Abstract
Background
There is no treatment for cancer‐related cognitive impairment, an important adverse effect that negatively impacts quality of life (QOL). We conducted a 3‐arm randomized controlled trial to evaluate the impact of computer‐assisted cognitive rehabilitation (CR) on cognition, QOL, anxiety, and depression among cancer patients treated with chemotherapy.
Methods
Patients who reported cognitive complaints during or after completing chemotherapy were randomly assigned to 1 of 3 12‐week CR programs: computer‐assisted CR with a neuropsychologist (experimental group A), home cognitive self‐exercises (active control group B), or phone follow‐up (active control group C). Subjective cognition was assessed by the Functional Assessment of Cancer Therapy–Cognitive Function (FACT‐Cog), objective cognition was assessed by neuropsychological tests, QOL was assessed by the FACT‐General, and depression and anxiety were assessed by psychological tests. The primary endpoint was the proportion of patients with a 7‐point improvement in the FACT‐Cog perceived cognitive impairment (PCI) score.
Results
Among the 167 enrolled patients (median age, 51 years), group A had the highest proportion of patients with a 7‐point PCI improvement (75%), followed by groups B (59%) and C (57%), but the difference was not statistically significant (P = .13). Compared with groups B and C, the mean difference in PCI score was significantly higher in group A (P = .02), with better perceived cognitive abilities (P < .01) and a significant improvement in working memory (P = .03). Group A reported higher QOL related to cognition (FACT‐Cog QOL) (P = .01) and improvement in depression symptoms (P = .03).
Conclusions
These results suggest a benefit of a computer‐based CR program in the management of cancer‐related cognitive impairment and complaints.
Keywords: cancer, chemotherapy, cognitive impairment, cognitive rehabilitation, supportive care
Short abstract
In this 3‐arm randomized clinical trial of 167 patients, computer‐assisted cognitive rehabilitation improved cognitive complaints, with significant improvement in working memory, quality of life related to cognition, and depression symptoms. Computer‐assisted cognitive rehabilitation is a compelling approach toward the management of cancer‐related cognitive impairment and complaints.
Introduction
Cancer‐related cognitive impairment (CRCI) affects various domains of cognition, such as working memory, attention, and executive function. 1 , 2 This phenomenon is known as “chemobrain,” and it has been studied primarily in breast cancer patients. 3 Cancer‐related objective cognitive dysfunction affects approximately 30% of cancer patients, but cognitive complaints comprise one of the most important symptoms reported. 4 Up to 75% of cancer patients report cognitive complaints during or after chemotherapy, according to the few longitudinal studies conducted. 4 , 5 , 6 , 7 , 8 Due to improvements in therapeutic management and life expectancy among long‐term cancer survivors, the negative impact of chemobrain on posttreatment quality of life (QOL) remains an emerging area of research, and there is a high demand among patients for specific management of these symptoms. 4 Currently, no medication has been clearly established to prevent or manage CRCI, and the benefits of cognitive intervention programs need to be confirmed. 9 Despite the lack of standardized methods and large studies, cognitive intervention programs appear promising, with some demonstrating improvements in cognitive complaints. 10 , 11 , 12 , 13 However, additional data are required to establish the efficacy of such programs, particularly because the few randomized studies that have been performed had small samples and/or no active control group. 12 , 14 , 15 , 16 , 17 , 18 Moreover, some of the intervention studies are cognitive training programs. By definition, cognitive rehabilitation (CR) includes not only cognitive training, but also individualized intervention that focuses on a particular patient's needs and goals.
We conducted a 3‐arm randomized controlled trial (RCT) with 2 active control arms to evaluate the impact of computer‐assisted CR on subjective and objective cognition, QOL, anxiety, and depression among cancer patients treated with chemotherapy and reporting cognitive complaints.
Materials and Methods
Participants
This longitudinal, multicenter RCT recruited patients from 5 French centers. Eligible patients were diagnosed with solid or hematological cancer, were ≤18 years of age, had been treated with sequential chemotherapy, were in remission or in the midst of a therapeutic break, reported cognitive complaint (during or within 5 years of chemotherapy completion), and were able to read and write French fluently. Exclusion criteria were as follows: known primary or secondary central nervous system tumor, history of childhood cancer, noncontrolled psychiatric disorders, inability to perform cognitive tests, documented alcohol or drug abuse, and use of opioids or grade 3 analgesics. Patients were recruited during medical consultations (chemotherapy, supportive care, or monitoring with their doctor), and if a cognitive complaint was identified, they were referred to a neuropsychologist at their center. Only those with a Functional Assessment of Cancer Therapy–Cognitive Function (FACT‐Cog) QOL subscale score of ≥4/16 were selected to ensure that patients with complaints impacting their QOL were included in the study.
All participants provided written informed consent. This RCT was approved by a local ethics committee and the French Health Authority (ID‐RCB: 2011‐A00077‐34) and was conducted according to the provisions of the Declaration of Helsinki.
Study Design
Participants were randomly assigned using nQuery's mixed block size in a 1:1:1 ratio to computer‐assisted CR with a neuropsychologist (experimental group A) or 1 of the 2 active control groups: home cognitive self‐exercises (control group B) or phone follow‐up (control group C). Randomization was stratified according to time since chemotherapy completion (1‐18 months and 18 months to 5 years), type of tumor, and institution.
