Abstract
Aims
We compared the new use of sodium‐glucose cotransporter‐2 inhibitor (SGLT2i) versus dipeptidyl peptidase‐4 inhibitor (DPP4i) and the risk of cardiorenal disease, heart failure (HF) or chronic kidney disease (CKD), in patients with type 2 diabetes without a history of prevalent cardiovascular and renal disease, defined as cardiovascular and renal disease (CVRD) free, managed in routine clinical practice.
Materials and methods
In this observational cohort study, patients were identified from electronic health records from England, Germany, Japan, Norway, South Korea and Sweden, during 2012‐2018. In total, 1 006 577 CVRD‐free new users of SGLT2i or DPP4i were propensity score matched 1:1. Unadjusted Cox regression was used to estimate hazard ratios (HRs) for outcomes: cardiorenal disease, HF, CKD, stroke, myocardial infarction (MI), cardiovascular and all‐cause mortality.
Results
Baseline characteristics were well balanced between the treatment groups (n = 105 130 in each group) with total follow‐up of 187 955 patient years. Patients had a mean age of 56 years, 43% were women and they were indexed between 2013 and 2018. The most commonly used agents were dapagliflozin (91.7% of exposure time) and sitagliptin/linagliptin (55.0%), in the SGLT2i and DPP4i, groups, respectively. SGLT2i was associated with lower risk of cardiorenal disease, HF, CKD, all‐cause and cardiovascular mortality; HR (95% confidence interval), 0.56 (0.42‐0.74), 0.71 (0.59‐0.86), 0.44 (0.28‐0.69), 0.67 (0.59‐0.77), and 0.61 (0.44‐0.85), respectively. No differences were observed for stroke [0.87 (0.69‐1.09)] and MI [0.94 (0.80‐1.11)].
Conclusion
In this multinational observational study, SGLT2i was associated with a lower risk of HF and CKD versus DPP4i in patients with type 2 diabetes otherwise free from both cardiovascular and renal disease.
Keywords: dapagliflozin, diabetic nephropathy, DPP‐IV inhibitor, heart failure, observational study, SGLT2 inhibitor
1. INTRODUCTION
Heart failure (HF) and chronic kidney disease (CKD) have been shown to be reaching epidemic proportions, 1 , 2 , 3 particularly in patients with type 2 diabetes, with negative consequences on quality of life, concomitant disease risks and health care utilization. 1 , 3 , 4 , 5 , 6 The combination of high prevalence of type 2 diabetes, 425 million patients world‐wide 7 and associated elevated risks of HF and CKD puts these patients in a particularly vulnerable group. 8
Despite successful treatment strategies addressing prevention of atherosclerotic cardiovascular disease, 9 significant residual risks of HF and CKD in type 2 diabetes have been reported from several studies. 10 , 11 , 12 , 13 One study reported that optimal management of cardiovascular risk factors in type 2 diabetes might neutralize the excess risk of myocardial infarction (MI) and stroke, but not the risk for HF, which remained high when compared with patients without type 2 diabetes. 13 Other reports have shown that CKD prevalence and mortality risk in clinical practice remain high despite widely used renin‐angiotensin inhibition. 10 , 11 In addition, a large multinational study including both European and Asian countries, reported that HF and CKD are the most frequent first manifestations of cardiovascular and renal diseases (CVRD) in patients without prevalent cardiovascular or renal diseases (defined as CVRD‐free). 8 Findings from this study also demonstrated that these complications were associated with high risk of subsequent mortality, highlighting the prevalence and impact of cardiorenal diseases in the type 2 diabetes population. 8 Therefore, there is an unmet clinical need and urgent requirement for more effective cardiorenal risk prevention strategies for HF and CKD in type 2 diabetes. 14 DECLARE‐TIMI 58 and CANVAS are cardiovascular outcome trials (CVOTs) with sodium‐glucose transporter‐2 inhibitors (SGLT2i), which demonstrated preventive effects on cardiorenal disease in patients with type 2 diabetes with both atherosclerotic cardiovascular disease and cardiovascular risk factors, HF or overt CKD. 15 , 16 , 17 , 18 Recent trials have extended preventive effects to treatment effects to patients with HF with reduced ejection fraction and potentially in patients with CKD. 19 , 20
Little is known about how the primary preventative effects of SGLT2i on cardiorenal disease shown in randomized controlled trials translate into a broad real‐world population from a clinical practice setting and, further, how these effects may compare with other commonly used novel oral antihyperglycaemic agents. 15 , 17 , 21 Dipeptidyl peptidase 4 inhibitor (DPP4i) belongs to a class of widely used glucose‐lowering drugs (GLDs), which have been shown to be associated with cardiovascular and renal safety in several large clinical trials. 22 , 23 , 24 Both SGLT2i and DPP4i are oral drugs of comparative costs being recommended for use after metformin in patients with CVRD‐free type 2 diabetes, hence well suited to be compared as common treatment options in a real‐world clinical setting. 25
In this multinational and contemporary cohort study from Europe and Asia we evaluated cardiorenal disease risk with new initiation of SGLT2i compared with those with new initiation of DPP4i in propensity score matched patients with type 2 diabetes without prevalent CVRD.
2. MATERIALS AND METHODS
This study utilizes available linked electronic health care records across six countries: England, Germany, Japan, Norway, South Korea and Sweden. Additional details of the individual datasets can be found in Supporting Information (pp. 3‐5).
2.1. Study population
All patients with type 2 diabetes, defined by use of GLDs and/or diagnosis codes (Supporting Information, p. 6), 8 without any recorded pre‐index history of cardiovascular or renal disease, defined as stroke, MI, angina pectoris (including the use of nitrates), unstable angina pectoris, atrial fibrillation, HF, coronary revascularization, peripheral artery disease, peripheral artery revascularization and CKD (chronic/acute/unspecified kidney disease, hypertensive kidney failure and diabetic nephropathy), were eligible, hereinafter referred to as CVRD‐free type 2 diabetes patients (Table S1). 8 Diseases and treatments were searched in prescribed drug and hospital records in all countries except in England where additional general practice records were searched.
A new‐user event date (index date) was defined as the date of the initial filled prescription for an SGLT2i or a DPP4i preceded by a 12‐month period without any filled prescription for the same drug classes. 26 For the sensitivity analysis comparing SGLT2i with new use of any other GLD (oGLD), the index date for any initiation of oGLD was defined by all filled prescriptions of a new GLD class other than SGLT2i. 27 This oGLD definition allowed for several possible new‐user dates for a patient within the observation period, both within drug class and between classes.
