Abstract
Esophageal squamous cell carcinoma (ESCC) is one of the most common malignant tumors of the digestive tract in humans. Several studies have indicated that PAK4 is associated with the risk of ESCC and may be a potential druggable kinase for ESCC treatment. However, the underlying mechanism remains largely unknown. The aim of our study is to identify the functional role of PAK4 in ESCC. To determine the expression of PAK4 in ESCC, Western blot analysis and immunohistochemistry were performed, and the results showed that PAK4 is significantly upregulated in ESCC tissues and cell lines compared with normal controls and normal esophageal epithelial cell line. To further investigate the role of PAK4 in ESCC, cell viability assays, anchorage‐independent cell growth assays, wound healing assays, cellular invasion assays, in vivo xenograft mouse models, and metastasis assays were conducted, and the results showed that PAK4 can significantly facilitate ESCC proliferation and metastasis in vitro and in vivo. To determine the potential target of PAK4 in ESCC progression, a pull‐down assay was performed, and the results showed that LASP1 may be a potential target of PAK4. An immunoprecipitation assay and confocal microscopy analysis confirmed that PAK4 can bind to and colocalize with LASP1 in vitro and in cells. Notably, rescue experiments further illustrated the mechanistic network of PAK4/LASP1. Our research reveals the oncogenic roles of PAK4 in ESCC and preliminarily elucidates the mechanistic network of PAK4/LASP1 in ESCC.
Keywords: cell proliferation, esophageal squamous cell carcinoma, LASP1, metastasis, PAK4
1. INTRODUCTION
Esophageal cancer is one of the most common malignant tumors of the digestive tract in humans; it ranks as the sixth most common cause of cancer‐related death and is the seventh most commonly diagnosed cancer worldwide. 1 There are two main histological subtypes of esophageal cancer: esophageal squamous cell carcinoma (ESCC) and esophageal adenocarcinoma (EAC). ESCC, which is the predominant subtype of esophageal cancer, accounts for more than 90% of esophageal cancer cases in China. The etiology of ESCC is complex, and it has been found that alcohol consumption, tobacco use, hot food consumption, deficiency of selenium, zinc, or vitamin E, and high exposure to polycystic aromatic hydrocarbons are risk factors for ESCC. 2 Currently, the major treatment strategy for ESCC patients includes surgery, chemotherapy, radiotherapy, and a combination of them. 3 Although a great progress has been made in recent years, the prognosis of ESCC remains poor, and the average 5‐year survival rate of ESCC patients is only approximately 15%–20%. 4 , 5 One of the major problems faced during the course of ESCC is the lack of specific biomarkers for early detection and treatment. Therefore, finding specific biomarkers for ESCC patients is urgently needed.
p21‐activated kinases (PAKs) are a family of serine/threonine protein kinases that were originally discovered downstream of the small Rho GTPases Rac1 and Cdc42. 6 To date, a total of six PAK family members have been found in mammalian cells and have been classified into Type I PAKs (PAK1, PAK2, and PAK3) and Type II PAKs (PAK4, PAK5, and PAK6). 7 PAKs are involved in the regulation of many biological processes, such as cell survival, cell proliferation, cell apoptosis, cell migration, and skeleton rearrangement. 6 According to a number of studies, PAK1 and PAK4 are highly expressed in many tumor tissues, and PAK4 is the most closely associated with human tumors among other PAK family members, 8 such as gastric cancer, 9 prostate cancer, 10 and colorectal cancer. 11 Accumulating evidence indicates that PAK4 can regulate cancer cell proliferation, migration, and invasion by participating in the PI3K/AKT, 12 Wnt/β‐catenin, 13 , 14 , 15 LIMK1/confilin, 16 , 17 and MEK/ERK 18 , 19 , 20 , 21 signaling pathways. Recent studies using whole‐transcriptome sequencing and SNP analysis found that PAK4 was associated with the risk of ESCC and may be a potential druggable kinase for ESCC treatment. 22 , 23 However, the underlying mechanism remains unclear.
LIM and SH3 protein 1 (LASP1) was initially identified in the metastatic lymph nodes of human breast cancer. 24 In recent years, growing evidence has shown that LASP1, originally considered a structural actin‐binding protein, is a multifunctional protein in different cancers. It has been reported that LASP1 is highly expressed in a variety of cancers and that its overexpression contributes to cancer aggressiveness, suggesting the potential value of LASP1 as a new cancer prognostic biomarker. 25
In the present study, we show that PAK4 and p‐PAK4 are highly expressed in ESCC tissues and ESCC cell lines compared with normal adjacent tissues and esophageal epithelial cell line. PAK4 can promote cell proliferation, migration, and invasion of ESCC in vitro and in vivo, which suggests that PAK4 may be a key regulator in ESCC progression. In addition, pull‐down assays showed that LASP1 may be a potential target of PAK4, and binding assays revealed that PAK4 can bind to and colocalize with LASP1 in vitro and in cells. We explored the mechanism by which PAK4 affects cell proliferation and invasion of ESCC and found that PAK4 facilitates ESCC progression via LASP1. Taken together, these results suggest that PAK4 can promote ESCC through LASP1 and that PAK4 may be a potential target for the treatment of ESCC patients in the clinic.
2. MATERIALS AND METHODS
2.1. Reagents and antibodies
Dulbecco's modified Eagle's medium (DMEM), RPMI 1640 and fetal bovine serum (FBS) were purchased from Biological Industries (BI). Jet PRIME transfection reagent was purchased from Polyplus‐transfection, Inc. (Polyplus). Antibodies to detect total PAK4 and phosphorylated PAK4 (Ser474) were obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz). Antibodies to detect total AKT, phosphorylated AKT (Ser473), total ERK1/2, phosphorylated ERK1/2 (Thr202/Tyr204), total mTOR, phosphorylated mTOR (Ser2448), E‐cadherin, N‐cadherin, Vimentin, and Snail were purchased from Cell Signaling Technology, Inc. (Cell Signaling Technology). The antibody to specifically detect LASP1 was obtained from Proteintech Group, Inc. (Proteintech). The primary antibodies used in this study are listed in Table S1. G418 (Geneticin), puromycin, and HEPES were purchased from Solarbio Life Sciences, Inc.
2.2. Cell culture and transfection
The SHEE (Shantou human embryonic esophageal) cell line was a gift from Professor Enmin Li (Medical College of Shantou University 26 ). The human embryonic kidney 293T cell line, KYSE30, and 150, 450 human esophageal cancer cell lines were purchased from the Type Culture Collection of the Chinese Academy of Sciences, and the EC109 human esophageal cancer cell line was purchased from the National Laboratory Cell Resource Sharing Platform. HEK293T and KYSE450 cells were maintained in DMEM supplemented with 10% FBS at 37°C in 5% CO2. KYSE30, KYSE150 and EC109 cells were maintained in RPMI 1640 supplemented with 10% FBS at 37°C in 5% CO2. The full‐length pak4 and lasp1 constructs were purchased from Addgene, Inc. (Addgene) and subcloned into pcDNA.3.1‐3×Flag and pcDNA.3.1‐HA vectors, respectively. The small hairpin RNA (shRNA) constructs against pak4 (number 1 sense sequence, CGACCAGCACGAGCAGAAGTT; number 2 sense sequence, GACTCGATCCTGCTGACCCAT; and lasp1 sense sequence, ACCTGCGACAGCTTGTGATTC) used in this study were synthesized by Genewiz Inc. (Genewiz) and subcloned into the pLKO.1‐puro vector. All constructs were verified by DNA sequencing. For transfection, cells were seeded until they reached 70% confluency, at which point they were transfected with Jet PRIME according to the manufacturer's instructions.
