Abstract
Objective
This post hoc study investigated the relationship between patient response in terms of migraine headache day reduction and patient‐reported outcomes of health‐related quality of life (HRQoL) and disability categories.
Background
Migraine causes considerable disease‐related disability and negatively impacts HRQoL of patients. Calcitonin gene‐related peptide inhibitors improve these outcomes and may eliminate disability due to migraine in some patients.
Methods
Analyses used data from 3 double‐blind, placebo (PBO)‐controlled, phase 3 studies in adults with episodic migraine (EM) (EVOLVE‐1: N = 858 and EVOLVE‐2: N = 915) or chronic migraine (CM) (REGAIN: N = 1113). Patients were randomized 2:1:1 to subcutaneous injection of PBO, galcanezumab (GMB) 120 mg, or GMB 240 mg once monthly for 6 months in EVOLVE‐1 and ‐2 and for 3 months in REGAIN. Primary endpoint was overall mean change from baseline in monthly migraine headache days. Patients were divided into 4 response‐level groups based on percent change from baseline (<30%, ≥30% to <50%, ≥50% to <75%, ≥75%). Patient‐reported outcomes included the 14‐item Migraine‐Specific Quality of Life Questionnaire version 2.1 (MSQ) and Migraine Disability Assessment (MIDAS) questionnaire.
Results
Among patients with migraine, mean improvements from baseline in MSQ domain scores increased with each successive level of migraine headache day response. On a 100‐pt scale, increases in Role Function‐Restrictive score in EM were 16.8 and 36.0 at the <30% and ≥75% response levels, respectively, and for CM were 10.7 and 46.5. Similar patterns in scores were observed for the Role Function‐Preventive and Emotional Function domains. Examination of improvement in MSQ item score by treatment group showed that, in patients with EM, approximately 10 to 20% more GMB‐treated patients (N = 796 for GMB 120 mg and GMB 240 mg) had improvements in all 14 MSQ items compared with PBO‐treated patients (N = 773) (all P < .001). In patients with CM, 3 to 16% more GMB‐treated patients (N = 507) had improvements in the 14 MSQ items compared with PBO (N = 494), though differences were statistically significant in only 19 of 28 comparisons. At baseline, mean MIDAS scores (EM, 33.1; CM, 67.2) indicated severe mean disability for patients with EM and very severe disability for patients with CM. Among patients with EM, 215 of 425 (50.6%) of those treated with GMB 120 mg and 212 of 413 (51.3%) treated with 240 mg had little/no disability due to migraine after 6 months (PBO: 277 of 832 (33.3%), P < .001 for both). Among patients with CM, 50 of 254 (19.7%) of those treated with GMB 120 mg and 54 of 258 (20.9%) treated with 240 mg reached the level of little/no disability after 3 months of treatment (PBO: 70 of 504 (13.9%), P = .045 for 120 mg, P = .017 for 240 mg).
Conclusions
Because migraine greatly impairs an individual’s ability to participate in activities of daily living, measurements of HRQoL are essential in clinical research. This study showed that function in daily life, as measured by MSQ score, improved as migraine headache days were reduced and that GMB‐treated patients were more likely to see improvement in MSQ item scores compared with PBO‐treated patients. Elimination of migraine‐related disability was also more frequent in GMB‐treated patients compared with placebo‐treated patients.
Keywords: galcanezumab, calcitonin gene‐related peptide, episodic migraine, chronic migraine, patient‐reported outcomes
Introduction
Episodic migraine (EM) and chronic migraine (CM) disorders result in substantial disease‐related disability and strongly impact health‐related quality of life (HRQoL) of patients by limiting their ability to perform activities of daily living. 1 Migraine is the second major cause of disability worldwide, 2 leading to a loss of an average of more than 10 days of paid work per year as well as missed domestic and social activities. 3 Not unexpectedly, individuals with more frequent migraine attacks, such as those with CM, experience greater disability and have larger reductions in HRQoL. 4 , 5
The harmful effects of migraine on HRQoL are extensive, with adverse effects on work as well as physical, emotional, and social aspects of daily living. 6 , 7 While HRQoL is negatively affected by headache‐related disability, 6 HRQoL can also be lowered by migraine‐related symptoms experienced between headache days. 8 Furthermore, HRQoL is impacted by economic burdens due to migraine such as the cost of healthcare and diminished productivity at work 3 , 9 as well as the effects of comorbid conditions common among people with migraine, including cardiovascular diseases, psychiatric conditions, and neurologic disorders. 6 , 9 , 10
The negative effects of migraine are not limited to the acute symptoms of a migraine attack, including headache pain, nausea and vomiting, and sensitivity to light and sound, but also include the burdens caused by migraine attacks due to limitations in daily activities such as work, household and childcare duties, and time with friends and family. In addition, between‐attack limitations, known as interictal burden, affect people even on symptom‐free days and combine with the more apparent ictal burden of migraine to have a substantial, negative impact on HRQoL. 1 , 3 , 8 Because the harmful effects of migraine on HRQoL are extensive, goals for migraine preventive treatment are not limited to decreasing migraine attack frequency, but also include improving function and reducing disability in people with migraine. 11 , 12 , 13 Trials of a new class of migraine preventive medication, calcitonin gene‐related peptide (CGRP) inhibitors, have yielded monthly migraine headache day reduction rates of ≥50%, ≥75%, and up to 100% from baseline, 14 , 15 and improvements in patient functioning have been demonstrated across these studies. 16 The consistency of these results across multiple studies indicates that meaningful reductions and even the elimination of disability due to migraine are a real possibility for some patients.
The Migraine‐Specific Quality of Life Questionnaire version 2.1 (MSQ), a reliable and validated 14‐item instrument, is often used to measure the impact of migraine on HRQoL. This survey covers the preceding 4 weeks across 3 domains including role function‐restrictive (RF‐R), role function‐preventive (RF‐P), and emotional function (EF) domains. 17 , 18 , 19 , 20 The reliability and validity of the MSQ have been specifically evaluated in patients with CM and those undergoing migraine preventive treatment, confirming that the instrument is appropriate for evaluating changes in the patient population enrolled in these studies. 20 , 21
To assess headache‐related disability in people with migraine, the Migraine Disability Survey (MIDAS) is frequently used to quantify missed days of paid work, chores at home, and social activity over a recall period of 3 months. The MIDAS includes 5 scored questions and 2 additional questions covering monthly headache days and headache severity. 22 It has been verified as reliable and scientifically sound in population‐based studies. 23 The MIDAS provides a simple way to gather meaningful information on the disability associated with migraine and improves communication between doctors and patients. 24
Galcanezumab (GMB) is a humanized monoclonal antibody that potently and selectively binds to CGRP and is used for the prevention of migraine. 25 , 26 , 27 Phase 3 trials with approximately 2900 patients enrolled demonstrated the efficacy and safety of monthly subcutaneous injections of GMB 120 and 240 mg for the preventive treatment of EM and CM. 28 , 29 , 30 In all studies, patients treated with GMB had statistically significantly greater reductions in the average number of monthly migraine headache days vs placebo (PBO)‐treated patients. 28 , 29 , 30 In addition, substantially greater percentages of GMB‐treated patients achieved ≥50%, ≥75%, and 100% reductions in monthly migraine headache days over 6 months in the EVOLVE trials 28 , 29 and ≥30% and ≥50% reductions in monthly migraine headache days over 3 months in the REGAIN trial, 30 , 31 compared to PBO‐treated patients. All of these phase 3 trials used the MSQ v2.1 and MIDAS as tools to measure the effects of migraine on patient functioning and disability. 28 , 29 , 30
The objective of this study was to test our hypothesis that improvement in migraine headache day response was associated with meaningful improvements in patient functioning for all 3 domains of the MSQ as well as each of the items. We also hypothesized that the proportion of patients with MSQ item‐level improvements and shifts to lower disability were greater for the GMB treatment group vs PBO. The post hoc analyses of 3 phase 3 trials, EVOLVE‐1, EVOLVE‐2, and REGAIN, described in this article evaluated the relationship between response in terms of migraine headache day reduction and patient functioning as measured by the MSQ domains and items. The effect of GMB compared with PBO was also evaluated for MSQ items and categorical shifts in MIDAS‐determined levels of disability to the category of “little/no disability.”
