Abstract
The transient receptor potential cation channel subfamily V member 1 (TRPV1) receptor is an important mediator of nociception and its expression is enriched in nociceptive neurons. TRPV1 signaling has been implicated in bladder pain and is a potential analgesic target. Resiniferatoxin is the most potent known agonist of TRPV1. Acute exposure of the rat bladder to resiniferatoxin has been demonstrated to result in pain-related freezing and licking behaviors that are alleviated by virally encoded IL-4. The interleukin-4-inducing principle of Schistosoma mansoni eggs (IPSE) is a powerful inducer of IL-4 secretion, and is also known to alter host cell transcription through a nuclear localization sequence-based mechanism. We previously reported that IPSE ameliorates ifosfamide-induced bladder pain in an IL-4- and nuclear localization sequence-dependent manner. We hypothesized that pre-administration of IPSE to resiniferatoxin-challenged mice would dampen pain-related behaviors. IPSE indeed lessened resiniferatoxin-triggered freezing behaviors in mice. This was a nuclear localization sequence-dependent phenomenon, since administration of a nuclear localization sequence mutant version of IPSE abrogated IPSE’s analgesic effect. In contrast, IPSE’s analgesic effect did not seem IL-4-dependent, since use of anti-IL-4 antibody in mice given both IPSE and resiniferatoxin did not significantly affect freezing behaviors. RNA-Seq analysis of resiniferatoxin- and IPSE-exposed bladders revealed differential expression of TNF/NF-κb-related signaling pathway genes. In vitro testing of IPSE uptake by urothelial cells and TRPV1-expressing neuronal cells showed uptake by both cell types. Thus, IPSE’s nuclear localization sequence-dependent therapeutic effects on TRPV1-mediated bladder pain may act on TRPV1-expressing neurons and/or may rely upon urothelial mechanisms.
Keywords: Bladder, pain, analgesic, parasite, schistosoma, immune modulation
Introduction
The bladder is a heavily innervated organ.1 The high density of afferent nerve endings in the bladder partly accounts for its sensitivity to noxious stimuli (reviewed by de Groat and Yoshimura2). Diverse stimuli cause bladder-based nociception, including urinary tract infections, catheterization, surgical manipulation, ureteral stents, hemorrhagic cystitis, and bladder pain syndromes such as interstitial cystitis.2 Despite the importance of bladder-based nociception in clinical medicine, there are few therapeutic options that directly target afferent nerve endings in the bladder.
One potential set of therapeutics for bladder pain are the proteins encoded by the interleukin-4-inducing principle of Schistosoma mansoni eggs (IPSE) genes.3 As the name indicates, IPSE is a potent inducer of IL-4 secretion by host cells. IPSE also features a nuclear localization sequence (NLS) which facilitates its entry into host cell nuclei and subsequent modulation of transcription.4,5 Macedo et al. have reported that administration of IL-4 to mice with ifosfamide-induced hemorrhagic cystitis alleviates bladder injury.6 This led us to test IPSE in this model. A single dose of IPSE reduced spontaneous pain behaviors in ifosfamide-challenged mice in an IL-4- and NLS-dependent manner.7
Other investigators have reported that administration of virally-encoded IL-4 reduces resiniferatoxin-induced, bladder pain-related behaviors.8 Thus, we hypothesized that IPSE may likewise dampen bladder pain caused by resiniferatoxin. Herein we describe the ability of IPSE to ameliorate resiniferatoxin-triggered bladder pain behaviors. This property of IPSE is NLS-dependent, and possibly weakly IL-4-dependent. RNA-Seq analysis of resiniferatoxin- and IPSE-exposed bladders indicates that IPSE reduces gene expression related to the TNF signaling via NF-κB pathway. These effects occur in the context of uptake of IPSE by both urothelial and neuronal cells.
Materials and methods
Study approval
All animal work was conducted according to relevant U.S. and international guidelines. Specifically, animal experimental work was reviewed and approved as protocol 14–03 by the Institutional Animal Care and Use Committee of the Biomedical Research Institute (Rockville, Maryland, USA). Our Institutional Animal Care and Use Committee guidelines comply with the U.S. Public Health Service Policy on Humane Care and Use of Laboratory Animals.
Mice
Female 6- to 8-wk-old C57BL/6 mice (Charles River Laboratories, Wilmington, MA, USA) were housed under 12 h light- dark cycles in temperature-controlled holding rooms with unlimited access to dry mouse chow and water. Newly received mice were acclimated to the animal facility for at least one week prior to experimental use.
IPSE protein production and labeling
Recombinant H06 H-IPSE (one of the major Schistosoma haematobium IPSE orthologs) and an NLS mutant of H06 H-IPSE were produced in HEK293-6E cells as previously described.9 H06 IPSE was conjugated to Alexa Fluor 488 using a Alexa Fluor 488 antibody labeling kit (Thermofisher Scientific, Waltham, MA) according to manufacturer instructions; however, the pH was kept at 7.4 throughout the reaction to enrich for labeling of the terminal amine (pKa of 7.4). The efficiency of conjugation was confirmed by Nanodrop. The typical degree of labeling was one mole of dye per mole of IPSE, which suggested IPSE was only labeled on the terminal amine. Low labeling efficiently minimized the potential interference of the dye with IPSE’s functional domains.
IPSE administration
One day prior to resiniferatoxin or vehicle challenge, mice underwent tail vein injection with phosphate-buffered saline or 25 µg of H06 H-IPSE (or its NLS mutant) in phosphate-buffered saline.
Recombinant IL-4 administration
Intraperitoneal injection of recombinant mouse IL-4 is used for systemic delivery of this cytokine.10–13 Recombinant mouse IL-4 was obtained from Peprotech Laboratories (Rocky Hill, NJ, USA). A subset of mice underwent i.p. injection with 10 ng of IL-4 one hour prior to resiniferatoxin challenge.
Anti-IL-4 antibody administration
Neutralizing anti-IL-4 antibody (11B11 clone) was purchased from BioXcell (West Lebanon, NH, USA). A subset of mice underwent i.p. injection with 100 µg of anti-IL-4 antibody 30 minutes before resiniferatoxin challenge.
Resiniferatoxin administration and assessment of freezing behavior
Intravesically administered resiniferatoxin has been previously reported to induce bladder nociception in rodents.14–18 Mice were anesthetized, treated, and evaluated one by one. Anesthesia was achieved using vaporized isoflurane and mice kept on a heating blanket to maintain body temperature. Lubricated sterile catheters (Excel Safelet Cath 24 G x 3/4“) attached to 1 mL-syringes were gently inserted into the mouse urethra. Phosphate-buffered saline (50 µL), vehicle (50 µL of 10% v/v ethanol, 10% v/v Tween 80 and 80% v/v phosphate-buffered saline) or resiniferatoxin (3 µM in 50 µL of 10% v/v ethanol, 10% v/v Tween 80 and 80% v/v phosphate-buffered saline) was slowly pushed into the mouse bladder and held in place for 1 minute.