Experimental group A: computer‐assisted cognitive rehabilitation with a neuropsychologist
Computer‐assisted CR was performed using RehaCom software (http://www.rehacom.fr), a modular interactive program designed to train cognitive abilities. The system design includes compensatory strategies, controlled stimuli, and immediate feedback to train and improve attention, memory, visuospatial processing, and executive functions, all of which are usually impacted by chemotherapy. The neuropsychologist could choose some modules according to the specific deficits of each patient, meaning that deficits could be targeted and specifically trained. The program is autoadaptive, so the activity could become easier or more difficult depending on the performance of the patient. Participants completed 9 standardized sessions (45‐60 minutes) over 3 months at their institution in the presence of a neuropsychologist who could advise and encourage them during the exercises and give them feedback at the end of the session on the progress made.
Active control group B: home cognitive self‐exercises
Participants were given a workbook and were instructed to perform the exercises therein for 9 standardized sessions (30‐60 minutes per week) over 3 months. The workbook proposed 8 different exercises to work different cognitive domains, including memory and executive functions. Participants were called every month to ensure that the exercises were performed and to provide motivation for completing them. The completed workbook was collected at the end of the CR program.
Active control group C: phone follow‐up
Participants were called 9 times over 3 months; standardized questions were used to collect information on the evolution of cognitive disorders and their impact on each participant's daily life.
Measurements
Subjective cognitive assessment
Cognitive complaints were evaluated using the French version of the FACT‐Cog, which had good internal consistency reliability (Cronbach's α = 0.74‐0.91). 19 , 20 This 37‐item self‐administered questionnaire evaluates memory, attention, concentration, language, and thinking abilities in cancer patients 21 and contains 4 subscales: perceived cognitive impairment (PCI) (Chronbach's α = 0.91), perceived cognitive abilities (PCA) (Chronbach's α = 0.90), impact on QOL (Chronbach's α = 0.88), and comments from others (Chronbach's α = 0.74). It includes negatively and positively worded items. Negatively worded items are reverse‐scored to create subscale scores, with higher scores reflecting fewer cognitive problems and better QOL. FACT‐Cog PCI score was used as the primary measure of subjective cognition.
Objective cognitive assessment
A neuropsychologist performed a standardized battery of tests evaluating the following cognitive domains: Grober and Buschke test for anterograde episodic memory, 22 D2 test for attention and concentration, 23 verbal fluency test, 24 Trail Making Test for executive functioning and processing speed, 25 and Wechsler Adult Intelligence Scale IV (WAIS IV) for working memory and short‐term memory. 26
Quality of life and fatigue assessment
QOL was evaluated using the 28‐item FACT‐General (Chronbach's α = 0.9 for the global FACT‐G scale and >0.75 for subscales) 27 which assesses four dimensions of QOL (physical, functional, emotional, and social well‐being). 28 Symptoms of fatigue were measured with the 20‐item FACT‐Anemia (Chronbach's α = 0.96). 29
Anxiety and depression assessment
The Spielberger State‐Trait Anxiety Inventory 30 and the Center for Epidemiologic Studies Depression Scale 31 were used to assess anxiety and depression, respectively, with higher scores representing higher levels of anxiety or depression.
Data Collection
Subjective and objective cognitive assessments were completed at baseline (T0) and at the end of the CR program 3 months later (T3) at the center with the neuropsychologist. A subjective cognitive assessment was also completed 1 month (T1) and 2 months (T2) after initiating the CR program.
Endpoints
The primary endpoint was the proportion of participants showing a 7‐point improvement in the FACT‐Cog PCI score (range, 0‐72) in group A compared with groups B or C, between T0 and T3. The choice of 7 points is based on some data, and a variation of 10% seems clinically important. 32 Data from 24 (14%) participants could not be analyzed for the primary endpoint because cognitive complaints were not assessed at T3 for 7 (13%) participants from group A, 10 (21%) participants from group B, and 5 (9%) participants from group C.
The main secondary endpoints were: improvement of objective cognition and QOL. The relationships between subjective and objective cognition and between cognitive impairment and QOL, as well as the evolution of cognitive scores, were also evaluated.
Statistical Analysis
Sample size calculations were based on 2‐by‐2 comparisons of the primary endpoint, a 7‐point improvement in FACT‐Cog PCI score between T0 and T3. The bilateral Fisher's exact test at α = 0.05/3 = 0.017 and β = 0.2 was considered to account for multiple test correction. We assumed that the proportion of participants with a 7‐point improvement in the FACT‐Cog PCI score would be 75% in experimental group A and 40% and 5% in control groups B and C, respectively. In this context, 46 patients per group were required. To compensate for potential dropouts, we increased this number by 20% and planned to include 56 patients per group, for a total of 168 patients. The Kolmogorov‐Smirnov test was used to test for normality of the distributions. Normally distributed continuous data were expressed as mean values (±SD). Differences between groups were analyzed for statistical significance using a paired t test, Kruskal‐Wallis test, chi‐square test, or Fisher's exact test as appropriate. Categorical variables were reported as percentages. We further used a repeated measures mixed model, with the patient as the “cluster variable,” using the normally distributed PCI as the main outcome and the group and interaction group*time to reflect the changes over time. The model was stratified by center and contrast, and margins were used in Stata version 15 (Stata Corp., College Station, Texas). We performed complete case analyses after describing the pattern of missing data.
Results
Between September 2012 and July 2017, 167 patients were enrolled in the RCT: 55 in group A, 56 in group B, and 56 in group C (Fig. 1). Baseline demographic and clinical characteristics were well balanced between the 3 groups, without statistically significant differences (Table 1). The median age was 51 years, 96% of participants were women, and 140 (84%) participants had breast cancer, including 98 (70%) with previous hormone therapy. Only 15 (9%) participants had metastatic disease. The median time from chemotherapy completion was 10.6 months (range, 6.0‐18.2 months). The completion rate of all 9 sessions was 87% for group A, 72% for group B, and 84% for group C.
