Abstract
Background
Migraine preventive medications are used to reduce headache frequency, severity, and duration. In patients with chronic migraine (CM), reversion to episodic migraine (EM) is an important treatment goal.
Objective
To evaluate the effect of fremanezumab on the rate of reversion from CM to EM.
Methods
This phase 3, randomized, double‐blind, placebo‐controlled, parallel‐group trial included a 28‐day pretreatment period and a 3‐month treatment period. Patients with CM received subcutaneous fremanezumab quarterly (675 mg at baseline) or monthly (675 mg at baseline; 225 mg at Weeks 4 and 8), or placebo. Post hoc analyses evaluated the proportion of patients who reverted from CM to EM, defined as either a reduction to an average of <15 headache days per month during the 3‐month treatment period or a reduction to <15 headache days per month in all 3 months of the treatment period.
Results
This analysis included data from 1088 CM patients (quarterly, n = 366; monthly, n = 365; placebo, n = 357). More fremanezumab‐treated patients with CM reverted to EM using either the monthly average number of headache days criteria for reversion (quarterly: 50.5% [185/366], P = .108; monthly: 53.7% [196/365], P = .012; vs placebo: 44.5% [159/357]) or the monthly headache day count at Months 1, 2, and 3 criteria for reversion (quarterly: 31.2% [114/366], P = .008; monthly: 33.7% [123/365], P = .001; vs placebo: 22.4% [80/357]). Patients with CM who reported previous topiramate or onabotulinumtoxinA use, concomitant preventive medication use, or medication overuse were less likely to revert to EM.
Conclusions
Fremanezumab may offer the benefit of reversion from CM to EM, based on a reduction in the number of headache days over 3 months of treatment.
Keywords: migraine, all headache, all pain, clinical trials randomized controlled
Introduction
Migraine affects approximately 1 billion people and is the second‐leading cause of years lived with disability worldwide. 1 Migraine is classified as episodic migraine (EM), <15 headache days per month; or chronic migraine (CM), ≥15 headache days per month for >3 months, with ≥8 migraine days. 2 CM and EM have distinct features beyond headache frequency. Compared with EM, CM generally results in higher rates of disability, allodynia, medication overuse, and comorbidities, as well as greater reductions in health‐related quality of life (HRQoL). 3 , 4 , 5 , 6 , 7 The burden of CM extends to society, with greater direct and indirect medical costs. 8
Migraine preventive medication may be recommended to reduce headache frequency, severity, and duration in patients with CM. 9 As a natural course of the disease, CM may revert to EM, with estimated reversion rates of 15% (3‐month estimate in hospitalized patients) to 26% (2‐year rate based on population survey). 10 , 11 While headache frequency varies from month to month within individuals, 12 reversion from CM to EM represents an important treatment goal that may reduce disability and improve HRQoL. Reversion, however, is not clearly defined in the literature; reversion has been defined based on varying criteria (eg, International Classification of Headache Disorders [ICHD] criteria for CM and EM, study‐specified monthly reductions) evaluated at disparate or unidentified time points. 10 , 11
Here, we report a post hoc analysis of reversion from CM to EM using data from the phase 3 HALO CM trial of fremanezumab, a fully humanized monoclonal antibody (IgG2Δa) that selectively targets calcitonin gene‐related peptide and is approved in the United States, the European Union, and Australia for the preventive treatment of migraine in adults. 13 , 14 , 15 Reversion from CM to EM was examined using 2 definitions. First, reversion from CM to EM was defined based on the ICHD diagnostic criteria for a patient having CM vs having EM (≥15 headache days at baseline [28‐day pretreatment period] and having a monthly average of <15 headache days during the 3‐month treatment period). Given the lack of a clear definition for reversion from CM to EM, a second, more stringent, definition for reversion from CM to EM was also used. This was defined as ≥15 headache days in the baseline and <15 headache days during each month of the treatment period (Months 1, 2, and 3). We hypothesized that in CM patients, fremanezumab treatment would be associated with significant reductions in the frequency of headache and migraine days and a reversion from a CM classification to an EM classification.
Methods
Standard Protocol Approvals, Registrations, and Patient Consents
This trial was conducted in accordance with the study protocol of HALO CM (ClinicalTrials.gov identifier NCT02621931) and the International Conference for Harmonization guidelines for Good Clinical Practice, the Declaration of Helsinki, and relevant national and local regulations. The study protocol was approved by relevant ethics committees and institutional review boards. Informed written consent was obtained from each participant before any study procedure or assessments were performed.