Mice were awakened from anesthesia and allowed to recover by leaving them on the warm pad for 5 min. Subsequently, mice were transferred to a transparent cage and a continuous video footage were recorded for 15 minutes following resiniferatoxin administration. Nociception-related freezing behaviors were scored for each individual mouse over 5 minute periods (5 min, 10 min and 15 min) in a blinded fashion.8,14,16,17,19
RNA purification
RNA was isolated from mouse bladders using TRIzol Reagent and PureLink RNA Mini Kit (Invitrogen), according to manufacturer instructions. Briefly, aseptically excised bladders were homogenized in 1 mL TRIzol Reagent by bead-beating using ceramic beads (Omni International) and a mini-bead beater (Biospec). Following a 5-min incubation, 0.2 mL chloroform was added and the solution was incubated for 3 min before centrifugation at 12,000 × g for 15 min to separate homogenates into aqueous and organic phases. The aqueous supernatant (∼400 μL) was mixed with an equal volume of 70% ethanol before binding the mixture to RNA binding columns by centrifugation. On-column DNase digestion (Invitrogen) was performed for 30 minutes, according to manufacturer instructions. After column washes and drying, RNA was eluted in RNase-free water, quantified and its quality checked using a NanoDrop 1000 spectrophotometer (Thermo Scientific) and Bioanalyzer 2100 (Agilent).
RNA sequencing and RNA-seq analysis pipeline
RNA sequencing was performed using the Illumina-HiSeq 4000 NGS platform at a depth of >20 million reads. Analyses were conducted using the RNA analysis tools of the Galaxy platform (https://usegalaxy.org). Raw sequence reads were aligned to the mouse genome (mm10) by HISAT2 (version 2.1.0+galaxy4). The resulting alignment files, along with the most recent mouse genome annotation file in the Illumina iGenomes UCSC mm10 mouse genome collection (http://igenomes.illumina.com.s3-website-us-east-1.amazonaws.com/Mus_musculus/UCSC/mm10/Mus_musculus_UCSC_mm10.tar.gz), were used as the input for HTSeq-count (version 0.9.1). DESeq2 (Galaxy version 2.11.40.6+galaxy1; DESeq2 version 1.22.1) was used to determine differentially expressed genes across all treatment groups. Principal component analysis was performed by DESeq2.
Functional and pathway analysis, statistics and plots
Treatment-to-pathway association was performed with the Gene Set Enrichment Analysis (GSEA) software package (version 4.0.3) (https://www.gsea-msigdb.org/gsea/index.jsp), using the DESeq2 normalized read counts file from which genes that showed zero read counts for any sample were removed, the hallmark gene set (version 7.1) (ftp://ftp.broadinstitute.org/distribution/gsea/gene_sets/h.all.v7.1.symbols.gmt), and the mouse gene symbol remapping file (version 7.1) (ftp://ftp.broadinstitute.org/distribution/gsea/annotations_versioned/Mouse_Gene_Symbol_Remapping_to_Human_Orthologs_MSigDB.v7.1.chip), with “Permutation type” set to “Gene_set”, “Create GCT files” set to “True”, and other analysis options set to default values.20 The volcano plot was generated using the EnhancedVolcano software package (version 1.4.0 from the bioconda distribution channel) for R.21 The heat maps were generated using the Morpheus software package (https://software.broadinstitute.org/morpheus). Other data analyses and plots were generated using GraphPad Prism v 6.00, and ggplot2 and plotly packages in R. For comparisons among groups, one-way analysis of variance (ANOVA) was performed and if significant, was followed by post hoc Student t-tests for pairwise comparisons after confirming a normal distribution. Plotted data show individual data points with error bars representing means and standard deviation.
Endocytosis assays
Cath.a mouse brain-derived neuronal cells (ATCC CRL-11179) were obtained from ATCC (Manassas, VA) and were grown in RPMI-1640 (Sigma-Aldrich, St. Louis, MO) with 8% horse serum (Sigma-Aldrich, St. Louis, MO) and 4% fetal bovine serum (Sigma-Aldrich, St. Louis, MO). HCV-29 human derived urothelial cells were obtained as a gift from Paul Brindley and grown in MEM (Thermofisher Scientific, Waltham, MA) with 10% fetal bovine serum (Sigma-Aldrich, St. Louis, MO). For internalization assays, floating cells and adherent cells (released via 0.12% trypsin (Sigma-Aldrich, St. Louis, MO) without EDTA) were washed in fresh medium, and aliquoted into 24 well plates at 200,000 cells/mL in 1 mL. The cells were incubated with Alexa 488-labeled H06 at 1 μg/mL or Alexa 488-labeled transferrin at 4 μg/mL (Thermofisher Scientific, Waltham, MA) for 16 hours at 37° C. Cells were released via 0.12% trypsin without EDTA and washed 3 times with PBS (Sigma-Aldrich, St. Louis, MO). 0.4% trypan blue (Thermofisher Scientific, Waltham, MA) was added to the cells (1:4) to quench extracellular Alexa 488 signal. The cells were analyzed by flow cytometry (Beckman Coulter, CytoFLEX) to measure the intracellular Alexa 488 signal. Data were analyzed using FlowJo and GraphPad.
Results
IPSE reduces resiniferatoxin-induced, bladder pain-associated behaviors in an IL-4- and nuclear localization sequence-dependent fashion
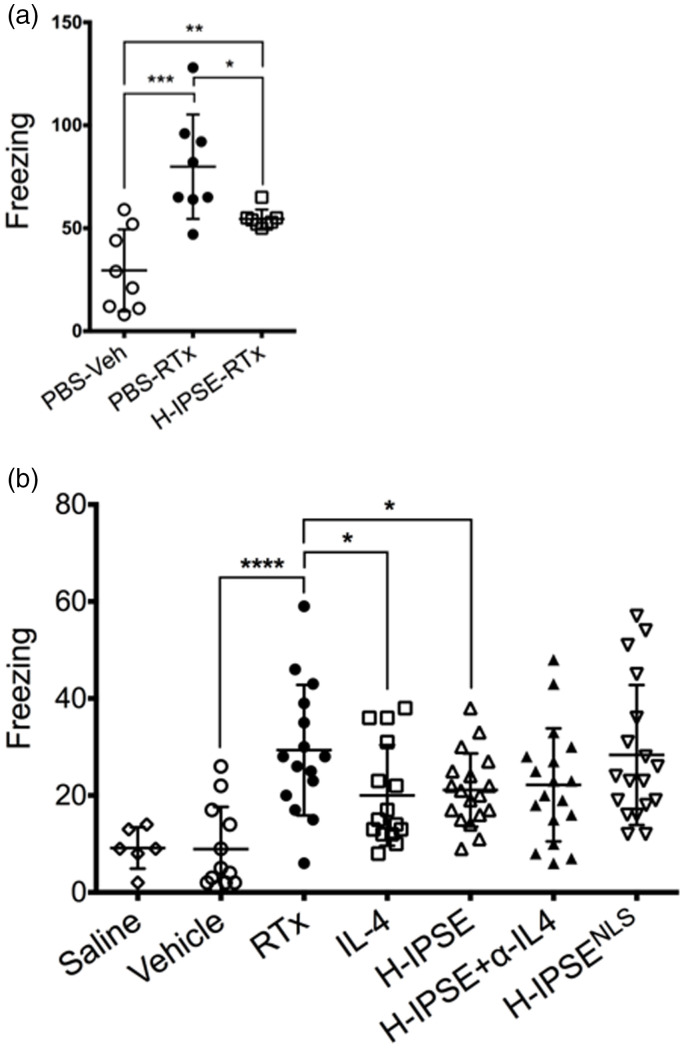
When mice were given intravesical resiniferatoxin, they exhibited a significant increase in pain-associated freezing behaviors (Figure 1(a)). Administration of a single intravenous dose of IPSE 24 hours prior to resiniferatoxin challenge resulted in significantly decreased freezing episodes. However, IPSE did not bring freezing episodes down to the levels of vehicle-treated mice (e.g., no resiniferatoxin exposure).
