Abstract
Background
As the first non‐factor replacement therapy for persons with congenital hemophilia A (PwcHA), emicizumab's safety profile is of particular interest to the community.
Objectives
We applied an algorithm for categorization of fatal events contemporaneous to emicizumab using reporter‐assessed causality documented in the Roche Emicizumab Global Safety Database.
Patients/Methods
All fatalities in PwcHA reported to the database (from clinical trials, pre‐market access, and spontaneous post‐marketing reports) were categorized into: associated with hemophilia A—hemorrhagic, thrombotic, human immunodeficiency virus (HIV)/hepatitis C virus (HCV), hepatic (non‐HCV); associated with general population—trauma/suicide, non‐HA‐associated conditions; or, unspecified. Reported cause of death was not reassessed.
Results
As of cut‐off May 15, 2020, 31 fatalities in PwcHA taking emicizumab were reported. Median age at death was 58 years; 51% had factor VIII inhibitors. Fifteen fatalities were considered associated with HA; overall, the most frequent category was hemorrhage (11/31). Of these, six had a history of life‐threatening bleeds, and four had a history of intracranial hemorrhage. The remaining HA‐associated fatalities were related to HIV/HCV (3/31) and other hepatic causes (1/31). No cases were categorized as thrombotic. Of 10 cases considered not associated with HA, two were categorized as cardiovascular (non‐thrombotic), five as infection/sepsis, and one each of trauma/suicide, pulmonary, and malignancy. Six cases were unspecified.
Conclusions
No unique risk of death was associated with emicizumab prophylaxis in PwcHA. The data reveal that mortality in PwcHA receiving emicizumab was primarily associated with hemorrhage or non‐HA‐associated conditions, and was not reported by treaters to be related to emicizumab treatment.
Keywords: benchmarking, cause of death, hemophilia A, mortality, safety
Essentials.
The safety profile of emicizumab is of particular interest to the hemophilia A (HA) community.
Fatalities in people with congenital HA were assessed through the mortality framework.
Causes of death were primarily hemorrhage or non‐HA conditions, and unrelated to emicizumab.
No unique risk of death was associated with emicizumab prophylaxis.
1. BACKGROUND
In recent years, the treatment landscape for hemophilia A (HA) has rapidly evolved. Innovation in therapeutic approaches addressing factor VIII (FVIII) deficiency has led to the continued development of non‐factor replacement and gene therapies, which offer improved efficacy 1 and have the potential to reduce treatment burden compared with standard replacement therapies. While these emerging therapies have started to shift the treatment paradigm for persons with congenital hemophilia A (PwcHA), the community has limited experience with the safety profiles of these newer agents but a keen interest in gaining a comprehensive understanding. 2
Emicizumab is the first commercially available non‐factor replacement therapy for the prevention of bleeds in PwcHA. 3 , 4 Emicizumab is a bispecific, humanized, monoclonal antibody that replaces the function of missing activated FVIII by bridging activated factor IX (FIX) and factor X (FX), 5 , 6 thereby improving hemostasis in PwcHA. 7 , 8 , 9 , 10 As of March 31, 2020, more than 6500 people worldwide have been treated with emicizumab.
The risk–benefit profile of emicizumab was established through the HAVEN clinical trial program, which included PwcHA of all ages (range 1–77 years) both with and without FVIII inhibitors. 7 , 8 , 9 , 10 Emicizumab prophylaxis has demonstrated statistically significant and clinically meaningful annualized bleeding rate reductions across multiple bleed‐related endpoints throughout the HAVEN clinical trials compared with on‐demand FVIII and bypassing agents. 7 , 8 , 9 Similar improvements in bleed rates have been observed in intra‐individual comparisons of emicizumab with prophylactic FVIII or bypassing agents. 8 , 9 Long‐term median follow‐up from these studies of more than 2 years demonstrates that emicizumab prophylaxis continues to provide sustained bleed protection regardless of FVIII inhibitor status, age, or dosing regimen. 11
Across HAVEN 1‐4 studies, emicizumab was shown to have an acceptable safety profile, with the most common treatment‐related adverse events (AEs) being local injection‐site reactions. Importantly, no new safety concerns were observed in the HAVEN studies with long‐term emicizumab prophylaxis. 11 However, an interaction between emicizumab and activated prothrombin complex concentrates (aPCC) was observed during the HAVEN 1 clinical trial, during which three cases of thrombotic microangiopathy (TMA) and two cases of thrombotic events (TEs) were reported in PwcHA with FVIII inhibitors who received, on average, a cumulative amount of >100 U/kg/24 hours of aPCC for 24 hours or more while receiving emicizumab prophylaxis. 7 Additional safety data on emicizumab are currently being collected in the ongoing STASEY study (N = 195), 12 in numerous real‐world and investigator‐initiated studies, 13 and via national and international registries.
Review of the existing literature demonstrates an evolving mortality profile in PwcHA throughout time. 14 In recent decades, an observed reduction in mortality rates and ratios has been accompanied by an altered profile of common causes of death reflecting an aging population, an ebbing role of viral infections (namely human immunodeficiency virus [HIV] and hepatitis C virus [HCV]), 15 , 16 and improved treatment accessibility and outcomes. However, reporting of serious AEs, including fatalities, remains heterogeneous, limiting the characterization of these emerging trends. To facilitate thorough interpretation of such mortality data, a unified approach (“framework”) for assessing fatal events and causes of death has been developed. 17
In order to gain a comprehensive understanding of reported causes of death for PwcHA receiving emicizumab, here we apply the framework 17 to fatal cases captured in the Roche Emicizumab Global Safety Database.
2. METHODS
2.1. Identification of fatal events
Fatal case reports from the emicizumab clinical trials, pre‐market access, and spontaneous post‐marketing reports in PwcHA were collated from the Roche Emicizumab Global Safety Database by the Roche Safety Team, and the case narratives were distributed in their entirety to the authors for review. The Roche Emicizumab Global Safety Database captures all fatal events contemporaneous to emicizumab use in PwcHA reported to the company—no additional fatal events occurring contemporaneous to emicizumab use are known up to the time of data cut‐off. Due to national registry reporting criteria, it is possible that not all cases presented in the Roche Emicizumab Global Safety Database are captured in publicly available databases, such as European Haemophilia Safety Surveillance (EUHASS), EudraVigilance, and the US Food and Drug Administration (FDA) Adverse Event Reporting System (FAERS). However, information stored in the Roche Emicizumab Global Safety Database is regularly shared with relevant regulatory authorities worldwide according to regulator obligations.
The Medical Dictionary for Regulatory Activities (MedDRA) version 23.0 event terms were used for event queries. Fatal cases with a confirmed diagnosis of congenital hemophilia A, or cases for which the diagnosis was not specified beyond “hemophilia A,” were included in this analysis, and are referred to as “PwcHA” throughout. Fatalities in persons with acquired HA are not assessed within this analysis as there is no established risk–benefit profile for emicizumab in persons with acquired HA, and emicizumab is not approved for use in persons with acquired HA.
Where provided, demographics such as age, FVIII inhibitor status, comorbidities, indication, and reported causality were aggregated from individual case reports for assessment. By doing so, the authors aimed to protect patient confidentiality while also providing sufficient information to allow the establishment of informed conclusions by the wider community. Legal requirements (such as the Health Insurance Portability and Accountability Act Privacy Rule 18 in the United States and others across the world) and ethical obligations require that patient information shared in confidence cannot be disclosed in a way that allows identification of the individual.
Every reasonable effort was made to collect a full case history for each individual. As expected with spontaneous reports, however, not all case details could be obtained. Reasons for this include lack of reporter contact information, refusal to provide consent for follow‐up, or lack of response to multiple information requests. In some cases with limited information, follow‐up with the reporter was ongoing at the time of data cut‐off, thereby limiting the information available.
2.2. Mortality framework assessment
Using all information made available in the submitted case reports, fatalities in PwcHA reported to the Roche Emicizumab Global Safety Database were categorized to one of the subgroups identified in the mortality algorithm. 17 The known details of each case were provided to all authors of this article, including the causes of death as reported by the treating physician. Causes of death were not reassessed. However, authors analyzed the reports from the treating physicians and identified the key event leading to death (“primary cause”) and then this was classified into one of the following categories defined in the HA mortality framework: associated with hemophilia A—hemorrhagic, thrombotic, HIV/HCV, hepatic (non‐HCV) and associated with the general population—trauma/suicide, non‐HA‐associated conditions. All other contributing factors to the primary HA‐associated cause of death were identified using the secondary questions in the mortality algorithm and then reflected in a “heat map,” thus representing clinically complex cases. Non‐HA‐associated condition categories represent leading causes of death in the United States and worldwide general populations identified by the Centers for Disease Control and Prevention (CDC) and World Health Organization (WHO), such as cardiovascular (CV; non‐thrombotic), infection/sepsis, malignancy, dementias, and pulmonary disease–related deaths. 19 , 20 In cases in which the information provided in the report was not sufficient to identify the primary cause of death, the case is reported herein as “unspecified.” The reporter's assessment of emicizumab causality was not reassessed by the authors of this article.
3. RESULTS
3.1. Demographics and clinical characteristics
As of the May 15, 2020 cut‐off date, 31 fatalities were reported among PwcHA taking emicizumab. This is an all‐inclusive total intended to ensure absence of selection bias; even cases with incomplete information have been included.
The median (interquartile range) age at time of death was 58 (49‐‐67) years, and the majority of individuals who died were male (28/31; one female, two unknown sex; Table 1). Of the 31 individuals who died, 16 had FVIII inhibitors and 11 did not; in four cases FVIII inhibitor status information was not available. The majority of reports (24/31) provided to the Roche Emicizumab Global Safety Database were spontaneous post‐marketing reports; three were from clinical studies (including the HAVEN program), and the remaining four were from pre‐market access programs, including compassionate use. Five of the 31 individuals reported in this population were receiving palliative/hospice care at the time of death.
Table 1.
Demographic and clinical characteristics of the 31 persons receiving emicizumab who experienced fatal events
| Characteristic |
Total N = 31 |
|---|---|
| Age | |
| Median (IQR), y | 58 (49‐67) |
| <18, n (%) | 0 (0) |
| 18‐39, n (%) | 5 (16) |
| 40‐65, n (%) | 14 (45) |
| >65, n (%) | 9 (29) |
| Age not reported, n (%) | 3 (10) |
| Sex, n (%) | |
| Male | 28 (90) |
| Female | 1 (3) |
| Not reported | 2 (7) |
| Treatment setting, n (%) | |
| Clinical study a | 3 (10) |
| Access program b | 4 (13) |
| Post‐marketing | 24 (77) |
| Indication, n (%) | |
| Hemophilia A without FVIII inhibitors | 11 (35) |
| Hemophilia A with FVIII inhibitors | 16 (51) |
| Hemophilia not otherwise specified | 2 (7) |
| Not reported | 2 (7) |
| Relatedness to emicizumab, n (%) | |
| Related | 0 (0) |
| Not related | 22 (71) |
| Unknown | 1 (3) |
| Not reported | 7 (23) |
| Multiple cause c | 1 (3) |
| Past or concurrent medical history, n (%) | |
| HIV | 1 (3) |
| HCV | 12 (39) |
| HIV and HCV | 4 (13) |
| ≥1 cardiovascular risk factor d | 24 (77) |
| Cirrhosis | 7 (23) |
| Malignancy (historic or present) | 9 (29) |
Abbreviations: FVIII, factor VIII; HCV, hepatitis C virus; HIV, human immunodeficiency virus; IQR, interquartile range.
Trials the individuals were participating in: HAVEN 1 (n = 1), STASEY (n = 2).
Includes compassionate use and expanded access program, among others. The inclusion criteria for compassionate use are outlined in Table S4 in supporting information.
In one case (infection/sepsis), the relatedness for the causes of death listed were not aligned: one event was considered “not related” to emicizumab; the other was “not reported.” All other cases to which multiple causes of death were assigned consistently listed the relatedness as either “not related” or “not reported.”
A list of cardiovascular risk factors considered in the analysis is outlined in Table S1.
The majority of PwcHA who experienced fatal events while receiving emicizumab prophylaxis, and for whom case details were available to make an assessment, represent a population with complex clinical characteristics and comorbidities (Table 1). The majority of individuals (24/31) had at least one existing CV risk factor (Table S1 in supporting information). Twelve individuals were reported to have HCV, one had HIV, and four individuals had coinfection of HIV and HCV at the time of death. Furthermore, seven individuals were reported to have cirrhosis, and nine had a current or previous history of malignancy.
An assessment of causality for the fatal event relative to emicizumab was reported in 24 of the 31 cases (Table 1). Where relatedness was reported, 22 cases reported the fatality as “not related” to emicizumab treatment; one listed relatedness as “unknown.” In this case, the elderly individual was found to have experienced sudden death at his home. No autopsy was performed, and the cause of death was reported as “unknown.” One additional case had more than one cause of death listed: one was assessed as “not related” to emicizumab, while the other's relatedness was “not reported.” In all other cases in which multiple causes of death were reported, the relatedness was consistently listed as either “not related” or “not reported.” The remaining seven cases did not report the relatedness of the fatal event to emicizumab prophylaxis.
3.2. Framework categorization
As per the mortality algorithm, 17 the cause of death was assessed as being associated with HA in 15 of 31 cases. In 10 cases, the cause of death was associated with one of the leading causes observed within the general population; of these, one was associated with trauma/suicide, and the other nine cases were attributed to non‐HA‐associated conditions. The remaining six cases were assessed as having insufficient information to assign to a category, and are listed as “unspecified” (Figures 1 and 2, Table S2 in supporting information).
Figure 1.
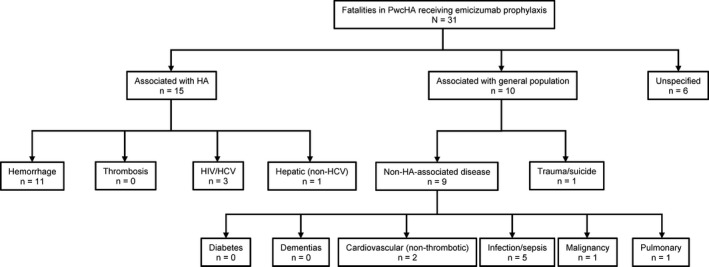
Category disposition of 31 fatalities in PwcHA receiving emicizumab prophylaxis using a contemporary algorithm for understanding mortality in PwcHA. HA, hemophilia A; HCV, hepatitis C virus; HIV, human immunodeficiency virus; PwcHA, persons with congenital hemophilia A
Figure 2.
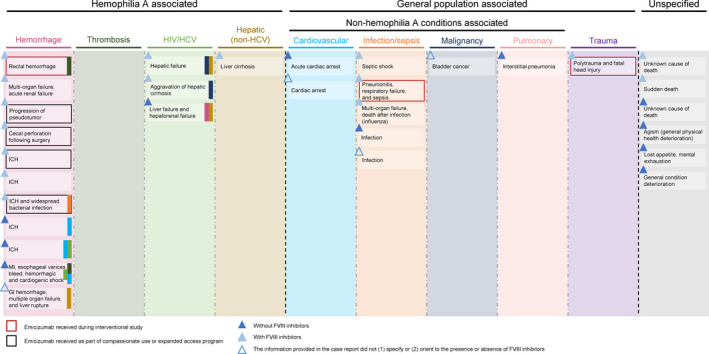
Brief cause of death of 31 fatalities in PwcHA receiving emicizumab prophylaxis by framework category. Information provided for each case reflects the cause of death as reported to the Roche Global Emicizumab Safety Database. Where multiple causes of death are given, colored bands are used to denote any cross‐over with additional framework categories. GI, gastrointestinal; HCV, hepatitis C virus; HIV, human immunodeficiency virus; ICH, intracranial hemorrhage; MI, myocardial infarction; PwcHA, persons with congenital hemophilia A
Of the 15 cases associated with HA, the most frequently attributed category for the primary cause of death was hemorrhage, with 11 cases assigned to this category. Intracranial hemorrhage (ICH) was indicated as the cause of death by the reporting physician in five of the eleven hemorrhage cases; three of these individuals were reported as having FVIII inhibitors, and four had a reported history of previous ICH.
When applying the secondary questions from the mortality algorithm 17 to these eleven cases, six reported a history of prior life‐threatening bleeds. Of these, four cases reported a past medical history of ICH prior to the initiation of emicizumab prophylaxis (Table S3 in supporting information), although it was not reported whether these prior events were spontaneous or traumatic. Furthermore, seven individuals had an increased risk of hemorrhage due to concomitant medical conditions or use of additional medications. In some of these individuals, medications known to be associated with risk of ICH (eg aspirin and enoxaparin [doses and schedules not available]) were reported as part of the record (Table S3 in supporting information). Five of the eleven cases were considered to be in receipt of adequate hemostatic factor for the treatment of the fatal event, and four cases had insufficient information to answer this question. In the remaining two cases, adequate hemostatic protection could either not be assessed or was not indicated by the reported treatment plan; in the first case, additional treatments at the time of death were not clearly reported in the case narrative and life support was removed from the patient due to a “do not resuscitate” order; in the second case, hemostatic treatment was stopped when the individual entered palliative care, and so was not present at the time of death. Neutralizing antibodies can potentially result in loss of efficacy and hemorrhage, and are important to consider. None of the hemorrhage cases contained objective corroborating evidence of the development of neutralizing antibodies such as neutralizing antibody titers or absence (or loss) of measurable emicizumab drug levels.
Four of the eleven hemorrhage cases were in individuals receiving emicizumab through pre‐market access programs inclusive of compassionate use. These individuals were required to meet a range of inclusion criteria (Table S4 in supporting information), including a history of ≥2 life‐ or limb‐threatening events in any prior consecutive 12‐month period, plus one additional such event at the time of request. One of the individuals who died of hemorrhage was participating in a clinical trial (HAVEN 1). 7 , 21
While a thrombotic event was not the primary cause of death in any of the 31 fatalities reported, in 2 deaths caused by hemorrhage, thrombosis was identified as an additional complication. One of these individuals experienced a TMA subsequent to aPCC administration for the treatment of catastrophic rectal bleeding, which was considered by the treating physician to be the primary cause of death in this individual. 21 At the time of death, the TMA was reported to be resolving. 21 The second individual experienced an electrocardiogram‐diagnosed myocardial infarction and is described in more detail below. Infection/sepsis was also identified as an additional complication of one case categorized as associated with hemorrhage (Figure 2).
Beyond the hemorrhage cases, the remaining HA‐associated cases were considered to be associated with HIV and/or HCV infection (3/31) and other hepatic causes (1/31). In the case associated with other hepatic causes, no medical history (such as HCV infection) was reported as the cause for the pre‐existing liver dysfunction. Two of the cases assigned to HIV/HCV had liver cancer. While one of these cases had achieved a sustained virologic response against HCV infection, hepatic cancer is still a potential downstream complication of previous infection, 22 hence the assigned classification for this case.
In total, 10 fatalities were from causes not associated with HA. Specifically, one case was from trauma/suicide, and the remaining cases were associated with non‐HA conditions.
Two cases were categorized as “cardiovascular (non‐thrombotic).” In the case details provided to the sponsor for one of these events, potential TE causes including stroke, pulmonary embolism, and myocardial infarction were specifically ruled out as contributing to the cause of death in the case narrative. Five fatalities were categorized as infection/sepsis (other than HCV or HIV). Of the additional cases categorized as non‐HA‐associated conditions, one case was categorized as malignancy, which was pre‐existing at the time emicizumab prophylaxis was initiated, and one was categorized as pulmonary. The remaining six cases were considered to have insufficient information to attribute a category and are listed here as unspecified.
Due to the complex nature of the cases reported, 9 of the 31 cases were reported by the treating physician as having multiple causes of death. In one such case, an individual with advanced liver disease, esophageal varices, and CV risk factors died of a severe gastrointestinal bleed with hemorrhagic shock. This individual had hemorrhagic shock, evidence of hypoperfusion, and experienced an electrocardiogram‐diagnosed myocardial infarction. Coronary artery obstruction could not be confirmed based on the limited reported information; the reporting physician did not rule it out nor include a coronary angiogram for this case. Overall, the treating physician in this case listed seven distinct causes of death for this individual, including myocardial infarction, metabolic acidosis, and cardiogenic shock. The cause of this fatality was attributed to “hemorrhage” as opposed to “thrombosis” given that the cardiac event was considered secondary to hypovolemic shock. Additional considerations such as those listed above are captured through the application of the framework secondary questions.
In a second case classified as hemorrhage, the individual was severely immunosuppressed (potentially, according to the reporter, due to experimental immunosuppressive therapies and extreme caloric restriction), with suspicion of septic emboli, in addition to being malnourished. This individual later developed a fatal ICH likely caused by disseminated methicillin‐susceptible Staphylococcus aureus infection.
A further example of the complex nature of the cases reported herein is an individual with confirmed coinfection with HIV and HCV who was diagnosed with lymphoma (type not reported). After successful treatment of the lymphoma with chemotherapy, the individual contracted influenza and later died due to multiple organ failure. Here, the death was considered to be associated with “infection/sepsis”. The individual's medical history and previous and concomitant medications were not reported, and therefore cannot add further insight into contributions of other factors, such as comorbidities.
In another case considered associated with “infection/sepsis,” an elderly individual with multiple comorbidities (including HCV‐induced liver cirrhosis) developed bilateral cellulitis. On the day this individual commenced emicizumab treatment, they were hospitalized for infection and bilateral cellulitis of the legs. In the following days, the individual developed apnea and cyanosis, and died due to infection and acute pulmonary arrest. The cause of infection was reported as being present at the time of first emicizumab treatment, although the reporter was unsure as to whether existing liver infection or infection of the legs was the primary cause of death.
Together, these example cases demonstrate the complexity and diversity of the cases reported herein.
4. DISCUSSION
At the time of data cut‐off May 15, 2020, 31 fatalities in PwcHA receiving emicizumab have been reported to the Roche Emicizumab Global Safety Database. At the closest cut‐off available to our analysis cut‐off, March 31, 2020, more than 6500 people worldwide had been treated with emicizumab. Because these two data sets are not contemporaneous and due to the nature of the post‐marketing setting, it is not possible to establish an accurate denominator for the assessment of mortality in persons receiving emicizumab, although these events provide useful insights into the safety profile of this emerging therapy. Of the 31 events reported, half (15/31) are considered to be associated with hemophilia A, one third (10/31) are considered to be associated with causes commonly seen in the general population, and the remaining six (19%) are considered “unspecified” due to incomplete case information. This is largely aligned with those previously reported for the general population of PwcHA. 14 , 17
In line with existing literature 14 and a previous analysis of fatality data across a range of coagulation products reported to the FAERS database, 17 the most common cause of death in fatalities reported to the Roche Emicizumab Global Safety Database is hemorrhage, accounting for approximately one third of all fatalities (11/31).
Approximately half (5/11; 45%) of the hemorrhage‐associated cases listed ICH as the cause of death, which is lower than in the literature reporting mortality attributed to ICH relative to all hemorrhage‐related deaths (54%‐90%). 23 , 24 , 25 , 26 The individuals reported to have died due to ICH in this population were also reported as having known risk factors for ICH, 27 , 28 specifically, prior medical history of life‐threatening ICH, and presence of FVIII inhibitors at the time of death.
Overall, the population reported here were medically complex. At the time of death, it was reported that 17 individuals had a diagnosis of HIV and/or HCV, 16 individuals had FVIII inhibitors, 24 had at least one CV risk factor, 5 individuals were in receipt of palliative/hospice care, and 4 individuals were in receipt of emicizumab through enrollment in a pre‐market access program. Within the 11 fatalities categorized as hemorrhage alone, approximately two‐thirds were at increased risk of hemorrhage due to medical conditions other than congenital HA (eg, FVIII inhibitors) or the reported use of medications other than emicizumab, and just over half had a medical history of prior life‐threatening bleeds. While the number of medically complex patients likely represents an expected channeling bias regularly observed within the first 2 years following the commercialization of therapeutic agents, this also demonstrates the challenges in assessing the events leading to the cause of death in these individuals, and, consequently, in the singular categorization of events per the algorithm used. In addition, these hemorrhagic deaths require consideration of the limitations of emicizumab prophylaxis as applied in these situations, given the evidence that emicizumab at therapeutic plasma levels converts persons with severe HA to a mild disease phenotype rather than fully normalizing hemostasis. 29 , 30
As in the FAERS analysis, in which emicizumab was the only coagulation treatment without any thrombosis‐associated deaths, 17 none of the 31 fatalities contemporaneous to emicizumab use reported to the Roche Emicizumab Global Safety Database have thrombosis as the primary cause of death. As therapies that bring the hemostatic potential of PwcHA in closer alignment with the non‐HA population are developed and become available, the incidence of thromboses—from which PwcHA have traditionally been considered to be relatively protected—warrants further exploration and monitoring. Especially in the context of an aging population with the increasing development of associated comorbidities, the rare but established risk of thrombotic events 31 , 32 , 33 , 34 , 35 remains an area of keen interest for the HA community. In a recent analysis of mortality data reported to FAERS, deaths associated with thrombosis were reported in PwcHA receiving FVIII, activated recombinant factor VII, and aPCC products, and represented 10% of all fatalities reported to the platform, further representing the need for continued monitoring of developing risk signals. 17
To sufficiently characterize any evolution of the relationship between thrombotic events and emerging hemostatic agents as development continues, stringent post‐registration monitoring should be applied to all therapies, both novel and established, and robust, standardized data collection in association with independent registries should be adopted. 14 , 17
In this population, more than half of individuals reportedly had HCV, HIV, or both, demonstrating the ongoing burden of these infections within the HA community decades after the first reports of the viral contamination of blood products. 36 Ten percent of deaths reported contemporaneous to emicizumab use were associated with HIV/HCV and these were identified as the primary cause, demonstrating that both HCV and HIV continue to contribute to mortality outcomes in PwcHA either directly or indirectly despite advancements in the therapeutic armamentarium. 37 , 38 , 39 , 40 , 41
Overall, one third of deaths were associated with causes consistent with those commonly seen in the general population according to the WHO and the CDC, including CV disease (other than demonstrably thrombotic in nature), malignancy, and infections.
Six of the 31 cases reported to the Roche Emicizumab Global Safety Database are considered “unspecified” due to insufficient case information for the determination of the primary cause of death. This demonstrates the need for improved reporting of fatal events—and all AEs—to enable clinical contextualization and mortality assessment.
Where causality of the fatal event was available (24/31), the vast majority of events were assessed as not related to emicizumab treatment by the reporter. In the remaining causes, causality of the event was reported as unknown in one case, and in one case the relatedness was reported separately for the two causes of death given (one not related, one not reported). The relatedness of the fatality to emicizumab was not reported in seven cases despite attempts to collect this information. Importantly, when cross‐examining fatal events in PwcHA receiving emicizumab in the context of this framework, there were no new or emerging safety concerns.
Despite the singular classification of cases outlined in this assessment, the complexity of cases reported in the post‐approval setting should not be underestimated. As outlined (Table 1), these cases are contextualized by complex case histories, including existing comorbidities (29% with multiple causes of death), and for which additional treatment modalities were either unavailable or refused by the patient. 21 Notably, with this retrospective assessment, CV risk factors may have been under‐reported particularly for the cases that did not involve CV‐related death, ultimately underestimating the complexity of these clinical cases.
The case details presented here demonstrate that changes in quality of life associated with emicizumab prophylaxis may bias use toward PwcHA with complex medical histories, or those that need end‐of‐life or palliative care. Indeed, in more than one case PwcHA were seen to switch to emicizumab in association with admission to palliative care facilities. While this could indicate a potential need for flexible treatment options for aging PwcHA, it also supports the hypothesis that the data herein may be subject to channeling biases that can lead to inaccurate conclusions about the safety of a therapy. 42 , 43
Limitations of this analysis include the heterogeneity of the information included in case reports, the depth of information included in reports, and the lack of a standardized approach to data collection before it is reported to the company. Ideally, surveillance systems would ensure the collection of all data relevant to the most frequently asked questions in the field of hemostasis. Detailed, timely case information is essential for the evaluation of risk in emerging therapies, and a harmonization of mortality reporting is required to enable accurate adjudication of reports and consistent analysis. Assessment of these cases is further limited by the retrospective nature of the categorization by professionals other than the treating physician. Without full knowledge of the clinical circumstances leading to the cause of death—such knowledge as is typically held by the treating physician—the authors of this article are thus limited in their assessment of categorization where incomplete information is provided. For example, in one case assigned to the hepatic (non‐HCV) category, the authors were unable to determine whether this case should, in fact, be considered as related to HIV/HCV infection. This demonstrates that the categorization framework 17 should ideally be applied by the treating physician who has the benefit of the patient's full medical history and clinical conditions prior to the fatal event, allowing for a more accurate judgment of the primary categorization of each case and, therefore, making such assessments more valuable contributions to the developing safety profile of the given therapeutic agent. Furthermore, the collection of more detailed information regarding the circumstances surrounding fatalities should ideally be submitted to regulatory authorities upon the reporting of such events by physicians, and our community should make every effort to report all AEs—both in established and novel therapies—comprehensively.
The analyses herein are also limited by the lack of a mortality denominator (ideally person‐years with time of exposure), without which the formal calculation and comparison of mortality rates across established treatments is not possible. This limitation is not unique to the current analysis, but is relevant to all such AE reporting databases including FAERS and EUHASS. Furthermore, comparisons of drugs at different stages in their life cycle are likely to be skewed by the Weber effect, which sees a peak in AE reporting in the 2 years following a treatment being made available to the market 44 and channeling bias, by which clinically complex populations could preferentially receive a new‐to‐market product. 43 Both of these are potential sources of bias in the current analysis, given that emicizumab has been recently approved and has demonstrated reduced burden of administration 10 and potential quality of life improvements compared with older, established treatment methods that have been available for many years and even decades. 7 , 8 , 9 , 10 , 45 This has the potential to lead to the reporting of a cluster of fatalities not linked to the therapy itself. Unlike traditional, well‐established treatment options, the safety events concurrent to emicizumab are likely reported more stringently due to its recent approval.
In order to facilitate the comparison of mortality rates between different treatment options, the appropriate studies, along with consistent data collection, 17 a comprehensive platform for post‐marketing reporting, and comprehensive long‐term follow‐up (several years or until death) 46 are required, as previously suggested. 47 As we continue to experience an evolution in the HA treatment landscape, we should expect to also see a shift in the causes of death reported in PwcHA toward those reported in the non‐HA population. This trend will only become visible with long‐term follow‐up and extended time on the market.
5. CONCLUSIONS
In conclusion, the application of the mortality framework 17 to fatal events reported to the Roche Emicizumab Global Safety Database from initiation of its clinical development up to May 15, 2020 contextualizes these data and demonstrates an absence of unique mortality risks associated with the administration of emicizumab prophylaxis in PwcHA. The framework applied herein has enabled critical and objective evaluation of fatal events contemporaneous to emicizumab use, and similar analyses may be applied for other hemostatic treatments—both established and coming to market. Only through such a diligent and rigorous approach to pharmacovigilance can individual risk–benefit assessments be applied when deciding on treatment options. Moreover, in rare diseases such as HA, isolated reports of fatalities are not useful for a broad understanding of safety; rather, a framework must be applied prospectively to consistently track trends over time and enable comparisons across treatments.
CONFLICTS OF INTEREST
None of the authors received honoraria or fees for their contribution to the development of this article or supplement. P.K. is a current employee of and holds shares in Genentech, Inc. Outside of this manuscript, most authors received fees for participation in activities funded by F. Hoffmann‐La Roche Ltd and/or Genentech, Inc., as described in the following disclosures, with the exception of G.F.P.
F.P. has received honoraria for participating as a speaker at satellite symposia organized by F. Hoffmann‐La Roche Ltd, Sanofi, Sobi, Spark, and Takeda, and has participated in advisory boards for F. Hoffmann‐La Roche Ltd, Sanofi, and Sobi. J.N.M. has received consultancy from CSL Behring, Catalyst Biosciences, Freeline Therapeutics, Novo Nordisk, F. Hoffmann‐La Roche Ltd, Sanofi, Spark, and Takeda, has received research funding from BioMarin, CSL Behring, Freeline Therapeutics, Novo Nordisk, Novartis, Pfizer, Sanofi, F. Hoffmann‐La Roche Ltd, and uniQure; has participated in a speakers’ bureau for CSL Behring, Catalyst Biosciences, Novo Nordisk, F. Hoffmann‐La Roche Ltd, Sanofi, Spark, and Takeda; and sits on the board of directors/advisory committee for the South Africa Medical Research Council, the Wits Health Consortium, and Colleges of Medicine of South Africa. S.W.P. has received consultancy for Apcintex, Bayer, BioMarin, Catalyst Biosciences, CSL Behring, HEMA Biologics, Freeline Therapeutics, Novo Nordisk, Pfizer, F. Hoffmann‐La Roche Ltd/Genentech, Inc., Sangamo Therapeutics, Sanofi, Takeda, Spark Therapeutics, and uniQure; has received research funding from Siemens; and is a member of the board of directors/advisory committee for the Medical and Scientific Advisory Council to the National Hemophilia Foundation and the Medical Advisory Board to World Federation of Hemophilia. C.R.M.H. has received consultancy and honoraria from Shire, Alnylam, Novo Nordisk, Sobi, and F. Hoffmann‐La Roche Ltd; has participated in speakers’ bureaus for Shire, Sobi, F. Hoffmann‐La Roche Ltd, and Pfizer; has received research funding from Bayer, Shire, Sobi, Novo Nordisk, and Pfizer; and has received travel and accommodation expenses from F. Hoffmann‐La Roche Ltd, Sobi, CSL Behring, and Shire. G.F.P. is a leader of WFH and NHF; has received honoraria from Griffols; has received fees for consultancy/advice for BioMarin, Takeda, Pfizer, Geneception, Generation Bio, CRISPR Therapeutics, and Third Rock Ventures; and has patent fees from Sanofi. P.K. is a current employee of and holds shares in Genentech, Inc. R.K.‐J. has received consultancy from Chugai Pharmaceutical Co., BioMarin, CSL Behring, CRISPR Therapeutics, Genentech, Inc., has received research funding from CSL Behring, Genentech, Inc., and Spark; has received honoraria from Chugai Pharmaceutical Co., BioMarin, CSL Behring, CRISPR Therapeutics, and Genentech, Inc., and has participated in speakers’ bureaus for F. Hoffmann‐La Roche Ltd. M.S. has received consultancy and honoraria from Chugai Pharmaceutical Co.; has received research funding from Chugai Pharmaceutical Co., F. Hoffmann‐La Roche Ltd, Sanofi, CSL Behring, KM Biologics, Novo Nordisk, Shire/Takeda; holds patents related to anti‐FIXa/FX bispecific antibodies; has participated in speakers’ bureaus for Chugai Pharmaceutical Co., Sanofi, Bayer, and Sysmex; and is a member of the board of directors/advisory committee for Chugai Pharmaceutical Co., F. Hoffmann‐La Roche Ltd, BioMarin, Bayer, and Sanofi.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to the conception and design of the work, as well as the analysis and interpretation of data for the work. All authors revised the manuscript critically and provided final approval of the version to be published. They all agree to be accountable for all aspects of the work.
Supporting information
Table S1‐S4
ACKNOWLEDGMENTS
The authors would like to thank Michelle Rice of the National Hemophilia Foundation, USA; Sonji Wilkes of the Hemophilia Federation of America, USA; Mark Skinner of the Institute for Policy Advancement Ltd, USA; Brian O'Mahony of Irish Haemophilia Society, Ireland; and Declan Noone of the European Haemophilia Consortium for initial guidance and advice. The authors would also like to thank the members of the hemophilia community and reporters who have contributed to these data and who continue to report adverse events in a timely and comprehensive manner, and the Emicizumab Global Medical Team at F. Hoffmann‐La Roche Ltd for the provision of data and insights, without whom this analysis would not have been possible. Medical writing support was provided by Sophie Nobes, BSc and Rebecca A. Bachmann, PhD of Gardiner‐Caldwell Communications, and was funded by F. Hoffmann‐La Roche Ltd.
Peyvandi F, Mahlangu JN, Pipe SW, et al. Application of a hemophilia mortality framework to the Emicizumab Global Safety Database. J Thromb Haemost. 2021;19(Suppl. 1):32–41. 10.1111/jth.15187
Manuscript handled by: David Lillicrap
Final decision: David Lillicrap, 16 November 2020
REFERENCES
- 1. Reyes A, Révil C, Niggli M, et al. Efficacy of emicizumab prophylaxis versus factor VIII prophylaxis for treatment of hemophilia A without inhibitors: network meta‐analysis and sub‐group analyses of the intra‐patient comparison of the HAVEN 3 trial. Curr Med Res Opin. 2019;35:2079‐2087. [DOI] [PubMed] [Google Scholar]
- 2. Pierce GF, Hart DP, Kaczmarek R. Safety and efficacy of emicizumab and other novel agents in newborns and infants. Haemophilia. 2019;25:e334‐e335. [DOI] [PubMed] [Google Scholar]
- 3. Food and Drug Administration . HEMLIBRA® (emicizumab‐kxwh) injection for subcutaneous use, prescribing information. Initial U.S. approval: 2017. 2018; https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/761083s000lbl.pdf. Accessed April 28, 2020.
- 4. European Medicines Agency . HEMLIBRA® solution for injection: emicizumab piIEa. Initial EU approval: 2018. 2019. https://www.ema.europa.eu/en/documents/product‐information/hemlibra‐epar‐product‐information_en.pdf. Accessed April 28, 2020.
- 5. Kitazawa T, Igawa T, Sampei Z, et al. A bispecific antibody to factors IXa and X restores factor VIII hemostatic activity in a hemophilia A model. Nat Med. 2012;18:1570‐1574. [DOI] [PubMed] [Google Scholar]
- 6. Shima M, Hanabusa H, Taki M, et al. Factor VIII‐mimetic function of humanized bispecific antibody in hemophilia A. N Engl J Med. 2016;374:2044‐2053. [DOI] [PubMed] [Google Scholar]
- 7. Oldenburg J, Mahlangu JN, Kim B, et al. Emicizumab prophylaxis in hemophilia A with inhibitors. N Engl J Med. 2017;377:809‐818. [DOI] [PubMed] [Google Scholar]
- 8. Young G, Liesner R, Chang T, et al. A multicenter, open‐label, phase 3 study of emicizumab prophylaxis in children with hemophilia A with inhibitors. Blood. 2019;134:2127‐2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mahlangu J, Oldenburg J, Paz‐Priel I, et al. Emicizumab prophylaxis in patients who have hemophilia A without inhibitors. N Engl J Med. 2018;379:811‐822. [DOI] [PubMed] [Google Scholar]
- 10. Pipe S, Shima M, Lehle M, et al. Efficacy, safety and pharmacokinetics of emicizumab prophylaxis given every 4 weeks in people with haemophilia (HAVEN 4): a multicentre, open‐label, non‐randomised phase 3 study. Lancet Haematol. 2019;6:E295‐E305. [DOI] [PubMed] [Google Scholar]
- 11. Callaghan MU, Négrier C, Paz‐Priel I, et al. Long‐term outcomes with emicizumab prophylaxis for hemophilia A with/without FVIII inhibitors from the HAVEN 1–4 studies. Blood. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jiménez‐Yuste V, Klamroth R, Castaman G, et al. A single‐arm, multicentre, open‐label, phase III clinical trial to evaluate the safety and tolerability of prophylactic emicizumab in persons with haemophilia A (PwHA) with FVIII inhibitors (STASEY): interim analysis results. Res Pract Thromb Haemost. 2019;3:116‐117. [Google Scholar]
- 13. ATHN 7: Hemophilia Natural History Study (ATHN 7). 2020. https://clinicaltrials.gov/ct2/show/NCT03619863. Accessed August 20, 2020.
- 14. Hay CRM, Nissen F, Pipe SW. Mortality in congenital hemophilia A ‐ a systematic literature review. J Thromb Haemost. 2021;19:(Suppl. 1):6‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Marcus JL, Hurley LB, Chamberland S, et al. Life expectancy of insured people with and without hepatitis C virus infection. Open Forum Infect Dis. 2020;7:2007‐2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. The Antiretroviral Therapy Cohort Collaboration . Life expectancy of individuals on combination antiretroviral therapy in high‐income countries: a collaborative analysis of 14 cohort studies. Lancet. 2008;372:293‐299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pipe SW, Kruse‐Jarres R, Mahlangu JN, et al. Establishment of a framework for assessing mortality in persons with congenital hemophilia A and its application to an adverse event reporting database. J Thromb Haemost. 2021;19:(Suppl. 1):21‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. U.S. Department of Health & Human Services . The HIPAA Privacy Rule. 2002. https://www.hhs.gov/hipaa/for‐professionals/privacy/laws‐regulations/index.html. Accessed August 12, 2020.
- 19. Centers for Disease Control and Prevention . Leading causes of death. 2020. https://www.cdc.gov/nchs/fastats/leading‐causes‐of‐death.htm. Accessed August 19, 2020.
- 20. World Health Organization . The top 10 causes of death. 2020. https://www.who.int/news‐room/fact‐sheets/detail/the‐top‐10‐causes‐of‐death. Accessed August 19, 2020.
- 21. Khoo L, Matthews S, Kershaw G, et al. Case report of a fatal rectal haemorrhage in a person with severe haemophilia A receiving emicizumab and high‐dose bypassing agents in the HAVEN 1 study. Haemophilia. 2020;00:1‐4. [DOI] [PubMed] [Google Scholar]
- 22. Hiramatsu N, Oze T, Takehara T. Suppression of hepatocellular carcinoma development in hepatitis C patients given interferon‐based antiviral therapy. Hepatol Res. 2015;45:152‐161. [DOI] [PubMed] [Google Scholar]
- 23. Eckhardt CL, Loomans JI, van Velzen AS, et al. Inhibitor development and mortality in non‐severe hemophilia A. J Thromb Haemost. 2015;13:1217‐1225. [DOI] [PubMed] [Google Scholar]
- 24. Jardim LL, van der Bom JG, Caram‐Deelder C, Gouw SC, Leal Cherchiglia M, Meireles RS. Mortality of patients with haemophilia in Brazil: first report. Haemophilia. 2019;25:e146‐e152. [DOI] [PubMed] [Google Scholar]
- 25. Walsh CE, Soucie JM, Miller CH. United States Hemophilia Treatment Center Network. Impact of inhibitors on hemophilia A mortality in the United States. Am J Hematol. 2015;90:400‐405. [DOI] [PubMed] [Google Scholar]
- 26. Yoo KY, Kim SK, Kwon SS, et al. Life expectancy of Korean haemophiliacs, 1991–2012. Haemophilia. 2014;20:e356‐e358. [DOI] [PubMed] [Google Scholar]
- 27. Witmer C, Presley R, Kulkarni R, Soucie JM, Manno CS, Raffini L. Associations between intracranial haemorrhage and prescribed prophylaxis in a large cohort of haemophilia patients in the United States. Br J Haematol. 2011;152:211‐216. [DOI] [PubMed] [Google Scholar]
- 28. Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics–2016 update. A report from the American Heart Association. Circulation. 2016;133:e38‐e360. [DOI] [PubMed] [Google Scholar]
- 29. Ferrière S, Peyron I, Christophe OD, et al. A hemophilia A mouse model for the in vivo assessment of emicizumab function. Blood. 2020;136:740‐748. [DOI] [PubMed] [Google Scholar]
- 30. Schmitt C, Adamkewicz JI, Xu J, et al. Pharmacokinetics and pharmacodynamics of emicizumab in persons with hemophilia A with factor VIII inhibitors: HAVEN 1 Study. Thromb Haemost. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tiede A. Thromboembolic risks of non‐factor replacement therapies in hemophilia. Hamostaseologie. 2017;37:307‐310. [DOI] [PubMed] [Google Scholar]
- 32. Novo Nordisk . Press release: Novo Nordisk resumes the phase 3 clinical trials investigating concizumab (anti‐TFPI mAB) in haemophilia A and B with or without inhibitors. 2020. https://ml‐eu.globenewswire.com/Resource/Download/32302d78‐439f‐4a97‐a655‐3f7b96e2cc76. Accessed November 30, 2020.
- 33. Lee L, Moreno K, Kuebler P, et al. Summary of thromboembolic (TE) or thrombotic microangiopathy (TMA) events in persons taking emicizumab. Haemophilia. 2020;26:95‐96. [Google Scholar]
- 34. Food and Drug Administration . NovoSeven highlights of prescribing information. [Last updated: July 2014]. 1999. https://www.fda.gov/media/70442/download. Accessed August 12, 2020.
- 35. Food and Drug Administration . FEIBA highlights of prescribing information. [Last updated: November 2013]. 1986. https://www.fda.gov/media/78852/download. Accessed August 12, 2020.
- 36. Evatt BL. The tragic history of AIDS in the hemophilia population, 1982–1984. J Thromb Haemost. 2006;4:2295‐2301. [DOI] [PubMed] [Google Scholar]
- 37. Montessori V, Press N, Harris M, Akagi L, Montaner JS. Adverse effects of antiretroviral therapy for HIV infection. CMAJ. 2004;170:229‐238. [PMC free article] [PubMed] [Google Scholar]
- 38. UK Haemophilia Centre Doctors’ Organisation . The impact of HIV on mortality rates in the complete UK haemophilia population. AIDS. 2004;18:525‐533. [DOI] [PubMed] [Google Scholar]
- 39. Tatsunami S, Mimaya J, Nakamura I, Yago N, Meguro T, Yamada K. Life time expectancy of hemophilia patients infected with HIV‐1 with the risk of hepatocellular carcinoma after HCV infection. MedInfo. 1995;8(Pt 2):912. [PubMed] [Google Scholar]
- 40. Maor Y, Schapiro J, Bashari D, Marinowitz U. Survival of hepatitis C‐infected haemophilia patients is predicted by presence of cirrhosis but not by anti‐viral treatment. Ann Hepatol. 2014;13:753‐761. [PubMed] [Google Scholar]
- 41. Holmström M, Nangarhari A, Öhman J, Duberg A‐S, Majeed A, Aleman S. Long‐term liver‐related morbidity and mortality related to chronic hepatitis C virus infection in Swedish patients with inherited bleeding disorders. Haemophilia. 2016;22:e494‐e501. [DOI] [PubMed] [Google Scholar]
- 42. Petri H, Urquhart J. Channeling bias in the interpretation of drug effects. Stat Med. 1991;10:577‐581. [DOI] [PubMed] [Google Scholar]
- 43. Choy EH, Bernasconi C, Aassi M, Molina JF, Epis OM. Treatment of rheumatoid arthritis with anti‐atumor necrosis factor or tocilizumab therapy as first biologic agent in a global comparative observational study. Arthritis Care Res (Hoboken). 2017;69:1484‐1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hoffman KB, Dimbil M, Erdman CB, Tatonetti NP, Overstreet BM. The Weber effect and the United States Food and Drug Administration's Adverse Event Reporting System (FAERS): analysis of sixty‐two drugs approved from 2006 to 2010. Drug Saf. 2014;37:283‐294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Oldenburg J, Mahlangu JN, Bujan W, et al. The effect of emicizumab prophylaxis on health‐related outcomes in persons with haemophilia A with inhibitors: HAVEN 1 Study. Haemophilia. 2019;25:33‐44. [DOI] [PubMed] [Google Scholar]
- 46. Gliklich R, Dreyer N, Leavy M, Editors. Registries for Evaluating Patient Outcomes: A User's Guide [Internet]. (3rd ed.). Rockville, MD: Agency for Healthcare Research and Quality (US); 2014. [PubMed] [Google Scholar]
- 47. Peyvandi F, Makris M, Collins P, et al. Minimal dataset for post‐registration surveillance of new drugs in hemophilia: communication from the SSC of the ISTH. J Thromb Haemost. 2017;15:1878‐1881. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1‐S4