Figure 1.
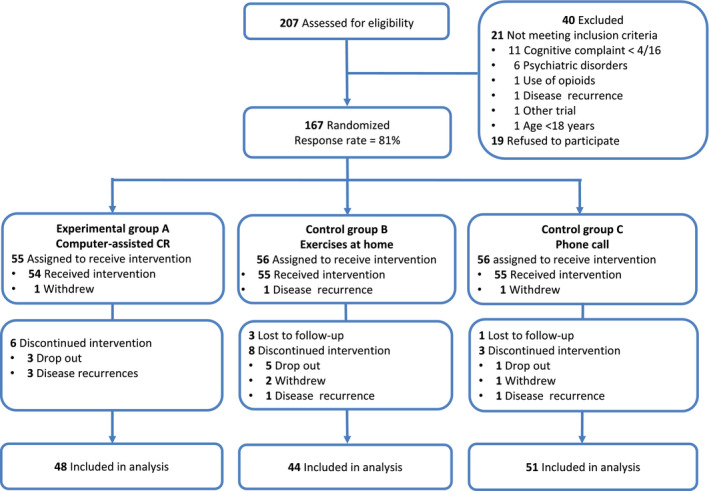
CONSORT flow diagram. CR, cognitive rehabilitation.
TABLE 1.
Baseline Characteristics of Study Participants
| Participant Characteristics | Group A: Computerized CR (n = 55) | Group B: Exercises at Home (n = 56) | Group C: Phone Call (n = 56) |
|---|---|---|---|
| Age, y, median (range) | 51.7 (35‐72) | 50.9 (28‐78) | 50.7 (24‐77) |
| Sex, n (%) | |||
| Women | 53 (96.4) | 53 (94.6) | 54 (96.4) |
| Men | 2 (3.6) | 3 (5.4) | 2 (3.6) |
| Education level, n (%) | |||
| Primary school | 4 (7.3) | 3 (5.4) | 2 (3.6) |
| Middle school | 8 (14.55) | 9 (16.1) | 10 (17.8) |
| High school | 8 (14.55) | 11 (19.6) | 9 (16.1) |
| University | 28 (50.9) | 25 (44.6) | 31 (55.4) |
| Unknown | 7 (12.7) | 8 (14.3) | 4 (7.1) |
| Cancer variables | |||
| Cancer type, n (%) | |||
| Breast | 47 (85.5) | 48 (85.7) | 45 (80.3) |
| Digestive | 4 (7.3) | 2 (3.6) | 1 (1.8) |
| Hematologic | 2 (3.6) | 3 (5.35) | 2 (3.6) |
| Urologic/Gynecologic | 1 (1.8) | 3 (5.35) | 7 (12.5) |
| Other | 1 (1.8) | 0 | 1 (1.8) |
| Presence of metastases, n (%) | 7 (12.7) | 2 (3.6) | 6 (10.7) |
| Time since chemotherapy completion, mo, median (range) | 11.7 (7.7‐18.3) | 9.5 (6.2‐20.8) | 10.5 (5.7‐19.3) |
| Prior anticancer therapies | |||
| Surgery, n (%) | 50 (90.9) | 51 (91.1) | 51(91.1) |
| Radiotherapy, n (%) | 44 (80.0) | 43 (76.8) | 45 (80.3) |
| Hormone therapy, n (%) | 29 (51.8) | 37 (66.1) | 32 (57.1) |
| Patient‐reported outcomes | |||
| FACT‐Cog, mean (SD) | |||
| PCI | 33.2 (13.2) | 35.4 (15.6) | 34.1 (13.6) |
| PCA | 11.0 (5.0) | 12.2 (4.5) | 11.2 (4.3) |
| FACT impact on QOL | 6.0 (4.0) | 6.8 (3.6) | 5.9 (3.4) |
| FACT comments from others | 11.5 (4.3) | 12.3 (3.5) | 11.9 (3.9) |
| Depression CES‐D, mean (SD) | 21.7 (9.9) | 20.4 (9.7) | 22.4 (9.1) |
| Anxiety STAI‐Trait, mean (SD) | 45.6 (12.1) | 45.4 (11.5) | 47.5 (10.4) |
| Fatigue FACT‐An, mean (SD) | 50.2 (12.2) | 50.1 (12.4) | 46.7 (13.5) |
| QOL FACT‐G, mean (SD) | 71.2 (15.2) | 70.3 (15.4) | 67.4 (14.8) |
Abbreviations: CES‐D, Center for Epidemiologic Studies Depression Scale; CR, cognitive rehabilitation; FACT‐An, Functional Assessment of Cancer Therapy–Anemia; FACT‐Cog, Functional Assessment of Cancer Therapy–Cognitive Function; FACT‐G, Functional Assessment of Cancer Therapy–General; PCA, perceived cognitive abilities; PCI, perceived cognitive impairments; QOL, quality of life; STAI‐Trait, Spielberger State‐Trait Anxiety Inventory.
