Abstract
Serial muscle biopsies within clinical trials for Duchenne muscular dystrophy (DMD) are critical to document therapeutic responses. Less invasive means of sampling muscle are needed. We analyzed a retrospective consecutive case‐series cohort of vacuum‐assisted core needle muscle biopsy procedures performed on healthy and dystrophic individuals at a single institution assessing for safety and reliability of obtaining sufficient high‐quality biopsy tissue for histologic assessment in adult and pediatric subjects. Of 471 muscle cores from 128 biopsy procedures, 377‐550 mg of total muscle tissue was obtained per procedure with mean core weight of 129 mg (SD, 25.1 mg). All biopsies were adequate for histological assessment. There were no significant adverse events. This core needle biopsy approach, when combined with improved sample processing, provides a safe means to consistently obtain muscle samples for diagnostic and clinical trial applications.
Keywords: clinical trials, Duchenne muscular dystrophy, muscle biopsy, needle biopsy, skeletal muscle
Abbreviations
- BB
biceps brachii
- BMD
Becker muscular dystrophy
- BSA
bovine serum albumin
- CDMD
Center for Duchenne Muscular Dystrophy
- DAPI
4′,6‐diamidino‐2‐phenylindole
- DMD
Duchenne muscular dystrophy
- GN
gastrocnemius
- H&E
hematoxylin and eosin
- IHC
immunohistochemistry
- IRB
Institutional Review Board LN2, liquid nitrogen
- NSAA
North Star Ambulatory Assessment
- PBS
phosphate buffered saline
- TA
tibialis anterior
- UCLA
University of California at Los Angeles
- VL
vastus lateralis
1. INTRODUCTION
Prior to the advent of genetic testing, open muscle biopsy was a mainstay of diagnosis for Duchenne muscular dystrophy (DMD). The first documented biopsies of living subjects were performed by an ingenious needle devised over 150 y ago to identify the histopathologic cause of pseudohypertrophy. 1 Since the discovery of the genetic basis of DMD and highly accurate and robust genetic testing from blood, there has been a dramatic reduction in the need for diagnostic muscle biopsies. 2 , 3 , 4 Even in the context of more rare forms of muscular dystrophy, broad‐based genetic testing is often an efficient means of diagnosis. 5
Despite the decreased reliance on muscle biopsy for clinical diagnosis, clinical trials for investigational new drugs for DMD are increasingly mandating muscle biopsies to obtain target tissue, especially in the case of therapeutic interventions intended to induce dystrophin expression in DMD skeletal muscle, such as exon skipping, nonsense readthrough, or microdystrophin replacement or dystrophin correction. 6 , 7 , 8 Successful observation of dystrophin induction in humans thus far has relied on open muscle biopsies in clinical trials, because of the need for sufficient material of high quality. 2 , 7 Core needle biopsies have not been used substantially in clinical trials because there is a perceived risk that these muscle biopsies may be too small or distorted to allow robust observation of myofibers in cross‐section for various quantitative assays. However, clinical trials often require multiple sequential muscle biopsies to demonstrate drug response, and when open biopsies are employed, the sites of muscle biopsy are rotated because of the invasive nature of open muscle biopsy. Thus, sampling the same muscle group on the same side of the body is more challenging with open biopsy and may introduce unknown variations from comparison of dominant versus non‐dominant muscle. As an example of the need for biopsy, most pediatric patients enrolled in the phase II trial for eteplirsen underwent four open muscle biopsies. 8 , 9 We should seek to minimize risks of these necessary samplings especially in the context of therapies of unknown benefit. Thus, there is ample reason to develop, and to evaluate the safety and success, of less invasive means for serial muscle sampling in adults and children with muscle disease.
Many muscle disease clinicians have some experience using Bergstrom needles to obtain sufficient muscle cores for histologic diagnosis, but these needle biopsies are typically not selected for clinical trials as they can be technically challenging to perform and because there is high risk of distortion of the histologic appearance and a small and highly variable amount of muscle obtained. 10 , 11 , 12 In contrast, vacuum‐assisted core biopsy systems offer attractive features for the study of muscle: they require only one individual to perform the procedure, and have resulted in larger muscle core biopsies. 13 , 15 , 16 OʼSullivan et al. reported that 80% of 40 muscle biopsies from individuals with suspected neuromuscular disease were able to provide a diagnosis using a 14‐gauge vacuum‐assisted core needle without any complications. 15 Gallo et al. reported that 92% of biopsies from the biceps brachii (BB) muscles of 102 adults using a 10‐gauge vacuum‐assisted core needle were sufficient for diagnosis. 13 Thus, there is experience using core needle biopsy systems for muscle disease diagnosis; however, the ability of core needle biopsies to produce consistent muscle specimens of sufficient quantity and quality for assessments needed in pediatric DMD clinical trials has not been adequately described. Here, we describe a retrospective consecutive case‐series cohort analysis of core needle biopsies performed to assess to the reliability of obtaining sufficient muscle cores using a 10‐gauge core needle biopsy coupled with a simplified cryopreservation system.