Methods
Clinical Trials
These analyses were conducted using data obtained from 3 double‐blind, randomized, PBO‐controlled phase 3 studies of similar design comparing GMB with PBO in patients with EM 28 , 29 or CM. 30 All trials used in these analyses followed the International Council for Harmonization on Good Clinical Practices guidelines as well as all relevant laws and regulations and complied with the Declaration of Helsinki. Ethical review boards approved the studies at each institution, and written, informed consent was given by all patients prior to enrollment. Study participants were adults, 18 to 65 years, with at least a 1‐year history of migraine (EVOLVE‐1 and ‐2), migraine onset before 50 years of age, and diagnosis of migraine (EVOLVE‐1 and ‐2) or CM (REGAIN) using the International Headache Society 2013 criteria. 32
Detailed descriptions of the study designs of EVOLVE‐1, EVOLVE‐2, and REGAIN have been published previously 28 , 29 , 30 and Table 1 presents key points from these 3 trials. Sample sizes for individual phase 3 trials, shown in Table 1, were determined based on numbers needed to power significance calculations for the primary endpoint (a reduction in mean migraine headache days per month) and predicted rates of discontinuation. No sample size calculations were made for the purpose of this post hoc analysis and the sample sizes were dictated by the number of patients in the original studies EVOLVE‐1, EVOLVE‐2, and REGAIN.
Table 1.
Clinical Trials Included in Analyses
| EVOLVE‐1 | EVOLVE‐2 | REGAIN | |
|---|---|---|---|
| NCT number | NCT02614183 | NCT02614196 | NCT02614261 |
| Number of patients randomized and treated (ITT) | 858 | 915 | 1113 |
| Study centers | 90 in United States and Canada | 109 in Asia, Europe, North, and South America | 116 in Asia, Europe, North, and South America |
| Headache frequency | 4‐14 migraine headache days/month (episodic migraine) | 4‐14 migraine headache days/month (episodic migraine) | ≥15 headache days/month† (chronic migraine) |
| Baseline period | 30‐40 days | 30‐40 days | 30‐40 days |
| Double‐blind period | 6 months | 6 months | 3 months |
| Follow‐up period | 4 months | 4 months | 4 months |
| Additional migraine preventive medications | Not permitted | Not permitted | Stable doses of allowed treatments permitted‡ |
| Trial phase | 3 | 3 | 3 |
| PBO‐controlled | Yes | Yes | Yes |
| Treatment groups | GMB 120 mg with 240 mg loading dose | GMB 120 mg with 240 mg loading dose | GMB 120 mg with 240 mg loading dose |
| GMB 240 mg | GMB 240 mg | GMB 240 mg | |
| PBO | PBO | PBO | |
| Treatment regimen | Monthly subcutaneous injection | Monthly subcutaneous injection | Monthly subcutaneous injection |
At least 8 of the monthly headache days were migraine headache days.
Permitted migraine preventive medications included topiramate and propranolol.
GMB = galcanezumab; ITT = intent‐to‐treat population; NCT = national clinical trial; PBO = placebo.
Outcome Measures
MSQ v2.1 and MIDAS
The MSQ v2.1 and MIDAS are self‐administered health status instruments that were collected from patients using electronic patient‐reported outcome with a slate device.
The MSQ v2.1 consists of 14 items that address 3 domains: RF‐R, RF‐P, and EF. 20 The restrictive (RF‐R) domain specifically measures disability related to the effects of migraine on the performance of work or activities of daily living, relationships with family and friends, leisure time, productivity, concentration, energy, and tiredness. The preventive (RF‐P) domain addresses complete functional impairment due to migraine and the emotional (EF) domain assesses feelings related to the effects of monthly migraine headache days. Patients were asked to respond to items using a 6‐point scale: “none of the time,” “a little bit of the time,” “some of the time,” “a good bit of the time,” “most of the time,” and “all of the time,” which were assigned scores of 1 to 6, respectively. Raw dimension scores for domains and total were computed as a sum of final item values (recoded 6 to 1) and rescaled to a 0 to 100 scale such that higher scores indicate better functioning. 17 , 18 MSQ was assessed at baseline and monthly for all trials.
The MIDAS questionnaire consists of 5 items used to assess disability in terms of days of missed or significantly reduced activity as a result of migraine headache. Items address 3 areas: work (school or employment), home (work and chores), and social events. Each item has a numeric response range from 0 to 90 days. If days are missed from work or home they are not counted as days with reduced productivity at work or home; therefore, the total score ranges from 0 to 270, with higher values indicative of more disability. Disability related to migraine is categorized into different grades based on the MIDAS score (Table 2). 9 , 33 Each score reflects patient disability over the previous 3 months, and this outcome was assessed at baseline and at Month 3 (EVOLVE‐1 and ‐2 and REGAIN) and Month 6 (EVOLVE‐1 and ‐2 only).
Table 2.
Migraine Disability Grades and Levels Based on the MIDAS Score
| Grade | Disability Level | MIDAS Total Score Range |
|---|---|---|
| Grade I | Little or no disability | 0‐5 |
| Grade II | Mild disability | 6‐10 |
| Grade III | Moderate disability | 11‐20 |
| Grade IV‐A | Severe disability | 21‐40 |
| Grade IV‐B | Very severe disability | >40 |
MIDAS = Migraine Disability Assessment.
Statistical Analyses
General Considerations
These post hoc analyses evaluated 2886 patients enrolled in EVOLVE‐1 (n = 858), EVOLVE‐2 (n = 915), and REGAIN (n = 1113), who had been randomized to receive GMB 120 or 240 mg (n = 1434) or PBO (n = 1452). For all analyses, data from EVOLVE‐1 and ‐2 were pooled and data from REGAIN were analyzed separately. All demographic characteristics and outcomes were summarized using mean and standard deviation (SD) or standard error (SE) for numeric or interval level variables and frequency and percentages for categorical variables. All statistical analyses were conducted using SAS Enterprise Guide Version 7.1 (Copyright © 2017 SAS Institute Inc., Cary NC) and included all randomized patients who received at least 1 dose of study drug, and who had non‐missing baseline value and at least 1 non‐missing post‐baseline value. All statistical tests conducted were post hoc assuming a 2‐sided significance level of 5%. No adjustments were made for multiple testing or for multiple comparisons.
Analyses of Changes in MSQ Scores by Response Categories
To analyze the association between reduction in migraine headache days and the corresponding improvements in MSQ domain scores and individual item scores, patients were grouped into 4 response categories based on their response rate as follows: First, the overall change in monthly migraine headache days was calculated as the difference between a patient’s baseline monthly migraine headache days and their average monthly migraine headache days across all months of the double‐blind period. Then, regardless of their assigned treatment group, patients were classified into the following 4 response categories based on their percent reduction in overall monthly migraine headache days compared with baseline: <30%, ≥30% to <50%, ≥50% to <75%, and ≥75%.