At baseline (T0), the 3 groups had comparable FACT‐Cog PCI scores (Table 2). The proportion of participants with a 7‐point improvement in PCI scores between T0 and T3 was highest in group A (75%), followed by group B (59%) and group C (57%). However, the difference between these groups did not reach statistical significance (P = .12 between groups A and B; P = .09 between groups A and C). Nevertheless, the mean difference in PCI scores between T0 and T3 was statistically significant in group A compared with groups B and C (P = .02), with differences in scores of 16, 11, and 9, respectively (group A versus B, P = .049; group A versus C, P = .009), whereas the difference in scores was not statistically significant between groups B and C (P = .62). Moreover, there was a linear increase in FACT‐Cog PCI scores over time for group A that was not observed in group B or C (P trend = .01). Group A also reported better scores in the other FACT‐Cog subscales: PCA scores improved significantly, with a mean difference in scores between T0 and T3 of 5.3 in group A, compared with 1.8 and 2.7 for groups B and C, respectively (P < .01). CR also had a significant impact on the FACT‐Cog QOL score in group A (5.1 vs 2.6 and 3.8, respectively, P = .01). Hormone therapy was not associated with any FACT‐Cog subscale scores. There was also no link between completion rate and FACT‐Cog PCI scores.
TABLE 2.
Mean Scores for Subjective Cognition With FACT‐Cog Assessment
| Group A: Computerized CR (n = 48) | Group B: Exercises at Home (n = 44) | Group C: Phone Call (n = 51) | P a | |
|---|---|---|---|---|
| PCI T0 | 33.2 (13.2) | 35.4 (15.6) | 34.1 (13.6) | .66 |
| PCI T1 | 40.8 (11.8) | 44.0 (16.1) | 44.4 (12.2) | |
| PCI T2 | 47.5 (11.8) | 47.4 (17.1) | 44.2 (12.5) | |
| PCI T3 b | 49.0 (12.9) | 45.2 (16.5) | 43.5 (12.9) | |
| 7‐Point improvement in PCI score between T0 and T3, n (%) b | 36 (75.0) | 26 (59.1) | 29 (56.9) | .13 |
| PCI: difference between T0 and T3 b | 16.3 (14.7) | 11.1 (14.8) | 9.1 (12.6) | .02 |
| PCA: difference between T0 and T3 b | 5.3 (5.6) | 1.8 (5.3) | 2.7 (4.3) | <.01 |
| FACT impact on QOL: difference between T0 and T3 b | 5.1 (5.4) | 2.6 (4.1) | 3.8 (3.9) | .01 |
| FACT comments from others: difference between T0 and T3 b | 2.7 (3.6) | 1.0 (4.1) | 1.6 (2.8) | .05 |
Abbreviations: CR, cognitive rehabilitation; FACT‐Cog, Functional Assessment of Cancer Therapy–Cognitive Function; PCA, perceived cognitive abilities; PCI, perceived cognitive impairments; QOL, quality of life; T0, baseline; T1, at 1 month; T2, at 2 months; T3, at the end of the 3‐month program.
All values are presented as mean (SD) unless noted otherwise.
Kruskal‐Wallis or Fisher's exact test. Significant values appear in boldface type.
Missing = 24 (14.4%).
Results of objective cognitive assessments showed a positive impact of CR on working memory, with significant improvement in group A (P = .03) and a significant relation between 7‐point improvement in PCI scores and working memory with higher scores of working memory (+0.9; 95% CI, 0.6‐1.21; P = .04). No significant difference was demonstrated for the other domains of objective cognition.
Participants in group A also presented improved depression scores compared with groups B and C (P = .03) (Table 3). There was no impact of CR on anxiety, fatigue, or any dimensions of the FACT‐G.
TABLE 3.
Mean Scores for Objective Cognition and Patient‐Reported Outcomes
| Domain | Test | Outcome Measure | Group A: Computerized CR (n = 48) Mean difference T0‐T3 (SD) | Group B: Exercises at Home (n = 44) Mean difference T0‐T3 (SD) | Group C: Phone call (n = 51) Mean difference T0‐T3 (SD) | P a |
|---|---|---|---|---|---|---|
| Objective cognitive function | ||||||
| Working memory | Digit span backward WAIS IV | Total score (max. 16) | 1.3 (1.8) | 1.1 (2.1) | 0.4 (1.6) | .03 |
| Short‐term memory | Digit span forward WAIS IV | Total score (max. 16) | 0.7 (1.9) | 0.6 (1.4) | 0.4 (1.6) | .54 |
| Processing speed | TMT A | Speed | −3.3 (10.5) | 0.0 (10.1) | −5.9 (13.5) | .04 |
| Total errors | −0.02 (0.5) | 0.14 (0.7) | 0.02 (0.5) | .60 | ||
| Executive function | TMT B | Speed | −9.4 (25.3) | −6.0 (25.6) | −1.9 (22.9) | .15 |
| Total perseverative errors | −0.13 (0.7) | −0.1 (0.9) | 0.07 (0.6) | .32 | ||
| Verbal fluency | Verbal fluency | Total no. of animals | 3.3 (6.8) | 0.6 (6.9) | 2.1 (6.4) | .22 |
| Total no. of words | 1.0 (4.7) | 1.9 (5.0) | 0.8 (6.2) | .60 | ||
| Attention | D2 test | GZ score | 31.8 (53.2) | 38.4 (86.2) | 27.9 (48.3) | .46 |
| GZ‐F score | 35.7 (55.8) | 38.6 (66.3) | 28.4 (53.3) | .43 | ||
| Episodic memory | Grober‐Buschke | Free recall score (max. 16) | 1.3 (2.3) | 1.6 (2.2) | 0.7 (2.1) | .13 |
| Delayed recall score (max. 16) | 1.3 (2.2) | 0.6 (1.7) | 0.8 (1.8) | .35 | ||
| Patient‐reported outcomes | ||||||
| Depression | CES‐D | Total score (max. 60) | −6.5 (10.3) | −1.7 (7.9) | −2.3 (8.3) | .03 |
| Anxiety | STAI‐Trait | Total score (max. 80) | −1.9 (8.4) | −0.8 (8.3) | −2.0 (9.1) | .76 |
| Fatigue | FACT‐An | Total score (max. 80) | 5.8 (11.9) | 4.1 (10.6) | 3.2 (12.2) | .59 |
| QOL | FACT‐G | Total score (max. 108) | 5.4 (11.3) | 4.2 (10.6) | 3.7 (12.3) | .56 |
| Physical well‐being | Total score (max. 28) | 1.1 (4.5) | 1.4 (4.0) | 2.1 (4.6) | .55 | |
| Social well‐being | Total score (max. 28) | 0.7 (3.9) | −0.5 (4.3) | −0.1 (3.5) | .36 | |
| Emotional well‐being | Total score (max. 24) | 2.3 (4.7) | 1.5 (3.6) | 1.0 (4.2) | .37 | |
| Functional well‐being | Total score (max. 28) | 1.7 (3.8) | 1.2 (3.1) | 0.5 (4.3) | .33 |
Abbreviations: CES‐D, Center for Epidemiologic Studies Depression Scale; CR, cognitive rehabilitation; FACT‐An, Functional Assessment of Cancer Therapy–Anemia; FACT‐G, Functional Assessment of Cancer Therapy–General; GZ, total number of marked items; GZ‐F, net performance quality; max., maximum; QOL, quality of life; STAI‐Trait, Spielberger State‐Trait Anxiety Inventory; T0, at baseline; T3, at the end of the 3‐month program; TMT, Trail Making Test; WAIS, Wechsler Adult Intelligence Scale.