2.2. Baseline
Patient characteristics were described at index, including age, sex, index date, drug treatment and comorbidities (see Table S2 for detailed definitions). Comorbidities were searched for in all available data before and including the index date, with the exception of severe hypoglycaemia (up to 12 months before the index date) and cancer (up to 5 years before the index date) (see Table S1 for detailed definitions). 8 Previous medications were defined as any drugs received within the 12 months preceding and including the index date (see Table S2 for detailed definitions).
2.3. Outcomes
First event of recorded hospital diagnoses of cardiorenal disease (diagnosis of HF or CKD), HF (including hypertensive HF), CKD (including diabetic nephropathy, acute kidney failure, unspecified kidney disease, hypertensive kidney failure and dialysis), stroke (including ischaemic and haemorrhagic stroke), MI, and all‐cause and cardiovascular mortality were analysed as outcomes (Table S3).
2.4. Statistical analysis
Baseline characteristics were described using standard statistical measures such as mean and standard deviations for numerical variables, and frequencies and percentages for categorical variables. An imbalance in baseline characteristics was considered if a >10% standardized difference occurred between the two groups. The CVRD‐free populations are described separately by country and the overall summary is weighted according to the number of patients from each country. The proportion of exposure time contributed by individual agents was summarized both overall and by country.
To avoid immortal time bias, only the first incident episode during the inclusion period of either SGLT2i or DPP4i treatment was eligible for inclusion. 28 One year of not using/filling prescriptions of both SGLT2i and DPP4i was required prior to index. Patients initiated on an SGLT2i and a DPP4i on the same date were excluded.
A propensity score for initiating SGLT2i was developed (separately within each country) for each episode of new treatment initiation using an extensive number of variables (Supporting Information, pp. 6‐7). Based on propensity scores, patients initiating SGLT2i were matched 1:1 with patients initiating DPP4i. The adequacy of matching was assessed by evaluating post‐match standardized differences in patient characteristics (Table 1).
TABLE 1.
Baseline characteristics of patients with type 2 diabetes free from cardiovascular and renal disease
| SGLT2i | DPP4i | Standardized difference (%) a | |
|---|---|---|---|
| Number of patients | 105 130 | 105 130 | n/a |
| Age, years (mean ± SD) | 55.7 ± 11.9 | 55.4 ± 12.6 | 2.5 |
| Females, n (%) | 45 254 (43.0) | 45 095 (42.9) | 0.3 |
| Microvascular complications, n (%) | 25 830 (24.6) | 24 870 (23.7) | 2.1 |
| Frailty, n (%) | 11 146 (10.6) | 10 980 (10.4) | 0.5 |
| CVD prevention, n (%) | |||
| Statins | 56 688 (53.9) | 56 318 (53.6) | 0.7 |
| Antihypertensives | 59 559 (56.7) | 58 776 (55.9) | 1.5 |
| ACE inhibitors | 17 358 (16.5) | 17 111 (16.3) | 0.6 |
| ARBs | 35 705 (34.0) | 35 398 (33.7) | 0.6 |
| Beta blockers | 15 478 (14.7) | 15 248 (14.5) | 0.6 |
| Loop diuretics | 4490 (4.3) | 4343 (4.1) | 0.7 |
| Aldosterone antagonists | 1400 (1.3) | 1339 (1.3) | 0.5 |
| Glucose‐lowering drugs, n (%) | |||
| Metformin | 81 010 (77.1) | 81 435 (77.5) | 1.0 |
| Sulphonylurea | 31 804 (30.3) | 31 068 (29.6) | 1.5 |
| GLP1‐RA | 4747 (4.5) | 3955 (3.8) | 3.8 |
| Thiazolidinediones | 7507 (7.1) | 7105 (6.8) | 1.5 |
| Insulin | 18 012 (17.1) | 17 395 (16.5) | 1.6 |
| Index year | |||
| 2013 | 1657 (2.6) | 1593 (2.5) | 0.6 |
| 2014 | 11 231 (10.7) | 10 839 (10.3) | 1.2 |
| 2015 | 24 681 (23.5) | 24 715 (23.5) | 0.1 |
| 2016 | 33 295 (31.7) | 33 102 (31.5) | 0.4 |
| 2017 | 16 178 (25.5) | 16 300 (25.7) | 0.4 |
| 2018 | 18 081 (28.5) | 18 578 (29.3) | 1.7 |
Note: All numbers in parenthesis are percentages if not stated otherwise. Frailty, three or more consecutive days in hospital within the year before the index.
Abbreviations: ACEi, angiotensin‐converting enzyme inhibitor; ARBs, angiotensin receptor blockers; CVD, cardiovascular disease; DPP4i, dipeptidyl‐peptidase‐4 inhibitors; GLP‐1RA, glucagon‐like peptide‐1 receptor agonist; SD, standard deviation; SGLT2i, sodium‐glucose cotransporter‐2 inhibitors.
An imbalance in baseline characteristics was considered when standardized difference >10%.
The time to first event was compared between groups using Cox proportional hazards models, presented as hazard ratios [HR; 95% confidence interval (CI)] for each outcome separately by country. Patients were observed from the index date until discontinuation of the index drug (where 6 months grace time after the last filled prescription was allowed), death or end of study. To explore the effects of off‐treatment time, patients were followed from the start of index treatment until either occurrence of the first outcome event, or the censoring date (whichever came first), regardless of whether index treatment was discontinued. When calculating the off‐treatment time, the added effects of grace time were not possible to assess.
The HRs for each endpoint from each individual country were then pooled for an overall weighted summary, 29 with random‐effects models with inverse variance weighting for each country implemented. 30 Forest plots displaying country‐specific HRs and pooled overall HR were produced. Analyses were also repeated in the same cohort regardless of whether index treatment was discontinued. In addition, to test the robustness of our results, an analysis that removed the data from one country at a time was performed. Subgroup analyses were multiple adjusted and tested for interactions. Multiple adjustments were performed using the following variables: age, gender, frailty (at least one hospitalization of three consecutive days within 1 year before index), duration of diabetes (if available), use of angiotensin‐converting enzyme inhibitors or angiotensin receptor blockers, β‐blockers, Ca2 +‐channel blockers and aldosterone antagonists.
In a few of the countries (England, Japan, Norway and Sweden), where estimated glomerulus filtration rate (eGFR, mL/min/1.73 m2) measurements were available for a subset of patients, the validity of the CKD definition was tested using a simplistic method where all patients with type 2 diabetes were classified as CKD yes/no based on all available data (one diagnosis), and the latest available eGFR measurement was used. The predictive probabilities of eGFR for CKD diagnosis were tested using receiver operating characteristic curves (ROC), including area under the curve of the receiver operating characteristic curve. In addition, the optimal cut‐off was estimated using the Youden index. The validity of CKD diagnoses only set in primary care and outpatient hospital visits were tested separately.