Analyses of changes in MSQ scores, for both domain and individual items, by migraine headache day response group used the last available observation of MSQ scores to determine the change from baseline. In analyses of MSQ scores, domain scores were on a 100‐point scale while individual items were examined as final item values on a 6‐point scale. We used an analysis of variance model (ANOVA) to evaluate whether the mean changes in the MSQv2.1 RF‐R, RF‐P, and EF domains as well as changes in individual MSQ items were different across the 4 levels of response. P values comparing the means of the 4 response groups were also calculated for each domain and item score. While no specific tests were conducted to test the assumption of normality of residuals for the ANOVA, sample sizes used were very large, ensuring the robustness of statistical tests comparing means between groups. In the analysis of changes in MSQ domain scores, standardized response means (SRMs) for each of the 4 response groups were calculated as mean change from baseline divided by the standard deviation of change from baseline in order to provide an estimate of the effect size of change from baseline for each response category and to facilitate comparison across multiple response categories. Relevant data on MSQ scores were missing for 200 patients in the EVOLVE‐1 and ‐2 trials and 37 patients in the REGAIN trial, so these patients were excluded from the analysis.
Analyses of Improvement in MSQ Item Scores
To investigate the effect of GMB treatment on improvement in MSQ item scores, changes from baseline to the average score of months 4 to 6 in MSQ item scores were calculated for patients in each treatment group. The percentages of patients with the improvement of ≥1 point on a 6‐point scale in each MSQ item were calculated and compared between pairs of treatment groups using a 2‐sided Fisher’s exact test. Therapeutic gain of GMB treatment was calculated as the difference between percentages of GMB‐treated and PBO‐treated patients experiencing improvement in item score. Relevant data on MSQ scores were missing for 204 patients in the EVOLVE‐1 and ‐2 trials and 112 patients in the REGAIN trial, so these patients were excluded from the analysis.
Analyses of Shifts to Little or No MIDAS Disability
To examine the effect of GMB treatment on the reduction of migraine headache‐related disability, the percentages of patients in each treatment group ending the trials in the little or no MIDAS disability level were determined. Patients were grouped by baseline disability level using their total MIDAS score at baseline. Total MIDAS scores at Month 6 for EVOLVE‐1 and ‐2 and at Month 3 for REGAIN were used to determine the percentage of patients who had little or no disability at the endpoint.
The percentages of patients ending the trial at the little or no disability level after treatment were calculated for both the overall populations and by baseline disability level grouping for each treatment. P values comparing percentages across pairs of treatment groups were calculated using a 2‐sided Fisher's exact test. Relevant data on MIDAS scores were missing for 103 patients in the EVOLVE‐1 and ‐2 trials and 97 patients in the REGAIN trial, so these patients were excluded from the analysis.
Results
Patient Disposition and Baseline Characteristics
Patients with migraine had considerable functional impairment and disability prior to randomization. Table 3 shows the baseline patient and migraine characteristics. On average, patients were diagnosed with migraine 20 to 21 years prior to study enrollment. Mean monthly migraine headache days at baseline were 9 for EVOLVE‐1 and ‐2 and 19 for REGAIN. In both EM and CM, patients began the trial with a relatively high level of migraine‐related disability. Patients with EM had a mean baseline MIDAS score of 33.1, indicating severe disability, and the mean baseline MIDAS score for patients with CM was 67.2, indicating very severe disability. More than half of all patients reported using prior preventive treatment for migraine during the past 5 years, and 328 of 1113 (29.5%) patients in the REGAIN study had experienced failure by at least 2 preventive medications during the same time period. As expected, MSQ and MIDAS scores reflected greater functional impairment and disability due to migraine for patients with CM compared with patients in the EM trials. In all trials, baseline RF‐R scores were the lowest (most impacted) of the MSQ domains.
Table 3.
Demographic and Baseline Clinical Characteristics: Intent‐to‐Treat Population
| EVOLVE‐1 (N = 858) | EVOLVE‐2 (N = 915) | REGAIN (N = 1113) | |
|---|---|---|---|
| Patient demographics | |||
| Mean age, years (SD) | 40.7 (11.6) | 41.9 (11.1) | 41.0 (12.1) |
| Female, n (%) | 718 (83.7) | 781 (85.4) | 946 (85.0) |
| White, n (%) | 690 (80.4) | 643 (70.3) | 879 (79.1) |
| Clinical characteristics | |||
| Mean duration of migraine diagnosis, years (SD) | 20.1 (12.4) | 20.6 (12.4) | 21.1 (12.8) |
| Mean number of monthly migraine headache days, days (SD) | 9.1 (3.0) | 9.1 (2.9) | 19.4 (4.5) |
| Mean severity of migraine headaches per month (SD)† | 2.1 (0.4) | 2.1 (0.4) | 2.2 (0.4) |
| Mean number of monthly migraine headache days with acute medication use, days (SD) | 7.4 (3.5) | 7.5 (3.3) | 15.2 (6.4) |
| Prior preventive treatment in past 5 years, n (%) | 515 (60.0) | 599 (65.5) | 866 (77.8) |
| Failed ≥2 preventives in past 5 years, n (%) | 42 (4.9) | 131 (14.3) | 328 (29.5) |
| Mean number of comorbidities other than migraine (SD)‡ | 4.7 (3.6) | 3.6 (3.1) | 4.3 (3.5) |
| Patient‐reported outcomes § | |||
| MIDAS, mean total score (SD) | 33.2 (27.7) | 33.0 (29.7) | 67.2 (57.3) |
| MIDAS disability¶: | |||
| little or no, n (%) | 68 (8.4) | 92 (10.7) | 48 (4.7) |
| Mild, n (%) | 75 (9.3) | 75 (8.7) | 43 (4.2) |
| Moderate, n (%) | 163 (20.2) | 196 (22.7) | 94 (9.3) |
| Severe, n (%) | 274 (34.0) | 252 (29.2) | 223 (21.9) |
| Very severe, n (%) | 226 (28.0) | 249 (28.8) | 608 (59.8) |
| MSQ total, mean (SD) | 57.6 (16.8) | 58.4 (16.4) | 44.9 (18.0) |
| MSQ role function‐restrictive, mean (SD) | 51.5 (16.0) | 51.7 (15.6) | 38.7 (17.2) |
| MSQ role function‐preventive, mean (SD) | 67.0 (18.9) | 67.6 (19.3) | 55.7 (21.1) |
| MSQ emotional function, mean (SD) | 59.4 (24.6) | 61.9 (24.0) | 44.9 (26.3) |
Severity ratings defined as 0 = none, 1 = mild, 2 = moderate, 3 = severe.
Numbers of patients from whom the mean number of comorbidities were collected were 772 for EVOLVE‐1, 718 for EVOLVE‐2, and 937 for REGAIN.
Numbers of patients from whom patient‐reported outcomes were collected were 851 for EVOLVE‐1, 909 for EVOLVE‐2, and 1090 for REGAIN.
MIDAS disability category was determined only for patients with both a baseline and ending MIDAS score (806 for EVOLVE‐1, 864 for EVOLVE‐2, and 1016 for REGAIN).
MIDAS = Migraine Disability Assessment; MSQ = Migraine‐Specific Quality of Life Questionnaire v2.1; SD = standard deviation.