All values are presented as the mean (SD) difference between T0 and T3.
Significant values appear in boldface type.
Sensitivity analyses were performed by adjusting for age, education level, and baseline cognitive performance and did not change the results.
The effect sizes between T0 and T3 showed a strong impact of CR on cognitive functioning in group A, with an effect size of 0.49 on PCI score and 0.53 on working compared with group C (Fig. 2).
Figure 2.
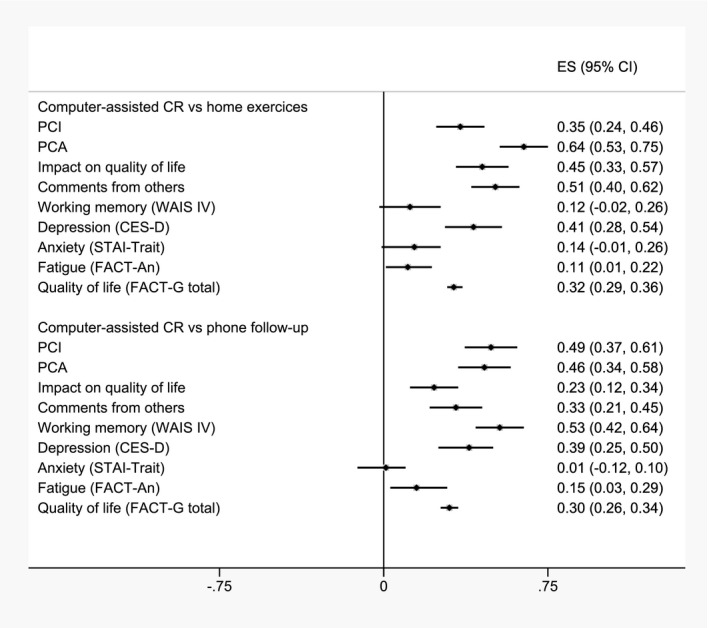
Forest plot of effect sizes and 95% CIs for computer‐assisted cognitive rehabilitation compared with the 2 active control groups. CES‐D, Center for Epidemiologic Studies Depression Scale; CR, cognitive rehabilitation; ES, effect size; FACT‐An, Functional Assessment of Cancer Therapy–Anemia; FACT‐G, Functional Assessment of Cancer Therapy–General; PCA, perceived cognitive abilities; PCI, perceived cognitive impairments; STAI‐Trait, Spielberger State‐Trait Anxiety Inventory; WAIS, Wechsler Adult Intelligence Scale.
Discussion
This large, multicenter RCT investigated the impact of 3 months of computer‐assisted CR on subjective and objective cognition in a sample of cancer patients reporting cognitive complaints during or after chemotherapy completion. The computer‐assisted CR program led to an improvement in all of the subjective FACT‐Cog subscales, which evaluate cognitive complaints and their impact on QOL. There was also a positive impact on objective cognition, specifically with improved working memory, and lower levels of depression.
The primary endpoint was defined as the proportion of patients with a 7‐point improvement in the FACT‐Cog PCI score (range, 0‐72) between T0 and T3. This corresponded to an improvement of 10%, which seems clinically important, but no cutoff had been clearly established at the time of our study. The primary endpoint did not reach statistical significance, although the proportion of patients with a 7‐point improvement in PCI score was higher among group A participants, who received computer‐assisted CR (75% vs 59% and 57% in groups B and C, respectively). A recent work estimated that 7.4 is a clinically important difference for the PCI postintervention in cancer survivors with cognitive impairment after adjuvant chemotherapy. 33 Using this definition instead of the proportion of patients, we found a clear mean difference in PCI score with significant results: a 16‐point improvement in experimental group A, which was well above the clinically important difference, compared with an 11‐point improvement in the control group B, where participants performed home cognitive self‐exercises (P = .049) and a 9‐point improvement for control group C, where participants received phone follow‐up (P = .009). The other FACT‐Cog subscales also showed statistically significant improvements in experimental group A, especially PCA scores (P < .01) and QOL related to cognition (P = .01).