3. RESULTS
Before matching, the majority of variables between the SGLT2i and DPP4i group were similar, standardized difference <10%, but with differences in age, GLP1‐RA, metformin and insulin treatment (Figure S1). After matching (Figure 1), baseline characteristics were well balanced between the SGLT2i and DPP4i groups (n = 105 130 in each group) with mean follow‐up of 1.5 years (311 992 patient‐years) (Table 1 and Figure S1). The mean patient age was 56 years, 43% were women, and there was moderate use of statins (54%) and antihypertensives (57%). Similar findings were found when comparing SGLT2i versus oGLD (Table S4).
FIGURE 1.
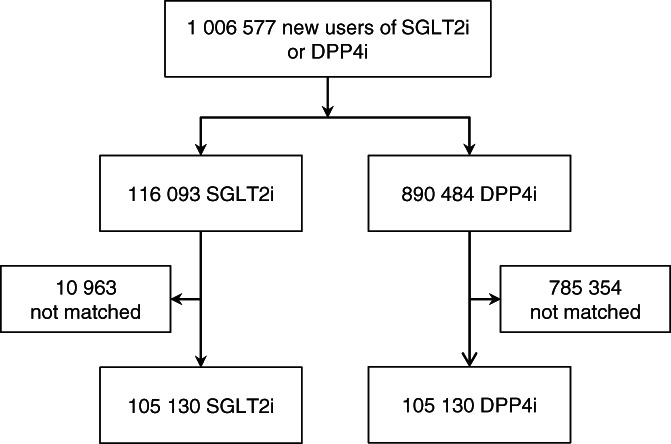
Patient flow‐chart of patients with type 2 diabetes without cardiovascular or renal disease. DPP4i, dipeptidyl peptidase‐4 inhibitor; SGLT2i, sodium‐glucose cotransporter‐2 inhibitor
Figure 2 shows the distribution of follow‐up time for SGLT2i and DPP4i types (details in Table S5). The SGLT2i group was dominated by dapagliflozin (91.7%), whereas the DPP4i group mainly consisted of sitagliptin (29%), linagliptin (26%), gemigliptin (14%), vildagliptin (11%) and alogliptin (8%). Event rates of cardiorenal disease in the SGLT2i and DPP4i groups were 6.4 and 11.5 events per 100 patient‐years, respectively (Figure 3). SGLT2i, compared with DPP4i, was associated with a lower incidence of cardiorenal disease; HR (95% CI) 0.56 (0.42‐0.74) (Figure 3).
FIGURE 2.
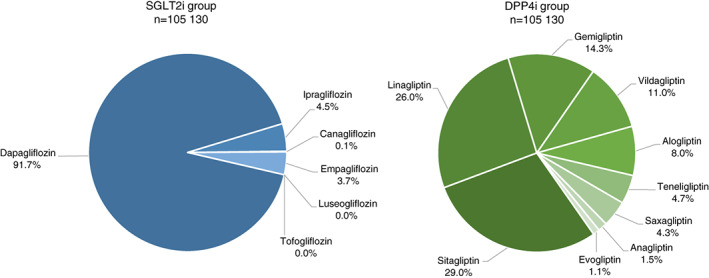
Distribution of follow‐up time for the different SGLT2i and DPP4i types. DPP4i, dipeptidyl peptidase‐4 inhibitor; SGLT2i, sodium‐glucose cotransporter‐2 inhibitor
FIGURE 3.
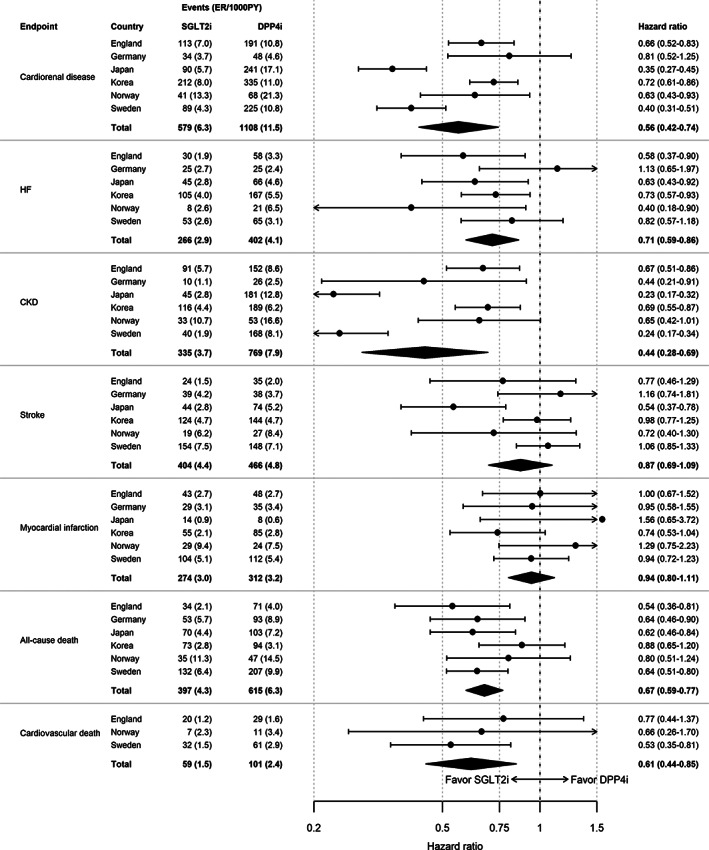
Risks of cardiorenal disease, cardiovascular disease and death in patients with type 2 diabetes free from cardiovascular and renal disease. ER, event rates; PY, patient‐years; CKD, chronic kidney disease; DPP4i, dipeptidyl peptidase‐4 inhibitor; HF, heart failure; SGLT2i, sodium‐glucose cotransporter‐2 inhibitor
Event rates of HF, CKD, all‐cause and cardiovascular death for SGLT2i/DPP4i were 2.9/4.1, 3.7/7.9, 4.3/6.3 and 1.5/2.4, respectively (Figure 3). Significant beneficial associations for SGLT2i were also observed for the individual components of HF [0.71 (0.59‐0.86)] and CKD [0.44 (0.28‐0.69)] and with the mortality outcomes of all‐cause death and cardiovascular death, 0.67 (0.59‐0.77) and 0.61 (0.44‐0.85), respectively (Figure 3). Neutral associations were observed for stroke and MI, 0.87 (0.69‐1.09) and 0.94 (0.80‐1.11), respectively.