Post Hoc Analyses
Change in MSQ Domain Scores in Relation to Monthly Migraine Headache Day Response
Data in Table 4 show changes in mean MSQ domain scores, each on a scale of 100 points, for response groups that include both PBO‐ and GMB‐treated patients. In both EM and CM, the mean changes in MSQ domain scores increased as migraine headache day response level increased, for all 3 domains (P < .001 for all MSQ domain scores in both EM and CM). SRMs were highest for changes observed in the RF‐R domain (up to 2.0 for EM and 2.6 for CM), indicating that scores for this domain were more strongly impacted by monthly migraine headache day frequency.
Table 4.
Mean CHANGE in MSQ Domain Scores by Monthly Migraine Headache Day Response Group
| Percent Improvement From Baseline in Monthly Migraine Headache Day Frequency† (Response Group) | n | Mean (SD) Change From Baseline in Domain Score‡ | |||||
|---|---|---|---|---|---|---|---|
| RF‐R | RF‐R SRM | RF‐P | RF‐P SRM | EF | EF SRM | ||
| Episodic migraine population | |||||||
| <30% | 467 | 16.8 (16.6) | 1.0 | 11.2 (18.3) | 0.6 | 13.7 (21.8) | 0.6 |
| ≥30% to <50% | 250 | 26.1 (17.0) | 1.5 | 18.6 (17.9) | 1.0 | 23.3 (24.3) | 1.0 |
| ≥50% to <75% | 317 | 30.3 (15.4) | 2.0 | 20.4 (16.9) | 1.2 | 25.6 (21.2) | 1.2 |
| ≥75% | 539 | 36.0 (18.9) | 1.9 | 26.0 (20.6) | 1.3 | 31.1 (24.2) | 1.3 |
| P value§ | <.001 | <.001 | <.001 | ||||
| Chronic migraine population | |||||||
| <30% | 570 | 10.7 (18.3) | 0.6 | 7.7 (19.6) | 0.4 | 8.6 (25.7) | 0.3 |
| ≥30% to <50% | 183 | 26.4 (18.8) | 1.4 | 19.3 (19.0) | 1.0 | 22.9 (23.0) | 1.0 |
| ≥50% to <75% | 200 | 34.5 (19.5) | 1.8 | 28.2 (19.7) | 1.4 | 33.9 (26.3) | 1.3 |
| ≥75% | 123 | 46.5 (17.6) | 2.6 | 34.0 (18.6) | 1.8 | 44.8 (26.1) | 1.7 |
| P value§ | <.001 | <.001 | <.001 | ||||
Endpoint was mean monthly migraine headache days over Months 1‐6 for EVOLVE‐1 and ‐2, and Months 1‐3 for REGAIN.
MSQ domain scores shown are on a 100‐point scale and analysis of change in MSQ score used the last available observation of MSQ score to calculate change from baseline.
P values for between‐category comparisons are from the analysis of variance.
EF = emotional function domain of MSQ; MSQ = Migraine‐Specific Quality of Life Questionnaire version 2.1; RF‐P = role function‐preventive domain of MSQ; RF‐R = role function‐restrictive domain of MSQ; SD = standard deviation; SRM = standardized response mean (mean change/standard deviation of change).
The greatest observed mean changes in domain scores were for the MSQ RF‐R domain among the response group of patients experiencing ≥75% decrease in monthly migraine headache days (36.0 points for EM and 46.5 points for CM).
Change From Baseline in MSQ Item Scores by Migraine Headache Day Response Group
Figure 1 shows the mean changes from baseline to endpoint on the 6‐point scale for each individual MSQ item score, by migraine headache day response group. Consistently, greater mean changes in item‐level scores (improvements) were observed as response rates increased across all 14 items (for both EM and CM). The only exception was that “fear of disappointing others due to migraine” in the ≥30% to <50% response group for EM was equivalent to that for the ≥50% to <75% group. P values (<.001 for all item scores) verified that item scores differed significantly by response group.
Fig. 1.
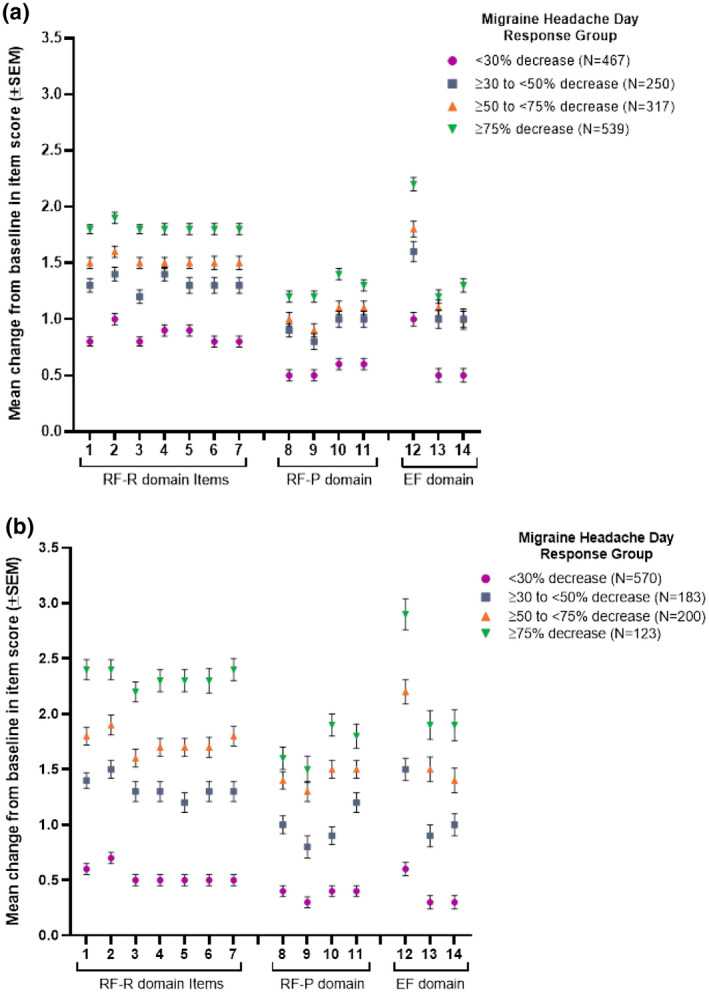
Patients were grouped by response rates based on their percent decrease from baseline in monthly migraine headache days. Response groups included both galcanezumab‐ and placebo‐treated patients. Mean change from baseline in unscaled Migraine‐Specific Quality of Life Questionnaire version 2.1 (MSQ) item scores (range 1‐6 for each item) were calculated for each response group. Points represent the mean change in score for individual items from the role function‐restrictive (RF‐R), role function‐preventive (RF‐P), and emotional function (EF) domains of the MSQ for response groups from EVOLVE‐1 and ‐2 (1a) and REGAIN (1b). Error bars show standard error of mean (SEM). Abbreviated content of MSQ items: 1. Migraine interfered with how dealt with family. 2. Migraine interfered with leisure activities. 3. Difficulty performing work or daily activities due to migraine. 4. Kept from getting much done at work or home due to migraine. 5. Migraine limited ability to concentrate at work or for an activity. 6. Migraine made too tired for work or activities. 7. Migraine limited the days felt energetic. 8. Skipped work or activity due to migraine. 9. Often needed help in handling routine tasks. 10. Stopped work or activity due to migraine. 11. Not gone to social activity due to migraine. 12. Felt frustrated due to migraine. 13. Felt like a burden due to migraine. 14. Afraid to disappoint others due to migraine.Pvalues across response groups were <.001 for all MSQ items in both episodic and chronic migraine populations.