All authors had access to the data and approved the final version of the manuscript prior to submission.
Patient Selection
The study design and patient selection criteria have been previously described. 16 Briefly, this was a randomized, double‐blind, placebo‐controlled, parallel‐group phase 3 clinical trial comprising a screening visit, a 28‐day pretreatment period, and a 3‐month treatment period, with a final evaluation at the end of Month 3.
Patients were eligible for participation in the study if they were 18‐70 years old, had a history of migraine (ICHD‐3 beta criteria) for ≥12 months, and had prospectively confirmed CM (headache on ≥15, and ≥8 days meeting ICHD‐3 beta criteria for migraine) during the 28‐day pretreatment period. Up to 30% of patients using a stable dosage of 1 preventive migraine medication for at least 2 months before the beginning of the pretreatment period were allowed to continue these medications. Patients were excluded from study participation if they had used onabotulinumtoxinA at any time during the 4 months preceding the screening visit, had received treatment with migraine interventions or devices such as nerve blocks or transcranial magnetic stimulation at any time during the 2 months before screening, used an opioid or barbiturate on >4 days during the pretreatment period, or had previously failed 2 or more out of 4 clusters of migraine‐preventive agents, as described in the study protocol.
Treatment and Evaluation
After the pretreatment period, patients were randomized 1:1:1 to 1 of 3 groups: fremanezumab quarterly (675 mg at baseline, and placebo at study visits for Weeks 4 and 8), fremanezumab monthly (675 mg at baseline, and 225 mg at study visits for Weeks 4 and 8), or placebo (at baseline, study visits for Weeks 4 and 8). Headache data, including occurrence, headache hours, pain severity, and migraine symptoms were captured using an electronic diary device.
Endpoints and Assessment
The primary endpoint was the mean change from baseline (the 28‐day pretreatment period) in the monthly average number of headache days of at least moderate severity during the 3‐month treatment period. A headache day of at least moderate severity was defined as a calendar day with ≥4 consecutive hours of headache pain and peak severity of at least a moderate level, or a day when acute migraine‐specific medication (triptan or ergot) was used to treat a headache of any severity or duration. A calendar day was considered a migraine day if headache pain lasted for at least 4 consecutive hours and met criteria for migraine or probable migraine, or if acute migraine‐specific medication (triptans or ergots) was used to treat a headache of any duration.
Reversion Status From CM to EM and Headache Days
Post hoc analyses were performed to determine the proportion of patients who reverted from CM to EM (CM‐EM) in each treatment group. Reversion was defined in 2 ways, first as having ≥15 headache days during the 28‐day pretreatment run‐in period (baseline) and having a monthly average of <15 headache days during the 3‐month period following initial treatment. The second, more stringent, definition required ≥15 headache days at baseline and <15 headache days each month of the treatment period (Months 1, 2, and 3). Patients who had ≥15 headache days at baseline but did not meet the criteria for reversion according to the definition used were considered to have not reverted (CM‐CM).
Baseline Demographics, Definition of Medication Overuse, and Reversion Status
To identify characteristics associated with CM to EM reversion, patients were stratified by their reversion status (defined using the monthly average number of headache days criteria for reversion) and by treatment group, and baseline demographic and clinical characteristics (including medication overuse) were examined. Medication overuse was defined as the use of acute headache medication on ≥15 days, use of migraine‐specific acute medication on ≥10 days, or use of combination medications for headache on ≥10 days based on ICHD‐3 criteria. 17 The mean change from baseline in the monthly average number of migraine days during the 3‐month treatment period was evaluated for patients who reverted or did not revert based on each reversion criteria (ie, monthly average over 3 months or monthly count at all 3 months).