3.1. Sensitivity analyses
When working stepwise and excluding one single country at a time, risk estimates remained similar and significant (Figure S3), indicating that the overall conclusion was not caused by one separate country. The multiple adjusted model showed near identical and significant results compared with the unadjusted model (Figure S4). When comparing SGLT2i versus oGLD, similar risk‐lowering effects were observed for cardiorenal disease, HF, CKD, all‐cause and cardiovascular death, HR (95% CI) 0.56 (0.50‐0.63), 0.69 (0.60‐0.80), 0.48 (0.38‐0.61), 0.54 (0.45‐0.64), 0.65 (0.52‐0.82), respectively (Figure S5). Subgroup analyses for cardiorenal disease were all in favour of SGLT2i and there were no significant interactions (Figure 4). For all the other endpoints, no interaction was observed except for age for the risk of HF (Figure S6).
FIGURE 4.
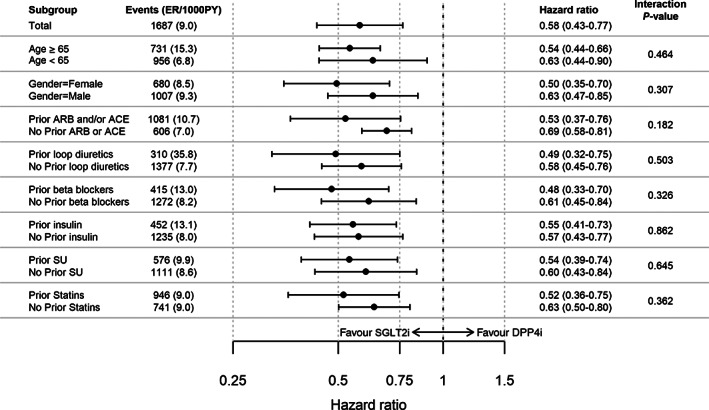
Subgroup analyses of cardiorenal disease in patients with type 2 diabetes free from cardiovascular and renal disease. ER, event rates; PY, patient‐years; ACE, angiotensin converting enzyme; ARB, angiotensin receptor blockers; DPP4i, dipeptidyl peptidase‐4 inhibitor; loop diuretics, high ceiling diuretics; RAASi, renin angiotensin aldosterone system inhibitor; SGLT2i, sodium‐glucose cotransporter‐2 inhibitor
When including the follow‐up time after discontinuation of index treatment, an added ‘off‐treatment’ follow‐up time by 66% was observed, similar in both the SGLT2i and DPP4i groups (Figure S7). Here, similar and significant results were observed for cardiorenal disease, HF, CKD, all‐cause and cardiovascular death, HR (95% CI) 0.68 (0.55‐0.84), 0.88 (0.79‐0.98), 0.56 (0.40‐0.78), 0.74 (0.67‐0.82) and 0.72 (0.56‐0.94), respectively (Figure S8).
Validation of the CKD definition using available eGFR data showed robust sensitivity and specificity results across multiple countries (Figure S9a). Detailed validation of CKD diagnosis registered in outpatient clinic and primary care visits, showed similar results (Figures S9b and S9c).
4. DISCUSSION
In this multinational study of 210 060 patients with CVRD‐free type 2 diabetes across six countries in Asia and Europe, we have shown a new use for SGLT2i associated with significant primary preventive effects of cardiorenal disease compared with a new use for DPP4i. Significant beneficial associations were also observed for the individual components of HF and CKD and with the mortality outcomes of all‐cause and CV mortality. The associations were consistent across all countries and subgroups. No association with MI and stroke risk was observed. When compared with new use of any other GLDs and multiple sensitivity tests, the results remained similar.
Whereas many CVOTs have shown beneficial SGLT2i effects on HF and kidney function in high‐risk populations (patients with type 2 diabetes with atherosclerotic cardiovascular disease), 15 , 16 , 17 , 31 a small number of trials have also reported on cardiorenal effects in low‐risk populations. 15 , 17 The DECLARE‐TIMI 58 trial included 17 160 patients with type 2 diabetes, of which 59% had no atherosclerotic cardiovascular disease, but multiple risk factors (defined as ≥2 cardiovascular risk factors). 1 , 17 One of the inclusion criteria for this CVOT was an eGFR >60 mL/min × 1.73 m2, resulting in a patient population with predominantly preserved renal function (92.1% had eGFR >60 mL/min × 1.73 m2) compared with other SGLT2i CVOTs. 16 , 31 , 32 In the multi‐risk factor group, dapagliflozin showed significant risk reduction of incident cardiorenal disease, 36% [HR 0.64 (0.46‐0.88)] and 49% [HR 0.51 (0.37‐0.69)] for HF and CKD, respectively. 15 , 17 Our results show that the beneficial cardiorenal disease prevention with SGLT2i seen in patients with multiple risk factors indeed translates into a real‐world setting of comparable CVRD‐free patients managed in routine clinical practice. 15 , 17 To the best of our knowledge, the present study including younger patients (mean age 55 years) and patients with CVRD‐free type 2 diabetes is the first to validate cardiorenal prevention in a real‐world setting shown in SGLT2i CVOTs when compared with DPP4i. 15 , 17
Large observational studies and CVOTs have consistently shown SGLT2i to reduce the risk of hospitalization for HF, with similar results to the present study, 16 , 17 , 26 , 31 , 33 , 34 , 35 , 36 , 37 , 38 , 39 of which some have compared SGLT2i with DPP4i but in patients with type 2 diabetes both with and without CVRD. 26 , 39 Two of the observational studies have shown beneficial HF risk reductions specifically with SGLT2i in both general‐ and CVOT‐like type 2 diabetes populations with similar results compared with SGLT2i CVOTs. 26 , 36 However, none of these studies have studied the prevention of cardiorenal disease with SGLT2i versus DPP4i in CVRD‐free patients. The findings in our study are directionally aligned with these reports and, hence, extend the SGLT2 effects to a real‐world primary cardiorenal prevention setting when compared with the commonly used DPP4i.
In many CVOTs, SGLT2i have been shown to prevent or delay worsening of renal function also in patients without CKD. 15 , 18 In general, strong beneficial SGLT2i risk reducing effects of approximately 50% on various renal function outcomes have been reported from both clinical trials and large observational studies. 15 , 18 , 32 , 40 Our results show similar beneficial SGLT2i effects on primary renal protection, translating the effects of the early treatment demonstrated in CVOTs into a real‐world clinical setting.
