Abstract
The Gleason grading system is one of the most important factors in clinical decision‐making for prostate cancer patients, and is entirely based on the classification of tumour growth patterns. In recent years it has become clear that some individual growth patterns themselves have independent prognostic value, and could be used for better personalised risk stratification. In this review we summarise recent literature on the clinicopathological value and molecular characteristics of individual prostate cancer growth patterns, and show how these, most particularly cribriform architecture, could alter treatment decisions for prostate cancer patients.
Keywords: cribriform, growth pattern, prostate cancer, three‐dimensional
Introduction
The Gleason grading system is important for determining prostate cancer prognosis and for clinical decision‐making. The Gleason score is entirely based on the classification of adenocarcinoma growth patterns. These patterns are assigned a Gleason grade from 1 to 5. As prostate cancer is such a heterogeneous disease, the Gleason score is determined by adding together the most common grade and the highest grade in biopsies, and the two most predominant grades in radical prostatectomy (RP) specimens. 1 , 2 Gleason patterns 1–3 encompass well‐delineated glandular structures with variable interglandular distances and nodular circumscription. As no practical and prognostic differences exist between these three Gleason grades, the International Society of Urological Pathology (ISUP) has recommended that Gleason scores 2–4 should rarely, if ever, be used for biopsy specimens. 2 Gleason pattern 4 comprises poorly formed, fused, glomeruloid and cribriform glandular structures, whereas growth patterns with essentially no glandular differentiation, such as single cells, cords, and solid fields, and the presence of comedonecrosis are classified as Gleason pattern 5 (Figure 1). 2 , 3 Individual growth patterns have, in general, not been specified in pathology reports or in molecular–biological investigations. However, recent studies have indicated that individual growth patterns have independent predictive value for clinical outcome, and facilitate more comprehensive interpretation of molecular–biological findings. The aims of this review are to summarise the clinicopathological impact of individual prostate cancer growth patterns beyond the Gleason score, and to investigate their molecular–biological background. We show how consideration of growth patterns could optimise decision‐making in clinical practice.
Figure 1.
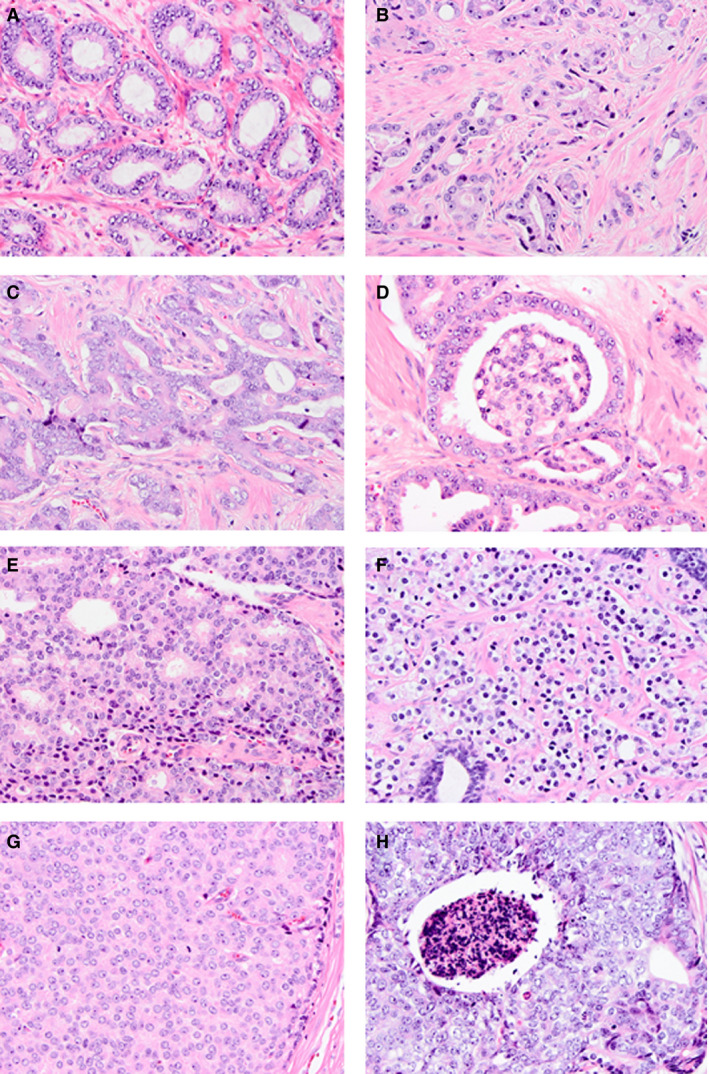
Overview of Gleason growth patterns. A, Gleason pattern 3 well‐delineated glands. B–E, Gleason pattern 4 poorly formed (B), fused (C), glomeruloid (D) and cribriform (E) architecture. F–H, Gleason pattern 5 single cells/cords (F), solid sheets (G), and comedonecrosis (H). Haematoxylin and eosin.
Clinicopathological impact of individual growth patterns
Individual tumour growth patterns have mainly been analysed in Gleason score 3 + 4 = 7 (Grade Group 2) prostate cancer, which is composed of variable quantities of well‐delineated Gleason pattern 3 glands and Gleason pattern 4 structures. In 2011, Iczkowski et al. were the first to report that a cribriform growth pattern had independent prognostic value for postoperative biochemical recurrence. 4 Many groups have since confirmed the independent predictive value of a cribriform pattern for adverse pathological features, biochemical recurrence‐free survival and disease specific survival in biopsy and RP specimens. 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 Whereas the value of cribriform architecture has mostly been investigated in Gleason score 7 prostate cancer, it also affects cancer‐specific survival for patients with Gleason score 8 (Grade Group 4) biopsies. 11 , 16 A limitation of many studies on cribriform growth pattern, however, is that it is not entirely clear whether, and if so how, an invasive Gleason pattern 4 pattern was distinguished from intraductal carcinoma (IDC) of the prostate.