Figure 1.
IPSE reduces resiniferatoxin-induced, pain-related freezing behaviors in an IL-4- and nuclear localization sequence-dependent manner. (a) Mice were administered intravenous phosphate-buffered saline (PBS) followed by intravesical PBS/Tween/ethanol vehicle (“PBS-Veh”), intravenous PBS with intravesical resiniferatoxin in vehicle (“PBS-RTx”), or one intravenous dose of the H06 H-IPSE ortholog of IPSE 24 hours before intravesical resiniferatoxin in vehicle (“H-IPSE-RTx”). (b) Mice were given intravenous PBS and intravesical PBS (“saline”), intravenous PBS and intravesical PBS/Tween/ethanol vehicle (“Vehicle”), intravenous PBS and intravesical resiniferatoxin in vehicle (“RTx”), recombinant IL-4 given intraperitoneally followed by intravesical resiniferatoxin in vehicle (“IL-4”), the H06 H-IPSE ortholog of IPSE given intravenously 24 hours before intravesical PBS with resiniferatoxin in vehicle (“H-IPSE”), the H06 H-IPSE ortholog of IPSE 24 hours given intravenously and anti-IL-4 antibody given intraperitoneally 30 minutes before resiniferatoxin in vehicle administered intravesically (“H-IPSE+α-IL4”), or a nuclear localization sequence (NLS) mutant of H06 H-IPSE given intravenously 24 hours before resiniferatoxin in vehicle administered intravesically (“H-IPSENLS”).
In an independent set of experiments we then tested the ability of an NLS mutant of IPSE, as well as IPSE combined with anti-IL-4 antibody, to reduce resiniferatoxin-induced pain behaviors compared to wild type IPSE and recombinant IL-4 (positive control) (Figure 1(b)). H06-IPSE had similar effects to recombinant IL-4. The treatment with an IL-4 blocking antibody half an hour before resiniferatoxin administration and 24 hours after IPSE treatment did not appear to have a strong effect on IPSE’s lessening of resiniferatoxin-induced, pain-related freezing behaviors, suggesting that the effects of IPSE are not dependent on IL-4 release. In contrast to wild type IPSE, the NLS mutant of IPSE did not seem to exert an analgesic effect on resiniferatoxin-exposed mice.
IPSE decreases resiniferatoxin-induced bladder expression of genes associated with TNF signaling via NF-κB
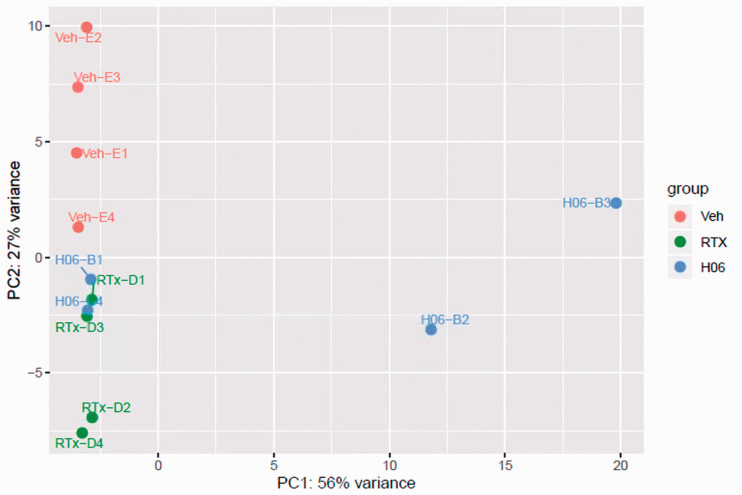
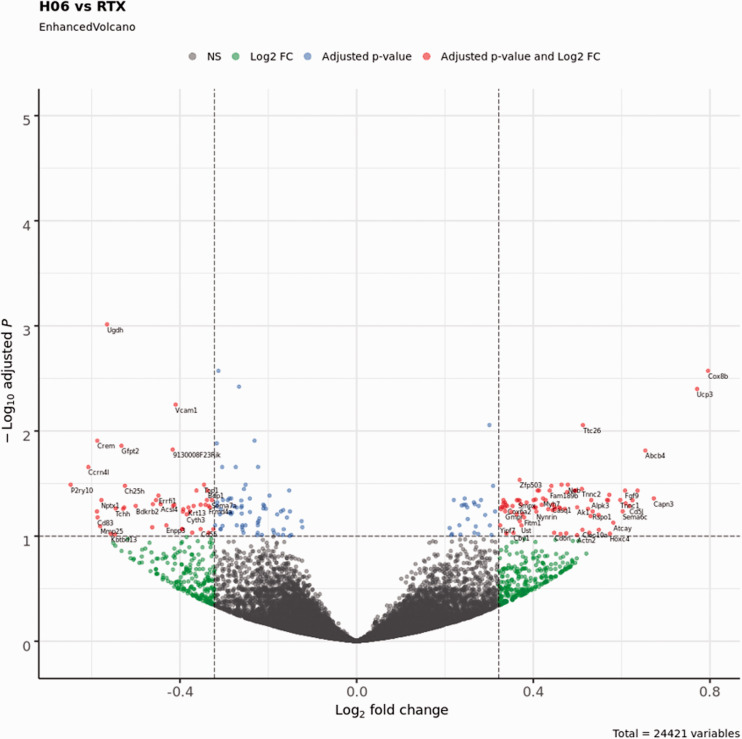
We next sought to determine the effects of IPSE and resiniferatoxin treatment on bladder transcription. Mice were administered H06 H-IPSE and resiniferatoxin, vehicle alone, or vehicle and resiniferatoxin, and their bladders harvested for RNA-Seq analysis. Principal component analysis (PCA) confirmed that resiniferatoxin-treated bladders clustered distinctly from vehicle-treated bladders (Figure 2). Likewise, PCA of IPSE combined with resiniferatoxin versus resiniferatoxin only-treated bladders also showed distinct clustering patterns, albeit less so (Figure 2). By analysis with DESeq2, we found 219 differentially expressed genes (adjusted p-value < 0.1), 592 genes with an absolute value of Log2 fold change > 0.322 (greater than 1.25-fold change in either direction), and 129 genes satisfying both conditions in the comparison between IPSE combined with resiniferatoxin versus resiniferatoxin treatment groups (Figure 3; Supplemental Table 1). To determine whether IPSE could restore or rescue the expression of genes perturbed by the resiniferatoxin treatment, we performed the following pairwise comparisons: resiniferatoxin versus vehicle (RTXvsVeh; Supplemental Table 2), IPSE versus resiniferatoxin (H06vsRTX; Supplemental Table 1), and IPSE versus vehicle (H06vsVeh; Supplemental Table 