While patients with <30% decrease in migraine headache days experienced the smallest changes in item scores (0.5 to 1 for EM and 0.3 to 0.7 for CM), patients with ≥75% decrease in migraine headache days had the highest mean increases (1.2 to 2.2 for EM and 1.5 to 2.9 for CM).
In both EM and CM, the greatest improvements were observed for items in the RF‐R domain, which is also the domain with the lowest (worst) baseline scores, as well as for 1 question in the EF domain about feeling frustrated due to migraine.
Percentages of Patients With Improvement in Individual MSQ Items
Table 5 shows the percentages of patients who experienced an improvement from baseline to endpoint of ≥1 point on the 6‐point scale of each individual MSQ item score, by treatment group. In patients with EM, statistically significantly greater percentages of patients experienced an improvement in scores for all items when treated with GMB compared with PBO (all P values <.001). The therapeutic gains for GMB‐treated patients with EM ranged from approximately 10 to 20% for the 14 MSQ items.
Table 5.
Percentages of Patients Who Experienced Improvement From Baseline in MSQ Item Scores
| MSQ Item | Episodic Migraine Population | ||||||
|---|---|---|---|---|---|---|---|
| PBO N = 773 | GMB 120 mg N = 402 | GMB 240 mg N = 394 | P Value vs PBO* | Therapeutic Gain (GMB % – PBO %) | |||
| n (%) | n (%) | n (%) | 120 mg | 240 mg | 120 mg (%) | 240 mg (%) | |
| RF‐R | |||||||
| 1 | 496 (64.2) | 313 (77.9) | 300 (76.1) | <.001 | <.001 | 13.7 | 12.0 |
| 2 | 511 (66.1) | 323 (80.3) | 302 (76.6) | <.001 | <.001 | 14.2 | 10.5 |
| 3 | 467 (60.4) | 308 (76.6) | 315 (79.9) | <.001 | <.001 | 16.2 | 19.5 |
| 4 | 505 (65.3) | 315 (78.4) | 315 (79.9) | <.001 | <.001 | 13.0 | 14.6 |
| 5 | 499 (64.6) | 307 (76.4) | 315 (79.9) | <.001 | <.001 | 11.8 | 15.4 |
| 6 | 476 (61.6) | 300 (74.6) | 288 (73.1) | <.001 | <.001 | 13.0 | 11.5 |
| 7 | 483 (62.5) | 301 (74.9) | 308 (78.2) | <.001 | <.001 | 12.4 | 15.7 |
| RF‐P | |||||||
| 8 | 367 (47.5) | 244 (60.7) | 239 (60.7) | <.001 | <.001 | 13.2 | 13.2 |
| 9 | 349 (45.1) | 231 (57.5) | 216 (54.8) | <.001 | <.001 | 12.3 | 9.7 |
| 10 | 403 (52.1) | 269 (66.9) | 250 (63.5) | <.001 | <.001 | 14.8 | 11.3 |
| 11 | 384 (49.7) | 243 (60.4) | 245 (62.2) | <.001 | <.001 | 10.8 | 12.5 |
| EF | |||||||
| 12 | 503 (65.1) | 318 (79.1) | 301 (76.4) | <.001 | <.001 | 14.0 | 11.3 |
| 13 | 334 (43.2) | 228 (56.7) | 227 (57.6) | <.001 | <.001 | 13.5 | 14.4 |
| 14 | 335 (43.3) | 218 (54.2) | 224 (56.9) | <.001 | <.001 | 10.9 | 13.5 |
| MSQ Item | Chronic Migraine Population | ||||||
|---|---|---|---|---|---|---|---|
| PBO N = 494 | GMB 120 mg N = 252 | GMB 240 mg N = 255 | P Value vs PBO* | Therapeutic Gain (GMB % – PBO %) | |||
| n (%) | n (%) | n (%) | 120 mg | 240 mg | 120 mg (%) | 240 mg (%) | |
| RF‐R | |||||||
| 1 | 323 (65.4) | 197 (78.2) | 188 (73.7) | <.001 | .059 | 12.8 | 8.3 |
| 2 | 317 (64.2) | 192 (76.2) | 196 (76.9) | .004 | <.001 | 12.0 | 12.7 |
| 3 | 291 (58.9) | 188 (74.6) | 178 (69.8) | <.001 | .013 | 15.7 | 10.9 |
| 4 | 307 (62.1) | 172 (68.3) | 179 (70.2) | .147 | .077 | 6.1 | 8.1 |
| 5 | 301 (60.9) | 161 (63.9) | 181 (71.0) | .131 | .025 | 3.0 | 10.0 |
| 6 | 288 (58.3) | 167 (66.3) | 174 (68.2) | .021 | .031 | 8.0 | 9.9 |
| 7 | 293 (59.3) | 174 (69.0) | 186 (72.9) | .018 | <.001 | 9.7 | 13.6 |
| RF‐P | |||||||
| 8 | 277 (56.1) | 164 (65.1) | 156 (61.2) | .051 | .392 | 9.0 | 5.1 |
| 9 | 232 (47.0) | 144 (57.1) | 142 (55.7) | .004 | .009 | 10.2 | 8.7 |
| 10 | 269 (54.5) | 166 (65.9) | 169 (66.3) | .012 | .007 | 11.4 | 11.8 |
| 11 | 263 (53.2) | 167 (66.3) | 149 (58.4) | <.001 | .075 | 13.0 | 5.2 |
| EF | |||||||
| 12 | 292 (59.1) | 182 (72.2) | 180 (70.6) | .002 | .006 | 13.1 | 11.5 |
| 13 | 252 (51.0) | 146 (57.9) | 153 (60.0) | .05 | .007 | 6.9 | 9.0 |
| 14 | 238 (48.2) | 156 (61.9) | 140 (54.9) | .002 | .191 | 13.7 | 6.7 |
Abbreviated content of items: 1. Migraine interfered with how dealt with family. 2. Migraine interfered with leisure activities. 3. Difficulty performing work or daily activities due to migraine. 4. Kept from getting much done at work or home due to migraine. 5. Migraine limited ability to concentrate at work or for an activity. 6. Migraine made too tired for work or activities. 7. Migraine limited the days felt energetic. 8. Skipped work or activity due to migraine. 9. Often needed help in handling routine tasks. 10. Stopped work or activity due to migraine. 11. Not gone to social activity due to migraine. 12. Felt frustrated due to migraine. 13. Felt like a burden due to migraine. 14. Afraid to disappoint others due to migraine.
P values determined using a 2‐sided Fisher’s exact test.
GMB = galcanezumab; EF = emotional function domain; MSQ = Migraine‐Specific Quality‐of‐Life Questionnaire v2.1; n = number of patients within each specific category; N = number of patients in each population; PBO = placebo; RF‐P = role function‐preventive domain; RF‐R = role function‐restrictive domain.
In patients with CM, greater percentages of GMB‐treated patients experienced improvements in scores for all individual items, compared with PBO‐treated patients, although the differences were statistically significant in only 19 of 28 pairwise comparisons (Table 5). In the CM group, the therapeutic gains for GMB‐treated patients ranged from approximately 3 to 16%.
Items in the RF‐R domain had larger percentages of patients with improvement in scores for individual items (EM: 73.1 to 80.3% with GMB, 60.4 to 66.1% with PBO) compared with scores for individual items in the RF‐P domain (EM: 54.8 to 66.9% with GMB, 45.1 to 52.1% with PBO). In patients with CM, a similar pattern was seen with higher percentages of patients experiencing improvements in items of the RF‐R domain (Table 5).