IDC is characterised by a cribriform or solid proliferation of atypical epithelial cells within distended pre‐existing prostate acini, either with or without comedonecrosis, and has also been associated with adverse clinical outcomes. 10 , 11 , 17 , 18 , 19 , 20 , 21 In the vast majority of patients, IDC occurs intermixed with invasive carcinoma, but rare cases of isolated IDC without invasive disease have been described. 22 These rare cases of isolated IDC should not be graded, but a comment should indicate their association with unsampled high‐grade carcinoma. 1 , 3 , 22 , 23 The finding of isolated IDC on needle biopsy should lead to immediate re‐biopsy, and some even advocate radical treatment in these cases. 22 The International Society of Urological Pathology (ISUP) recommended in 2014 and the World Health Organization (WHO) recommended in 2016 that the presence of IDC without invasive carcinoma should be specifically mentioned, but no recommendations were made for reporting of IDC admixed with invasive cancer. 1 , 3
Invasive cribriform carcinoma and IDC are often difficult, if not impossible, to distinguish without the application of basal cell immunohistochemistry. If no basal cells are present, a cribriform lesion is generally considered to be invasive Gleason pattern 4; if continuous, scattered or sporadic basal cells are observed, cribriform architecture is mostly regarded as IDC. Only a few studies have attempted to investigate invasive cribriform carcinoma and IDC separately by using extensive immunohistochemistry. 11 , 15 , 24 In a prostate biopsy screening cohort of 1031 men, Kweldam et al. found that the presence of invasive cribriform carcinoma and the presence of IDC were both associated with worse disease‐specific survival in univariate analyses. 11 The two combined showed the strongest association with outcome in this study. At the most recent ISUP consensus meeting in Nice, France, 2019, it was agreed that both invasive cribriform carcinoma and IDC should be specifically reported. 23
The grading of IDC intermixed with invasive carcinoma has been controversial. Whereas the 2014 ISUP meeting did not make a recommendation on this issue, in 2016 the WHO stated that it should not be factored into grading. 1 , 3 A consequence of the WHO recommendation is that basal cell immunohistochemistry should be performed in every case in which IDC cannot be distinguished from invasive disease and classification as either IDC or invasive carcinoma will alter the final Gleason score. Apart from additional turnaround time and costs, basal cell immunohistochemistry does not distinguish between IDC and invasive cribriform or solid carcinoma in every case. It is well known that foci of high‐grade prostate intraepithelial neoplasia can lack basal cells, probably because of sampling artefact; as IDC glands are, by definition, distended, the chance of a false‐negative basal cell immunohistochemical result is likely to be even larger, resulting in erroneous classification as an invasive cribriform pattern. On the other hand, large irregular cribriform tumour fields substantially exceeding the pre‐existing gland architecture and thus clearly invasive may have occasional basal cells, as has also been reported on rare occasions for low‐grade invasive adenocarcinoma. 25 As IDC is an adverse pathological parameter, and difficult or even impossible to distinguish from invasive carcinoma even with the use of basal cell immunohistochemistry, it was recommended at the latest 2019 ISUP consensus meeting that IDC intermixed with invasive carcinoma should be assigned a Gleason grade based on its underlying growth pattern, as if it were invasive carcinoma. 23 On the other hand, the Genitourinary Pathology Society (GUPS) recommends not factoring IDC into Gleason grading, and performing basal cell immunohistochemistry if classification as either invasive carcinoma or IDC would lead to a change in the final Gleason score. 26 Thus, a Gleason score 6 prostate cancer biopsy with a cribriform lesion is now classified as Grade Group 2 according to the 2019 ISUP recommendation, without immunohistochemistry; the GUPS recommends performing basal cell immunohistochemistry and grading the tumour as Grade Group 1 if basal cells are present and as Grade Group 2 if they are not. Both the ISUP and the GUPS recommend including a comment on the association of IDC with aggressive disease, whereas the vast majority of genitourinary pathologists consider the above‐mentioned case not to be eligible for active surveillance. 27 Inclusion or exclusion of IDC in tumour grading results in a global Grade Group shift in < 2% of prostate cancer biopsies. 28 , 29
Clinical implications
As both cribriform invasive carcinoma and IDC have independent predictive value, the presence of either of them should routinely be reported as ‘cribriform carcinoma’. The question arises as to what extent the absence or the presence of cribriform carcinoma can lead to optimisation of therapeutic decision‐making for individual prostate cancer patients. Patients with biopsy Gleason score 3 + 4 = 7 (Grade Group 2) disease will generally be offered definitive treatment, whereas those with Gleason score 3 + 3 = 6 (Grade Group 1) disease are often eligible for active surveillance. The recent identification of additional prognostic pathological parameters, such as the presence of invasive cribriform carcinoma and/or IDC and the quantity of Gleason pattern 4, allows for more detailed risk stratification of patients with Grade Group 2 disease. Patients with a small quantity of Gleason pattern 4 in the biopsy may be eligible for active surveillance, as their outcome is comparable to that of those with Grade Group 1 disease. The substantial interobserver variability for grade assignment for small foci of poorly formed and fused glands could be another argument for this strategy. 30 , 31 , 32 , 33 , 34 , 35 Disease‐specific and biochemical recurrence‐free survival are not statistically significantly different between patients with Grade Group 2 disease without cribriform carcinoma on biopsy and those with Grade Group 1 disease, and it has therefore been proposed that the absence of both invasive cribriform carcinoma and IDC may qualify a patient with Grade Group 2 disease on biopsy for active surveillance. 10 , 11 , 36 , 37 If the safety of this eligibility approach is demonstrated in prospective studies, it will have a major impact on the management of patients with Grade Group 2 disease. The presence of cribriform carcinoma may also affect other aspects of clinical decision‐making; the absence of cribriform architecture has been associated with a low risk of pelvic lymph node metastasis. 13 , 38 , 39 In a series of 627 RP specimens, 22 of 228 (10%) patients with Grade Group 2 disease with cribriform carcinoma developed metastases, whereas no metastases were observed among 192 patients with cribriform‐negative Grade Group 2 disease and 207 patients with Grade Group 1 disease. 38 Current guidelines for performing pelvic lymph node dissections (PLNDs) are based on clinicopathological nomograms that do not take into consideration cribriform architecture, but the future inclusion of invasive cribriform carcinoma and IDC may optimise these nomograms. A few studies have also found independent value for cribriform carcinoma with respect to radiation therapy or response to docetaxel, but the definitive clinical impact on these treatment modalities remains to be determined. 19 , 40 , 41
As invasive cribriform carcinoma and IDC may increasingly influence clinical decision‐making, the sensitivity for detection of these adverse features on biopsy should ideally be high. Concordance between Grade Group in RP specimens and in matched biopsies is relatively low, with tumour upgrading occurring in up to 40% of cases. As compared with RP specimens, the sensitivity and specificity for identification of invasive cribriform carcinoma and/or IDP in biopsy specimens vary from 43% to 56% and from 87% to 95%, respectively. 42 , 43 , 44 This indicates that approximately half of cribriform carcinoma lesions are missed in diagnostic biopsies. Detailed analysis of features potentially associated with false‐negative reporting of cribriform growth on biopsies did not reveal any association with the number of positive biopsies, the percentage of Gleason pattern 4 or the presence of glomeruloid architecture in a relatively small series. 43 On the other hand, multiparametric magnetic resonance imaging (MRI) may have added value for the identification of patients with prostatic invasive cribriform carcinoma and IDC that has not been identified on the biopsy, owing to sampling error. Many of these lesions show Prostate Imaging Reporting and Data System score 5 MRI abnormalities. 43 , 45 , 46 Finally, commercially available molecular tests might also play a role in the identification of patients with these adverse features; this is discussed in more detail later. 47 , 48 , 49
Invasive cribriform growth pattern delineation
As invasive cribriform carcinoma should be separately commented on in pathology reports and may increasingly affect therapeutic decision‐making, it is important to delineate this growth pattern and separate it from its microscopic mimics. 23 Here, we will only consider other invasive acinar tumour growth patterns that might be confused with a cribriform pattern; for the broad differential diagnosis of cribriform architecture including benign mimics, we refer to some excellent reviews on this subject. 50 , 51 , 52 The adjective ‘cribriform’ is a combination of the Latin words cribrum (sieve) and forma (likeness), and refers to sheets of epithelial cells punctuated by gland‐like spaces. Recognition of cribriform and glomeruloid growth patterns is better than for poorly formed and fused glands of Gleason pattern 4. 35 , 53 , 54 Nevertheless, tangentially sectioned tumour glands, complex fused glands, large glomeruloid structures and solid Gleason pattern 5 might all be confused with an invasive cribriform pattern. 54 As cribriform morphology might affect clinical decision‐making, and to allow for comparison in future studies, a clear definition of cribriform pattern is essential. Our group has defined cribriform architecture as an epithelial sheet (a) in which the majority of tumour cells do not contact the surrounding stroma, (b) with a gland‐like space surrounding less than half of the sheet circumference, and (c) with regular intercellular lumens clearly visible on haematoxylin and eosin (H&E)‐stained sections (Figure 2). 55 , 56 The first criterion (a) distinguishes a cribriform pattern from complex fused glands in which most if not all tumour cells are still in direct contact with subtle connective tissue cores present within the lesion. The second criterion (b) arbitrarily distinguishes a cribriform from a large glomeruloid pattern in which gland‐like spaces surround more than half of the central protrusion. The validity of this criterion is supported by the fact that the clinicopathological features and biochemical recurrence‐free survival of patients with large glomeruloid structures on RP were more comparable to those of small glomeruloid structures than of cribriform Gleason pattern 4. 57 The third criterion (c) distinguishes cribriform from solid Gleason pattern 5, in which essentially no glandular differentiation is visible on H&E‐stained sections. With respect to the latter, it should be noted that the presence of intracytoplasmic vacuoles should be ignored for tumour grading, in contrast to lumen formation between two or more individual tumour cells. 2 Although our H&E‐based description is also mirrored by the three‐dimensional architectural characteristics of individual prostate cancer growth patterns, a broad consensus on the definition of cribriform morphology and its unique features delineating it from its mimics needs to be achieved. 55
Figure 2.