3). In the first scenario, we filtered for genes whose expression was increased in the resiniferatoxin treatment compared to both vehicle and H06 treatment using the following conditions: Log2 fold change > 0.322 with adjusted p-value < 0.1 in RTXvsVeh; Log2 fold change < -0.322 with adjusted p-value < 0.1 in H06vsRTX; and adjusted p-value >= 0.1 in H06vsVeh. The filtering for this scenario yielded 21 genes (Supplemental Table 4). Similarly, in the second scenario, we filtered for genes whose expression was decreased in the resiniferatoxin treatment compared to both vehicle and H06 treatment using the following conditions: Log2 fold change < -0.322 with adjusted p-value < 0.1 in RTXvsVeh; Log2 fold change > 0.322 with adjusted p-value < 0.1 in H06vsRTX; and adjusted p-value >= 0.1 in H06vsVeh. This scenario yielded 23 genes (Supplemental Table 5).
Figure 2.
Principal component analysis of resiniferatoxin- and IPSE-treated bladder gene expression. Principal component analysis showed homogeneous clustering of gene expression among resiniferatoxin-treated mice (green symbols labeled with “RTx-D”) and vehicle-treated mice (red symbols labeled with “Veh-E”). There was some overlap of gene expression among resiniferatoxin-treated mice and mice treated with both H06 H-IPSE and resiniferatoxin (blue symbols labeled with “H06-B”).
Figure 3.
Volcano plot showing differentially expressed genes between bladders treated with IPSE and resiniferatoxin (H06) versus bladders treated with resiniferatoxin alone (RTX). The cutoff value for the adjusted p-value was set at < 0.1, and the cutoff for the absolute value of the Log2 fold change was set at > 0.322 (1.25-fold in either direction). Blue dots represent genes satisfying the adjusted p-value cutoff. Green dots represent genes satisfying the Log2 fold change cutoff. Red dots represent genes satisfying both of these cutoff conditions and are labeled with the corresponding gene symbols. Gray dots represent genes that do not satisfy either condition (NS, not significant).
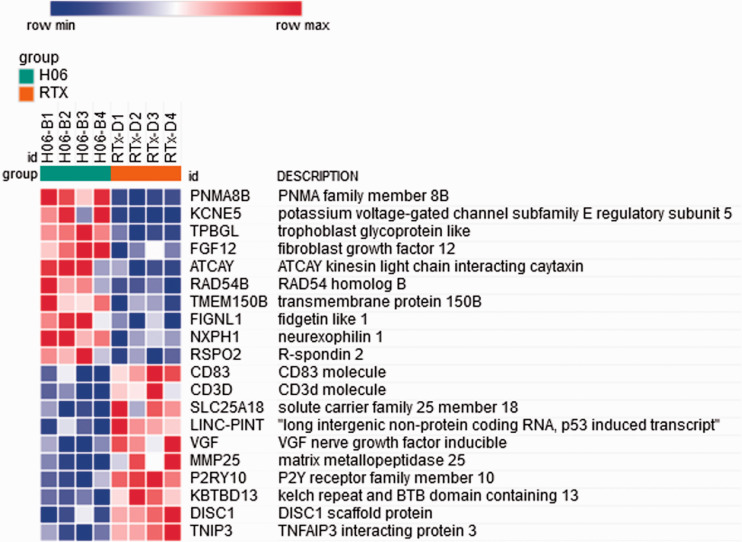
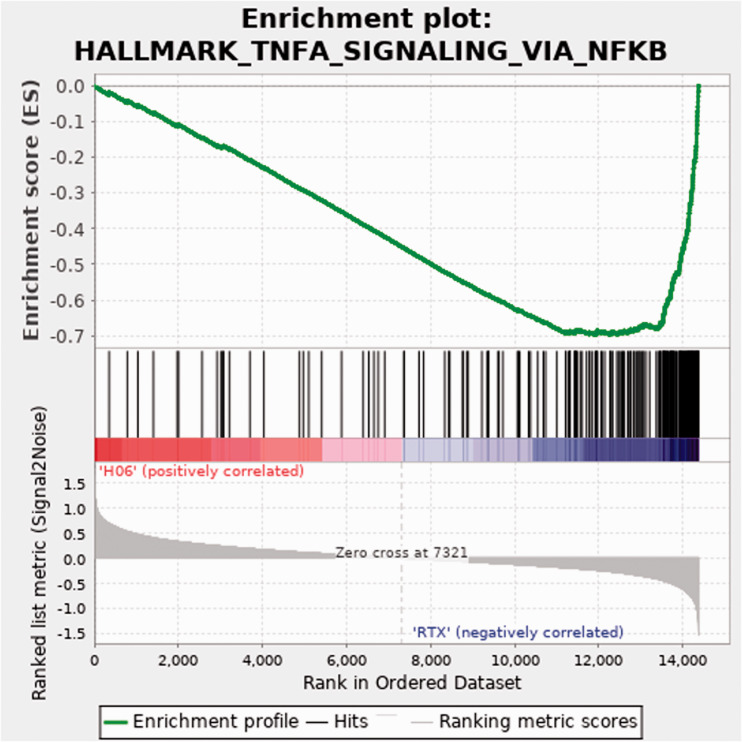
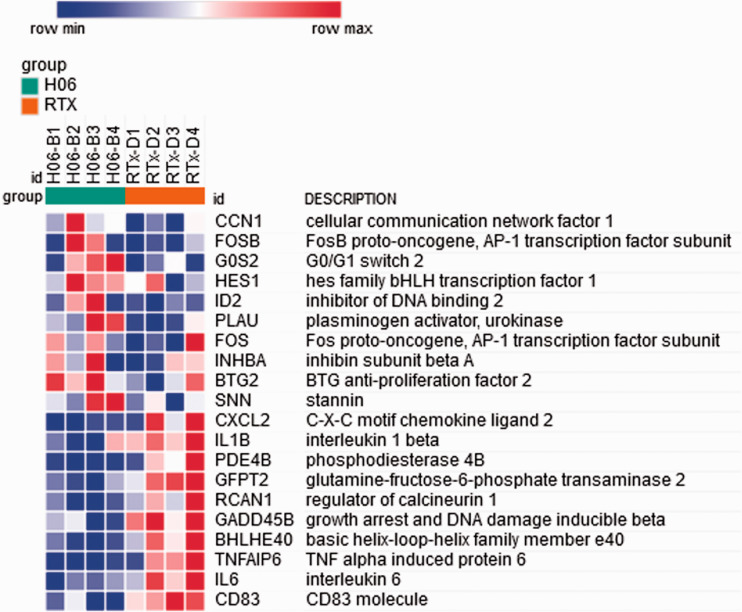
Using the normalized read counts file from DESeq2 (Supplemental Table 6) processed to remove genes for which any sample showed a zero read count, Gene Set Enrichment Analysis (GSEA) software, and Morpheus software, we then generated a heat map of differential gene expression in bladders treated with H06 H-IPSE and resiniferatoxin versus resiniferatoxin alone (Figure 4; Supplemental Table 7). Among the 50 hallmark gene sets in the Molecular Signatures Database (https://www.gsea-msigdb.org/gsea/msigdb/collections.jsp), many gene sets with a false discovery rate (FDR) q-value < 0.05 were enriched in the resiniferatoxin-alone treatment, including TNF signaling via NF-κB, inflammatory response, allograft rejection, interferon gamma response, and IL6/JAK-STAT3 signaling (Supplemental Table 8). Of these, the TNF signaling via NF-κB gene set showed the greatest normalized enrichment score in terms of absolute magnitude. The enrichment plot for the TNF signaling via NF-κB gene set shows a negative peak in the enrichment score, indicating a greater correlation of this gene set to the resiniferatoxin-alone treatment when compared to the IPSE combined with resiniferatoxin treatment (Figure 5). Notably, IL6 and IL1B were more strongly associated with the resiniferatoxin-alone treatment compared to the IPSE-resiniferatoxin treatment (Figure 6; Supplemental Table 9).
Figure 4.