Statistical Analyses
For the original study, a sample size of 867 patients was estimated to provide at least 90% power to detect a mean (standard deviation [SD]) difference of 1.7 (6.3) in the average number of headache days per month between monthly fremanezumab and placebo groups (2‐sided alpha level of .05). Assuming a 15% discontinuation rate, 1020 patients were planned for randomization in the trial. The analyses in this study were conducted in the full analysis set (FAS), which included all randomized patients who had received at least 1 dose of study drug and had at least 10 days of post‐baseline assessments. Assumptions required to interpret the statistics have been verified. For all efficacy endpoints using normal theory‐based methods in the HALO study, the normality assumption was checked using visual inspections of Q‐Q plots and histograms, as well as the Shapiro‐Wilk test. Where the validity of the assumption was suspected (for 2 endpoints), nonparametric method was used as a sensitivity analysis. As expected from the large‐sample normal approximation theory, the results from the sensitivity analyses and the main analyses were consistent, demonstrating the robustness of study results based on means and large‐sample normality approximations. Descriptive statistics (frequencies; means; SDs; proportions) were used to characterize the patients by reversion (CM‐EM) or no reversion (CM‐CM) based on the treatment group. Treatment differences in the percentages of responders and means of percent changes from baseline were calculated with corresponding 95% confidence intervals (CI). Patients who discontinued early were not imputed for reversion when using the monthly average number of headache days criteria or the monthly headache day count at Months 1, 2, and 3 criteria. A value of P < .05 was considered significant, and tests are 2‐tailed. All summaries and statistical analyses were generated using SAS® software (Version 9.4 of SAS System for Windows, SAS Institute Inc., Cary, NC, USA).
Data Availability Statement
Anonymized data will be shared upon request from any qualified investigator.
Results
Patients
A total of 1130 patients were randomly assigned to 1 of the 3 treatment groups (fremanezumab quarterly, n = 376; fremanezumab monthly, n = 379; placebo, n = 375). Of these patients, 1121 patients (fremanezumab quarterly, n = 375; fremanezumab monthly, n = 375; placebo, n = 371) were included in the FAS. This analysis included data from 1088 CM patients (fremanezumab quarterly, n = 366; fremanezumab monthly, n = 365; placebo, n = 357). As previously reported, baseline demographics and clinical characteristics were similar across all 3 treatment groups. 16 Overall, 21% (239/1130) of patients enrolled had been using a stable dosage of 1 migraine preventive medication for at least 2 months before the beginning of the pretreatment period and were allowed to continue this medication during the study. For the current post hoc analysis, 1088 patients were included, with 540 CM‐EM patients and 548 CM‐CM patients based on the monthly average number of headache days criteria for reversion (Table 1). Overall, of those who reverted from CM to EM, 18% (98/540) reported stable concomitant preventive medication use at baseline, whereas 24% (132/548) CM‐CM patients did.
Table 1.
Baseline Characteristics of Patients by Treatment Group and Reversion Status†
| Characteristic | Total | Fremanezumab | Placebo | |||||
|---|---|---|---|---|---|---|---|---|
| Quarterly | Monthly | |||||||
| CM–CM n = 548 | CM–EM n = 540 | CM–CM n = 181 | CM–EM n = 185 | CM–CM n = 169 | CM–EM n = 196 | CM–CM n = 198 | CM–EM n = 159 | |
| Age, mean (SD), year | 41.8 (12.3) | 41.1 (12.0) | 42.7 (12.6) | 40.9 (12.2) | 41.4 (12.2) | 40.7 (11.7) | 41.3 (12.1) | 41.7 (12.1) |
| BMI, mean (SD), kg/m2 | 26.3 (5.2) | 26.7 (5.2) | 26.6 (5.2) | 26.6 (5.5) | 26.4 (5.1) | 26.6 (5.1) | 26.0 (5.2) | 27.0 (5.0) |
| Sex, female, n (%) | 479 (87) | 472 (87) | 157 (87) | 165 (89) | 145 (86) | 172 (88) | 177 (89) | 135 (85) |
| Years since initial migraine diagnosis, mean (SD) | 20.2 (13.0) | 19.8 (12.2) | 20.2 (13.5) | 19.0 (12.1) | 20.9 (12.7) | 20.0 (11.4) | 19.6 (12.9) | 20.6 (13.3) |
| Concomitant preventive medication use, n (%) | 132 (24) | 98 (18) | 43 (24) | 30 (16) | 44 (26) | 40 (20) | 45 (23) | 28 (18) |
| Any acute headache medication use, n (%) | 516 (94) | 520 (96) | 171 (94) | 178 (96) | 157 (93) | 189 (96) | 188 (95) | 153 (96) |
| Medication overuse, n (%)‡ | 314 (57) | 268 (50) | 105 (58) | 94 (51) | 95 (56) | 102 (52) | 114 (58) | 72 (45) |
| Previous onabotulinumtoxinA use, n (%) | 108 (20) | 55 (10) | 44 (24) | 21 (11) | 31 (18) | 19 (10) | 33 (17) | 15 (9) |
| Previous topiramate use, n (%) | 182 (33) | 148 (27) | 58 (32) | 47 (25) | 54 (32) | 58 (30) | 70 (35) | 43 (27) |
Based on the <15 monthly average number of headache days for the 3‐month treatment period criteria for reversion.