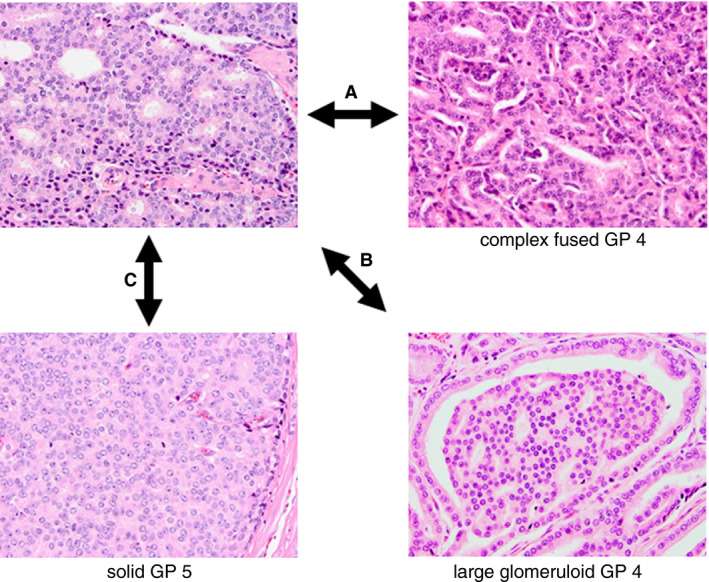
Delineating features of invasive cribriform carcinoma. In cribriform carcinoma: A, the majority of tumour cells do not contact the surrounding stroma, whereas, in complex fused glands, most epithelial cells are adjacent to subtle connective tissue cores; B, a gland‐like space surrounds less than half of the sheet circumference, whereas it encircles most of the protrusion in glomeruloid glands; and C, regular intercellular lumens are clearly visible as opposed to Gleason pattern 5 solid sheets.
Clinical relevance of non‐cribriform growth patterns
Whereas invasive cribriform carcinoma and IDC have independent value for predicting clinical outcome in patients with Grade Group 2 disease, it is not yet entirely clear to what extent the outcomes of patients with Grade Group 2 disease without cribriform architecture differ from those of patients with Grade Group 1 disease. With a median follow‐up of 13 years, Kweldam et al. did not find statistically different disease‐specific survival rates for 256 patients with biopsy cribriform‐negative Grade Group 2 disease and 486 patients with Grade Group 1 disease. 11 In a RP cohort, Hollemans et al. did not find any metastasis on PLND or during follow‐up in 207 patients with Grade Group 1 disease and in 197 patients with Grade Group 2 disease without invasive cribriform carcinoma and IDC. 38 The latter group, however, had significantly shorter biochemical recurrence‐free survival than the former group. These data suggest that cribriform architecture reflects an intrinsic capacity for the development of metastasis, whereas the risk of biochemical recurrence depends more on other factors, such as tumour volume, positive surgical margins, or non‐cribriform Gleason patterns.
Because of its morphological resemblance to, and frequent co‐occurrence with, a cribriform pattern, glomeruloid architecture has been classified as Gleason pattern 4 since the 2014 ISUP consensus meeting. 3 Some authors have postulated that it represents a precursor of cribriform morphology. 58 However, in 350 RP specimens with Gleason score 7, Choy et al. found that cribriform morphology independently increased the risk of biochemical recurrence, whereas glomeruloid architecture was significantly associated with a reduced risk. 5 Among 472 Grade Group 2 RP specimens, patients with cribriform morphology had significantly worse clinicopathological features and biochemical recurrence‐free survival than those with a glomeruloid pattern, irrespective of the size of the glomerulations. 57 These findings seem to be incompatible with the hypothesis that glomeruloid glands are precursors of cribriform architecture.
With increasing awareness of their clinical impact, proposals for the subclassification of some established growth patterns have been made. In a detailed study of 1275 RP specimens, McKenney et al. distinguished 20 different growth pattern features. 14 This group confirmed the adverse outcome associated with cribriform architecture as compared with poorly formed glands. They also found that a reactive stroma response was associated with worse recurrence‐free survival, whereas mucin extravasation was associated with a better prognosis. More detailed analysis of cribriform patterns has shown that the number of cribriform fields does not seem to affect clinical outcome in a negative way, whereas their maximal individual size does. 11 , 15 , 24 In 420 Grade Group 2 RP specimens, tumours with large expansile cribriform fields arbitrarily defined as exceeding at least two times the size of adjacent benign glandular structures showed seminal vesicle invasion in 32% of cases and pelvic node metastasis in 23% of cases. This was significantly higher than the 9% and 5%, respectively, found with small fields of invasive cribriform carcinoma. 24 Other groups have also separated cribriform patterns, but these studies are difficult to compare, as they applied other size criteria, such as the presence of at least 12 intercellular lumens or exceeding the average diameter of benign glands. 4 , 15 , 59 If the adverse outcome associated with large expansile cribriform architecture can be confirmed by further studies, and consensus can be reached on its definition, this could be another growth pattern that is independently associated with clinical outcome and that could potentially impact on treatment decisions.