Shift in MIDAS Disability Group to Little/No Disability
At baseline, 1001 of 1670 (59.9%) patients with EM and 831 of 1016 (81.8%) with CM were experiencing severe or very severe disability, according to their MIDAS total scores. Percentages of patients reaching the threshold for little or no disability due to migraine as measured by the MIDAS are shown in Figure 2.
Fig. 2.
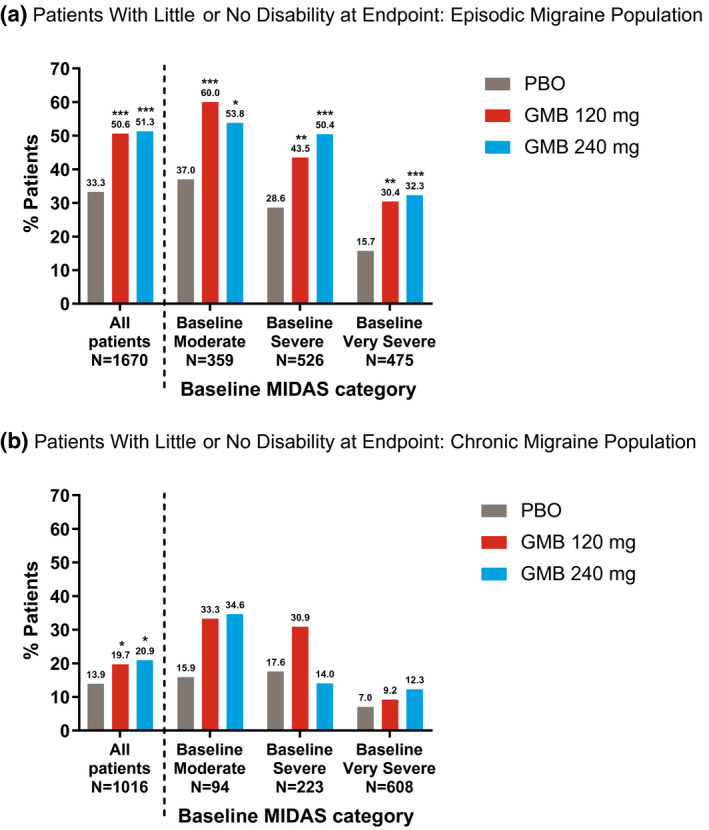
Patients from EVOLVE‐1 and ‐2 (3a) and REGAIN (3b) were grouped into baseline Migraine Disability Assessment (MIDAS) disability categories using baseline total MIDAS score. Bars show percentages of patients who were in the “little/no disability” category at the trial endpoint (Month 6 for EVOLVE‐1 and ‐2 and Month 3 for REGAIN) for baseline disability categories of “moderate,” “severe,” and “very severe,” as well as all patients together, by treatment. The “all patients” group also included patients with baseline MIDAS categories of “mild” and “little/no” disability. Galcanezumab (GMB).*P < .05, **P < .01, ***P < .001 vs placebo (PBO) by Fisher’s exact test.
In all patients, including those at any baseline level of disability, greater percentages of GMB‐treated patients ended the double‐blind treatment phase with little or no disability compared with PBO‐treated patients in both the EM (Fig. 2A) and CM (Fig. 2B) populations. Among all patients with EM, 215 of 425 patients (50.6%) in the GMB 120 mg group and 212 of 413 (51.3%) of those in the GMB 240 mg group had little or no disability due to migraine after 6 months, compared with 277 of 832 patients (33.3%) treated with PBO (P < .001 for both 120 and 240 mg groups). For patients with CM, 50 of 254 patients (19.7%) in the GMB 120 mg group and 54 of 258 (20.9%) of those treated with GMB 240 mg reached the level of little or no disability after 3 months of treatment compared with 70 of 504 patients (13.9%) treated with PBO (P = .045 for GMB 120 mg and P = .017 for GMB 240 mg).
Shifts to little or no disability were also observed within each population baseline disability group in patients with EM (Fig. 2A) and CM (Fig. 2B). For both EM and CM, the lower the baseline disability category, the greater the percentage of patients experiencing little or no disability at the endpoint. Percentages of patients ending the trials with little or no disability were greater for GMB‐treated groups, even after stratifying by baseline disability category. The only exception was in the group of patients with CM and severe baseline disability level. In this group, the percentage of GMB 240 mg‐treated patients experiencing little or no disability at the end of the trial was lower than that of PBO‐treated patients.
Discussion
In both EM and CM, patient function, as measured by mean changes in MSQ domain scores, increased as the migraine headache day response level improved. The greatest observed change was for the MSQ RF‐R domain among the subgroup of patients experiencing ≥75% response rates. On examining the item‐level results across the response groups, greater mean changes in item‐level scores (improvements) were observed as response rates increased, for both EM and CM. On evaluating item‐level improvements with GMB vs PBO, statistically significantly greater percentages of patients experienced an improvement across all 14 items for EM, and for 12 of the 14 items for CM in 1 or both dose groups.
It was notable that 50% of the patients with EM who were treated with GMB had little or no disability due to migraine after 6 months, as measured by the MIDAS, and approximately 20% of the patients with CM who were treated with GMB reached the level of little or no disability after 3 months of treatment.
With the emergence of CGRP monoclonal antibodies designed specifically to treat migraine, response rates exceeding 75% reduction in migraine headache days have been reported. 34 However, in addition to clinical measures of reduced migraine headache days, improvements in patient functioning are recognized in treatment guidelines as an important outcome to be evaluated. 35 , 36 The results of this analysis demonstrate that patients with higher response rates for migraine headache days are also experiencing greater improvements in HRQoL as measured by the MSQ. The findings of this research are important because the previous examination of the relationship between reduction in migraine headache days and improvement in patient functioning did not look beyond what is achieved with a 50% reduction in migraine headache days. 37 The current study went beyond the 50% response rate and showed that further gains in function could be realized when a response of ≥75% is achieved. Patients with migraine have described the broad negative impact of migraine on their lives including the inability to function normally, loss of time, diminished power over life, and experiencing temporary incapacitation. 38 Therefore, knowledge that achieving higher migraine headache day response rates equates to a meaningful increase in a patient’s ability to function provides evidence for healthcare providers and patients that they should continue to strive for greater reductions in monthly migraine headache days.
Patients with greater migraine headache day response experienced more improved performance in activities of daily living that were limited or completely interrupted due to migraine (RF‐R and RF‐P domains) and alleviation of the emotional impairments of migraine (EF domain). Evaluation at the MSQ item‐level revealed improvements in multiple areas of daily living, including but not limited to performance at work, concentration, and energy. In addition, MIDAS scores showed that meaningful proportions of GMB‐treated patients were experiencing little or no disability due to migraine by the end of the clinical trials for both EM and CM. These findings are highly relevant given that more than half of all patients with migraine have moderate to severe disability due to migraine, 6 and the average patient reports a reduction in work productivity due to migraine of 10.2 work‐equivalent days per year. 3
Improved patient functioning and decreased disability have been reported for clinical studies of other CGRP inhibitors, although without evaluation by the level of migraine headache response. 35 Evaluation of 2 clinical trials of onabotulinumtoxinA in people with CM reported MSQ domain score changes with response rates up to ≥50% and also found that greater headache day reduction was associated with greater MSQ score improvements, although there were a few differences. 37 The onabotulinumtoxinA trials examined headache days rather than migraine headache days and identification of a most‐impacted domain was less obvious. The SRM data showed a greater change in RF‐R for 1 of the 2 onabotulinumtoxinA trials and an equivalent effect for RF‐R and EF in the other, 37 whereas changes were greatest in the RF‐R domain in comparisons of both SRM and mean change in the GMB CM clinical trial. The results of these post hoc analyses provide more detailed evidence on improvements in patient functioning and disability that support previously reported changes in MSQ and MIDAS scores for GMB vs PBO. 28 , 29 , 30 , 39 , 40
Study Limitations
This report includes a comprehensive evaluation of the MSQ domain and item‐level changes in patient groups with response rates in multiple categories, including patients with ≥75% reductions in migraine headache days. In addition, it provides supporting evidence via analysis of changes in the MIDAS category to a level of little/no disability due to migraine. Both the MSQ and MIDAS are patient‐reported instruments with established validity and reliability among patients with migraine; however, reporting biases may have been introduced. Week‐to‐week changes could not be evaluated with either of these instruments due to their recall periods. The MIDAS, in particular, has a recall of 3 months and is not an independent assessment of patient performance.