2.3. Western blot analysis
Cells were lysed with RIPA lysis buffer (Beyotime) and cleared by centrifugation at 4°C, 15,000 rpm for 30 min. The protein concentration was determined with the BCA Protein Assay Kit (Solarbio Life Sciences). A total of 50 μg protein was resolved by SDS/PAGE and then transferred onto polyvinylidene difluoride (PVDF) membranes. The membranes were incubated with the appropriate specific primary antibody and a horseradish peroxidase‐conjugated secondary antibody. Protein bands were visualized by an enhanced chemiluminescence (ECL) reagent.
2.4. Immunohistochemistry
The human esophageal tissue array containing human esophageal in situ carcinoma and paired adjacent normal tissues was purchased from Shanghai Outdo Biotech Co., Ltd. Briefly, formalin‐fixed paraffin sections were stained with PAK4 and p‐PAK4 antibodies according to the antibody datasheets. The antigens were retrieved by boiling in 10 mM sodium citrate buffer for 10 min. Other experimental procedures were carried out according to the specifications of the immunohistochemistry kit (ZSGB‐Bio). The slides were photographed and analyzed by a panoramic tissue cell quantitative analysis system (TissueFAXS PLUS). The specimens were quantified based on the staining intensity and percentage of positive cells.
2.5. Cell viability assay
Cells (4 × 103/well) were plated in 0.1 ml of medium containing 10% FBS in 96‐well plates. At 0, 1, 2, 3, and 4 days after plating, the plates were removed from the incubator. Cells were fixed with 4% paraformaldehyde, and then 100 μl of 1 μg/ml DAPI was added to each well. Cells were incubated for 20 min at 37°C. Cells were counted by using a high content imaging system (GE Healthcare).
2.6. Anchorage‐independent cell growth assay
Cells (8 × 103) were suspended in 1 ml Basal Medium Eagle (BME) supplemented with 10% FBS and 0.3% agar and plated on 3 ml solidified BME with 10% FBS and 0.5% agar in each well of a 6‐well plate. After culturing at 37°C in 5% CO2 for 9 days, the colonies were photographed, and colonies were counted by using a high content imaging system (GE Healthcare).
2.7. Wound healing assay
Cells (1 × 106/well) were plated in 6‐well plates. After culturing at 37°C in 5% CO2 for 16 h, cells were wounded with a sterile 200 μl pipette tip. And then cells were cultured in serum‐free medium. Phase‐contrast microscopy images were taken at the same position of the wound every 6 h or 12 h. The width of the open areas was measured using Photoshop (Adobe) and averaged.
2.8. Cellular invasion assay
Cells (1 × 105) in 200 μl serum‐free medium were seeded into the upper chamber (Corning, #3422) with Matrix (Corning), and 800 μl media with 10% FBS were added into the lower chamber. After culturing at 37°C in 5% CO2 for 24 h, noninvaded cells remaining on the upper side of Transwell inserts were cleared with a cotton swab. The invaded cells on the bottom side of inserts were fixed with 10% TCA for 2 h at 4°C and stained with 0.1% crystal violet for 30 min at 37°C. Pictures were taken under a microscope, and cells were counted across five random microscopic fields and averaged.
2.9. Protein purification and pull‐down assay
To purify the 6×His‐PAK4 fusion protein, the pET46‐Ek/LIC vector encoding His6‐PAK4 was transformed into Escherichia coli BL21 bacteria at 25°C for 4 h by adding 0.25 mM isopropyl‐β‐D‐1 thiogalactopyranoside (IPTG). Cells were disrupted and resuspended in 15 ml of buffer containing 50 mM Tris (pH 7.4), 500 mM NaCl, phenylmethylsulfonyl fluoride, 1 mM NaVO3, 1 mM EDTA, and protease cocktail inhibitors. Cell lysates were vortexed, sonicated, and centrifuged for 1 h at 20,000g at 4°C. The lysates were added to 0.2 ml of 50% (v/v) Ni‐NTA bead slurry and gently rotated at 4°C overnight. The beads were then washed five times with lysis buffer containing 100 mM imidazole. The purity of 6×His‐PAK4 was determined by SDS‐PAGE, followed by Coomassie blue staining. For the pull‐down assay, 20 µl of PAK4 bead slurry was incubated with KYSE450 cell lysates for 6 h while rotating at 4°C. The beads were washed five times in lysis buffer, and three independent samples were analyzed by SDS‐PAGE. The different protein bands were excised and digested with sequencing grade trypsin. These digests were analyzed by microcapillary liquid chromatography/tandem mass spectrometry (LC‐MS/MS).
2.10. Immunoprecipitation assay
HEK293T cells were transfected with Flag‐pak4 and HA‐lasp1, and 36 h after transfection, cellular proteins were extracted by using 1% CHAPS buffer (30 mM Tris–HCl, pH 7.5, 150 mM NaCl, 1% CHAPS, and protease inhibitors). Cell lysates were first incubated with specific primary antibody and then combined with agarose–protein A/G beads (50% slurry) by gentle rocking at 4°C overnight. The beads were washed three times with high salt buffer (0.1% SDS, 1% Triton X‐100, 2 mM EDTA, 20 mM Tris–HCl, pH 8.1, and 500 mM NaCl), low salt buffer (0.1% SDS, 1% Triton X‐100, 2 mM EDTA, 20 mM Tris–HCl, pH 8.1, and 150 mM NaCl) and phosphate‐buffered saline (PBS). Proteins bound to beads were boiled and resolved by 10% SDS‐PAGE and subjected to Western blot analysis.
2.11. Confocal microscopy analysis
Cells were seeded on glass coverslips and incubated for 24 h in a 37°C incubator and then fixed with 4% paraformaldehyde for 30 min at room temperature. After washing two times with PBS, cells were blocked with 1% BSA‐PBST for 90 min at room temperature. Cells were then incubated with a 1:50 dilution of LASP1 rabbit antibody and a 1:50 dilution of p‐PAK4 mouse antibody in 3% BSA‐PBST at 4°C overnight and then incubated with secondary antibody for 90 min at room temperature. Finally, the nuclei of cells were stained with DAPI for 15 min at room temperature. The slides were viewed under a Nikon A1R+ confocal microscope system.
2.12. In vivo animal study
BALB/c‐nu/nu mice (6–8 weeks old) and NOD/SCID mice (5–6 weeks old) were purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd. For the in vivo xenograft mouse model, the mice were randomly divided into three groups: (i) the EC109 shmock group (n = 8); (ii) the EC109 shpak4‐1 group (n = 8); (iii) the EC109 shpak4‐2 group (n = 8). A total of 1 × 106 cells were inoculated subcutaneously into the left flank of each mouse in different groups. The mice were killed 80 days after cell injection. Tumor volume was measured perpendicularly every week and calculated based on the following formula: tumor volume (mm3) = (length × width2)/2. The mice were monitored until tumors reached 1 cm3 total volume, at which time mice were euthanized and tumors were extracted. For the metastasis assay, 1 × 106 KYSE30 cells stably overexpressing empty vector and Flag‐pak4 were suspended in PBS (200 μl) and injected via the tail vein of NOD/SCID mice. The mice were killed 8 weeks after injection. The lungs and livers were harvested and photographed. Lung tissues were stained with picric acid to count the metastatic nodules and eosin (HE) staining to confirm pathology. All animal protocols were approved by the Medical Ethics Committee of Zhengzhou University.