There is still little known about the clinical relevance of Gleason 5 growth patterns, which have been classified as single cells, cords, solid fields, or the presence of comedonecrosis. 1 This is mostly because tumours with primary, secondary or tertiary Gleason pattern 5 are very heterogeneous, with variable quantities of Gleason patterns 3, 4, and 5, several different growth patterns, and the occurrence of IDC. Meaningful statistical analysis including all relevant covariates requires inclusion of a large number of these patients. Nevertheless, the presence of comedonecrosis and the presence of solid sheets were found to be adverse parameters among Gleason 5 patterns in two relatively small series. 60 , 61
Molecular aberrations in cribriform architecture
Molecular alterations in prostate cancer have mostly been examined according to the Gleason score, without underlying growth patterns being taken into account. Recently, a few groups have aimed to identify the molecular characteristics of invasive cribriform carcinoma and IDC. 62 , 63 , 64 As these bioinformatic analyses were performed retrospectively on publicly available databases with digitally scanned H&E‐stained reference slides, no reliable distinction between invasive cribriform carcinoma and IDC could be made. Cribriform carcinoma showed an increased percentage of genomic alterations, this being a sign of genomic instability. 62 , 63 Among others, deletions of 8p and 10q and amplification of 8q24 corresponding to PTEN loss and c‐MYC gain were significantly enriched in cribriform carcinoma, together with SPOP point mutations. 62 , 63 , 64 , 65 , 66 Molecular profiling and RNA in‐situ hybridisation revealed that the long non‐coding RNA SChLAP1 had more than three‐fold higher levels in cribriform architecture, and could serve as a potential marker for its detection in clinical practice. 63 , 67 Interestingly, some of the molecular aberrations associated with cribriform carcinoma have been linked to aggressive clinical behaviour of prostate cancer. 68 , 69 , 70 , 71 , 72 , 73 Together, these data indicate that cribriform carcinoma is a morphological substrate of increased genomic instability, and this brings histopathology, molecular aberrations and adverse clinical outcome together comprehensively.
Individual non‐cribriform growth patterns in patient samples have not been subjected to extensive molecular profiling. In a growth pattern‐based study on epithelial–mesenchymal transition (EMT) reflected by E‐cadherin to N‐cadherin switching in RP specimens, Kolijn et al. showed that E‐cadherin to N‐cadherin switching mainly occurred in poorly formed Gleason pattern 4 gland architecture. 74 As EMT is considered to be a reversible process, consecutive mesenchymal–epithelial transition might be associated with E‐cadherin up‐regulation, N‐cadherin down‐regulation, and the formation of mature Gleason pattern 3 tubules. With regard to the fact that patients with non‐cribriform Grade Group 2 disease have a comparable low risk for metastasis to that of patients with Grade Group 1 disease, it is of interest that molecular profiling did not reveal significant differences in copy number alterations between these two groups. 62 , 75 On the basis of these clinical and molecular data, we hypothesise that some growth patterns, such as poorly formed Gleason pattern 4, represent temporary morphological tumour states reflecting dynamic tubular branching and elongation, especially when they are admixed with Gleason pattern 3 structures. 76
Apart from the scientific point of view, identification of a ‘cribriform signature’ could have substantial relevance. As mentioned previously, the high rate of false cribriform‐negative biopsies might be a significant limitation to developing clinical decision models incorporating cribriform carcinoma. A clinically applicable molecular urine, serum or tissue test might identify patients at risk for unsampled cribriform carcinoma. In the past few years, RNA expression‐based tissue assays such as Decipher (GenomeDx Biosciences Inc., Vancouver, BC, Canada), Oncotype Dx (Genomic Health Inc., Redwood City, CA, USA) and Prolaris (Myriad Genetics, Salt Lake City, UT, USA) have become commercially available for clinical stratification of patients with intermediate‐risk prostate cancer. 77 , 78 Three recent studies have shown that higher risk scores obtained with both the Decipher and Oncotype Dx tests were significantly associated with the presence of cribriform carcinoma in the tissue sample analysed. 47 , 48 , 49 These studies underscore the importance of the recording of cribriform architecture in the pathology report, and suggest that cribriform morphology might substitute for some of the information gained from these tests. 47 It remains to be determined whether these molecular assays will still have added clinical value when cribriform carcinoma and the percentage of Gleason pattern 4 are taken into account, and whether they can identify patients with false cribriform‐negative prostate cancer biopsies.
Three‐dimensional architecture of prostate cancer growth patterns
Microscopic diagnostic pathology in everyday practice is performed with thin tissue slides representing two‐dimensional cross‐sections of a three‐dimensional structure. Little is known about the actual three‐dimensional architecture of prostate cancer growth patterns. Reconstruction of hundreds of consecutive slides has shown that poorly formed Gleason pattern 4 is continuous with Gleason pattern 3. 79 , 80 Recent improvements in tissue‐clearing techniques, long‐distance confocal laser scanning and light‐sheet microscopy have enabled the imaging of intact 1‐mm‐thick prostate tissues. 81 , 82 , 83 By detailed three‐dimensional analysis of formalin‐fixed paraffin‐embedded RP specimens, we were able to gain comprehensive insights into the three‐dimensional architecture of prostate adenocarcinoma growth patterns. 55 This revealed that Gleason pattern 3 three‐dimensionally represented tubules with local interconnections. This pattern was continuous with both poorly formed Gleason pattern 4, in which tubular size and lumen diameter were smaller and tubular interconnections occurred more frequently, and fused Gleason pattern 4, in which interconnections often occurred at distances smaller than the tubular diameter. In fact, Gleason pattern 5 single cells and cords formed a continuum with poorly formed glands, in which lumen size further decreased until lumens disappeared. On the other hand, cribriform Gleason pattern 4 and solid Gleason pattern 5 either with or without comedonecrosis consisted of serpentine fields of epithelial cells, with the majority of tumour cells not being in direct contact with the surrounding stroma. Both patterns formed a continuum with or without the presence of recognisable intercellular lumens. On the basis of these three‐dimensional features, we classified the growth patterns in two distinct subgroups, which both formed continua. The first group consisted of Gleason pattern 3 tubules, Gleason pattern 4 poorly formed and fused glands, and Gleason pattern 5 single cells and cords, which all consisted of cells directly contacting the surrounding stroma, but with variable gland diameter, lumen size, and number of interconnections. The second group encompassed cribriform Gleason pattern 4 and solid Gleason pattern 5 with or without comedonecrosis consisting of epithelial cells, in which the majority of cells did not contact the adjacent stroma and there were variable intercellular lumen frequencies and sizes (Figure 3). Glomeruloid structures formed a three‐dimensional intermediate between these two subgroups. They represented intraluminal protrusions of epithelial cells appearing within a background of Gleason pattern 3 tubules, and were mostly present at the sites of tubular interconnections. The three‐dimensional architectural continuity and transitions between growth patterns in both subgroups can, to a large extent, explain the interobserver variability in Gleason grading. 53 , 84 Whereas growth patterns are classified into separate Gleason grades in clinical practice, in fact they gradually transition into each other without the presence of clearly identifiable cut‐offs. Furthermore, the three‐dimensional architectural relationships support the definition and delineating characteristics of a cribriform growth pattern, as mentioned previously. Future studies need to determine whether this three‐dimensional dichotomisation is also reflected by clinical and molecular observations.