Medication overuse was defined as the use of acute headache medication on ≥15 days or use of migraine‐specific acute medication on ≥10 days or use of combination medications for headache on ≥10 days.
BMI = body mass index; SD = standard deviation.
A Priori Analyses of HALO CM
A priori analyses on the FAS population have been published. 16 Briefly, during the 3‐month treatment period, significant reductions from baseline (least‐squares mean change ± standard error) in the monthly average number of headache days of at least moderate severity were observed with fremanezumab (quarterly: −4.3 ± 0.3 days, monthly: −4.6 ± 0.3 days) compared with placebo (−2.5 ± 0.3 days; both comparisons, P < .001). The monthly average number of migraine days was also significantly reduced with fremanezumab (quarterly [least‐squares mean change ± standard error]: −4.9 ± 0.4 days, monthly: −5.0 ± 0.4 days) compared with placebo (−3.2 ± 0.4 days; both comparisons, P < .001).
Effect of Fremanezumab on Reversion From CM to EM and Headache Days
For reversion from CM to EM based on the monthly average number of headache days, a greater proportion of patients who received fremanezumab monthly reverted from CM to EM, compared with those who received placebo (53.7% [196/365] vs 44.5% [159/357]; difference [95% CI]: 9.2% [1.9%, 16.4%]; P = .012). A greater proportion of patients who received fremanezumab quarterly (50.5% [185/366]) reverted from CM to EM vs placebo (difference [95% CI]: 6.0% [−1.3%, 13.3%]; P = .108). The mean percentage change in the monthly average headache days in patients who reverted was −47.1% with fremanezumab quarterly (difference vs placebo [95% CI]: −6.5% [−10.5%, −2.5%]), −47.7% with fremanezumab monthly (difference vs placebo [95% CI]: −7.1% [−11.3%, −2.9%]), and −40.6% with placebo (Fig. 1).
Fig. 1.
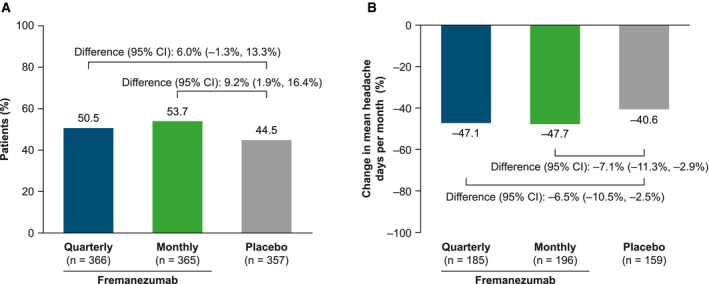
Reversion from chronic migraine (CM) to episodic migraine (EM) based on the monthly average number of headache days criteria. (A) Proportion of patients who reverted from CM to EM. (B) Percentage change in the monthly average number of headache days from baseline to Month 3 in patients who reverted from CM to EM. Missing data were not imputed.
For reversion based on the monthly headache day count at all 3 months (Months 1, 2, and 3), reversion rates were directionally similar but lower. There were higher rates of CM to EM reversion with fremanezumab (quarterly: 31.2% [114/366] patients, difference vs placebo [95% CI]: 8.7% [2.3%, 15.2%], P = .008; monthly: 33.7% [123/365] patients, difference vs placebo [95% CI]; 11.3% [4.8%, 17.8%], P = .001) compared with placebo (22.4% [80/357] patients). The mean percentage reduction in monthly average headache days in patients who reverted was 54.7% with fremanezumab quarterly (difference vs placebo [95% CI]: −5.1% [−9.9%, −0.3%]), 56.3% with fremanezumab monthly (difference vs placebo [95% CI]: −6.7% [−11.7%, −1.7%]), and 49.6% with placebo (Fig. 2). In these patients who reverted using the monthly headache day count at Months 1, 2, and 3 criteria, the monthly average number of headache days decreased from 18.0 to 8.1 days at Month 3 with fremanezumab quarterly, from 17.8 to 7.6 days with fremanezumab monthly, and 17.8 to 8.8 days with placebo.
Fig. 2.