Our study confirms the results of 2 recent RCTs that evaluated computer‐assisted CR and used the mean difference in PCI score as the primary endpoint, both of which showed a positive impact of the intervention on cognitive complaints. 14 , 15 However, our study is the only one in the literature to include active control groups. The bias linked to wait‐list groups is well documented in cognitive behavioral therapy, as these groups lead to bigger effect sizes estimates. 34 , 35 Using an intervention in our control groups allowed us to limit the overestimation of the intervention effect and make our results robust and closer to reality.
We found a statistically significant improvement in working memory, as evaluated with the Digit Span Backward of the WAIS IV. Alteration of working memory induces daily difficulties, and our results hold promise for easing them. 36 Our results confirm those of small previous studies. Like us, Cherrier et al 18 used the Digit Span Backward in an RCT that included 12 patients in the experimental group and 16 patients in a wait‐list group and found significant results of improved working memory. Von Ah et al 12 also found improvements in memory performance among 82 breast cancer survivors after 6 to 8 weeks of memory or processing intervention compared with a wait‐list group. However, memory scores were evaluated by the Rey Auditory Verbal Learning Test, which focuses more on episodic memory. 37 Some studies have not reported improvements in working memory after computer‐assisted CR; however, these studies often used a suboptimal cognitive test to explore working memory. For example, Bray et al 14 conducted an RCT with 242 cancer patients with cognitive complaints after chemotherapy who were randomly assigned to computer‐assisted CR or a control group and found no significant differences in objective cognitive assessment. The n‐back test was used for working memory, which is commonly used in functional neuroimaging studies. 38 Some results have demonstrated that the n‐back is not a pure measure of working memory, with no correlation between n‐back performance and Digit Span Backward. 39 , 40 Mihuta et al 15 did not observe a significant difference in objective cognition among 76 patients allocated to a 4‐week computer‐assisted CR or wait‐list group. Indeed, it was a very short CR program with an online evaluation (test battery Webneuro), and the study sample was small.
Depression scores improved significantly in our experimental group A, confirming previous results of a negative association between PCI scores and depression. 12 , 14 By acting on cognitive complaints, CR improves depression symptoms. There was no significant difference in fatigue scores or global QOL. Although cognitive disorders can be associated with cancer‐related fatigue, they remain a specific complaint.
Our study has some limitations. There was no long‐term follow‐up to determine whether the positive effect we observed was maintained over time. The impact of the presence of the neuropsychologist at CR sessions in the experimental group could also be questioned; on the other hand, this may have been a strength given that online CR programs make coaching achievable at home, which could improve feasibility in terms of resources, costs, and patient availability. Most of the participants had breast cancer, and 59% had received previous hormone therapy, the effect of which on cognition remains controversial. 41 , 42 In our study, hormone therapy did not affect cognitive functioning outcomes before or after the CR program. Finally, multiple test correction was performed for the primary analysis; however, several other statistical tests were performed, especially for objective cognition, possibly increasing the false‐positive error rate.
Cancer‐related cognitive impairment is a common adverse effect that remains a challenge for cancer patients. Our study is the first RCT with active control groups to show promising results for improving objective cognitive functioning, particularly working memory. It reinforces existing data on the beneficial effect of computer‐assisted CR programs on cognitive complaints, QOL related to cognition, and depression symptoms. Computer‐assisted CR appears to be an interesting option in the management of chemobrain for patients with cognitive complaints.
Funding Support
This study was supported by Ligue Nationale Contre le Cancer.
Conflict of Interest Disclosures
The authors made no disclosures.
Author Contributions
Mélanie Dos Santos: Data collection and assembly, data analysis and interpretation, writing‐review and editing. Isabelle Hardy‐Léger: Study conception and design, data collection and assembly. Olivier Rigal: Study conception and design, data collection and assembly. Idlir Licaj: Data analysis and interpretation, writing–review and editing. Sarah Dauchy: Data collection and assembly. Christelle Levy: Data collection and assembly. Sabine Noal: Data collection and assembly. Carine Segura: Data collection and assembly. Corinne Delcambre: Data collection and assembly. Djelila Allouache: Data collection and assembly. Aurélie Parzy: Data collection and assembly. Jérôme Barriere: Data collection and assembly. Thierry Petit: Data collection and assembly. Marie Lange: Data collection and assembly. Aurélie Capel: Study conception and design, data collection and assembly. Bénédicte Clarisse: Study conception and design, project administration. Jean Michel Grellard: Study conception and design, project administration, quality control of data. Johan Lefel: Study conception and design, data collection and assembly. Florence Joly: Study conception and design, data collection and assembly, data analysis and interpretation, funding acquisition, writing–review and editing.
Dos Santos M, Hardy‐Léger I, Rigal O, Licaj I, Dauchy S, Levy C, Noal S, Segura C, Delcambre C, Allouache D, Parzy A, Barriere J, Petit T, Lange M, Capel A, Clarisse B, Grellard JM, Lefel J, Joly F. Cognitive rehabilitation program to improve cognition of cancer patients treated with chemotherapy: A 3‐arm randomized trial. Cancer. 2020:126:5328‐5336. 10.1002/cncr.33186
Presented in poster form at the 2019 ASCO Annual Meeting; May 31 to June 4, 2019; Chicago, Illinois.
We acknowledge the Northwest Data Center for managing the data. We also thank all of the patients who participated in the study.
Clinical Trial registration number: NCT01788618.