2.13. Statistical analysis
Statistical analysis of the results was performed by one‐way analysis of variance, Student's t‐test, or Χ 2 test using SPSS version 17.0 (SPSS). The results are expressed as the mean ± standard deviation, and p < 0.05 were considered significant.
3. RESULTS
3.1. PAK4 is highly expressed in human esophageal cancer tissues and esophageal cancer cell lines
To determine the expression level of PAK4 in ESCC tissues, we performed immunohistochemistry of ESCC tissue microarrays (TMAs). The results revealed that the PAK4 expression level was higher in ESCC tissues, with a median positive rate of 63%, than the PAK4 expression level in paired adjacent normal tissues, which had a median positive rate of 38% (Figure 1A,B). By analyzing data from the public Gene Expression Profiling Interactive Analysis (GEPIA) database, we found that compared with normal tissues, PAK4 was significantly highly expressed in esophageal carcinoma tissues (Figure 1C). In addition, PAK4 activity, as revealed by p‐PAK4 (Ser474) staining, was also highly expressed in ESCC tissues compared with normal controls and adjacent normal tissues (Figure 1D,E). Moreover, we also detected the expression levels of PAK4 and p‐PAK4 in different ESCC cell lines, and the results showed that PAK4 and p‐PAK4 were highly expressed in most ESCC cell lines compared with SHEE, a human normal esophageal epithelial cell line (Figure 1F,G). Overall, these results indicated that PAK4 and p‐PAK4 were highly expressed in esophageal cancer tissues and esophageal cancer cell lines.
Figure 1.
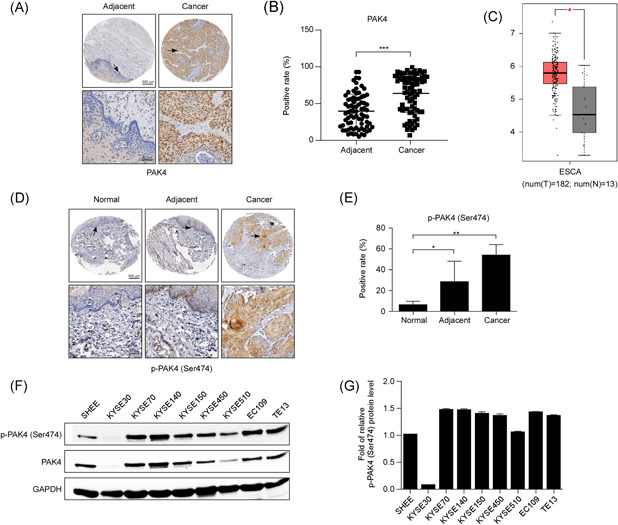
PAK4 is overexpressed in ESCC tissues and cell lines. (A) Representative images of immunohistochemistry staining for PAK4 in 77 ESCC cancer tissues and their corresponding adjacent normal tissues are shown. (B) Statistical analysis of PAK4 expression was performed. The positive rate of PAK4 expression from each sample was determined using ImageScope. The asterisks (***) indicate that the level of PAK4 in ESCC cancer tissues was significantly (p < .0001) higher than that in adjacent normal tissues. (C) Expression of PAK4 in esophageal carcinoma (ESCA) from the public GEPIA database is shown. (D) Representative images of immunohistochemistry staining of p‐PAK4 (Ser474) in esophageal normal tissues, adjacent normal tissues, and ESCC cancer tissues are shown. (E) Statistical analysis of p‐PAK4 (Ser474) expression was performed. The positive rate of p‐PAK4 expression from each sample was determined using ImageScope. The asterisks indicate that the level of p‐PAK4 in ESCC cancer tissues was significantly higher than that in esophageal normal tissues and adjacent normal tissues (*p < .05, **p < .01). (F, G) Expression of PAK4 and p‐PAK4 (Ser474) in eight different ESCC cell lines compared with that in a human normal esophageal epithelial cell line (SHEE cell), as examined by Western blot analysis. The data shown are representative of results from triplicate independent experiments. ESCC, esophageal squamous cell carcinoma [Color figure can be viewed at wileyonlinelibrary.com]
3.2. Knockdown of PAK4 can suppress cell proliferation, migration, and invasion of human ESCC
To explore the effect of PAK4 on cell proliferation, migration, and invasion of human ESCC, we designed shRNA (1 and 2) against PAK4 and introduced the pLKO.1 constructs into EC109 and KYSE450 cell lines. Stable PAK4 knockdown cell lines were established by puromycin selection. The knockdown efficiency was detected by Western blot analysis, and the results showed that the PAK4 expression level was significantly decreased in cells transfected with two individual PAK4 shRNAs (shpak4‐1 and shpak4‐2) compared with control cells that expressed GFP‐shRNAs (shmock; Figure 2A). Next, we used cell viability and anchorage‐independent cell growth assays to assess whether PAK4 exerted effects on cell proliferation and colony formation in ESCC. Our results indicated that knockdown of PAK4 slowed cell growth and led to the formation of fewer and smaller colonies in both EC109 and KYSE450 cells compared with control shmock cells (Figure 2B,C). To further validate the effects of PAK4 on the migration and invasion of ESCC cells, we performed wound healing and Transwell invasion assays. The wound healing assay is an economical method used to assess and quantify collective cell migration in vitro, and the Transwell invasion assay is used to simulate the invasion process of tumor cells in vitro with a gel composed of extracellular matrix and examine the invasive potential of cells. The results showed that the rate of cell migration and the number of invading cells in both EC109 and KYSE450 shpak4 cells were significantly lower than those in control cells (Figure 2D,E). Overall, the results indicated that a reduction in PAK4 expression led to a dramatic inhibition of cell proliferation, migration and invasion of ESCC cells.
Figure 2.