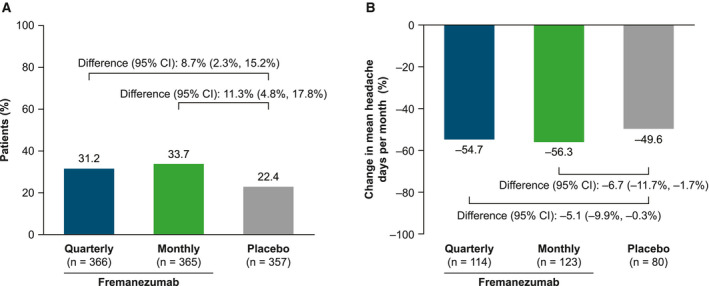
Reversion from chronic migraine (CM) to episodic migraine (EM) based on the monthly headache day count at Months 1, 2, and 3 criteria. (A) Proportion of patients in the full analysis set (FAS) population who reverted from CM to EM. (B) Percentage change in the monthly average number of headache days from baseline to Month 3 in patients who reverted from CM to EM. Missing data were not imputed.
Baseline Disease Status in CM‐EM Patients vs CM‐CM Patients
Baseline disease characteristics were examined in patients stratified by reversion status (reversion [CM‐EM] or no reversion [CM‐CM]), using the stringent definition of reversion (<15 monthly average number of headache days for the 3‐month treatment period). A total of 1088 patients were included in this analysis, with 540 CM‐EM patients and 548 CM‐CM patients. Baseline demographic characteristics (eg, age, gender, and body mass index) were similar in CM‐EM and CM‐CM patients. Average times since initial migraine diagnosis were also similar in CM‐EM (19.8 years) and CM‐CM (20.2 years) patients.
Overall, pooling across all 3 treatment groups, a higher proportion of CM‐CM patients (57%, 314/548) had medication overuse, as compared with CM‐EM patients (50%, 268/540). Among people who reverted from CM to EM, medication overuse at baseline was present in 51% (94/185), 52% (102/196), and 45% (72/159) of patients treated with fremanezumab quarterly, fremanezumab monthly, and placebo, respectively. For those who did not revert to EM (CM‐CM), medication overuse rates were 58% (105/181), 56% (95/169), and 58% (114/198) for patients treated with fremanezumab quarterly, fremanezumab monthly, and placebo, respectively.
Current and prior preventive treatment use differed between the CM‐EM and CM‐CM groups. In the total population, prior use of most preventive medications was associated with persistent CM. For example, previous use of onabotulinumtoxinA was substantially higher in CM‐CM patients (20%, 108/548) than CM‐EM patients (10%, 55/540) patients. Similarly, previous use of topiramate was more common in CM‐CM patients than in CM‐EM patients overall (total: 33% [182/548] vs 27% [148/540]) and in each treatment group. In addition, concomitant preventive medication use was more common in the CM‐CM patients than in the CM‐EM patients (total: 24% [132/548] vs 18% [98/540]) and in each treatment group.
Overall, CM‐EM patients experienced fewer monthly average headache days at baseline than CM‐CM patients. At baseline in CM‐EM patients, the monthly average number (mean ± SD) of headache days was 18.9 ± 3.2 in the quarterly group, 18.1 ± 2.9 in the monthly group, and 18.1 ± 2.6 in the placebo group; whereas CM‐CM patients experienced approximately 4 more headache days per month at baseline (quarterly: 22.3 ± 3.7; monthly: 23.1 ± 3.9; placebo: 22.5 ± 4.0) than CM‐EM patients.
Similar results were observed with monthly headache days of at least moderate severity; at baseline, CM‐EM patients experienced approximately 3 fewer headache days of at least moderate severity per month (quarterly: 12.1 ± 5.0; monthly: 11.5 ± 4.8; placebo: 11.2 ± 4.5), as compared with CM‐CM patients (quarterly: 14.4 ± 5.7; monthly: 14.7 ± 6.3; placebo: 15.1 ± 6.2) in all study arms. Similarly, CM‐EM patients experienced fewer migraine days per month at baseline (quarterly: 15.0 ± 4.3; monthly: 14.7 ± 4.0; placebo: 14.4 ± 3.8) than CM‐CM patients (quarterly: 17.6 ± 5.1; monthly: 17.9 ± 5.8; placebo: 18.2 ± 5.5).