Cardiovascular death event rates were lower in the SGLT2i group compared with the DPP4i group [HR 0.61 (0.44‐0.81)], and these results were directionally similar with overlapping CIs when compared with the SGLT2i CVOT meta‐analysis by Zelniker et al. [0.84 (0.75‐0.94)], and consistent with multiple large observational studies. 18 , 33 , 36 , 41 However, results from the same meta‐analysis of patients without atherosclerotic cardiovascular disease were less convincing for cardiovascular death. However, real‐world populations are in general more frail compared with CVOT populations because of unselected inclusion and having less structured follow‐up, 42 which might be associated with a lower likelihood of surviving preventable events such as HF. 36 , 43 This is also seen when comparing CVOTs; the cardiovascular mortality SGLT2i effects seem greater in more frail populations 31 , 41 , 44 , 45 compared with less frail populations. 16 , 17 , 36 , 43 Hence, the SGLT2i mortality results in a real‐world setting might be different compared with a CVOT setting, including less frail patient populations and having a more structured follow‐up. 36 , 43 Moreover, the SGLT2 effect on HF [0.71 (0.59‐0.86)] and CKD [0.44 (0.28‐0.69)] and neutral effects on MI [0.94 (0.80‐1.11)] and stroke [0.87 (0.69‐1.09)] in the present study are similar to multiple CVOTs [0.69 (0.61‐0.79), 0.55 (0.48‐0.64), 0.89 (0.80‐0.98) and 0.97 (0.86‐1.10), respectively] and observational studies, making confounding less likely. 16 , 17 , 18 , 26 , 31 , 33 , 34 , 36 Consequently, these findings support that the observed significant risk‐reducing effects with SGLT2i on cardiovascular and all‐cause mortality are reasonable in a real‐world setting where patients are probably frailer.
In the present study, DPP4i is a relevant comparator frequently used for early glucose treatment, having similar costs to SGLT2i in many countries and both being recommended options for treatment of patients with CVRD‐free type 2 diabetes. 25 DPP4i, in contrast to SGLT2i, has no cardiovascular and cardiorenal protective effects. 22 , 46 To the best of our knowledge, there are no trials assessing the relative effectiveness of cardiorenal risk reduction with SGLT2i and DPP‐4i in a primary preventative setting, thus our results represent the first and most comprehensive assessment of this comparison to date.
The primary 15 , 16 , 17 , 18 and secondary 20 , 47 preventive effects on HF and CKD seem to appear very early after initiating treatment with an SGLT2i, and are not explained by the drug's effect on known risk factors such as glycated haemoglobin, blood pressure and body weight. A plethora of mechanistic hypotheses that may mediate these effects have been raised; some of the most interesting may be the natriuretic/diuretic effects and improved renal haemodynamics, a reduction in ventricular mass and improved diastolic function and myocardial energy metabolism. 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 Nevertheless, the explanation of the beneficial cardiorenal effects of SGLT2is is unclear and further translational research is needed. 50
In summary, recent results have shown that cardiorenal disease is a very common and serious complication in, initially, patients with CVRD‐free type 2 diabetes and that it can be prevented with SGLT2i both in CVOTs and in a real‐world clinical setting. 8 , 15 , 16 , 17 , 18 These data further support increased clinical focus on identifying and implementing early cardiorenal prevention strategies in the treatment of patients with type 2 diabetes at risk for cardiorenal events.
The strength of this study is that, to the best of our knowledge, this is the first analyses of the primary prevention of cardiorenal disease with SGLT2i versus DPP4i in patients with CVRD‐free type 2 diabetes. The current analyses include populations across numerous well‐established health care registries in countries and regions covering several ethnicities, resulting in a large, widely representative data set yielding a large number of events. 8 Robust propensity score matching using multiple variables, and well‐established methods to minimize risks of immortal time bias were used. 26 , 27 , 33 , 34 , 35 , 36 , 43 , 56 The large study size of >200 000 patients resulted in a substantial number of outcome events, for example 1683 cardiovascular disease events during follow‐up for up to 6 years (mean 1.5 years), warranting robust statistical power to assess risk differences. Following several sensitivity tests with multiple adjustments, step‐wise removal of countries, subgroup analyses and including time after treatment discontinuation, the results remained similar and significant. Similar treatment persistence during follow‐up was shown in both groups (Figure S7). Validation of the study CKD definition showed high sensitivity and specificity. 8
This study does have some limitations. Despite the large size of the study, observational comparative effectiveness studies can never replace randomized clinical trials and our results should be interpreted with several potential limitations in mind. The results are only representative of patients who have initiated treatment with SGLT2i or DPP4i with similar characteristics (e.g. treatments and comorbidities), and cannot be extended to all patients with type 2 diabetes. This study provides no information on laboratory measurements (e.g. blood pressure, serum cholesterol, body weight), lifestyle parameters, or socio‐economic data, and consequently there could be residual confounding factors. The close matching on many essential variables ensures that some confounding factors are controlled, but even propensity score matching does not remedy all confounding; for example, residual confounding by indication, to the extent that prescribers will probably use more information about their patients than we have available. Measurements of eGFR albuminuria were not provided in the registries. However, a recent comparative effectiveness study by Heerspink et al., 40 including eGFR in the propensity score, showed similar risk reducing effects on renal outcomes with SGLT2i between 51% and 67% [0.49 (0.35‐0.67) and 0.33 (0.16‐0.68)], compared with the 56% [0.44 (0.28‐0.69)] in the present study, suggesting that renal function in part could be reflected by the extensive number of variables included in the propensity score calculations, for example age, gender, disease history (e.g. microvascular complications) and use of drugs (e.g. renin‐angiotensin‐aldosterone system inhibitor, sulphonylurea, metformin, insulin). Furthermore, there is no information available about the duration of diabetes in these patients. However, a robust proxy for diabetes duration is the inclusion of variables associated with diabetes duration in the propensity score, such as index date and the date of first‐line initiation (i.e. diabetes treatment duration), GLD use, and cardiovascular and microvascular disease burden. Moreover, recent studies indicate that HF and CKD reduction of SGLT2i is present regardless of diabetes duration. 57 Duration of hypertension was not available. However, based on similar disease progression concerning glucometabolic (similar use of GLDs), hypertensive (similar use of antihypertensive drugs) and CVRD (CVRD‐free patients) risk in both groups, it is probable that the hypertensive duration is similar in both groups. We did not examine safety. Another important limitation is that dapagliflozin was much more widely used than other SGLT2i drugs in our study population; thus, potential differences between different SGLT2i could not be assessed.