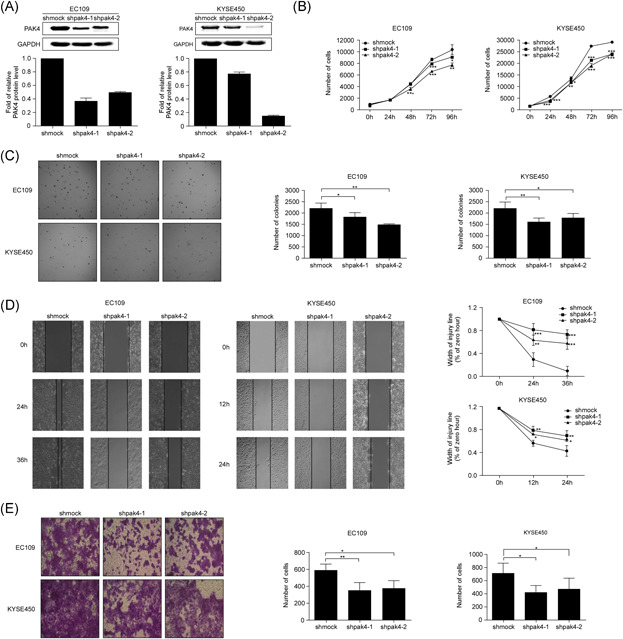
Knockdown of PAK4 can suppress cell proliferation, migration, and invasion of ESCC. (A) Endogenous PAK4 expression was efficiently decreased by shRNA targeting PAK4 (shpak4‐1 and shpak4‐2) in EC109 and KYSE450 cells, as detected by Western blot analysis. The proliferation of EC109 and KYSE450 cells stably infected with shpak4‐1, shpak4‐2, or shmock was examined using a cell viability assay (B) and a soft agar colony formation assay (C). The asterisks indicate that the rate of cell proliferation in shpak4 EC109 and KYSE450 cells was significantly lower than that in shmock EC109 and KYSE450 cells (*p < .05, **p < .01, ***p < .001). Data are shown as the mean ± SD from triplicate experiments. The migration and invasion of EC109 and KYSE450 cells infected with shpak4‐1, shpak4‐2, or shmock were examined by wound healing assay (D) and Transwell invasion assay (E). The asterisks indicate that the cell migration and invasion in shpak4 EC109 and KYSE450 cells were significantly lower than that in shmock EC109 and KYSE450 cells (*p < .05, **p < .01, ***p < .001). Data are shown as the mean ± SD from triplicate experiments. ESCC, esophageal squamous cell carcinoma [Color figure can be viewed at wileyonlinelibrary.com]
3.3. Overexpression of PAK4 can promote cell proliferation, migration, and invasion of human ESCC
To provide definitive proof of concept that PAK4 expression plays an active role in ESCC, we further assessed whether overexpression of PAK4 could promote cell proliferation, migration, and invasion in ESCC cells. First, we subcloned the PAK4 plasmid into the pcDNA.3.1‐3×Flag vector and introduced the constructs into KYSE30 and KYSE150 cells. Stable PAK4 overexpressing cell lines were established with G418 selection. The overexpression efficiency was assessed by Western blot analysis, and the results showed that the expression of PAK4 in both KYSE30 and KYSE150 cells was significantly higher than that in control cells that expressed the empty pcDNA.3.1‐3×Flag vector (Figure 3A). As expected, overexpression of PAK4 significantly promoted the cell growth and colony formation in KYSE30 and KYSE150 cells compared with those in control cells (Figure 3B,C), and the rate of cell migration and the number of invading cells also increased in both KYSE30 and KYSE150 overexpressing cells compared with control cells (Figure 3D,E). Our results indicated that overexpression of PAK4 promotes cell proliferation, migration, and invasion of ESCC cells.
Figure 3.
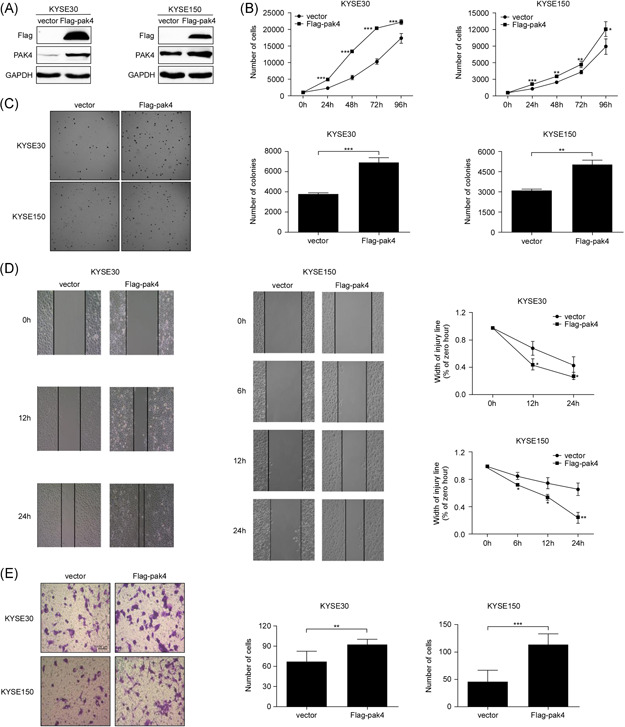
Overexpression of PAK4 can promote cell proliferation, migration, and invasion of ESCC. (A) The expression level of PAK4 was examined after stable transfection with flag empty vector or Flag‐pak4 in KYSE30 and KYSE150 cells. The overexpression efficiency was detected by Western blot analysis. The proliferation of KYSE30 and KYSE150 cells transfected with empty vector or flag‐pak4 was examined using a cell viability assay (B) and a soft agar colony formation assay (C). The asterisks indicate that the rate of cell proliferation in Flag‐pak4 KYSE30 and KYSE150 cells was significantly higher than that in control KYSE30 and KYSE150 cells (*p < .05, **p < .01, ***p < .001). Data are shown as the mean ± SD from triplicate experiments. The migration and invasion of KYSE30 and KYSE150 cells transfected with empty vector or Flag‐pak4 were examined by wound healing assay (D) and Transwell invasion assay (E). The asterisks indicate that the rate of cell migration and invasion in Flag‐pak4 KYSE30 and KYSE150 cells was significantly higher than that in control KYSE30 and KYSE150 cells (*p < .05, **p < .01, ***p < .001). Data are shown as the mean ± SD from triplicate experiments. ESCC, esophageal squamous cell carcinoma [Color figure can be viewed at wileyonlinelibrary.com]
3.4. PAK4 physically interacts with LASP1
Based on our previous results, PAK4 affects the progression of ESCC. To identify the potential target and mechanism of PAK4 in ESCC, we expressed and purified PAK4 protein and carried out pull‐down assay. First, the 6×His‐PAK4 protein was purified from E. coli BL21 by using Ni‐NTA agarose beads. The purified 6×His‐PAK4 protein was separated by SDS‐PAGE. Next, a pull‐down assay was used to detect the 6×His‐PAK4 interacting proteins in KYSE450 cell line. By performing mass spectrometry analysis of the proteins obtained from the pull‐down experiment, we screened some potential proteins that could bind with PAK4 in ESCC. Finally, 38 specific genes were obtained. Gene ontology analysis of these 38 genes showed that LASP1, TWF1, and JUP were closely related to cell adhesion, connection, and adhesion function (Figure 4A,B). The correlations between the expression of PAK4 and the expression of the above three genes in esophageal carcinoma and normal esophageal tissues was analyzed in the cancer genome atlas (TCGA) database, and the results showed that there was a strong positive correlation between PAK4 and LASP1 (Figure 4C). To further determine whether PAK4 can directly interact with LASP1, HEK293T cells were transiently transfected with pc‐DNA3.1‐3×Flag‐pak4 and pc‐DNA3.1‐HA‐lasp1. Anti‐Flag was used to immunoprecipitate Flag‐PAK4, and anti‐HA was used to detect HA‐lasp1 by Western blot analysis. The results indicated that PAK4 can combine with LASP1 in vitro and vice versa. (Figure 4D). To further detect whether PAK4 and LASP1 can colocalize in ESCC cells, immunocytofluorescence analysis was performed. The results showed that p‐PAK4, the active form of PAK4, can colocalize with LASP1 in ESCC cells and mainly colocalize in the cytoplasm (Figure 4E). These results confirmed that PAK4 can bind with LASP1 in vitro and in cells.
Figure 4.