Heat map of genes from the 50 hallmark gene sets from the Molecular Signatures Database with enriched differential expression in bladders exposed to IPSE combined with resiniferatoxin or resiniferatoxin alone. The 10 most strongly differentially expressed genes associated with each treatment are shown. Each column shows gene expression for an individual mouse bladder. Green and red column coloring indicates IPSE combined with resiniferatoxin (H06) versus resiniferatoxin only (RTX)-treated bladders, respectively. Genes are sorted by signal-to-noise scores, and their symbols and names are listed in rows. Darkest blue to darkest red coloring represents lowest to highest gene expression, respectively, based on normalized read counts.
Figure 5.
Enrichment plot for the TNF signaling via NF-κB pathway. Top panel, green line indicates running enrichment score for the TNF signaling via NF-κB pathway as the gene set enrichment analysis walks down the ranked list of genes. Middle panel depicts where the members of the TNF signaling via NF-κB pathway gene set appear in the ranked list of genes. Bottom panel shows the value of the ranking metric moving down the list of ranked genes. Positive values indicate correlation with the first phenotype (H06; H-IPSE combined with resiniferatoxin) and negative values indicate correlation with the second phenotype (RTX; resiniferatoxin alone).
Figure 6.
Heat map of gene members of the TNF signaling via NF-κB pathway with enriched differential expression in bladders exposed to IPSE combined with resiniferatoxin or resiniferatoxin alone. The 10 most strongly differentially expressed genes associated with each treatment are shown. Each column shows gene expression for an individual mouse bladder. Green and red column coloring indicates IPSE combined with resiniferatoxin (H06) versus resiniferatoxin only (RTX)-treated bladders, respectively. Genes are sorted by signal-to-noise scores, and their symbols and names are listed in rows. Darkest blue to darkest red coloring represents lowest to highest gene expression, respectively, based on normalized read counts.
IPSE is taken up by both neuronal and urothelial cells via endocytosis
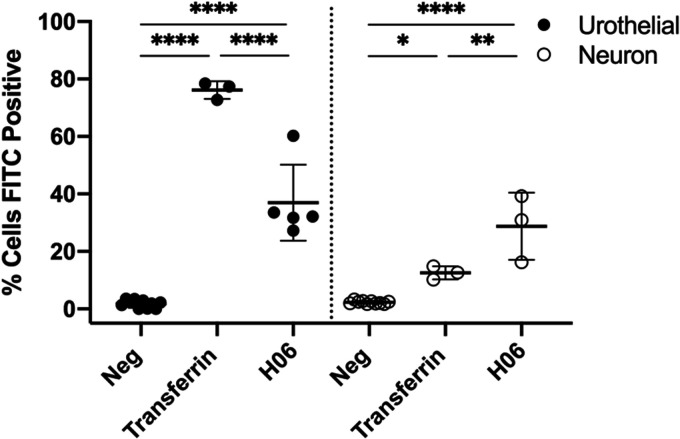
Resiniferatoxin is the most potent known agonist of the transient receptor potential cation channel subfamily V member 1 (TRPV1) receptor. Given that expression of this receptor is enriched in afferent neurons, we hypothesized that IPSE may mediate some or most of its effects on resiniferatoxin-induced pain through neuronal endocytosis and downstream modulation of neuronal transcription. To test this hypothesis, we sought to measure endocytosis of IPSE by Cath.a mouse neuronal cells versus HCV-29 urothelial cells (Figure 7). Despite differences in endocytosis of transferrin control between Cath.a cells and HCV-29 cells, we found that Cath.a endocytosis of IPSE was similar to that of urothelial cells, which are known to take up IPSE.22 This lends credence to the theory that IPSE may exert some of its analgesic effects through neuronal mechanisms, but also supports a possible urothelial role in IPSE’s bladder analgesic properties.
Figure 7.
Internalization of IPSE by neuronal and urothelial cells. Cath.a mouse neuronal and HCV-29 human urothelial cells were incubated for 16 hrs with Alexa 488 conjugated H06 H-IPSE (1 μg/ml) or transferrin (4 μg/ml) and analyzed by flow cytometry after trypan blue quenching of extracellular Alexa 488 signal. Data is representative of 2 experiments. *p = 0.0139, **p = 0.0025, ****p<0.0001.