References
- 1. Myers JS. Cancer‐ and chemotherapy‐related cognitive changes: the patient experience. Semin Oncol Nurs. 2013;29:300‐307. doi: 10.1016/j.soncn.2013.08.010 [DOI] [PubMed] [Google Scholar]
- 2. Joly F, Giffard B, Rigal O, et al. Impact of cancer and its treatments on cognitive function: advances in research from the Paris International Cognition and Cancer Task Force Symposium and update since 2012. J Pain Symptom Manage. 2015;50:830‐841. doi: 10.1016/j.jpainsymman.2015.06.019 [DOI] [PubMed] [Google Scholar]
- 3. Vardy J, Dhillon H. The fog hasn't lifted on “chemobrain” yet: ongoing uncertainty regarding the effects of chemotherapy and breast cancer on cognition. Breast Cancer Res Treat. 2010;123:35‐37. doi: 10.1007/s10549-009-0719-0 [DOI] [PubMed] [Google Scholar]
- 4. Lange M, Licaj I, Clarisse B, et al. Cognitive complaints in cancer survivors and expectations for support: results from a web‐based survey. Cancer Med. 2019;8:2654‐2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jansen CE, Cooper BA, Dodd MJ, Miaskowski CA. A prospective longitudinal study of chemotherapy‐induced cognitive changes in breast cancer patients. Support Care Cancer. 2011;19:1647‐1656. doi: 10.1007/s00520-010-0997-4 [DOI] [PubMed] [Google Scholar]
- 6. Janelsins MC, Kohli S, Mohile SG, Usuki K, Ahles TA, Morrow GR. An update on cancer‐ and chemotherapy‐related cognitive dysfunction: current status. Semin Oncol. 2011;38:431‐438. doi: 10.1053/j.seminoncol.2011.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brezden CB, Phillips K‐A, Abdolell M, Bunston T, Tannock IF. Cognitive function in breast cancer patients receiving adjuvant chemotherapy. J Clin Oncol. 2000;18:2695‐2701. doi: 10.1200/JCO.2000.18.14.2695 [DOI] [PubMed] [Google Scholar]
- 8. Wefel JS, Lenzi R, Theriault RL, Davis RN, Meyers CA. The cognitive sequelae of standard‐dose adjuvant chemotherapy in women with breast carcinoma: results of a prospective, randomized, longitudinal trial. Cancer. 2004;100:2292‐2299. doi: 10.1002/cncr.20272 [DOI] [PubMed] [Google Scholar]
- 9. Chan RJ, McCarthy AL, Devenish J, Sullivan KA, Chan A. Systematic review of pharmacologic and non‐pharmacologic interventions to manage cognitive alterations after chemotherapy for breast cancer. Eur J Cancer. 2015;51:437‐450. doi: 10.1016/j.ejca.2014.12.017 [DOI] [PubMed] [Google Scholar]
- 10. Kesler S, Hadi Hosseini SM, Heckler C, et al. Cognitive training for improving executive function in chemotherapy‐treated breast cancer survivors. Clin Breast Cancer. 2013;13:299‐306. doi: 10.1016/j.clbc.2013.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Duan YP, Liang W, Guo L, Wienert J, Si GY, Lippke S. Evaluation of a web‐based intervention for multiple health behavior changes in patients with coronary heart disease in home‐based rehabilitation: pilot randomized controlled trial. J Med Internet Res. 2018;20:e12052. doi: 10.2196/12052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Von Ah D, Carpenter JS, Saykin A, et al. Advanced cognitive training for breast cancer survivors: a randomized controlled trial. Breast Cancer Res Treat. 2012;135:799‐809. doi: 10.1007/s10549-012-2210-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mihuta ME, Green HJ, Shum DHK. Efficacy of a web‐based cognitive rehabilitation intervention for adult cancer survivors: a pilot study. Eur J Cancer Care. 2018;27:e12805. doi: 10.1111/ecc.12805 [DOI] [PubMed] [Google Scholar]
- 14. Bray VJ, Dhillon HM, Bell ML, et al. Evaluation of a web‐based cognitive rehabilitation program in cancer survivors reporting cognitive symptoms after chemotherapy. J Clin Oncol. 2017;35:217‐225. doi: 10.1200/JCO.2016.67.8201 [DOI] [PubMed] [Google Scholar]
- 15. Mihuta ME, Green HJ, Shum DHK. Web‐based cognitive rehabilitation for survivors of adult cancer: a randomised controlled trial. Psychooncology. 2018;27:1172‐1179. doi: 10.1002/pon.4615 [DOI] [PubMed] [Google Scholar]
- 16. Ercoli LM, Petersen L, Hunter AM, et al. Cognitive rehabilitation group intervention for breast cancer survivors: results of a randomized clinical trial: cognitive rehabilitation intervention for breast cancer survivors. Psychooncology. 2015;24:1360‐1367. doi: 10.1002/pon.3769 [DOI] [PubMed] [Google Scholar]
- 17. Damholdt M, Mehlsen M, O’Toole M, Andreasen R, Pedersen A, Zachariae R. Web‐based cognitive training for breast cancer survivors with cognitive complaints‐a randomized controlled trial: web‐based cognitive training for breast cancer survivors. Psychooncology. 2016;25:1293‐1300. doi: 10.1002/pon.4058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cherrier MM, Anderson K, David D, et al. A randomized trial of cognitive rehabilitation in cancer survivors. Life Sci. 2013;93:617‐622. doi: 10.1016/j.lfs.2013.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Joly F, Lange M, Rigal O, et al. French version of the Functional Assessment of Cancer Therapy–Cognitive Function (FACT‐Cog) version 3. Support Care Cancer. 2012;20:3297‐3305. doi: 10.1007/s00520-012-1439-2 [DOI] [PubMed] [Google Scholar]