Figure 3.
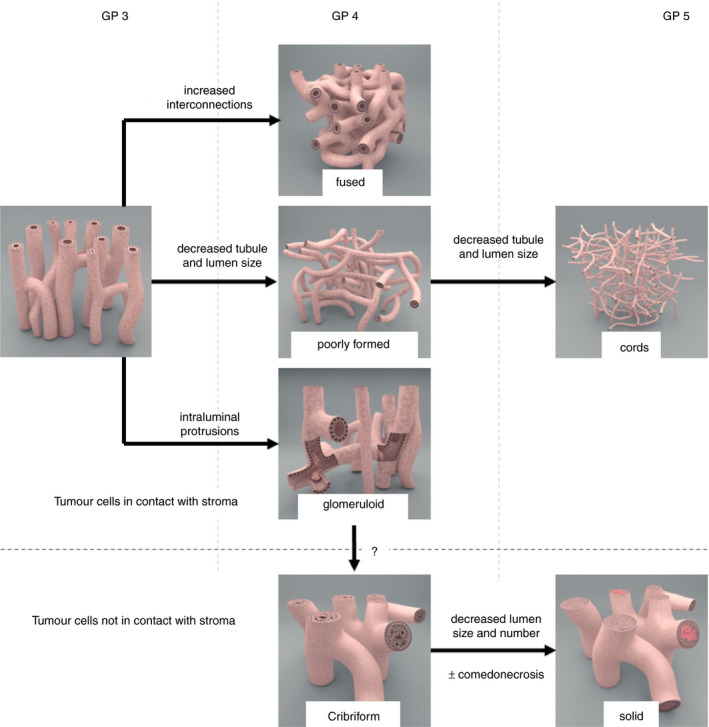
Schematic overview of the Gleason growth patterns in prostate cancer. Three‐dimensionally, two morphological subgroups of growth pattern are observed. Gleason pattern 3, poorly formed and fused Gleason pattern 4 and Gleason pattern 5 cords form the first subgroup, in which the vast majority of the tumour cells make direct contact with the surrounding stroma. The second subgroup consists of cribriform Gleason pattern 4 and solid Gleason pattern 5; the vast majority of tumour cells do not make contact with the surrounding stroma. Comedonecrosis might be present in this subgroup. Glomeruloid structures represent a morphological intermediate pattern between the two subgroups.
Future perspectives
During the last decade, invasive cribriform carcinoma, IDC, the percentage of Gleason pattern 4 and the presence of minor/tertiary high‐grade Gleason patterns have all been shown to be pathological factors with independent prognostic value. 23 In most studies, these parameters have been investigated as solitary features without inclusion of the other factors as covariates. It remains to be determined whether each of these factors has independent prognostic value in multivariable analysis. In a study of 370 Grade Group 2 biopsies, Kweldam et al. showed that 44% of patients with 25–50% Gleason pattern 4 had invasive cribriform carcinoma or IDC, whereas these patterns were present in only 6% of patients with 1–10% Gleason pattern 4. 85 In multivariable analysis, cribriform carcinoma was an independent parameter for postoperative biochemical recurrence‐free survival, whereas the percentage of Gleason pattern 4 was not. Future multivariable analyses need to elucidate the independent value of these recently established prognostic pathological factors. After identification of the independent, most influential and most reproducible factors, modification of the current Gleason grading/Grade Group systems could even be considered to increase the discriminative value of tumour grading. 56 , 86 Furthermore, prospective studies including cribriform carcinoma in clinical decision‐making, e.g. when considering eligibility for active surveillance, should indicate to what extent growth pattern specification can optimise patient management.
In conclusion, incorporation of individual growth patterns in pathology reporting and clinical decision‐making has the potential to optimise personalised treatment of prostate cancer patients. Investigation of individual growth patterns beyond heterogeneous Gleason groups allows for more comprehensive linkage of pathological, clinical and molecular–biological tumour features.
Conflicts of interest
The authors declare no conflicts of interest.
Author contributions
The manuscript was written by GvL, EV and EH.
Acknowledgements
We would like to thank Dr Patricia C. Ewing for her support with the writing of the manuscript. Use of the schematic growth patterns (Figure 3) was licensed by Medical Visuals.
van Leenders G J L H, Verhoef E I & Hollemans E. (2020) Histopathology 77, 850–861. 10.1111/his.14214 Prostate cancer growth patterns beyond the Gleason score: entering a new era of comprehensive tumour grading
References
- 1. Moch HE, Humphrey PA, Ulbright TM, Reuter VE eds. World Health Organization classification of tumours of the urinary system and male genital organs. 4th ed Geneva: WHO Press, 2016. [Google Scholar]
- 2. Epstein JI, Allsbrook WC Jr, Amin MB, Egevad LL, ISUP Grading Committee . International Society of Urological Pathology (ISUP) consensus conference on Gleason grading of prostatic carcinoma. Am. J. Surg. Pathol. 2005; 2005(29); 1228–1242. [DOI] [PubMed] [Google Scholar]
- 3. Epstein JI, Egevad L, Amin MB et al The 2014 International Society of Urological Pathology (ISUP) consensus conference on Gleason grading of prostatic carcinoma: definition of grading patterns and proposal for a new grading system. Am. J. Surg. Pathol. 2016; 40; 244–252. [DOI] [PubMed] [Google Scholar]
- 4. Iczkowski KA, Torkko KC, Kotnis GR et al Digital quantification of five high‐grade prostate cancer patterns, including the cribriform pattern, and their association with adverse outcome. Am. J. Clin. Pathol. 2011; 136; 98–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Choy B, Pearce SM, Anderson BB et al Prognostic significance of percentage and architectural types of contemporary Gleason pattern 4 prostate cancer in radical prostatectomy. Am. J. Surg. Pathol. 2016; 40; 1400–1406. [DOI] [PubMed] [Google Scholar]
- 6. Dong F, Yang P, Wang C et al Architectural heterogeneity and cribriform pattern predict adverse clinical outcome for Gleason grade 4 prostatic adenocarcinoma. Am. J. Surg. Pathol. 2013; 37; 1855–1861. [DOI] [PubMed] [Google Scholar]
- 7. Haffner MC, Salles DC, Gao G, Epstein JI. Gleason pattern 4 with cribriform morphology on biopsy is associated with adverse clinicopathological findings in a prospective radical prostatectomy cohort. Hum. Pathol. 2020; 98; 74–80. [DOI] [PubMed] [Google Scholar]
- 8. Keefe DT, Schieda N, El Hallani S et al Cribriform morphology predicts upstaging after radical prostatectomy in patients with Gleason score 3 + 4 = 7 prostate cancer at transrectal ultrasound (TRUS)‐guided needle biopsy. Virchows Arch. 2015; 467; 437–442. [DOI] [PubMed] [Google Scholar]
- 9. Kir G, Sarbay BC, Gümüş E, Topal CS. The association of the cribriform pattern with outcome for prostatic adenocarcinomas. Pathol. Res. Pract. 2014; 210; 640–644. [DOI] [PubMed] [Google Scholar]
- 10. Kweldam CF, Kummerlin IP, Nieboer D et al Prostate cancer outcomes of men with biopsy Gleason score 6 and 7 without cribriform or intraductal carcinoma. Eur. J. Cancer 2016; 66; 26–33. [DOI] [PubMed] [Google Scholar]
- 11. Kweldam CF, Kummerlin IP, Nieboer D et al Disease‐specific survival of patients with invasive cribriform and intraductal prostate cancer at diagnostic biopsy. Mod. Pathol. 2016; 29; 630–636. [DOI] [PubMed] [Google Scholar]