Discussion
Bladder pain can be caused by infection, inflammation, instrumentation, or poorly understood conditions such as bladder pain syndrome. Regardless of etiology, there is a lack of therapeutics that target bladder pain. One study estimates that 3.3–7.9 million women in the US suffer from bladder pain symptoms.23 In the United States alone, interstitial cystitis/bladder pain syndrome costs ∼$20–40 billion per annum to treat.24 The high costs of this condition reflect limitations in available efficacious treatments. Although the pathophysiology of interstitial cystitis/bladder pain syndrome is not completely understood, prior bladder infection, stress, and changes to neural pathways may play roles in the nociception associated with this condition.2
Novel analgesics have attempted to target TRPV1 (capsaicin receptor)-expressing afferent neurons. TRPV1-expressing neurons have been implicated in chemotherapy-induced hemorrhagic cystitis as well as inflammation associated with interstitial cystitis/bladder pain syndrome.25,26 Hence, analgesics targeting TRPV1-expressing neurons may be a promising therapeutic approach for bladder pain caused by disparate noxious stimuli.
Parasite-derived molecules hold promise as non-opioid analgesics. Parasites have closely co-evolved with humans, and in the process have evolved the ability to produce molecules which modulate host inflammation to prevent parasite death. This observation has led to “helminth therapy”, including administration of helminth eggs to patients with inflammatory bowel disease to decrease disease flares and symptoms.27 A likely safer approach to helminth therapy would be to generate recombinant parasite-derived proteins and administer these single proteins to patients based on known disease mechanisms.
One set of parasite proteins with significant therapeutic potential is the group of homologs of the Interleukin-4 inducing Principle of Schistosoma mansoni Eggs (IPSE).28 IPSE, also known as α-13, has multiple host immune modulatory functions. Firstly, IPSE ligates Fcε receptor-bound IgE on the surface of basophils and mast cells to induce IL-4.7,28–30 It is also able to bind to immunoglobulins on the surface of B regulatory cells (Bregs) and thereby activate these cells.31 The S. mansoni ortholog of IPSE called S. mansoni chemokine-binding protein (smCKBP) can neutralize chemokines.32 Finally, IPSE contains a nuclear localization sequence which directs the protein to host cell nuclei,4,9 where it modulates transcription.5,22
IPSE’s IL-4-influencing properties led us to test its ability to lessen IL-4-dependent, ifosfamide-induced hemorrhagic cystitis.5,7,33 Besides verifying that IPSE indeed could dampen ifosfamide-triggered hemorrhagic cystitis in an IL-4-dependent manner, we also found that many of IPSE’s effects in this model relied upon an intact nuclear localization sequence.7,33 In a subsequent RNA-Seq-based analysis, we confirmed that gene transcription related to TNF signaling is upregulated in ifosfamide-induced hemorrhagic cystitis,34 as reported by others using alternative experimental approaches.35,36 Moreover, through this analysis we discovered that IPSE reduces expression of ifosfamide-induced genes related to the TNF pathway.
TNF signaling has also been implicated in promotion of resiniferatoxin-induced nociception.37 Resiniferatoxin is the most potent known agonist for the nociception-associated TRPV1 receptor. TRPV1 stimulation by resiniferatoxin causes this ion channel to become permeable to cations, including calcium. The influx of calcium and other cations causes TRPV1-expressing neurons to depolarize, transmitting strong nociceptive signals. Acute resiniferatoxin stimulation is followed by desensitization and analgesia, in part because nerve endings die from calcium overload.38,39
Oguchi et al. reported that resiniferatoxin-induced bladder pain could be alleviated by virally delivered IL-4.8 Considering IPSE’s IL-4-inducing properties, we postulated that IPSE could also lessen resiniferatoxin-triggered bladder pain through IL-4-related pathways. Although we did not definitively confirm IPSE could decrease resiniferatoxin-induced bladder pain via IL-4-dependent signaling, we did verify IPSE exerts analgesia through nuclear localization sequence-dependent mechanisms (Figure 1(b)), similar to our observations in the ifosfamide-induced hemorrhagic cystitis model.7,33 Furthermore, bladder transcriptional profiling revealed a role for TNF pathways in resiniferatoxin-triggered bladder pain (Figures 4 and 5 and Supplemental Table 1), parallel to our findings in ifosfamide-induced hemorrhagic cystitis.5 Lastly, we discovered that IPSE decreases gene transcription of TNF-associated pathways induced by resiniferatoxin (Figures 4 and 5 and Supplemental Table 1), again mirroring our observations using the ifosfamide-triggered model of hemorrhagic cystitis-associated bladder pain.5 It remains to be determined whether IPSE’s ability to alleviate resiniferatoxin-induced nociception acts at the level of the spinal cord and/or bladder.
Our work has noteworthy limitations. Although a single dose of IPSE prior to resiniferatoxin exposure greatly decreased bladder pain-associated behaviors, it did not abolish them completely (Figure 1). In addition, IPSE did not alleviate licking, another set of resiniferatoxin-induced nociceptive behaviors (data not shown). Future work will examine the effects of repeated doses of H06 H-IPSE, as well as other wild type and mutant orthologs of IPSE. Despite an apparent analgesic phenotype, H06 H-IPSE did not lead to radical changes in the transcriptome of the resiniferatoxin-exposed bladder (Figure 2). However, the observed differential expression of TNF-associated genes is consistent with known effects of resiniferatoxin (Figures 4 and 5 and Supplemental Table 1), and are also well-aligned with our observations in ifosfamide-induced hemorrhagic cystitis.5,37 Finally, it is possible that trypsinization of the neuronal and urothelial cells prior to IPSE uptake experiments may have affected cellular endocytosis. Nonetheless, HCV-29 urothelial cells demonstrated 80% transferrin uptake 16 hours following trypsinization, suggesting that transferrin receptor function recovers well after trypsin exposure. Cath.a neuronal cells primarily grow buoyant in suspension, and only a minority of cells are adherent and require trypsinization to release them. Assuming the majority of buoyant Cath.a cells have intact transferrin receptors (due to lack of exposure to trypsin), the low transferrin and higher IPSE uptake by these cells indicates that Cath.a endocytosis of IPSE may not be inhibited by trypsin exposure.
In summary, a single intravenous dose of H06 H-IPSE ameliorates bladder pain induced by resiniferatoxin, the most potent known agonist for TRPV1, an ion channel widely expressed by nociceptive neurons. H06 H-IPSE exerts this effect through nuclear localization sequence-linked pathways and does so in the context of endocytosis by both neurons and urothelial cells. This indicates that H06 H-IPSE’s analgesic features may depend on the molecule’s multiple host modulatory functions. Additionally, these functions may act upon neurons, but may also be executed through effects on other cell types that express TRPV1 and/or that modulate neuronal properties. For example, human leukocytes have been reported to express TRPV1,40 as well as urothelial cells (reviewed by Andersson41) Ongoing efforts will help identify IPSE’s mechanisms of effect on TRPV1-associated nociception and may contribute to development of IPSE as a novel analgesic.