There were variable ratios of PBO‐treated to GMB‐treated patients in the migraine headache day response groups, and groups with better monthly migraine headache day responses had higher percentages of GMB‐treated patients. It has been shown that other headache characteristics, including pain intensity, headache duration, and headache frequency can affect an individual’s HRQoL, 6 and it is possible that GMB treatment could have influenced patient MSQ responses through changes other than decreases in migraine headache days. Analyses were not controlled for the ratio of PBO‐ to GMB‐treated patients nor were adjustments made for other headache characteristics.
At baseline, approximately 60% of trial patients with CM were in the very severe disability category established by total MIDAS score. The mean baseline MIDAS score for all patients with CM was 67.2, which is 27 points above the 40‐point minimum score for the very severe disability category. From this starting point, the average patient with CM would need to improve by more than 60 points to end the study in the category of “little or no disability.” For this reason, evaluating an improvement to moderate disability may have been more clinically appropriate for the CM population. Furthermore, the duration of the CM clinical trial was only 3 months, meaning that the final MIDAS score covered all 3 months of the double‐blind period, whereas the EM trials lasted 6 months and the final MIDAS score covered Months 4 to 6 of treatment. It is also possible that 3 months of treatment might not have been enough time for many patients with CM to make the considerable improvements required to reach the level of little or no disability.
In addition, the analyses conducted were post hoc in nature and were not powered to detect a statistically significant difference between GMB and the PBO groups. Thus, a non‐statistically significant difference could be due to a lack of power to detect a difference, while statistically significant differences may have been due to chance. Given the post hoc nature of these analyses and the lack of adjustments for multiple testing/comparisons, results should be interpreted with caution. Last, there are limitations to the generalizability of these findings as this research was specific to patients from 3 different clinical trials, who may not be representative of the general migraine population.
Conclusion
Treatment of migraine should address not only the headache pain, nausea, and heightened sensitivities associated with a migraine attack, but also the impact that migraine attacks have on the daily activities of a patient with migraine. The results of this research demonstrate that the MSQ, and particularly the RF‐R domain, can be used to reliably measure the impact of migraine headache across the spectrum of headache frequency and response, including ≥75% response rates for monthly migraine headache days in both EM and CM. Item‐level analyses of the MSQ also demonstrated a strong relationship with monthly migraine headache day response levels. With regard to treatment‐specific effect, patients with EM or CM who were treated with GMB were not only more likely to experience greater reductions in migraine headache days compared with PBO, but also experienced statistically significantly greater mean improvements across MSQ items and were more likely to experience a reduction in headache‐related disability, as measured by MIDAS score. Specifically, patients were more likely to experience improvements in functioning across multiple areas of daily life, including but not limited to performance at work, concentration, energy, social activities, and emotional health. Furthermore, MIDAS scores showed that GMB treatment increases the likelihood of a patient experiencing a level of little or no headache‐related disability due to migraine.
Statement of Authorship
Category 1
(a) Conception and Design
Janet Ford, David Ayers, Dustin Ruff
(b) Analysis and Interpretation of Data
Dustin Ruff, Mallikarjuna Rettiganti, Janet Ford, Tobias Kurth, Amaal Starling, David Ayer, Linda Wietecha, Martha Port
Category 2
(a) Drafting the Manuscript
Janet Ford, Martha Port, Dustin Ruff
(b) Revising It for Intellectual Content
Janet Ford, Tobias Kurth, Amaal Starling, David Ayer, Linda Wietecha, Martha Port, Mallikarjuna Rettiganti, Dustin Ruff
Category 3
(a) Final Approval of the Completed Manuscript
Janet Ford, Tobias Kurth, Amaal Starling, David Ayer, Linda Wietecha, Martha Port, Mallikarjuna Rettiganti, Dustin Ruff
Data Availability Statement
Lilly provides access to all individual participant data collected during the trial, after anonymization, with the exception of pharmacokinetic or genetic data. Data are available to request 6 months after the indication studied has been approved in the US and EU and after primary publication acceptance, whichever is later. No expiration date of data requests is currently set once data are made available. Access is provided after a proposal has been approved by an independent review committee identified for this purpose and after receipt of a signed data sharing agreement. Data and documents, including the study protocol, statistical analysis plan, clinical study report, blank or annotated case report forms, will be provided in a secure data sharing environment. For details on submitting a request, see the instructions provided at www.vivli.org.
References
- 1. Freitag FG. The cycle of migraine: Patients' quality of life during and between migraine attacks. Clin Ther. 2007;29:939‐949. [DOI] [PubMed] [Google Scholar]
- 2. GBD Headache Collaborators . Global, regional, and national burden of migraine and tension‐type headache, 1990‐2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018;17:954‐976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Leonardi M, Raggi A. A narrative review on the burden of migraine: When the burden is the impact on people's life. J Headache Pain. 2019;20:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bigal ME, Rapoport AM, Lipton RB, Tepper SJ, Sheftell FD. Assessment of migraine disability using the migraine disability assessment (MIDAS) questionnaire: A comparison of chronic migraine with episodic migraine. Headache. 2003;43:336‐342. [DOI] [PubMed] [Google Scholar]
- 5. Guitera V, Munoz P, Castillo J, Pascual J. Quality of life in chronic daily headache: A study in a general population. Neurology. 2002;58:1062‐1065. [DOI] [PubMed] [Google Scholar]
- 6. Abu Bakar N, Tanprawate S, Lambru G, Torkamani M, Jahanshahi M, Matharu M. Quality of life in primary headache disorders: A review. Cephalalgia. 2016;36:67‐91. [DOI] [PubMed] [Google Scholar]
- 7. Chaushev N, Milanov I. Impact of migraine and migraine treatment on patient's capacity to work and quality of life. J Clin Med. 2009;2:26‐31. [Google Scholar]
- 8. Brandes JL. The migraine cycle: Patient burden of migraine during and between migraine attacks. Headache. 2008;48:430‐441. [DOI] [PubMed] [Google Scholar]
- 9. Blumenfeld AM, Varon SF, Wilcox TK, et al. Disability, HRQoL and resource use among chronic and episodic migraineurs: Results from the International Burden of Migraine Study (IBMS). Cephalalgia. 2011;31:301‐315. [DOI] [PubMed] [Google Scholar]
- 10. Burch RC, Buse DC, Lipton RB. Migraine: Epidemiology, burden, and comorbidity. Neurol Clin. 2019;37:631‐649. [DOI] [PubMed] [Google Scholar]