Discussion
In the 3‐month phase 3 HALO CM trial of fremanezumab as a preventive treatment for patients with CM, both quarterly and monthly dosing resulted in significant reductions in the frequency of headache and migraine days. 16 In this post hoc analysis, reversion from CM to EM after fremanezumab treatment was assessed. A higher proportion of fremanezumab‐treated patients reverted from CM to EM relative to patients who received placebo, when reversion was defined either as <15 monthly headache days per month over 3 months on average or, more stringently, as <15 headache days during each month (measured at Months 1, 2, and 3) of the treatment period. Patients who met the criteria of the more stringent definition of reversion from CM to EM had an average reduction in monthly headache days of 55% with fremanezumab quarterly and 56% with fremanezumab monthly. Of note, patients with CM who were randomized to fremanezumab quarterly received only 1 dose of fremanezumab 675 mg. Approximately 50% of these patients had reversion from CM to EM, and one‐third met the more stringent criteria with <15 headache days at Months 1, 2, and 3. To our knowledge, reversion from CM to EM after only 1 dose of preventive migraine therapy has not previously been reported.
Using the less stringent definition of reversion, more than half of fremanezumab‐treated patients in either the quarterly (51%) or monthly (54%) treatment arms reverted from CM to EM during the 3‐month treatment period, though only fremanezumab monthly achieved statistical significance vs placebo. The findings with the monthly dose suggest that in comparison with placebo, monthly treatment with fremanezumab is associated with reversion from CM to EM. The lack of significant improvement with fremanezumab quarterly vs placebo may be due to small size limitations or the short duration of the study.
Prior data on reversion from CM to EM are limited. A study of patients with medication overuse who were hospitalized for the treatment of CM reported a reversion rate of 15%. 10 In a web‐survey of persons with CM who took various medications (nonsteroidal anti‐inflammatory drug, triptan, ergotamine, barbiturate, opiate, and preventive medications), a reversion rate of 26% was reported using a different and more restrictive definition of reversion. 11 In the current analysis, using the more stringent CM‐EM definition, the reversion rate in patients who received placebo (22%) was within the range of the previously reported CM reversion rate (15‐26%); 10 , 11 of note, a significantly higher proportion of fremanezumab‐treated patients (31‐34%) achieved reversion from CM to EM. Although the studies differed in duration and design, and therefore preclude a direct comparison, these results support the conclusion of this post hoc analysis on reversion rates from CM to EM following treatment with fremanezumab vs placebo.
In these analyses, medication overuse was associated with lower rates of reversion from CM to EM. This observation is consistent with a previous systematic review that identified medication overuse as a factor for poor outcomes from preventive treatment in chronic headache. 18 Future studies with fremanezumab should evaluate whether certain acute medications are more likely to predict failure to revert than others, though this may represent a treatment‐resistant population. Further work would be needed to elucidate the relationship between medication overuse and reversion from CM to EM. Fewer monthly average headache days at baseline was strongly correlated with reversion from CM to EM, indicating that baseline headache days is a potential predictor of reversion.
Although this study was not designed to identify predictors of reversion of CM to EM, across all treatment arms, more patients from the CM‐CM vs the CM‐EM groups had previously used onabotulinumtoxinA or topiramate or were concomitantly using preventive medications. Perhaps patients who tried and failed previous preventives had more refractory migraine and a lower reversion rate. Further studies are needed to characterize the natural history of CM and to identify predictors of CM to EM reversion, including ascertaining which patients are more likely to benefit from fremanezumab and other preventive therapies and potentially experience reversion.
This analysis has several limitations, including the relatively brief duration of the study and the small sample size. Because a key finding of the CaMEO Study is that the migraine classification of a patient fluctuated over the course of 1 year, 12 data from a long‐term clinical study may help to better understand the durability of CM to EM reversion resulting from fremanezumab treatment. 19 Other limitations are those inherent to the post hoc analysis and to the imprecisely defined nature of reversion.
Classification of Evidence
This randomized, placebo‐controlled study provides Class II evidence 20 that fremanezumab, in both quarterly and monthly subcutaneous dosing regimens, is associated with reversion from CM to EM. Greater proportions of patients treated with fremanezumab quarterly or monthly reverted from CM to EM compared with placebo, when reversion was defined as ≥15 headache days at baseline (28‐day pretreatment period) and either a monthly average of <15 headache days during the 3‐month treatment period (quarterly: 50.5%, difference vs placebo [95% CI]: 6.0% [−1.3%, 13.3%]; monthly: 53.7%, difference vs placebo [95% CI]: 9.2% [1.9%, 16.4%], or <15 headache days per month at Months 1, 2, and 3 (quarterly: 31.2%, difference vs placebo [95% CI]: 8.7% [2.3%, 15.2%]; monthly: 33.7%, difference vs placebo [95% CI]: 11.3% [4.8%, 17.8%]).