Supplementary Material
Acknowledgment
We thank Paul Brindley for the gift of HCV-29 cells. We are grateful for support of this work by NIH R01DK113504.
Footnotes
Author Contributions: KI: performed experiments, conducted data analysis, assisted with manuscript editing. ECM: designed and performed experiments, conducted data analysis, assisted with manuscript editing. LL: performed experiments. OL: performed experiments. LFP: participated in experiment design, assisted with manuscript editing. JCF: assisted with manuscript editing. TSJ: provided key reagents, conducted data analysis. FHF: provided key reagents, conducted data analysis, assisted with manuscript editing. MHH: designed experiments, provided funding, wrote manuscript.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by NIH R01DK113504.
ORCID iD: Michael H Hsieh https://orcid.org/0000-0002-9650-2691
References
- 1.Elliott TR. The innervation of the bladder and urethra. J Physiol 1907; 35: 367–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.De Groat WC, Yoshimura N. Afferent nerve regulation of bladder function in health and disease. Handb Exp Pharmacol 2009; 194: 91–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schramm G, Gronow A, Knobloch J, Wippersteg V, Grevelding CG, Galle J, Fuller H, Stanley RG, Chiodini PL, Haas H, Doenhoff MJ. IPSE/alpha-1: a major immunogenic component secreted from Schistosoma mansoni eggs. Mol Biochem Parasitol 2006; 147: 9–19. [DOI] [PubMed] [Google Scholar]
- 4.Kaur I, Schramm G, Everts B, Scholzen T, Kindle KB, Beetz C, Montiel-Duarte C, Blindow S, Jones AT, Haas H, Stolnik S, Heery DM, Falcone FH. Interleukin-4-inducing principle from Schistosoma mansoni eggs contains a functional C-terminal nuclear localization signal necessary for nuclear translocation in mammalian cells but not for its uptake. Infect Immun 2011; 79: 1779–1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mbanefo EC, Le L, Zee R, Banskota N, Ishida K, Pennington LF, Odegaard JI, Jardetzky TS, Alouffi A, Falcone FH, Hsieh MH. IPSE, a urogenital parasite-derived immunomodulatory protein, ameliorates ifosfamide-induced hemorrhagic cystitis through downregulation of pro-inflammatory pathways. Sci Rep 2019; 9: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Macedo FYB, Mourão LTC, Freitas HC, Lima RCP, Wong DVT, Oriá RB, Vale ML, Brito GAC, Cunha FQ, Ribeiro RA. Interleukin-4 modulates the inflammatory response in ifosfamide-induced hemorrhagic cystitis. Inflammation 2012; 35: 297–307. [DOI] [PubMed] [Google Scholar]
- 7.Mbanefo EC, Le L, Pennington LF, Odegaard JI, Jardetzky TS, Alouffi A, Falcone FH, Hsieh MH. Therapeutic exploitation of IPSE, a urogenital parasite-derived host modulatory protein, for chemotherapy-induced hemorrhagic cystitis. FASEB J 2018; 32: fj.201701415R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oguchi T, Funahashi Y, Yokoyama H, Nishizawa O, Goins WF, Goss JR, Glorioso JC, Yoshimura N. Effect of herpes simplex virus vector-mediated interleukin-4 gene therapy on bladder overactivity and nociception. Gene Ther 2013; 20: 194–200. [DOI] [PubMed] [Google Scholar]
- 9.Pennington LF, Alouffi A, Mbanefo EC, Ray D, Heery DM, Jardetzky TS, Hsieh MH, Falcone FH. H-IPSE is a pathogen-secreted host nucleus-infiltrating protein (infiltrin) expressed exclusively by the schistosoma haematobium egg stage. Infect Immun 2017; 85: e00301–e00317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xiong J, Lin YH, Bi LH, Wang J, De Bai Y, Liu SD. Effects of interleukin-4 or interleukin-10 gene therapy on trinitrobenzenesulfonic acid-induced murine colitis. BMC Gastroenterol 2013; 13:165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li T, Zhai X, Jiang J, Song X, Han W, Ma J, Xie L, Cheng L, Chen H, Jiang L. Intraperitoneal injection of IL-4/IFN-γ modulates the proportions of microglial phenotypes and improves epilepsy outcomes in a pilocarpine model of acquired epilepsy. Brain Res 2017; 1657: 120–129. [DOI] [PubMed] [Google Scholar]
- 12.Haikal SM, Abdeltawab NF, Rashed LA, Abd El-Galil TI, Elmalt HA, Amin MA. Combination therapy of mesenchymal stromal cells and interleukin-4 attenuates rheumatoid arthritis in a collagen-induced murine model. Cells 2019; 8: 823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kalinichenko LS, Pertsov SS, Koplik EV. Effect of interleukin-4 on antioxidant protection of the brain in rats during acute emotional stress. Bull Exp Biol Med 2013; 156: 7–10. [DOI] [PubMed] [Google Scholar]
- 14.Yokoyama H, Oguchi T, Goins WF, Goss JR, Nishizawa O, de Groat WC, Wolfe D, Krisky DM, Glorioso JC, Yoshimura N. Effects of herpes simplex virus vector–mediated enkephalin gene therapy on bladder overactivity and nociception. Hum Gene Ther 2013; 24: 170–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Malykhina AP, Qin C, Lei Q, Pan X-Q, Greenwood-Van Meerveld B, Foreman RD. Differential effects of intravesical resiniferatoxin on excitability of bladder spinal neurons upon Colon–bladder cross-sensitization. Brain Res 2013; 1491: 213–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Majima T, Funahashi Y, Takai S, Goins WF, Gotoh M, Tyagi P, Glorioso JC, Yoshimura N. Herpes simplex virus vector-mediated gene delivery of poreless TRPV1 channels reduces bladder overactivity and nociception in rats. Hum Gene Ther 2015; 26: 734–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yoshikawa S, Oguchi T, Funahashi Y, de Groat WC, Yoshimura N. Glycine transporter type 2 (GlyT2) inhibitor ameliorates bladder overactivity and nociceptive behavior in rats. Eur Urol 2012; 62: 704–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Craft RM, Carlisi VJ, Mattia A, Herman RM, Porreca F. Behavioral characterization of the excitatory and desensitizing effects of intravesical capsaicin and resiniferatoxin in the rat. Pain 1993; 55: 205–215. [DOI] [PubMed] [Google Scholar]
- 19.Funahashi Y, Oguchi T, Goins WF, Gotoh M, Tyagi P, Goss JR, Glorioso JC, Yoshimura N. Herpes simplex virus vector mediated gene therapy of tumor necrosis factor-α blockade for bladder overactivity and nociception in rats. J Urol 2013; 189: 366–373. [DOI] [PubMed] [Google Scholar]
- 20.Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES, Mesirov JP. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A 2005; 102: 15545–15550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blighe K, Rana S, Lewis M. EnhancedVolcano: publication-ready volcano plots with enhanced colouring and labeling, https://github.com/kevinblighe/EnhancedVolcano (2018, accessed 21 October 2020).