5. CONCLUSION
In this large multinational observational study of patients with type 2 diabetes free from cardiovascular and renal disease, we have shown that the cardiorenal disease risk‐lowering effects of SGLT2i reported in clinical trials translate to a multinational real‐world setting.
CONFLICT OF INTEREST
KIB has received grants to his institution from AstraZeneca for this study and for lectures and consulting from Novo Nordisk, Sanofi, Lilly, Boehringer Ingelheim and Merck Sharp & Dohme. JB holds a full‐time position at AstraZeneca as an epidemiologist. AB acknowledges research support from NIHR, BMA, UKRI, HDR UK, EU and AstraZeneca. DJK has received research grant support from LG Life Sciences, Handok, Boehringer Ingelheim, and AstraZeneca; and has received speaker fees from Novo Nordisk, Novartis Korea, Boehringer Ingelheim, Lilly Korea, Handok, MSD, Hanmi and AstraZeneca. AN has received honoraria from MSD, Astra Zeneca, Eli Lilly, Boehringer Ingelheim and Novo Nordisk. JWE has received honoraria or research grants from AstraZeneca, NovoNordisk, Bayer, Sanofi and MSD. MT is employed by an independent statistical consultant company, Statisticon AB, Uppsala, Sweden, of which AstraZeneca Nordic‐Baltic is a client. SO is a full‐time employee of AstraZeneca. KHH has received research grant support from LG Life Sciences, Handok, Boehringer Ingelheim and AstraZeneca. NK is an employee of Wissenschaftliches Institut für Gesundheitsökonomie und Gesundheitssystemforschung and conducted work on behalf of Kantar Health. JBM, RZ and TY are full‐time employees of AstraZeneca. IK received grants from Astellas Pharma Inc., Boehringer Ingelheim Japan, Kowa Pharmaceutical Co. Ltd, Daiichi Sankyo Company Limited., Mitsubishi Tanabe Pharma Corporation, Shionogi & Co., Ltd, Sumitomo Dainippon Pharma Co., Ltd, Pfizer Japan Inc., Takeda Pharmaceutical Company Limited., Toa Eiyo Ltd, Honorarium: Astellas Pharma Inc., Boehringer Ingelheim Japan, Kowa Pharmaceutical Co. Ltd, Daiichi Sankyo Company Limited., Mitsubishi Tanabe Pharma Corporation, Shionogi & Co., Ltd, Sumitomo Dainippon Pharma Co., Ltd, Pfizer Japan Inc., Takeda Pharmaceutical Company Limited., Toa Eiyo Ltd. TK received grants from Asahi Mutual Life Insurance Co., Boehringer Ingelheim Japan, Daiichi Sankyo Company Limited, Kowa Pharmaceutical Co. Ltd, Mitsubishi Tanabe Pharma Corporation, MSD K.K., Novo Nordisk Pharma Ltd, Sanofi K.K., Takeda Pharmaceutical Company Limited. Lecture/other fees: AstraZeneca K.K., Astellas Pharma Inc., Boehringer Ingelheim Japan, Daiichi Sankyo Company Limited., Eli Lilly Japan K.K., Kowa Pharmaceutical Co. Ltd, Kyowa Hakko Kirin Co., Ltd, Mitsubishi Tanabe Pharma Corporation, MSD K.K., Ono Pharmaceutical Company, Ltd, Sanofi K.K., Sumitomo Dainippon Pharma Co., Ltd, Sanwa Kagaku Kenkyusho Co. Ltd, Taisho Pharmaceutical Co., Ltd and Takeda Pharmaceutical Company Limited.
AUTHOR CONTRIBUTIONS
All authors participated in the research design. Marcus Thuresson performed the data management and statistical analyses for all countries after discussion with all authors. Statistical analyses were performed in Japan, Norway and Sweden by Marcus Thuresson, Germany by Nils Kossack, England by Ruiqi Zhang and South Korea by Kyoung Hwa Ha. All authors participated in data interpretation and in writing the manuscript. All authors took final responsibility in the decision to submit for publication.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/dom.14189.
Supporting information
Appendix S1: Supporting Information
ACKNOWLEDGMENTS
We are grateful to Mizue Ogi, Masayoshi Takeda, Susanna Jerström and Helena Goike at AstraZeneca for logistic support and valuable comments on the manuscript. Urban Olsson, Statisticon AB, is acknowledged for database management. All authors are guarantors of the manuscript. Data from the Norwegian Patient Register have been used in this publication. The interpretation and reporting of these data are the sole responsibility of the authors, and no endorsements by the Norwegian patient register are intended nor should be inferred. This work was sponsored by AstraZeneca.
Birkeland KI, Bodegard J, Banerjee A, et al. Lower cardiorenal risk with sodium‐glucose cotransporter‐2 inhibitors versus dipeptidyl peptidase‐4 inhibitors in patients with type 2 diabetes without cardiovascular and renal diseases: A large multinational observational study. Diabetes Obes Metab. 2021;23:75–85. 10.1111/dom.14189
Funding information AstraZeneca
DATA AVAILABILITY STATEMENT
Data underlying the findings described in this manuscript may be obtained in accordance with AstraZeneca's data sharing policy described at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure
REFERENCES
- 1. Birkeland KI, Bodegard J, Norhammar A, et al. How representative of a general type 2 diabetes population are patients included in cardiovascular outcome trials with SGLT2 inhibitors? A large European observational study. Diabetes Obes Metab. 2019;21(4):968‐974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Thrainsdottir IS, Aspelund T, Thorgeirsson G, et al. The association between glucose abnormalities and heart failure in the population‐based Reykjavik study. Diabetes Care. 2005;28(3):612‐616. [DOI] [PubMed] [Google Scholar]
- 3. Collaboration GBDCKD . Global, regional, and national burden of chronic kidney disease, 1990–2017: a systematic analysis for the global burden of disease study 2017. Lancet. 2020;395(10225):709‐733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Braunwald E. Diabetes, heart failure, and renal dysfunction: the vicious circles. Prog Cardiovasc Dis. 2019;62(4):298‐302. [DOI] [PubMed] [Google Scholar]
- 5. Savarese G, Lund LH. Global public health burden of heart failure. Card Fail Rev. 2017;3(1):7‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Seferovic PM, Petrie MC, Filippatos GS, et al. Type 2 diabetes mellitus and heart failure: a position statement from the heart failure Association of the European Society of Cardiology. Eur J Heart Fail. 2018;20(5):853‐872. [DOI] [PubMed] [Google Scholar]
- 7. IDF Diabetes Atlas Eighth Edition 2017: International Diabetes Federation; 2017. Sixth edition. http://www.idf.org/diabetesatlas.