- 11. Silberstein SD. Practice parameter: Evidence‐based guidelines for migraine headache (an evidence‐based review): Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2000;55:754‐762. [DOI] [PubMed] [Google Scholar]
- 12. Estemalik E, Tepper S. Preventive treatment in migraine and the new US guidelines. Neuropsychiatr Dis Treat. 2013;9:709‐720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Silberstein SD, Goadsby PJ. Migraine: Preventive treatment. Cephalalgia. 2002;22:491‐512. [DOI] [PubMed] [Google Scholar]
- 14. Lambru G, Andreou AP, Guglielmetti M, Martelletti P. Emerging drugs for migraine treatment: An update. Expert Opin Emerg Drugs. 2018;23:301‐318. [DOI] [PubMed] [Google Scholar]
- 15. Tepper SJ. History and review of anti‐calcitonin gene‐related peptide (CGRP) therapies: From translational research to treatment. Headache. 2018;58(Suppl. 3):238‐275. [DOI] [PubMed] [Google Scholar]
- 16. Torres‐Ferrus M, Alpuente A, Pozo‐Rosich P. How much do calcitonin gene‐related peptide monoclonal antibodies improve the quality of life in migraine? A patient's perspective. Curr Opin Neurol. 2019;32:395‐404. [DOI] [PubMed] [Google Scholar]
- 17. Jhingran P, Davis SM, LaVange LM, Miller DW, Helms RW; MSQ: Migraine‐Specific Quality‐of‐Life Questionnaire . Further investigation of the factor structure. Pharmacoeconomics. 1998;13:707‐717. [DOI] [PubMed] [Google Scholar]
- 18. Martin BC, Pathak DS, Sharfman MI, et al. Validity and reliability of the migraine‐specific quality of life questionnaire (MSQ Version 2.1). Headache. 2000;40:204‐215. [DOI] [PubMed] [Google Scholar]
- 19. Jhingran P, Osterhaus JT, Miller DW, Lee JT, Kirchdoerfer L. Development and validation of the Migraine‐Specific Quality of Life Questionnaire. Headache. 1998;38:295‐302. [DOI] [PubMed] [Google Scholar]
- 20. Cole JC, Lin P, Rupnow MF. Validation of the Migraine‐Specific Quality of Life Questionnaire version 2.1 (MSQ v. 2.1) for patients undergoing prophylactic migraine treatment. Qual Life Res. 2007;16:1231‐1237. [DOI] [PubMed] [Google Scholar]
- 21. Bagley CL, Rendas‐Baum R, Maglinte GA, et al. Validating Migraine‐Specific Quality of Life Questionnaire v2.1 in episodic and chronic migraine. Headache. 2012;52:409‐421. [DOI] [PubMed] [Google Scholar]
- 22. Stewart WF, Lipton RB, Whyte J, et al. An international study to assess reliability of the Migraine Disability Assessment (MIDAS) score. Neurology. 1999;53:988‐994. [DOI] [PubMed] [Google Scholar]
- 23. Stewart WF, Lipton RB, Kolodner K, Liberman J, Sawyer J. Reliability of the migraine disability assessment score in a population‐based sample of headache sufferers. Cephalalgia. 1999;19:107‐114. [DOI] [PubMed] [Google Scholar]
- 24. D'Amico D, Usai S, Grazzi L, Leone M, Rigamonti A, Nespolo C, Bussone G. Disability and migraine: MIDAS. J Headache Pain. 2001;2:S25‐S27. [Google Scholar]
- 25. Raffaelli B, Reuter U. The biology of monoclonal antibodies: Focus on calcitonin gene‐related peptide for prophylactic migraine therapy. Neurotherapeutics. 2018;15:324‐335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Paemeleire K, MaassenVanDenBrink A. Calcitonin‐gene‐related peptide pathway mAbs and migraine prevention. Curr Opin Neurol. 2018;31:274‐280. [DOI] [PubMed] [Google Scholar]
- 27. Reichert JM. Antibodies to watch in 2017. mAbs. 2017;9:167‐181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Stauffer VL, Dodick DW, Zhang Q, Carter JN, Ailani J, Conley RR. Evaluation of galcanezumab for the prevention of episodic migraine: The EVOLVE‐1 randomized clinical trial. JAMA Neurol. 2018;75:1080‐1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Skljarevski V, Matharu M, Millen BA, Ossipov MH, Kim BK, Yang JY. Efficacy and safety of galcanezumab for the prevention of episodic migraine: Results of the EVOLVE‐2 Phase 3 randomized controlled clinical trial. Cephalalgia. 2018;38:1442‐1454. [DOI] [PubMed] [Google Scholar]
- 30. Detke HC, Goadsby PJ, Wang S, Friedman DI, Selzler KJ, Aurora SK. Galcanezumab in chronic migraine: The randomized, double‐blind, placebo‐controlled REGAIN study. Neurology. 2018;91:e2211‐e2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Forderreuther S, Zhang Q, Stauffer VL, Aurora SK, Lainez MJA. Preventive effects of galcanezumab in adult patients with episodic or chronic migraine are persistent: Data from the phase 3, randomized, double‐blind, placebo‐controlled EVOLVE‐1, EVOLVE‐2, and REGAIN studies. J Headache Pain. 2018;19:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Headache Classification Committee of the International Headache Society (IHS) . The International Classification of Headache Disorders, 3rd edition. Cephalalgia. 2018;38:1‐211. [DOI] [PubMed] [Google Scholar]
- 33. Lipton RB, Stewart WF, Sawyer J, Edmeads JG. Clinical utility of an instrument assessing migraine disability: The Migraine Disability Assessment (MIDAS) questionnaire. Headache. 2001;41:854‐861. [PubMed] [Google Scholar]
- 34. Tepper SJ. CGRP and headache: A brief review. Neurol Sci. 2019;40:99‐105. [DOI] [PubMed] [Google Scholar]
- 35. Sacco S, Bendtsen L, Ashina M, et al. European headache federation guideline on the use of monoclonal antibodies acting on the calcitonin gene related peptide or its receptor for migraine prevention. J Headache Pain. 2019;20:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. American Headache Society . The American Headache Society position statement on integrating new migraine treatments into clinical practice. Headache. 2019;59:1‐18. [DOI] [PubMed] [Google Scholar]
- 37. Rendas‐Baum R, Bloudek LM, Maglinte GA, Varon SF. The psychometric properties of the Migraine‐Specific Quality of Life Questionnaire version 2.1 (MSQ) in chronic migraine patients. Qual Life Res. 2013;22:1123‐1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rutberg S, Ohrling K. Migraine–more than a headache: Women's experiences of living with migraine. Disabil Rehabil. 2012;34:329‐336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ford JH, Ayer DW, Zhang Q, et al. Two randomized migraine studies of galcanezumab: Effects on patient functioning and disability. Neurology. 2019;93:e508‐e517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ford J, Tassorelli C, Leroux E, et al. Changes in patient functioning and disability: Results from a phase 3, double‐blind, randomized, placebo‐controlled clinical trial evaluating galcanezumab for chronic migraine prevention (REGAIN). Qual Life Res. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Lilly provides access to all individual participant data collected during the trial, after anonymization, with the exception of pharmacokinetic or genetic data. Data are available to request 6 months after the indication studied has been approved in the US and EU and after primary publication acceptance, whichever is later. No expiration date of data requests is currently set once data are made available. Access is provided after a proposal has been approved by an independent review committee identified for this purpose and after receipt of a signed data sharing agreement. Data and documents, including the study protocol, statistical analysis plan, clinical study report, blank or annotated case report forms, will be provided in a secure data sharing environment. For details on submitting a request, see the instructions provided at www.vivli.org.