- 12. Kweldam CF, Wildhagen MF, Steyerberg EW, Bangma CH, van der Kwast TH, van Leenders GJ. Cribriform growth is highly predictive for postoperative metastasis and disease‐specific death in Gleason score 7 prostate cancer. Mod. Pathol. 2015; 28; 457–464. [DOI] [PubMed] [Google Scholar]
- 13. Luo X, Khurana JS, Jhala N, Zhao H, Wang H. The association of invasive cribriform lesions with adverse prostatic adenocarcinoma outcomes: an institutional experience, systematic review, and meta‐analysis. Arch. Pathol. Lab. Med. 2019; 143; 1012–1021. [DOI] [PubMed] [Google Scholar]
- 14. McKenney JK, Wei W, Hawley S et al Histologic grading of prostatic adenocarcinoma can be further optimized: analysis of the relative prognostic strength of individual architectural patterns in 1275 patients from the Canary retrospective cohort. Am. J. Surg. Pathol. 2016; 40; 1439–1456. [DOI] [PubMed] [Google Scholar]
- 15. Trudel D, Downes MR, Sykes J, Kron KJ, Trachtenberg J, van der Kwast TH. Prognostic impact of intraductal carcinoma and large cribriform carcinoma architecture after prostatectomy in a contemporary cohort. Eur. J. Cancer 2014; 50; 1610–1616. [DOI] [PubMed] [Google Scholar]
- 16. Harding‐Jackson N, Kryvenko ON, Whittington EE et al Outcome of Gleason 3 + 5 = 8 prostate cancer diagnosed on needle biopsy: prognostic comparison with Gleason 4 + 4 = 8. J. Urol. 2016; 196; 1076–1081. [DOI] [PubMed] [Google Scholar]
- 17. Kimura K, Tsuzuki T, Kato M et al Prognostic value of intraductal carcinoma of the prostate in radical prostatectomy specimens. Prostate 2014; 74; 680–687. [DOI] [PubMed] [Google Scholar]
- 18. Van der Kwast T, Al Daoud N, Collette L et al Biopsy diagnosis of intraductal carcinoma is prognostic in intermediate and high risk prostate cancer patients treated by radiotherapy. Eur. J. Cancer 2012; 48; 1318–1325. [DOI] [PubMed] [Google Scholar]
- 19. Yamamoto A, Kato M, Matsui H et al Efficacy of docetaxel in castration‐resistant prostate cancer patients with intraductal carcinoma of the prostate. Int. J. Clin. Oncol. 2018; 23; 584–590. [DOI] [PubMed] [Google Scholar]
- 20. Zhao J, Liu J, Sun G et al The prognostic value of the proportion and architectural patterns of intraductal carcinoma of the prostate in patients with de novo metastatic prostate cancer. J. Urol. 2019; 201; 759–768. [DOI] [PubMed] [Google Scholar]
- 21. Zhao J, Shen P, Sun G et al The prognostic implication of intraductal carcinoma of the prostate in metastatic castration‐resistant prostate cancer and its potential predictive value in those treated with docetaxel or abiraterone as first‐line therapy. Oncotarget 2017; 8; 55374–55383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Guo CC, Epstein JI. Intraductal carcinoma of the prostate on needle biopsy: histologic features and clinical significance. Mod. Pathol. 2006; 19; 1528–1535. [DOI] [PubMed] [Google Scholar]
- 23. van Leenders G, van der Kwast TH, Grignon DJ et al The 2019 International Society of Urological Pathology (ISUP) consensus conference on grading of prostatic carcinoma. Am. J. Surg. Pathol. 2020; 44; e87–e99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hollemans E, Verhoef EI, Bangma CH et al Large cribriform growth pattern identifies ISUP grade 2 prostate cancer at high risk for recurrence and metastasis. Mod. Pathol. 2019; 32; 139–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Oliai BR, Kahane H, Epstein JI. Can basal cells be seen in adenocarcinoma of the prostate?: an immunohistochemical study using high molecular weight cytokeratin (clone 34betaE12) antibody. Am. J. Surg. Pathol. 2002; 26; 1151–1160. [DOI] [PubMed] [Google Scholar]
- 26. Epstein JI, Amin MB, Fine SW et alThe 2019 Genitourinary Pathology Society (GUPS) white paper on contemporary grading of prostate cancer. Arch. Pathol. Lab. Med. 2020. E‐pub ahead of print. [DOI] [PubMed]
- 27. Gandhi JS, Smith SC, Paner GP et al Reporting practices and resource utilization in the era of intraductal carcinoma of the prostate: a survey of genitourinary subspecialists. Am. J. Surg. Pathol. 2020; 44; 673–680. [DOI] [PubMed] [Google Scholar]
- 28. Chen‐Maxwell D, Prendeville S.Grading of prostate cancer: the impact of including intraductal carcinoma on the overall grade group assigned in diagnostic biopsies. Histopathology 2020. E‐pub ahead of print. [DOI] [PubMed]
- 29. Rijstenberg LL, Hansum T, Hollemans E et alIntraductal carcinoma has minimal impact on Grade Group assignment in prostate cancer biopsy and radical prostatectomy specimens. Histopathology 2020. E‐pub ahead of print. [DOI] [PMC free article] [PubMed]
- 30. Amin MB, Lin DW, Gore JL et al The critical role of the pathologist in determining eligibility for active surveillance as a management option in patients with prostate cancer: consensus statement with recommendations supported by the College of American Pathologists, International Society of Urological Pathology, Association of Directors of Anatomic and Surgical Pathology, the New Zealand Society of Pathologists, and the Prostate Cancer Foundation. Arch. Pathol. Lab. Med. 2014; 138; 1387–1405. [DOI] [PubMed] [Google Scholar]
- 31. Egevad L, Algaba F, Berney DM et al Interactive digital slides with heat maps: a novel method to improve the reproducibility of Gleason grading. Virchows Arch. 2011; 459; 175–182. [DOI] [PubMed] [Google Scholar]
- 32. McKenney JK, Simko J, Bonham M et al The potential impact of reproducibility of Gleason grading in men with early stage prostate cancer managed by active surveillance: a multi‐institutional study. J. Urol. 2011; 186; 465–469. [DOI] [PubMed] [Google Scholar]
- 33. Morash C, Tey R, Agbassi C et al Active surveillance for the management of localized prostate cancer: guideline recommendations. Can. Urol. Assoc. J. 2015; 9; 171–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sato S, Kimura T, Yorozu T et al Cases having a Gleason score 3+4=7 with <5% of Gleason pattern 4 in prostate needle biopsy show similar failure‐free survival and adverse pathology prevalence to Gleason score 6 cases in a radical prostatectomy cohort. Am. J. Surg. Pathol. 2019; 43; 1560–1565. [DOI] [PubMed] [Google Scholar]
- 35. Zhou M, Li J, Cheng L et al Diagnosis of ‘poorly formed glands’ Gleason pattern 4 prostatic adenocarcinoma on needle biopsy: an interobserver reproducibility study among urologic pathologists with recommendations. Am. J. Surg. Pathol. 2015; 39; 1331–1339. [DOI] [PubMed] [Google Scholar]
- 36. McKenney JK. The present and future of prostate cancer histopathology. Curr. Opin. Urol. 2017; 27; 464–468. [DOI] [PubMed] [Google Scholar]
- 37. Truong M, Frye T, Messing E, Miyamoto H. Historical and contemporary perspectives on cribriform morphology in prostate cancer. Nat. Rev. Urol. 2018; 15; 475–482. [DOI] [PubMed] [Google Scholar]
- 38. Hollemans E, Verhoef EI, Bangma CH et al Clinical outcome comparison of Grade Group 1 and Grade Group 2 prostate cancer with and without cribriform architecture at the time of radical prostatectomy. Histopathology 2020; 76; 755–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kryvenko ON, Gupta NS, Virani N et al Gleason score 7 adenocarcinoma of the prostate with lymph node metastases: analysis of 184 radical prostatectomy specimens. Arch. Pathol. Lab. Med. 2013; 137; 610–617. [DOI] [PubMed] [Google Scholar]
- 40. Tom MC, Nguyen JK, Luciano R et al Impact of cribriform pattern and intraductal carcinoma on Gleason 7 prostate cancer treated with external beam radiotherapy. J. Urol. 2019; 202; 710–716. [DOI] [PubMed] [Google Scholar]
- 41. Yamamoto A, Kato M, Hattori K et al Propensity score‐matched comparison of docetaxel and androgen receptor axis‐targeted agents in patients with castration‐resistant intraductal carcinoma of the prostate. BJU Int. 2020; 125; 702–708. [DOI] [PubMed] [Google Scholar]
- 42. Ericson KJ, Wu S, Lundy SD, Thomas LJ, Klein EA, McKenney JK. Diagnostic accuracy of prostate biopsy for detecting cribriform Gleason pattern 4 carcinoma and intraductal carcinoma in paired radical prostatectomy specimens: implications for active surveillance. J. Urol. 2020; 203; 311–319. [DOI] [PubMed] [Google Scholar]
- 43. Hollemans E, Verhoef EI, Bangma CH et al Concordance of cribriform architecture in matched prostate cancer biopsy and radical prostatectomy specimens. Histopathology 2019; 75; 338–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Masoomian M, Downes MR, Sweet J et al Concordance of biopsy and prostatectomy diagnosis of intraductal and cribriform carcinoma in a prospectively collected dataset. Histopathology 2019; 74; 474–482. [DOI] [PubMed] [Google Scholar]
- 45. Gao J, Zhang Q, Fu Y et al Combined clinical characteristics and multiparametric MRI parameters for prediction of cribriform morphology in intermediate‐risk prostate cancer patients. Urol. Oncol. 2020; 38; 216–224. [DOI] [PubMed] [Google Scholar]
- 46. Prendeville S, Gertner M, Maganti M et al Role of magnetic resonance imaging targeted biopsy in detection of prostate cancer harboring adverse pathological features of intraductal carcinoma and invasive cribriform carcinoma. J. Urol. 2018; 200; 104–113. [DOI] [PubMed] [Google Scholar]
- 47. Greenland NY, Zhang L, Cowan JE, Carroll PR, Stohr BA, Simko JP. Correlation of a commercial genomic risk classifier with histologic patterns in prostate cancer. J. Urol. 2019; 202; 90–95. [DOI] [PubMed] [Google Scholar]
- 48. Greenland NY, Cowan JE, Zhang L et al Expansile cribriform Gleason pattern 4 has histopathologic and molecular features of aggressiveness and greater risk of biochemical failure compared to glomerulation Gleason pattern 4. Prostate 2020; 80; 653–659. [DOI] [PubMed] [Google Scholar]
- 49. Taylor AS, Morgan TM, Wallington DG, Chinnaiyan AM, Spratt DE, Mehra R. Correlation between cribriform/intraductal prostatic adenocarcinoma and percent Gleason pattern 4 to a 22‐gene genomic classifier. Prostate 2020; 80; 146–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lee TK, Ro JY. Spectrum of cribriform proliferations of the prostate: from benign to malignant. Arch. Pathol. Lab. Med. 2018; 142; 938–946. [DOI] [PubMed] [Google Scholar]
- 51. Shah RB, Zhou M. Atypical cribriform lesions of the prostate: clinical significance, differential diagnosis and current concept of intraductal carcinoma of the prostate. Adv. Anat. Pathol. 2012; 19; 270–278. [DOI] [PubMed] [Google Scholar]
- 52. Wobker SE, Epstein JI. Differential diagnosis of intraductal lesions of the prostate. Am. J. Surg. Pathol. 2016; 40; e67–e82. [DOI] [PubMed] [Google Scholar]
- 53. Egevad L, Ahmad AS, Algaba F et al Standardization of Gleason grading among 337 European pathologists. Histopathology 2013; 62; 247–256. [DOI] [PubMed] [Google Scholar]
- 54. Kweldam CF, Nieboer D, Algaba F et al Gleason grade 4 prostate adenocarcinoma patterns: an inter‐observer agreement study among genitourinary pathologists. Histopathology 2016; 69; 441–449. [DOI] [PubMed] [Google Scholar]
- 55. Verhoef EI, van Cappellen WA, Slotman JA et al Three‐dimensional analysis reveals two major architectural subgroups of prostate cancer growth patterns. Mod. Pathol. 2019; 32; 1032–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. van Leenders G, Kweldam CF, Hollemans E et al Improved prostate cancer biopsy grading by incorporation of invasive cribriform and intraductal carcinoma in the 2014 grade groups. Eur. Urol. 2020; 77; 191–198. [DOI] [PubMed] [Google Scholar]
- 57. Hollemans E, Verhoef EI, Bangma CH et al Clinicopathological characteristics of glomeruloid architecture in prostate cancer. Mod. Pathol. 2020; 33; 1618–1625. [DOI] [PubMed] [Google Scholar]
- 58. Lotan TL, Epstein JI. Gleason grading of prostatic adenocarcinoma with glomeruloid features on needle biopsy. Hum. Pathol. 2009; 40; 471–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Flood TA, Schieda N, Keefe DT et al Utility of Gleason pattern 4 morphologies detected on transrectal ultrasound (TRUS)‐guided biopsies for prediction of upgrading or upstaging in Gleason score 3 + 4 = 7 prostate cancer. Virchows Arch. 2016; 469; 313–319. [DOI] [PubMed] [Google Scholar]