- 8. Birkeland KI, Bodegard J, Eriksson JW, et al. Heart failure and chronic kidney disease manifestation and mortality risk associations in type 2 diabetes: a large multinational cohort study. Diabetes Obes Metab. 2020;22(9):1607‐1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gregg EW, Zhuo X, Cheng YJ, Albright AL, Narayan KM, Thompson TJ. Trends in lifetime risk and years of life lost due to diabetes in the USA, 1985‐2011: a modelling study. Lancet Diabetes Endocrinol. 2014;2(11):867‐874. [DOI] [PubMed] [Google Scholar]
- 10. Afkarian M, Sachs MC, Kestenbaum B, et al. Kidney disease and increased mortality risk in type 2 diabetes. J Am Soc Nephrol. 2013;24(2):302‐308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. de Boer IH, Rue TC, Hall YN, Heagerty PJ, Weiss NS, Himmelfarb J. Temporal trends in the prevalence of diabetic kidney disease in the United States. JAMA. 2011;305(24):2532‐2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gregg EW, Li Y, Wang J, et al. Changes in diabetes‐related complications in the United States, 1990‐2010. N Engl J Med. 2014;370(16):1514‐1523. [DOI] [PubMed] [Google Scholar]
- 13. Rawshani A, Rawshani A, Franzen S, et al. Risk factors, mortality, and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2018;379(7):633‐644. [DOI] [PubMed] [Google Scholar]
- 14. Greene SJ, Butler J. Primary prevention of heart failure in patients with type 2 diabetes mellitus. Circulation. 2019;139(2):152‐154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mosenzon O, Wiviott SD, Cahn A, et al. Effects of dapagliflozin on development and progression of kidney disease in patients with type 2 diabetes: an analysis from the DECLARE‐TIMI 58 randomised trial. Lancet Diabetes Endocrinol. 2019;7(8):606‐617. [DOI] [PubMed] [Google Scholar]
- 16. Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377(7):644‐657. [DOI] [PubMed] [Google Scholar]
- 17. Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380(4):347‐357. [DOI] [PubMed] [Google Scholar]
- 18. Zelniker TA, Wiviott SD, Raz I, et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta‐analysis of cardiovascular outcome trials. Lancet. 2019;393(10166):31‐39. [DOI] [PubMed] [Google Scholar]
- 19. McMurray JJV, Docherty KF, Jhund PS. Dapagliflozin in patients with heart failure and reduced ejection fraction. Reply. N Engl J Med. 2020;382(10):973. [DOI] [PubMed] [Google Scholar]
- 20. Farxiga Phase III DAPA‐CKD trial will be stopped early after overwhelming efficacy in patients with chronic kidney disease [press release]. 2020. AstraZeneca. 30 March 2020.
- 21. Mahaffey KW, Jardine MJ, Bompoint S, et al. Canagliflozin and cardiovascular and renal outcomes in type 2 diabetes and chronic kidney disease in primary and secondary cardiovascular prevention groups: results from the randomized CREDENCE trial. Circulation. 2019;140:739‐750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Green JB, Bethel MA, Armstrong PW, et al. Effect of Sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;373(3):232‐242. [DOI] [PubMed] [Google Scholar]
- 23. Scirica BM, Bhatt DL, Braunwald E, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013;369(14):1317‐1326. [DOI] [PubMed] [Google Scholar]
- 24. White WB, Cannon CP, Heller SR, et al. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med. 2013;369(14):1327‐1335. [DOI] [PubMed] [Google Scholar]
- 25. Buse JB, Wexler DJ, Tsapas A, et al. 2019 update to: Management of Hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2020;43(2):487‐493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Persson F, Nystrom T, Jorgensen ME, et al. Dapagliflozin is associated with lower risk of cardiovascular events and all‐cause mortality in people with type 2 diabetes (CVD‐REAL Nordic) when compared with dipeptidyl peptidase‐4 inhibitor therapy: a multinational observational study. Diabetes Obes Metab. 2018;20(2):344‐351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Thuresson M, Cavender MA, Fu AZ, et al. Comment on Suissa. Lower risk of death with SGLT2 inhibitors in observational studies: real or bias? Diabetes Care. 2018;41(6):e106‐e108. [DOI] [PubMed] [Google Scholar]
- 28. Thuresson M, Cavender MA, Fu AZ, et al. Comment to lower risk of death with SGLT2 inhibitors in observational studies: real or bias? Diabetes Care. 2018;41(6):e106‐e108. [DOI] [PubMed] [Google Scholar]
- 29. DerSimonian R, Laird N. Meta‐analysis in clinical trials. Control Clin Trials. 1986;7(3):177‐188. [DOI] [PubMed] [Google Scholar]
- 30. Viechtbauer W. Conducting meta‐analyses in R with the metafor package. J Stat Softw. 2010;36(3):48. [Google Scholar]
- 31. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117‐2128. [DOI] [PubMed] [Google Scholar]
- 32. Mahaffey KW, Jardine MJ, Bompoint S, et al. Canagliflozin and cardiovascular and renal outcomes in type 2 diabetes mellitus and chronic kidney disease in primary and secondary cardiovascular prevention groups. Circulation. 2019;140(9):739‐750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Birkeland KI, Jorgensen ME, Carstensen B, et al. Cardiovascular mortality and morbidity in patients with type 2 diabetes following initiation of sodium‐glucose co‐transporter‐2 inhibitors versus other glucose‐lowering drugs (CVD‐REAL Nordic): a multinational observational analysis. Lancet Diabetes Endocrinol. 2017;5(9):709‐717. [DOI] [PubMed] [Google Scholar]
- 34. Kosiborod M, Cavender MA, Fu AZ, et al. Lower risk of heart failure and death in patients initiated on sodium‐glucose Cotransporter‐2 inhibitors versus other glucose‐lowering drugs: the CVD‐REAL study (comparative effectiveness of cardiovascular outcomes in new users of sodium‐glucose Cotransporter‐2 inhibitors). Circulation. 2017;136(3):249‐259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kosiborod M, Lam CSP, Kohsaka S, et al. Cardiovascular events associated with SGLT‐2 inhibitors versus other glucose‐lowering drugs: the CVD‐REAL 2 study. J Am Coll Cardiol. 2018;71(23):2628‐2639. [DOI] [PubMed] [Google Scholar]