- 22.Roderfeld M, Padem S, Lichtenberger J, Quack T, Weiskirchen R, Longerich T, Schramm G, Churin Y, Irungbam K, Tschuschner A, Windhorst A, Grevelding CG, Roeb E. Schistosoma mansoni egg-secreted antigens activate hepatocellular carcinoma-associated transcription factors c-Jun and STAT3 in hamster and human hepatocytes. Hepatology 2020; 72: 626–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berry SH, Elliott MN, Suttorp M, Bogart LM, Stoto MA, Eggers P, Nyberg L, Clemens JQ. Prevalence of symptoms of bladder pain syndrome/interstitial cystitis among adult females in the United States. J Urol 2011; 186: 540–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pierce AN, Christianson JA. Stress and chronic pelvic pain. Prog Mol Biol Transl Sci 2015; 131: 509–535. [DOI] [PubMed] [Google Scholar]
- 25.DeBerry JJ, Schwartz ES, Davis BM. TRPA1 mediates bladder hyperalgesia in a mouse model of cystitis. Pain 2014; 155: 1280–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu B-l, Yang F, Zhan H-l, Feng Z-y, Zhang Z-g, Li W-b, Zhou X-f. Increased severity of inflammation correlates with elevated expression of TRPV1 nerve fibers and nerve growth factor on interstitial cystitis/bladder pain syndrome. Urol Int 2014; 92: 202–208. [DOI] [PubMed] [Google Scholar]
- 27.Broadhurst MJ, Leung JM, Kashyap V, McCune JM, Mahadevan U, McKerrow JH, Loke P. IL-22+ CD4+ T cells are associated with therapeutic Trichuris trichiura infection in an ulcerative colitis patient. Sci Transl Med 2010; 2: 60ra88. [DOI] [PubMed] [Google Scholar]
- 28.Schramm G, Falcone FH, Gronow A, Haisch K, Mamat U, Doenhoff MJ, Oliveira G, Galle J, Dahinden CA, Haas H. Molecular characterization of an interleukin-4-inducing factor from Schistosoma mansoni eggs. J Biol Chem 2003; 278: 18384–18392. [DOI] [PubMed] [Google Scholar]
- 29.Knuhr K, Langhans K, Nyenhuis S, Viertmann K, Kildemoes AMO, Doenhoff MJ, Haas H, Schramm G. Schistosoma mansoni egg-released IPSE/alpha-1 dampens inflammatory cytokine responses via basophil interleukin (IL) -4 and IL-13. 2018; 9: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meyer NH, Mayerhofer H, Tripsianes K, Blindow S, Barths D, Mewes A, Weimar T, Köhli T, Bade S, Madl T, Frey A. A crystallin fold in the interleukin-4-inducing principle of Schistosoma mansoni eggs (IPSE/alpha-1) mediates IgE binding for antigen-independent basophil activation. J Biol Chem 2015; 290: 22111–22126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haeberlein S, Obieglo K, Ozir-Fazalalikhan A, Chayé MAM, Veninga H, van der Vlugt LEPM, Voskamp A, Boon L, den Haan JMM, Westerhof LB, Wilbers RHP, Schots A, Schramm G, Hokke CH, Smits HH. Schistosome egg antigens, including the glycoprotein IPSE/alpha-1, trigger the development of regulatory B cells. PLoS Pathog 2017; 13: e1006539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith P, Fallon RE, Mangan NE, Walsh CM, Saraiva M, Sayers JR, McKenzie ANJ, Alcami A, Fallon PG. Schistosoma mansoni secretes a chemokine binding protein with antiinflammatory activity. J Exp Med 2005; 202: 1319–1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zee RS, Mbanefo EC, Le LH, Pennington LF, Odegaard JI, Jardetzky TS, Alouffi A, Akinwale J, Falcone FH, Hsieh MH. IPSE, a parasite-derived host immunomodulatory protein, is a potential therapeutic for hemorrhagic cystitis. Am J Physiol Renal Physiol 2019; 316: F1133–F1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mbanefo E, Le L, Zee R, Banskota N, Ishida K, Pennington LF, Odegaard JI, Jardetzky TS, Alouffi A, Falcone FH, Hsieh MH. IPSE, a urogenital parasite-derived immunomodulatory protein, ameliorates ifosfamide-induced hemorrhagic cystitis through downregulation of pro-inflammatory pathways. bioRxiv [Internet]. Cold Spring Harbor Laboratory. 2018; 381764. https://www.biorxiv.org/content/early/2018/07/31/381764 [DOI] [PMC free article] [PubMed]
- 35.Leite CAVG, Alencar VTL, Melo DLR, Mota JMSC, Melo PH, Mourão LTC, Wong DVT, Magalhães PJC, Santos AA, Brito GAC, Lima-Júnior RCP, Cunha FQ, Ribeiro RA. Target inhibition of IL-1 receptor prevents Ifosfamide-Induced hemorrhagic cystitis in mice. J Urol 2015; 194: 1777–1786. [DOI] [PubMed] [Google Scholar]
- 36.Ribeiro RA, Freitas HC, Campos MC, Santos CC, Figueiredo FC, Brito GAC, Cunha FQ. Tumor necrosis factor-alpha and interleukin-1beta mediate the production of nitric oxide involved in the pathogenesis of ifosfamide induced hemorrhagic cystitis in mice. J Urol 2002; 167: 2229–2234. [PubMed] [Google Scholar]
- 37.Lu SC, Chang YS, Kan HW, Hsieh YL. Tumor necrosis factor-α mediated pain hypersensitivity through ret receptor in resiniferatoxin neuropathy. Kaohsiung J Med Sci 2018; 34: 494–502. [DOI] [PubMed] [Google Scholar]
- 38.Olah Z, Szabo T, Karai L, Hough C, Fields RD, Caudle RM, Blumberg PM, Iadarola MJ. Ligand-induced dynamic membrane changes and cell deletion conferred by vanilloid receptor 1. J Biol Chem 2001; 276: 11021–11030. [DOI] [PubMed] [Google Scholar]
- 39.Szallasi A, Blumberg PM. Vanilloid receptor loss in rat sensory ganglia associated with long term desensitization to resiniferatoxin. Neurosci Lett 1992; 140: 51–54. [DOI] [PubMed] [Google Scholar]
- 40.Spinsanti G, Zannolli R, Panti C, Ceccarelli I, Marsili L, Bachiocco V, Frati F, Aloisi AM. Quantitative real-time PCR detection of TRPV1-4 gene expression in human leukocytes from healthy and hyposensitive subjects. Mol Pain 2008; 4: 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Andersson K-E. TRP channels as lower urinary tract sensory targets. Med Sci 2019; 7: 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.