- 60. Acosta AM, Al Rasheed MRH, Rauscher GH et al Tumor necrosis in radical prostatectomies with high‐grade prostate cancer is associated with multiple poor prognostic features and a high prevalence of residual disease. Hum. Pathol. 2018; 75; 1–9. [DOI] [PubMed] [Google Scholar]
- 61. Flood TA, Schieda N, Sim J et al Evaluation of tumor morphologies and association with biochemical recurrence after radical prostatectomy in grade group 5 prostate cancer. Virchows Arch. 2018; 472; 205–212. [DOI] [PubMed] [Google Scholar]
- 62. Böttcher R, Kweldam CF, Livingstone J et al Cribriform and intraductal prostate cancer are associated with increased genomic instability and distinct genomic alterations. BMC Cancer 2018; 18; 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Chua MLK, Lo W, Pintilie M et al A prostate cancer ‘nimbosus’: genomic instability and SChLAP1 dysregulation underpin aggression of intraductal and cribriform subpathologies. Eur. Urol. 2017; 72; 665–674. [DOI] [PubMed] [Google Scholar]
- 64. Elfandy H, Armenia J, Pederzoli F et al Genetic and epigenetic determinants of aggressiveness in cribriform carcinoma of the prostate. Mol. Cancer Res. 2019; 17; 446–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Qian J, Jenkins RB, Bostwick DG. Detection of chromosomal anomalies and c‐myc gene amplification in the cribriform pattern of prostatic intraepithelial neoplasia and carcinoma by fluorescence in situ hybridization. Mod. Pathol. 1997; 10; 1113–1119. [PubMed] [Google Scholar]
- 66. Ronen S, Abbott DW, Kravtsov O et al PTEN loss and p27 loss differ among morphologic patterns of prostate cancer, including cribriform. Hum. Pathol. 2017; 65; 85–91. [DOI] [PubMed] [Google Scholar]
- 67. Böttcher R, Hoogland AM, Dits N et al Novel long non‐coding RNAs are specific diagnostic and prognostic markers for prostate cancer. Oncotarget 2015; 6; 4036–4050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Ahearn TU, Pettersson A, Ebot EM et al A prospective investigation of PTEN loss and ERG expression in lethal prostate cancer. J. Natl Cancer Inst. 2016; 108; djv346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Jamaspishvili T, Patel PG, Niu Y et alRisk stratification of prostate cancer through quantitative assessment of PTEN loss (qPTEN). J. Natl Cancer Inst. 2020. E‐pub ahead of print. [DOI] [PMC free article] [PubMed]
- 70. Liu W, Xie CC, Thomas CY et al Genetic markers associated with early cancer‐specific mortality following prostatectomy. Cancer 2013; 119; 2405–2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Mehra R, Salami SS, Lonigro R et al Association of ERG/PTEN status with biochemical recurrence after radical prostatectomy for clinically localized prostate cancer. Med. Oncol. 2018; 35; 152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Prensner JR, Iyer MK, Sahu A et al The long noncoding RNA SChLAP1 promotes aggressive prostate cancer and antagonizes the SWI/SNF complex. Nat. Genet. 2013; 45; 1392–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Ribeiro FR, Henrique R, Martins AT, Jerónimo C, Teixeira MR. Relative copy number gain of MYC in diagnostic needle biopsies is an independent prognostic factor for prostate cancer patients. Eur. Urol. 2007; 52; 116–125. [DOI] [PubMed] [Google Scholar]
- 74. Kolijn K, Verhoef EI, van Leenders GJ. Morphological and immunohistochemical identification of epithelial‐to‐mesenchymal transition in clinical prostate cancer. Oncotarget 2015; 6; 24488–24498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Williams JL, Greer PA, Squire JA. Recurrent copy number alterations in prostate cancer: an in silico meta‐analysis of publicly available genomic data. Cancer Genet. 2014; 207; 474–488. [DOI] [PubMed] [Google Scholar]
- 76. Andrew DJ, Ewald AJ. Morphogenesis of epithelial tubes: insights into tube formation, elongation, and elaboration. Dev. Biol. 2010; 341; 34–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Cucchiara V, Cooperberg MR, Dall’Era M, et al Genomic markers in prostate cancer decision making. Eur. Urol. 2018; 73; 572–582. [DOI] [PubMed] [Google Scholar]
- 78. Kristiansen G. Markers of clinical utility in the differential diagnosis and prognosis of prostate cancer. Mod. Pathol. 2018; 31; S143–S155. [DOI] [PubMed] [Google Scholar]
- 79. Boag AH, Kennedy LA, Miller MJ. Three‐dimensional microscopic image reconstruction of prostatic adenocarcinoma. Arch. Pathol. Lab. Med. 2001; 125; 562–566. [DOI] [PubMed] [Google Scholar]
- 80. Tolkach Y, Thomann S, Kristiansen G. Three‐dimensional reconstruction of prostate cancer architecture with serial immunohistochemical sections: hallmarks of tumour growth, tumour compartmentalisation, and implications for grading and heterogeneity. Histopathology 2018; 72; 1051–1059. [DOI] [PubMed] [Google Scholar]
- 81. Glaser AK, Reder NP, Chen Y et al Multi‐immersion open‐top light‐sheet microscope for high‐throughput imaging of cleared tissues. Nat. Commun. 2019; 10; 2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Reder NP, Glaser AK, McCarty EF, Chen Y, True LD, Liu JTC. Open‐top light‐sheet microscopy image atlas of prostate core needle biopsies. Arch. Pathol. Lab. Med. 2019; 143; 1069–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. van Royen ME, Verhoef EI, Kweldam CF et al Three‐dimensional microscopic analysis of clinical prostate specimens. Histopathology 2016; 69; 985–992. [DOI] [PubMed] [Google Scholar]
- 84. Allsbrook WC Jr, Mangold KA, Johnson MH, Lane RB, Lane CG, Epstein JI. Interobserver reproducibility of Gleason grading of prostatic carcinoma: general pathologist. Hum. Pathol. 2001; 32; 81–88. [DOI] [PubMed] [Google Scholar]
- 85. Kweldam CF, Kummerlin IP, Nieboer D, et al Presence of invasive cribriform or intraductal growth at biopsy outperforms percentage grade 4 in predicting outcome of Gleason score 3+4=7 prostate cancer. Mod. Pathol. 2017; 30; 1126–1132. [DOI] [PubMed] [Google Scholar]
- 86. Sauter G, Steurer S, Clauditz TS, et al Clinical utility of quantitative Gleason grading in prostate biopsies and prostatectomy specimens. Eur. Urol. 2016; 69; 592–598. [DOI] [PubMed] [Google Scholar]