- 36. Norhammar A, Bodegard J, Nystrom T, Thuresson M, Nathanson D, Eriksson JW. Dapagliflozin and cardiovascular mortality and disease outcomes in a population with type 2 diabetes similar to that of the DECLARE‐TIMI 58 trial: a nationwide observational study. Diabetes Obes Metab. 2019;21(5):1136‐1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kohsaka S, Takeda M, Bodegard J, et al. SGLT2 inhibitors compared with other glucose‐lowering drugs in Japan: subanalyses of the CVD‐REAL 2 study. J Diabetes Investig. 2020. [Online ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Komuro I, Kadowaki T, Bodegard J, Thuresson M, Okami S, Yajima T. Lower heart failure and chronic kidney disease risks associated with sodium–glucose Cotransporter‐2 inhibitor use in Japanese type 2 diabetes patients without established cardiovascular and renal diseases. Diabetes Obes Metab. 2020. 10.1111/dom.14119 [DOI] [PubMed] [Google Scholar]
- 39. Kohsaka S, Lam C, Kim J, et al. Risk of cardiovascular events and death associated with initiation of SGLT2 inhibitors compared with DPP‐4 inhibitors: an analysis from the CVD‐REAL 2 multinational cohort study. Lancet Diabetes Endocrinol. 2020;8(7):606‐615. [DOI] [PubMed] [Google Scholar]
- 40. Heerspink HJL, Karasik A, Thuresson M, et al. Kidney outcomes associated with use of SGLT2 inhibitors in real‐world clinical practice (CVD‐REAL 3): a multinational observational cohort study. Lancet Diabetes Endocrinol. 2020;8(1):27‐35. [DOI] [PubMed] [Google Scholar]
- 41. Clegg LE, Heerspink HJL, Penland RC, et al. Reduction of cardiovascular risk and improved estimated glomerular filtration rate by SGLT2 inhibitors, including Dapagliflozin, is consistent across the class: an analysis of the placebo arm of EXSCEL. Diabetes Care. 2019;42(2):318‐326. [DOI] [PubMed] [Google Scholar]
- 42. Rothwell PM. External validity of randomised controlled trials: “to whom do the results of this trial apply?”. Lancet. 2005;365(9453):82‐93. [DOI] [PubMed] [Google Scholar]
- 43. Norhammar A, Bodegard J, Nystrom T, et al. Dapagliflozin vs non‐SGLT‐2i treatment is associated with lower healthcare costs in type 2 diabetes patients similar to participants in the DECLARE‐TIMI 58 trial: a nationwide observational study. Diabetes Obes Metab. 2019;21(12):2651‐2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Furtado RHM, Bonaca MP, Raz I, et al. Dapagliflozin and cardiovascular outcomes in patients with type 2 diabetes mellitus and previous myocardial infarction. Circulation. 2019;139(22):2516‐2527. [DOI] [PubMed] [Google Scholar]
- 45. Kato ET, Silverman MG, Mosenzon O, et al. Effect of Dapagliflozin on heart failure and mortality in type 2 diabetes mellitus. Circulation. 2019;139(22):2528‐2536. [DOI] [PubMed] [Google Scholar]
- 46. Gallwitz B, Rosenstock J, Rauch T, et al. 2‐year efficacy and safety of linagliptin compared with glimepiride in patients with type 2 diabetes inadequately controlled on metformin: a randomised, double‐blind, non‐inferiority trial. Lancet. 2012;380(9840):475‐483. [DOI] [PubMed] [Google Scholar]
- 47. McMurray JJV, Solomon SD, Inzucchi SE, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381(21):1995‐2008. [DOI] [PubMed] [Google Scholar]
- 48. Herat LY, Magno AL, Rudnicka C, et al. SGLT2 inhibitor‐induced sympathoinhibition: A novel mechanism for cardiorenal protection. JACC Basic Transl Sci. 2020;5(2):169‐179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Mazer CD, Hare GMT, Connelly PW, et al. Effect of Empagliflozin on erythropoietin levels, iron stores, and red blood cell morphology in patients with type 2 diabetes mellitus and coronary artery disease. Circulation. 2020;141(8):704‐707. [DOI] [PubMed] [Google Scholar]
- 50. Verma S. Are the Cardiorenal benefits of SGLT2 inhibitors due to inhibition of the sympathetic nervous system? JACC Basic Transl Sci. 2020;5(2):180‐182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Verma S, Garg A, Yan AT, et al. Effect of Empagliflozin on left ventricular mass and diastolic function in individuals with diabetes: an important clue to the EMPA‐REG OUTCOME trial? Diabetes Care. 2016;39(12):e212‐e213. [DOI] [PubMed] [Google Scholar]
- 52. Verma S, Mazer CD, Yan AT, et al. Effect of Empagliflozin on left ventricular mass in patients with type 2 diabetes mellitus and coronary artery disease: the EMPA‐HEART CardioLink‐6 randomized clinical trial. Circulation. 2019;140(21):1693‐1702. [DOI] [PubMed] [Google Scholar]
- 53. Verma S, Rawat S, Ho KL, et al. Empagliflozin increases cardiac energy production in diabetes: novel translational insights into the heart failure benefits of SGLT2 inhibitors. JACC Basic Transl Sci. 2018;3(5):575‐587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Giugliano D, De Nicola L, Maiorino MI, Bellastella G, Esposito K. Type 2 diabetes and the kidney: insights from cardiovascular outcome trials. Diabetes Obes Metab. 2019;21(8):1790‐1800. [DOI] [PubMed] [Google Scholar]
- 55. Lopaschuk GD, Verma S. Mechanisms of cardiovascular benefits of sodium glucose co‐transporter 2 (SGLT2) inhibitors: A state‐of‐the‐art review. JACC Basic Transl Sci. 2020;5(6):632‐644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Nystrom T, Bodegard J, Nathanson D, Thuresson M, Norhammar A, Eriksson JW. Novel oral glucose‐lowering drugs are associated with lower risk of all‐cause mortality, cardiovascular events and severe hypoglycaemia compared with insulin in patients with type 2 diabetes. Diabetes Obes Metab. 2017;19(6):831‐841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Bajaj HS, Raz I, Mosenzon O, et al. Cardiovascular and renal benefits of dapagliflozin in patients with short and long‐standing type 2 diabetes: analysis from the DECLARE‐TIMI 58 trial. Diabetes Obes Metab. 2020;22(7):1122‐1131. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Supporting Information
Data Availability Statement
Data underlying the findings described in this manuscript may be obtained in accordance with AstraZeneca's data sharing policy described at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure