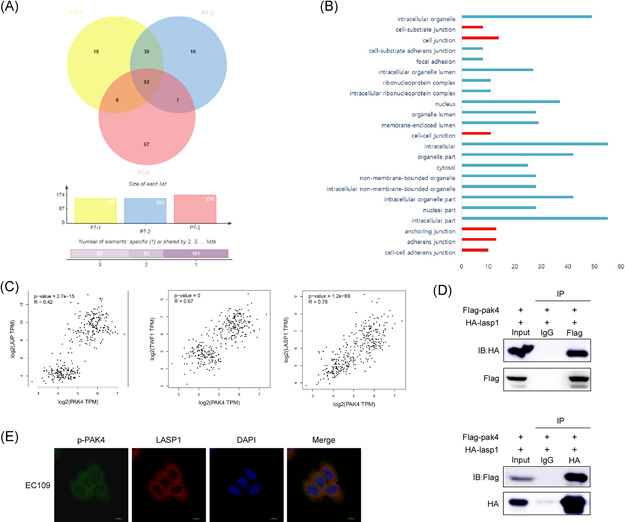
PAK4 interacts and colocalizes with LASP1. (A) The potential binding proteins of PAK4 were screened by pull‐down assay and mass spectrometry analysis. The Wayne diagram shows the 38 potential binding proteins. (B) Gene ontology functional analysis of 38 genes was performed. (C) The correlation between the expression of PAK4 and JUP, TWF1 and LASP1 in esophageal carcinoma and esophageal normal tissues was analyzed by the GEPIA database. Statistical analysis was performed using Spearman's nonparametric correlation test. (D) PAK4 binds with LASP1 in 293T cells. The pcDNA3.1‐Flag‐pak4 and pcDNA3.1‐HA‐LASP1 plasmids were transiently transfected into 293T cells, Flag‐PAK4 was immunoprecipitated by anti‐Flag, and coimmunoprecipitated HA‐LASP1 was detected by anti‐HA (top). Conversely, HA‐LASP1 was immunoprecipitated by anti‐HA, and coimmunoprecipitated Flag‐PAK4 was detected by anti‐Flag (bottom). (E) p‐PAK4 and LASP1 colocalize in EC109 cells. p‐PAK4 and LASP1 were detected by immunofluorescence staining with p‐PAK4 and LASP1 primary antibodies, a FITC‐conjugated goat anti‐mouse secondary antibody for p‐PAK4, and a TRITC‐conjugated goat anti‐rabbit antibody for detection of LASP1. The data shown are representative of results from triplicate independent experiments. IB, immunoblot; IP, immunoprecipitation [Color figure can be viewed at wileyonlinelibrary.com]
3.5. PAK4 facilitates the progression of ESCC via LASP1
The EGFR signaling pathways, including the RAS–RAF–MEK–ERK and PI3K–AKT–mTOR pathways, are well known pathways that regulate cell proliferation. 27 To further verify whether PAK4 is involved in the regulation of these two pathways in ESCC, immunoblot analysis of p‐ERK1/2 (Thr202/Tyr204), p‐AKT (Ser473), and p‐mTOR (Ser2448), and total ERKs, AKT, mTOR was performed; the results showed that the expression levels of p‐ERK, p‐AKT, and p‐mTOR were decreased after knocking down PAK4 (Figure 5A), whereas the expression levels were upregulated followed by PAK4 overexpression (Figure 5B), which indicated that PAK4 regulated the proliferation of ESCC cells by participating in ERK and AKT signaling pathways. Epithelial–mesenchymal transition (EMT), a process by which epithelial cells lose their cell polarity and cell–cell adhesion, is an important index used to judge the migration, invasion, and prognosis of cancer. 28 The hallmark of EMT is the acquisition of mesenchymal markers, including Vimentin and N‐cadherin, and the loss of epithelial surface markers, most notably E‐cadherin. To determine whether PAK4 was involved in the EMT process in ESCC, the expression levels of E‐cadherin and N‐cadherin were detected. The results showed that after knocking down PAK4, the expression level of E‐cadherin was increased, and the expression level of N‐cadherin was decreased (Figure 5A), while after overexpression of PAK4, the expression of E‐cadherin was decreased and the expression of N‐cadherin was increased (Figure 5B), which indicated that PAK4 was involved in the EMT process of ESCC cells. To further determine whether PAK4 promotes the development of ESCC through LASP1, rescue experiments were carried out. We first overexpressed PAK4 in KYSE150 cells and then downregulated LASP1 by using its shRNA. The results showed that downregulation of LASP1 could weaken the upregulation of p‐ERK, p‐AKT, N‐cadherin, Vimentin, and Snail caused by PAK4 overexpression (Figure 5C). Consistent with the immunoblotting results, the increase in the rate of cell migration and the number of invading cells in response to PAK4 overexpression was also blocked by knocking down LASP1 (Figure 5D,E). These results suggested that PAK4 promotes the progression of ESCC through LASP1.
Figure 5.
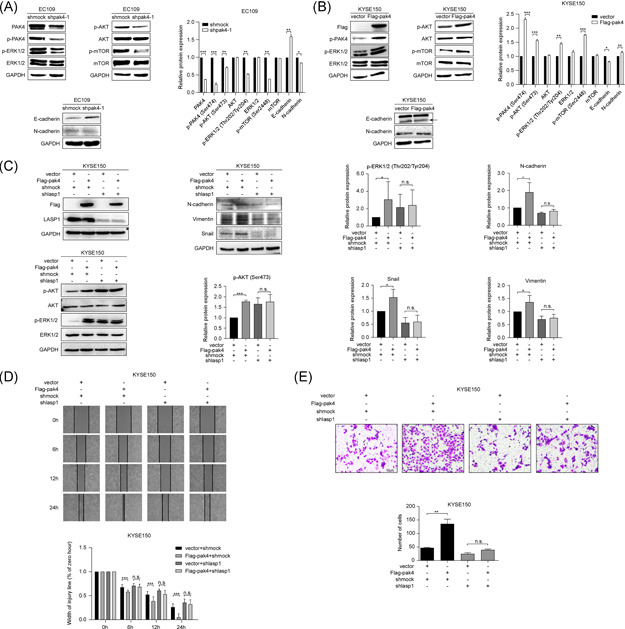
PAK4 promotes ESCC progression through LASP1. (A, B) PAK4 participates in the ERK, AKT, and EMT signaling pathways. After knocking down PAK4 in EC109 cells (A) or overexpressing PAK4 in KYSE150 cells (B), the expression of total ERKs, AKT, mTOR, E‐cadherin, N‐cadherin, vimentin, p‐mTOR, p‐ERKs, and p‐AKT was detected by Western blot analysis using specific antibodies. Data are representative of results from triplicate independent experiments. (C) PAK4 participates in ERK, AKT, and EMT signaling pathways through LASP1. After overexpressing PAK4 and downregulating LASP1 in KYSE150 cells, the expression of total ERKs, AKT, N‐cadherin, Vimentin, Snail, p‐ERKs, and p‐AKT was detected by Western blot analysis. The asterisks indicate that the expression level of N‐cadherin, vimentin, Snail, p‐ERKs and p‐AKT in Flag‐pak4 KYSE150 cells was significantly higher than that in control cells, but not in LASP1 knockdown cells (*p < .05, ***p < .001, n.s. means not significant). Data are shown as the mean ± SD from triplicate experiments. (D, E) PAK4 promotes ESCC migration and invasion through LASP1. Rescue experiments with wound healing assays (D) and Transwell assays (E) were performed in KYSE150 cells. The asterisks indicate that the rate of cell migration and invasion in Flag‐pak4 KYSE150 cells was significantly higher than that in control cells but not in knockdown LASP1 cells (**p < .01, ***p < .001, n.s. means not significant). Data are shown as the mean ± SD from triplicate experiments. ESCC, esophageal squamous cell carcinoma [Color figure can be viewed at wileyonlinelibrary.com]
3.6. PAK4 promotes ESCC proliferation and metastasis in vivo
Based on our previous data, the next step was to determine whether PAK4 could participate in ESCC proliferation and metastasis in vivo. First, an in vivo xenograft animal experiment was performed. The mice were randomly divided into three groups, and EC109 cells stably expressing shpak4 or shmock were implanted subcutaneously into the left flanks of nude mice. The first measurable tumors were observed on Day 14, and the results showed that the tumors in the shpak4 groups grew significantly more slowly and that the tumors were smaller than those in the control group (Figure 6A,B). Inoculated mice were euthanized 80 days after injection, and tumor weights were determined. The average tumor weights in the EC109‐shpak4‐1 and EC109‐shpak4‐2 groups were 0.04 g and 0.09 g, respectively, compared with a weight of 0.15 g in the control group (Figure 6C). These results indicated that knockdown of PAK4 can inhibit tumor growth in vivo. Taken with the results that PAK4 regulates the proliferation of ESCC cells by participating in ERK and AKT signaling pathways (Figure 5A,B), to determine whether the tumor inhibition effect was associated with EGFR and EMT signaling pathways, tumor extracts from each group were prepared for immunohistochemistry (IHC) analysis. Ki67 expression served as a marker of tumor proliferation and the expression of Ki67 was considerably decreased in the EC109‐shpak4 groups, suggesting that a reduction in PAK4 expression led to an inhibition of tumor proliferation. IHC staining results showed that the expression of p‐ERK1/2 (Thr202/Tyr204) was substantially decreased and the expression of E‐cadherin was significantly increased in the EC109‐shpak4 groups compared with the control group (Figure 6D). These results confirmed that the tumor inhibition caused by PAK4 deletion is related to EGFR and EMT signaling pathways in ESCC. To further confirm whether PAK4 can promote ESCC metastasis, KYSE30 cells stably expressing empty vector and Flag‐pak4 were injected into SCID mice via the tail vein. The mice were killed 8 weeks after injection, the lungs and livers were harvested, and the metastatic nodules were counted. The results showed that the mice injected with Flag‐pak4 KYSE30 cells exhibited more metastatic nodules in the lungs and livers than the control group (Figure 6E,F). Hematoxylin and eosin staining indicated metastatic tumors in lung tissues (Figure 6G). Overall, our results suggested that PAK4 can promote ESCC proliferation and metastasis in vivo.