- 20. Lange M, Heutte N, Morel N, Eustache F, Joly F, Giffard B. Cognitive complaints in cancer: the French version of the Functional Assessment of Cancer Therapy–Cognitive Function (FACT‐Cog), normative data from a healthy population. Neuropsychol Rehabil. 2016;26:392‐409. doi: 10.1080/09602011.2015.1036890 [DOI] [PubMed] [Google Scholar]
- 21. Wagner L, Sweet J, Butt Z, Lai JS, Cella D. Measuring patient self‐reported cognitive function: development of the Functional Assessment of Cancer Therapy–Cognitive Function instrument. J Support Oncol. 2009;7:W32‐W39. [Google Scholar]
- 22. Grober E, Buschke H. Genuine memory deficits in dementia. Dev Neuropsychol. 1987;3:13‐36. doi: 10.1080/87565648709540361 [DOI] [Google Scholar]
- 23. Brickenkamp R. Test d’Attention Concentrée: D2. Editions du Centre de Psychologie Appliquée; 1998. [Google Scholar]
- 24. Cardebat D, Doyon B, Puel M, Goulet P, Joanette Y. Formal and semantic lexical evocation in normal subjects. Performance and dynamics of production as a function of sex, age and educational level [in French]. Acta Neurol Belg. 1990;90:207‐217. [PubMed] [Google Scholar]
- 25. Reitan RM. Validity of trail making tests as an indicator of organic brain damage. Percept Mot Skills. 1958;8:271. doi: 10.2466/PMS.8.7.271-276 [DOI] [Google Scholar]
- 26. Wechsler D. Wechsler Adult Intelligence Scale. 4th ed. Pearson Assessment; 2008. [Google Scholar]
- 27. Costet N, Lapierre V, Benhamou E, Galès CL. Reliability and validity of the Functional Assessment of Cancer Therapy General (FACT‐G) in French cancer patients. Qual Life Res. 2005;14:1427‐1432. doi: 10.1007/s11136-004-5531-z [DOI] [PubMed] [Google Scholar]
- 28. Winstead‐Fry P, Schultz A. Psychometric analysis of the Functional Assessment of Cancer Therapy‐General (FACT‐G) scale in a rural sample. Cancer. 1997;79:2446‐2452. [PubMed] [Google Scholar]
- 29. Cella D. The Functional Assessment of Cancer Therapy‐Anemia (FACT‐An) Scale: a new tool for the assessment of outcomes in cancer anemia and fatigue. Semin Hematol. 1997;34(suppl 2):13‐19. [PubMed] [Google Scholar]
- 30. Spielberger SD. Manual for the State‐Trait Anxiety Inventory (STAI). Consulting Psychologists Press; 1983. [Google Scholar]
- 31. Radloff LS. The CES‐D scale: a self‐report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385‐401. [Google Scholar]
- 32. Cheung YT, Foo YL, Shwe M, et al. Minimal clinically important difference (MCID) for the functional assessment of cancer therapy: cognitive function (FACT‐Cog) in breast cancer patients. J Clin Epidemiol. 2014;67:811‐820. doi: 10.1016/j.jclinepi.2013.12.011 [DOI] [PubMed] [Google Scholar]
- 33. Bell ML, Dhillon HM, Bray VJ, Vardy JL. Important differences and meaningful changes for the Functional Assessment of Cancer Therapy‐Cognitive Function (FACT‐Cog). J Patient Rep Outcomes. 2018;2. doi: 10.1186/s41687-018-0071-4 [DOI] [Google Scholar]
- 34. Furukawa TA, Noma H, Caldwell DM, et al. Waiting list may be a nocebo condition in psychotherapy trials: a contribution from network meta‐analysis. Acta Psychiatr Scand. 2014;130:181‐192. [DOI] [PubMed] [Google Scholar]
- 35. Zhu Z, Zhang L, Jiang J, et al. Comparison of psychological placebo and waiting list control conditions in the assessment of cognitive behavioral therapy for the treatment of generalized anxiety disorder: a meta‐analysis. Shanghai Arch Psychiatry. 2014;26:319‐331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Baddeley A. Working memory and language: an overview. J Commun Disord. 2003;36:189‐208. [DOI] [PubMed] [Google Scholar]
- 37. Rey A. L’Examen Clinique en Psychologie [in French]. Presses Universitaires de France; 1964. [Google Scholar]
- 38. Ragland JD, Turetsky BI, Gur RC, et al. Working memory for complex figures: an fMRI comparison of letter and fractal n‐back tasks. Neuropsychology. 2002;16:370‐379. [PMC free article] [PubMed] [Google Scholar]
- 39. Miller KM, Price CC, Okun MS, et al. Is the n‐back task a valid neuropsychological measure for assessing working memory? Arch Clin Neuropsychol. 2009;24:711‐717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Jaeggi SM, Buschkuehl M, Perrig WJ, et al. The concurrent validity of the N‐back task as a working memory measure. Memory. 2010;18:394‐412. [DOI] [PubMed] [Google Scholar]
- 41. Schilder CM, Seynaeve C, Beex LV, et al. Effects of tamoxifen and exemestane on cognitive functioning of postmenopausal patients with breast cancer: results from the neuropsychological side study of the tamoxifen and exemestane adjuvant multinational trial. J Clin Oncol. 2010;28:1294‐1300. doi: 10.1200/JCO.2008.21.3553 [DOI] [PubMed] [Google Scholar]
- 42. Schilder CMT, Seynaeve C, Linn SC, et al. Self‐reported cognitive functioning in postmenopausal breast cancer patients before and during endocrine treatment: findings from the neuropsychological TEAM side‐study. Psychooncology. 2012;21:479‐487. doi: 10.1002/pon.1928 [DOI] [PubMed] [Google Scholar]