Conclusion
Fremanezumab is efficacious as a preventive therapy for CM, based on significantly reduced frequency of headache and migraine days compared with placebo. An additional clinical benefit of fremanezumab treatment may be the potential for reversion from a CM to an EM classification, a clinically relevant goal of preventive therapy.
Statement of Authorship
Category 1
(a) Conception and Design
Joshua M. Cohen, Kristen Bibeau, Maja Galic, Michael J. Seminerio, Verena Ramirez Campos
(b) Acquisition of Data
Joshua M. Cohen, Kristen Bibeau, Maja Galic, Michael J. Seminerio, Verena Ramirez Campos
(c) Analysis and Interpretation of Data
Richard B. Lipton, Joshua M. Cohen, Kristen Bibeau, Maja Galic, Michael J. Seminerio, Verena Ramirez Campos, Rashmi B. Halker Singh, Jessica Ailani
Category 2
(a) Drafting the Manuscript
Richard B. Lipton, Joshua M. Cohen, Kristen Bibeau, Maja Galic, Michael J. Seminerio, Verena Ramirez Campos, Rashmi B. Halker Singh, Jessica Ailani
(b) Revising It for Intellectual Content
Richard B. Lipton, Joshua M. Cohen, Kristen Bibeau, Maja Galic, Michael J. Seminerio, Verena Ramirez Campos, Rashmi B. Halker Singh, Jessica Ailani
Category 3
(a) Final Approval of the Completed Manuscript Richard B. Lipton, Joshua M. Cohen, Kristen Bibeau, Maja Galic, Michael J. Seminerio, Verena Ramirez Campos, Rashmi B. Halker Singh, Jessica Ailani
Acknowledgments
We thank the patients who have participated in this study and their families; all investigators, site personnel, and the coordinating investigators; and Norah Yudong Yao, PhD (Chameleon Communications International with funding from Teva Pharmaceutical Industries Ltd.) for editorial assistance in the preparation of this report. We also thank Ronghua Yang (Teva Pharmaceuticals, Frazer, PA, USA) and Kristen Bibeau (former employee of Teva Branded Pharmaceutical Products R&D, Inc, USA) for conducting statistical analysis.
Conflict of Interest: Richard B. Lipton: Edwin S. Lowe Professor of Neurology at the Albert Einstein College of Medicine in New York; receives research support from the NIH: 2PO1 AG003949 (program director), 5U10 NS077308 (PI), RO1 NS082432 (investigator), 1RF1 AG057531 (site PI), RF1 AG054548 (investigator), 1RO1 AG048642 (investigator), R56 AG057548 (investigator), K23 NS09610 (mentor), K23 AG049466 (mentor), 1K01 AG054700 (mentor); also receives support from the Migraine Research Foundation and the National Headache Foundation; serves on the editorial board of Neurology, as senior advisor of Headache, and as associate editor of Cephalalgia; has reviewed for the NIA and NINDS; holds stock options in eNeura Therapeutics and Biohaven Holdings; serves as consultant, advisory board member, or has received honoraria from American Academy of Neurology, Alder, Allergan, American Headache Society, Amgen, Autonomic Technologies, Avanir, Biohaven, Biovision, Boston Scientific, Dr. Reddy’s, ElectroCore, Eli Lilly, eNeura Therapeutics, GlaxoSmithKline, Merck, Pernix, Pfizer, Supernus, Teva, Trigemina, Vector, Vedanta; receives royalties from Wolff’s Headache 7th and 8th Edition, Oxford University Press, 2009, Wiley, Informa. Joshua M. Cohen: Employee of Teva Branded Pharmaceutical Products R&D, Inc. (USA). Kristen Bibeau: Former employee of Teva Branded Pharmaceutical Products R&D, Inc. (USA). Maja Galic: Employee of Teva Branded Pharmaceutical Products R&D, Inc. (NL). Michael J. Seminerio: Employee of Teva Branded Pharmaceutical Products R&D, Inc. (USA). Verena Ramirez Campos: Employee of Teva Branded Pharmaceutical Products R&D, Inc. (USA). Rashmi B. Halker Singh: Received honoraria from Current Neurology and Neuroscience Reports, MedLink, Supernus, Eli Lilly, and Amgen. Jessica Ailani: Received honoraria from Allergan (speaker/consultant, clinical trial), Eli Lilly (speaker/consultant, clinical trial), Teva Pharmaceuticals (speaker/consultant), Promius (speaker/consultant), Biohaven (consultant, clinical trial), Zosano (consultant), Revance (consultant), Current Pain and Headache Reports (section editor), ElectroCore (speaker/consultant), Amgen (speaker/consultant), Alder (speaker/consultant), Impel (consultant), Satsuma (consultant), Miller Communications (consultant), AlphaSights consulting (consultant), Aptus (consultant), Neurology Live (speaker), and ARMR (clinical trial).
Funding: This study was funded by Teva Pharmaceutical Industries Ltd., Petach Tikva, Israel.
ClinicalTrials.gov identifier: NCT02621931.
References
- 1. GBD 2016 Disease and Injury Incidence and Prevalence Collaborators . Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990‐2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390:1211‐1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Burstein R, Noseda R, Borsook D. Migraine: Multiple processes, complex pathophysiology. J Neurosci. 2015;35:6619‐6629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bigal ME, Ashina S, Burstein R, et al. Prevalence and characteristics of allodynia in headache sufferers: A population study. Neurology. 2008;70:1525‐1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lipton RB. Tracing transformation: Chronic migraine classification, progression, and epidemiology. Neurology. 2009;72:S3‐S7. [DOI] [PubMed] [Google Scholar]
- 5. Bigal ME, Serrano D, Reed M, Lipton RB. Chronic migraine in the population: Burden, diagnosis, and satisfaction with treatment. Neurology. 2008;71:559‐566. [DOI] [PubMed] [Google Scholar]
- 6. Buse DC, Manack A, Serrano D, Turkel C, Lipton RB. Sociodemographic and comorbidity profiles of chronic migraine and episodic migraine sufferers. J Neurol Neurosurg Psychiatry. 2010;81:428‐432. [DOI] [PubMed] [Google Scholar]
- 7. Aurora SK, Brin MF. Chronic migraine: An update on physiology, imaging, and the mechanism of action of two available pharmacologic therapies. Headache. 2017;57:109‐125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lanteri‐Minet M. Economic burden and costs of chronic migraine. Curr Pain Headache Rep. 2014;18:385. [DOI] [PubMed] [Google Scholar]
- 9. American Headache Society . The American Headache Society position statement on integrating new migraine treatments into clinical practice. Headache. 2019;59:1‐18. [DOI] [PubMed] [Google Scholar]
- 10. Ferrari A, Leone S, Vergoni AV, et al. Similarities and differences between chronic migraine and episodic migraine. Headache. 2007;47:65‐72. [DOI] [PubMed] [Google Scholar]
- 11. Manack A, Buse DC, Serrano D, Turkel CC, Lipton RB. Rates, predictors, and consequences of remission from chronic migraine to episodic migraine. Neurology. 2011;76:711‐718. [DOI] [PubMed] [Google Scholar]
- 12. Serrano D, Lipton RB, Scher AI, et al. Fluctuations in episodic and chronic migraine status over the course of 1 year: Implications for diagnosis, treatment and clinical trial design. J Headache Pain. 2017;18:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hoy SM. Fremanezumab: First global approval. Drugs. 2018;78:1829‐1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. AJOVY® (fremanezumab) [prescribing information]. In: Teva Pharmaceuticals USA, Inc.; 2018. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/761089s000lbl.pdf. [Google Scholar]
- 15. AJOVY (fremanezumab) [Summary of Product Characteristics]. In: Teva Pharmaceuticals GmbH; 2019. https://www.ema.europa.eu/en/documents/product‐information/ajovy‐epar‐product‐information_en.pdf. [Google Scholar]
- 16. Silberstein SD, Dodick DW, Bigal ME, et al. Fremanezumab for the preventive treatment of chronic migraine. N Engl J Med. 2017;377:2113‐2122. [DOI] [PubMed] [Google Scholar]
- 17. Headache Classification Committee of the International Headache Society . The International Classification of Headache Disorders, 3rd edition. Cephalalgia. 2013;33:629‐808. [DOI] [PubMed] [Google Scholar]
- 18. Probyn K, Bowers H, Caldwell F, et al. Prognostic factors for chronic headache: A systematic review. Neurology. 2017;89:291‐301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Efficacy and Safety of Subcutaneous Administration of TEV‐48125 for the Preventive Treatment of Migraine (HALO) – Full Text View – ClinicalTrials.gov. 2018. https://clinicaltrials.gov/ct2/show/NCT02638103.
- 20. Gronseth GS, Cox J, Gloss D, et al. Clinical Practice Guideline Process Manual, 2017 Edition. Minneapolis, MN: The American Academy of Neurology; 2017. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data will be shared upon request from any qualified investigator.