Figure 6.
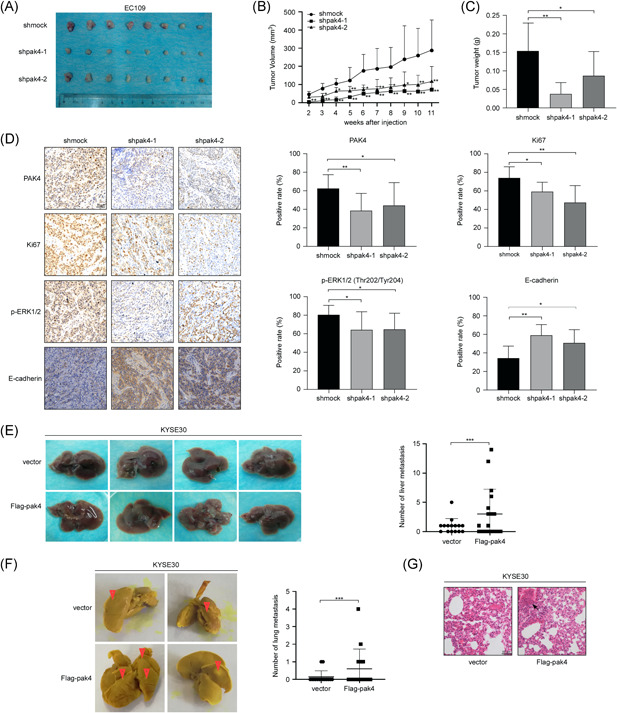
PAK4 promotes ESCC proliferation and metastasis in vivo. (A–C) Knockdown of PAK4 suppressed tumor growth in vivo. EC109 cells stably infected with shmock, shpak4‐1, or shpak4‐2 were subcutaneously injected into the left flank of each mouse. Tumor xenografts were measured as previously described, and mice were killed at day 80. Photographs of tumors from each group are shown at the end of the experiments (A). Tumor growth curves (B) and tumor weights (C) are shown as indicated. The asterisks indicate a significantly lower rate of tumor growth in the EC109 shpak4 groups compared with the vehicle group (*p < .05, **p < .01). Data are shown as the mean ± SD of eight mice. (D) Immunohistochemical analysis of the xenograft tumors. The expression of PAK4, Ki67, p‐ERKs, and E‐cadherin was stained using specific antibodies, and representative images are shown as indicated. Data are shown as the mean ± SD of eight mice. (E, F) PAK4 promotes ESCC metastasis in vivo. KYSE30 cells stably overexpressing empty vector and flag‐pak4 were suspended in PBS (200 μl) and injected via the tail vein of NOD/SCID mice. The mice were killed 8 weeks after injection. Liver (E) and lung (F) metastases were evaluated at the end of the experiments, and representative images are shown. The asterisks indicate that the rate of tumor metastasis in the KYSE30 Flag‐pak4 groups was significantly higher than that in the control group (***p < .001). Data are shown as the mean ± SD of eight mice. (G) Hematoxylin and eosin staining of lungs in each group and representative images are shown as indicated. ESCC, esophageal squamous cell carcinoma [Color figure can be viewed at wileyonlinelibrary.com]
4. DISCUSSION
PAK4, which belongs to the Type II PAK family, is closely associated with many types of human tumors. PAK4 can participate in the processes of tumor cell migration and invasion and is positively correlated with tumor grade and prognosis. Here, our data revealed that PAK4 is highly expressed in ESCC tissues and ESCC cell lines compared with adjacent normal tissue and esophageal epithelial cell lines (Figure 1A,D), and analysis of the public GEPIA database showed a similar result (Figure 1B), which suggests that PAK4 may be associated with the risk and progression of ESCC. In addition, knockdown of PAK4 significantly suppressed ESCC cell proliferation, migration, and invasion (Figure 2), whereas overexpression of PAK4 promoted ESCC progression (Figure 3). Moreover, we used a xenograft mouse model and metastasis assay to prove that PAK4 promotes ESCC proliferation and metastasis in vivo. These results demonstrated the oncogenic roles of PAK4 in ESCC. To further investigate the potential downstream target of PAK4 in ESCC, we conducted a pull‐down assay and identified LASP1 as a potential target of PAK4 (Figure 4A,B). TCGA database also showed that there is a strong positive correlation between PAK4 and LASP1 in esophageal carcinoma and normal esophageal tissues (Figure 4C). Immunoprecipitation assays and confocal microscopy analysis also confirmed that PAK4 can bind to and colocalize with LASP1 in vitro and in cells (Figure 4D,E).
LIM and SH3 protein 1 (LASP1) was initially identified from a cDNA library of the metastatic lymph nodes of human breast cancer, originally named MLN50, 24 located at chromosomes 17q11 and 21.3, encoding 261 amino acids. Because its N‐terminus is a typical LIM domain composed of two continuous zinc‐binding structures and its C‐terminus is composed of the SRC homologous region 3 (SH3) domain, MLN50 was renamed LIM and SH3 domain protein 1 (LASP1). The zinc finger domains in the LIM domain of the LASP1 N‐terminus can bind to chemokine receptors (CXCR2) and colocalize at the edge of the migrating cells, which plays an important role in the local movement and adhesion of cells. 29 At the adhesion point of the lesion, the C‐terminal SH3 domain of LASP1 is involved in binding to zyxin, 30 lipoma preferred partner (LPP), 31 and vasodilator stimulated phosphoprotein (VASP), 32 which regulates cell movement, adhesion, and shape changes. It has been found that LASP1 is mainly located at focal adhesions, 33 , 34 in podosomes, 35 and at the leading edges of lamellipodia, 36 and is closely related to the adhesion and dynamic actin assembly of cells.
