing or Dying from Subsequent Primary Cancers Among 5-Year Survivors of Other and Unspecified Primary Cancer Sites by Sex
eTable 6. Risks of Developing Specific Type of Subsequent Primary Cancer Among 5-Year Cancer Survivors in 12 SEER Registries: Incidence, Percent Contribution to the Total Incidence, Standardized Incidence Ratio by First Primary Cancer Types and Sex
eTable 7. Risks of Dying from a Specific Type of Subsequent Primary Cancer Among 5-Year Cancer Survivors in 12 SEER Registries: Mortality, Percent Contribution to the Total Mortality, Standardized Mortality Ratio by First Primary Cancer Types and Sex
eTable 8. Percentage Contribution of Each Subsequent Primary Cancer Types to the Total Incidence of Subsequent Primary Cancer and to the Total Subsequent Primary Cancer Mortality Among All 5-Year Cancer Survivors by Sex in 12 SEER Registries
eTable 9. Risk of Developing Any Smoking- or Any Obesity-Related Subsequent Primary Cancers Among Smoking or Obesity-Related Cancer Survivors
eTable 10. Standardized Incidence Ratios and Ratios of Standardized Ratios by Diagnosis Year (1975-1989, 1990-1999, 2000-2011) for the Risk of Select 6 Subsequent Primary Cancers Among 5-Year Survivors of 16 First Primary Cancer Types in 9 SEER Registries
eTable 11. Results Based on a Single Outcome Analysis for the Risk of Developing Subsequent Primary Cancer Overall Among 5-Year Cancer Survivors in 12 SEER Program Registries
eTable 12. Results Based on the Earlier Start of Follow-Up (Since 1 Year From First Primary Cancer Diagnosis) for the Risk of Developing Subsequent Primary Cancer Overall Among 1-Year Cancer Survivors in the 12 SEER Registries
eReferences.