Many studies have shown that LASP1 expression is closely related to the occurrence and development of several types of cancers. LASP1 is overexpressed in metastatic breast and ovarian cancer, and knockdown of LASP1 in metastatic breast cancer and ovarian cancer cell lines has a strong inhibitory effect on their migration and proliferation. 37 , 38 , 39 More studies have also found that LASP1 is overexpressed in metastatic colorectal cancer, 40 , 41 bladder cancer, 42 oral squamous cell carcinoma, 43 gastric cancer, 44 and pancreatic duct adenocarcinoma. 45 In addition, renal cell carcinoma patients with high expression of LASP1 have a poor prognosis. 46 Studies also show that LASP1 is related to the secretion of matrix metalloproteinases (MMP), 47 LASP1 goes deep into the cell layer through a structure similar to pseudopods, enhances the secretion of MMP in the extracellular matrix (ECM), and promotes the invasion of tumor cells. 48 Consistent with these reports, our data show that knockdown of LASP1 can block cell migration and invasion by PAK4 overexpression in ESCC (Figure 5) and that LASP1 is a potential target of PAK4 (Figure 4).
As we know, cancer is difficult to treat as it can metastasize and easily reoccur, which has become an important reason for the poor therapeutic effect of esophageal cancer treatment and the low 5‐year survival rate of patients with esophageal cancer. Generally speaking, the metastasis of tumors occurs through the cascade of invasion and metastasis, that is, through local invasion, breakthrough of the basement membrane, infiltration, extravasation, and colonization, to realize the metastasis of tumor cells to distal or other parts. The initiation of tumor cell migration is the premise of the metastasis cascade. Here, we found that PAK4 can promote cell migration and invasion through wound healing and Transwell assays (Figure 3D,E). Moreover, we also established a metastasis model to verify metastasis promotion by PAK4, and the results showed that PAK4 can facilitate ESCC metastasis in vivo (Figure 6E,F). Although we found that LASP1 may be a potential target of PAK4 in ESCC, the relationship between LASP1 and PAK4 and the detailed mechanism need to be further identified.
In summary, we discovered that PAK4 is highly expressed in ESCC tissues and cell lines compared with adjacent normal tissue and esophageal epithelial cell line. Moreover, our results indicate the pivotal role of PAK4 to facilitate the progression of ESCC in vitro and in vivo. Notably, we found that LASP1 may be a potential target of PAK4 that mediates its promoting effects on the metastasis of ESCC. Taken these together, our findings suggest that PAK4 may be a novel target for the clinical treatment of ESCC.
Supporting information
Supporting information.
ACKNOWLEDGMENTS
This study was supported by the National Natural Science Foundation of China (Grant no. 31301144); the Key Scientific Research Project Plan of Colleges and Universities in Henan Province (Grant no. 18A310034); the Science and Technology Project of Henan Province (Grant nos. 182102310324, 202102310206); the Training Plan for Young Backbone Teachers of Zhengzhou University (Grant no. 2018ZDGGJS037); and the Supporting Plan of Scientific and Technological Innovation Team in Universities of Henan Province (Grant no. 20IRTSTHN029).
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
Huang H, Xue Q, Du X, et al. p21‐activated kinase 4 promotes the progression of esophageal squamous cell carcinoma by targeting LASP1. Molecular Carcinogenesis. 2021;60:38–50. 10.1002/mc.23269
Hui Huang, Qianqian Xue, Xiaoge Du, and Jie Cui contributed equally to this study.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Bray F, Ferlay J, Laversanne M, et al. Cancer incidence in five continents: inclusion criteria, highlights from volume X and the global status of cancer registration. Int J Cancer. 2015;137(9):2060‐2071. [DOI] [PubMed] [Google Scholar]
- 2. Abnet CC, Arnold M, Wei WQ. Epidemiology of esophageal squamous cell carcinoma. Gastroenterology. 2018;154(2):360‐373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yamaguchi T, Kato K, Nagashima K, et al. Type of second primary malignancy after achieving complete response by definitive chemoradiation therapy in patients with esophageal squamous cell carcinoma. Int J Clin Oncol. 2018;23(4):652‐658. [DOI] [PubMed] [Google Scholar]
- 4. Pennathur A, Gibson MK, Jobe BA, Luketich JD. Oesophageal carcinoma. Lancet. 2013;381(9864):400‐412. [DOI] [PubMed] [Google Scholar]
- 5. Rustgi AK, El‐Serag HB. Esophageal carcinoma. N Engl J Med. 2014;371(26):2499‐2509. [DOI] [PubMed] [Google Scholar]
- 6. Bokoch GM. Biology of the p21‐activated kinases. Annu Rev Biochem. 2003;72:743‐781. [DOI] [PubMed] [Google Scholar]
- 7. Kumar R, Gururaj AE, Barnes CJ. p21‐activated kinases in cancer. Nat Rev Cancer. 2006;6(6):459‐471. [DOI] [PubMed] [Google Scholar]
- 8. Rane CK, Minden A. P21 activated kinase signaling in cancer. Semin Cancer Biol. 2019;54:40‐49. [DOI] [PubMed] [Google Scholar]
- 9. Wang C, Li Y, Zhang H, et al. Oncogenic PAK4 regulates Smad2/3 axis involving gastric tumorigenesis. Oncogene. 2014;33(26):3473‐3484. [DOI] [PubMed] [Google Scholar]
- 10. Park MH, Lee HS, Lee CS, et al. p21‐activated kinase 4 promotes prostate cancer progression through CREB. Oncogene. 2013;32(19):2475‐2482. [DOI] [PubMed] [Google Scholar]
- 11. Zhang X, Zhang X, Li Y, et al. PAK4 regulates G6PD activity by p53 degradation involving colon cancer cell growth. Cell Death Dis. 2017;8(5):e2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. He LF, Xu HW, Chen M, et al. Activated‐PAK4 predicts worse prognosis in breast cancer and promotes tumorigenesis through activation of PI3K/AKT signaling. Oncotarget. 2017;8(11):17573‐17585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yamada N, Noguchi S, Mori T, Naoe T, Maruo K, Akao Y. Tumor‐suppressive microRNA‐145 targets catenin delta‐1 to regulate Wnt/beta‐catenin signaling in human colon cancer cells. Cancer Lett. 2013;335(2):332‐342. [DOI] [PubMed] [Google Scholar]
- 14. Ryu BJ, Lee H, Kim SH, et al. PF‐3758309, p21‐activated kinase 4 inhibitor, suppresses migration and invasion of A549 human lung cancer cells via regulation of CREB, NF‐kappaB, and beta‐catenin signalings. Mol Cell Biochem. 2014;389(1–2):69‐77. [DOI] [PubMed] [Google Scholar]
- 15. Vershinin Z, Feldman M, Chen A, Levy D. PAK4 Methylation by SETD6 promotes the activation of the Wnt/beta‐catenin pathway. J Biol Chem. 2016;291(13):6786‐6795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dan C, Kelly A, Bernard O, Minden A. Cytoskeletal changes regulated by the PAK4 serine/threonine kinase are mediated by LIM kinase 1 and cofilin. J Biol Chem. 2001;276(34):32115‐32121. [DOI] [PubMed] [Google Scholar]
- 17. Ahmed T, Shea K, Masters JR, Jones GE, Wells CM. A PAK4‐LIMK1 pathway drives prostate cancer cell migration downstream of HGF. Cell Signal. 2008;20(7):1320‐1328. [DOI] [PubMed] [Google Scholar]
- 18. Tabusa H, Brooks T, Massey AJ. Knockdown of PAK4 or PAK1 inhibits the proliferation of mutant KRAS colon cancer cells independently of RAF/MEK/ERK and PI3K/AKT signaling. Mol Cancer Res. 2013;11(2):109‐121. [DOI] [PubMed] [Google Scholar]
- 19. Tyagi N, Bhardwaj A, Singh AP, McClellan S, Carter JE, Singh S. p‐21 activated kinase 4 promotes proliferation and survival of pancreatic cancer cells through AKT‐ and ERK‐dependent activation of NF‐kappaB pathway. Oncotarget. 2014;5(18):8778‐8789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lu X, Wang H, Su Z, Cai L, Li W. MicroRNA‐342 inhibits the progression of glioma by directly targeting PAK4. Oncol Rep. 2017;38(2):1240‐1250. [DOI] [PubMed] [Google Scholar]
- 21. Lu SX, Zhang CZ, Luo RZ, et al. Zic2 promotes tumor growth and metastasis via PAK4 in hepatocellular carcinoma. Cancer Lett. 2017;402:71‐80. [DOI] [PubMed] [Google Scholar]
- 22. Li WQ, Hu N, Wang Z, et al. Genetic variants in epidermal growth factor receptor pathway genes and risk of esophageal squamous cell carcinoma and gastric cancer in a Chinese population. PLOS One. 2013;8(7):e68999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jiang YY, Lin DC, Mayakonda A, et al. Targeting super‐enhancer‐associated oncogenes in oesophageal squamous cell carcinoma. Gut. 2017;66(8):1358‐1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tomasetto C, Moog‐Lutz C, Regnier CH, Schreiber V, Basset P, Rio MC. Lasp‐1 (MLN 50) defines a new LIM protein subfamily characterized by the association of LIM and SH3 domains. FEBS Lett. 1995;373(3):245‐249. [DOI] [PubMed] [Google Scholar]
- 25. Ruggieri V, Agriesti F, Tataranni T, Perris R, Mangieri D. Paving the path for invasion: the polyedric role of LASP1 in cancer. Tumour Biol. 2017;39(6):1393383907. [DOI] [PubMed] [Google Scholar]
- 26. Shen ZY, Xu LY, Chen XH, et al. The genetic events of HPV‐immortalized esophageal epithelium cells. Int J Mol Med. 2001;8(5):537‐542. [DOI] [PubMed] [Google Scholar]
- 27. Shaw RJ, Cantley LC. Ras, PI(3)K and mTOR signalling controls tumour cell growth. Nature. 2006;441(7092):424‐430. [DOI] [PubMed] [Google Scholar]
- 28. Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med. 2013;19(11):1423‐1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Raman D, Sai J, Neel NF, Chew CS, Richmond A. LIM and SH3 protein‐1 modulates CXCR2‐mediated cell migration. PLOS One. 2010;5(4):e10050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Li B, Zhuang L, Trueb B. Zyxin interacts with the SH3 domains of the cytoskeletal proteins LIM‐nebulette and Lasp‐1. J Biol Chem. 2004;279(19):20401‐20410. [DOI] [PubMed] [Google Scholar]
- 31. Grunewald TG, Pasedag SM, Butt E. Cell adhesion and transcriptional activity – defining the role of the novel protooncogene LPP. Transl Oncol. 2009;2(3):107‐116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kwiatkowski AV, Gertler FB, Loureiro JJ. Function and regulation of Ena/VASP proteins. Trends Cell Biol. 2003;13(7):386‐392. [DOI] [PubMed] [Google Scholar]
- 33. Schreiber V, Moog‐Lutz C, Régnier CH, et al. Lasp‐1, a novel type of actin‐binding protein accumulating in cell membrane extensions. Mol Med. 1998;4(10):675‐687. [PMC free article] [PubMed] [Google Scholar]
- 34. Chew CS, Chen X, Parente JJ, Tarrer S, Okamoto C, Qin HY. Lasp‐1 binds to non‐muscle F‐actin in vitro and is localized within multiple sites of dynamic actin assembly in vivo. J Cell Sci. 2002;115(Pt 24):4787‐4799. [DOI] [PubMed] [Google Scholar]
- 35. Stölting M, Wiesner C, van Vliet V, et al. Lasp‐1 regulates podosome function. PLOS One. 2012;7(4):e35340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nakagawa H, Terasaki AG, Suzuki H, Ohashi K, Miyamoto S. Short‐term retention of actin filament binding proteins on lamellipodial actin bundles. FEBS Lett. 2006;580(13):3223‐3228. [DOI] [PubMed] [Google Scholar]
- 37. Grunewald TGP, Kammerer U, Schulze E, et al. Silencing of LASP‐1 influences zyxin localization, inhibits proliferation and reduces migration in breast cancer cells. Exp Cell Res. 2006;312(7):974‐982. [DOI] [PubMed] [Google Scholar]
- 38. Grunewald TGP, Kammerer U, Winkler C, et al. Overexpression of LASP‐1 mediates migration and proliferation of human ovarian cancer cells and influences zyxin localisation. Br J Cancer. 2007;96(2):296‐305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Grunewald TG, Kammerer U, Kapp M, et al. Nuclear localization and cytosolic overexpression of LASP‐1 correlates with tumor size and nodal‐positivity of human breast carcinoma. BMC Cancer. 2007;7:198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhao L, Wang H, Liu C, et al. Promotion of colorectal cancer growth and metastasis by the LIM and SH3 domain protein 1. Gut. 2010;59(9):1226‐1235. [DOI] [PubMed] [Google Scholar]
- 41. Wang H, Shi J, Luo Y, et al. LIM and SH3 protein 1 induces TGFbeta‐mediated epithelial‐mesenchymal transition in human colorectal cancer by regulating S100A4 expression. Clin Cancer Res. 2014;20(22):5835‐5847. [DOI] [PubMed] [Google Scholar]
- 42. Chiyomaru T, Enokida H, Kawakami K, et al. Functional role of LASP1 in cell viability and its regulation by microRNAs in bladder cancer. Urol Oncol. 2012;30(4):434‐443. [DOI] [PubMed] [Google Scholar]
- 43. Shimizu F, Shiiba M, Ogawara K, et al. Overexpression of LIM and SH3 Protein 1 leading to accelerated G2/M phase transition contributes to enhanced tumourigenesis in oral cancer. PLOS One. 2013;8(12):e83187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zheng J, Yu S, Qiao Y, et al. LASP‐1 promotes tumor proliferation and metastasis and is an independent unfavorable prognostic factor in gastric cancer. J Cancer Res Clin Oncol. 2014;140(11):1891‐1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zhao T, Ren H, Li J, et al. LASP1 is a HIF1alpha target gene critical for metastasis of pancreatic cancer. Cancer Res. 2015;75(1):111‐119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Yang F, Zhou X, Du S, et al. LIM and SH3 domain protein 1 (LASP‐1) overexpression was associated with aggressive phenotype and poor prognosis in clear cell renal cell cancer. PLOS One. 2014;9(6):e100557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Endres M, Kneitz S, Orth MF, Perera RK, Zernecke A, Butt E. Regulation of matrix metalloproteinases (MMPs) expression and secretion in MDA‐MB‐231 breast cancer cells by LIM and SH3 protein 1 (LASP1). Oncotarget. 2016;7(39):64244‐64259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Jacob A, Linklater E, Bayless BA, Lyons T, Prekeris R. The role and regulation of Rab40b‐Tks5 complex during invadopodia formation and cancer cell invasion. J Cell Sci. 2016;129(23):4341‐4353. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