2. METHODS
2.1. Muscle biopsy consent
Healthy individuals with no history of muscle or other chronic disease and individuals genetically confirmed to have DMD or Becker muscular dystrophy (BMD) were biopsied as part of ongoing research studies in the Center for Duchenne Muscular Dystrophy (CDMD) on five different University of California at Los Angeles (UCLA) Institutional Review Board (IRB) protocols (18‐001366, 19‐000076, 19‐000090, 18‐001770, or 15‐001919). Informed consent was obtained for 128 procedures from 94 human subjects. Initial biopsies starting in June 2016 were performed under IRB 15‐001919 to assess the use of a vacuum‐assisted core needle muscle biopsy in healthy and dystrophic individuals. All biopsies were performed between June 2016 to February 2020 at UCLA by physician co‐authors S.F.N., J.D.W., or J.W. S.F.N. taught J.D.W. the procedure, which was readily transferred. Of the 128 procedure, 72 were performed during the final 12 mo of this case‐series.
2.2. Representative subject selection description
Eight individual biopsies were used to represent histologic appearance of muscle with a range of dystrophinopathy severity, including one healthy adult female (see Table 1).
TABLE 1.
Patient characteristics
| Subject ID | Biopsy (y) | Sex | Diagnosis | Age at diagnosis (y) | Loss of ambulation (y) | Steroid use | DMD mutation |
|---|---|---|---|---|---|---|---|
| 8020 | 22 | M | BMD | 3 | Ambulatory | No | Exon 2 duplication |
| 8010 | 27 | M | BMD | 27 | Ambulatory | No | Exons 51‐52 deletion |
| 8017 | 18 | M | DMD | 4 | 13 | Yes | c.649_650insAGTGAATTTCACACCTCTCCTTTTGAAAGATTCATTTCTATGAATTTGGGACAGCTTCCTAGTATGATATTCCATCT) 17 |
| 1612 | 7 | M | DMD | 6 | Ambulatory | Yes | c.10171C>T, p.Arg3391* |
| 1622 | 6 | M | DMD | 1.5 | Ambulatory | No | c.10402G>T, p.Glu3468* |
| 8009 | 14, 15, 17 | M | DMD | 5 | 17 | Yes | c.4100_4100delA, p.1367Gln>Arg*14 |
| 8021 | 57 | F | Healthy | Not relevant | Not relevant | No | None |
| 8022 | 20 | M | DMD | 2.5 | 11 | Yes | Exons 46‐47 deletion |
Abbreviations: BMD, Becker muscular dystrophy; DMD, Duchenne muscular dystrophy, F, female; M, male.
3. VACUUM‐ASSISTED CORE NEEDLE BIOPSY SYSTEM
The Vacora Breast Biopsy System Driver (VF2019, Bard Biopsy, USA) was used with the Vacora Biopsy Probe 10G x 118 mm needle (VB10118, Bard Biopsy, USA). A new probe was inserted into the driver for each biopsy session and covered in a sterile sleeve (Bard Finesse Ultra Biopsy Driver ‐ Sterile Instrument Cover – F01CVR, Bard Biopsy, USA).
4. CONSCIOUS SEDATION, ULTRASOUND, AND MUSCLE BIOPSY
In 23 instances (children between ages 2‐7 y), sedation was used with intravenous fentanyl and propofol at standard doses by intravenous (IV) push by a pediatric intensivist or an anesthesiologist with cardiorespiratory monitoring until alert.
Muscle biopsy was performed in a supine position (Figure 1B). Since, unlike open muscle biopsy, vacuum‐assisted needle biopsy does not allow for direct visualization of the anatomy, we used ultrasonography to target muscle and avoid neurovascular structures. 14 Ultrasonography of the GN and VL was always unremarkable, however, a branch of the anterior tibial artery was often observed by ultrasonography in the TA requiring adjustment of needle placement to lessen risks of hemorrhage. The VL was sampled at approximately two‐thirds of the length of the thigh distal from the hip with insertion through skin distal to the main mass of muscle. The TA biopsy site was approximately one‐third of the length of the tibia distal from the knee. The GN insertion site was just distal to the most distal aspect of the muscle at the most medial position of the gastrocnemius (GN). The BB insertion site was at the most distal edge of palpated biceps.
FIGURE 1.
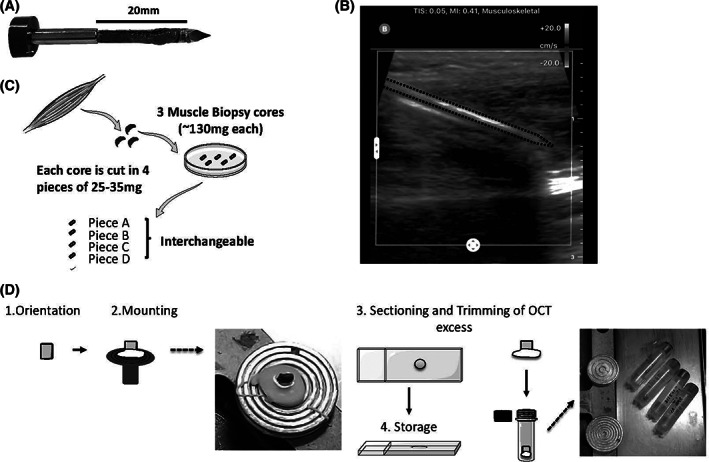
Overview of the core needle biopsy procedure, biopsy handling, and storage. Biopsies were obtained from different muscles including upper (BB) or lower limbs (vastus lateralis, tibialis anterior [TA], and gastrocnemius). Each pass of the 10‐gauge needle obtains a piece of muscle about 3 mm in diameter and about 15 mm in length; image is of a muscle core prior to removal of the muscle core from the needle chamber, A. A handheld ultrasound system is used prior to all procedures to ensure to avoid blood vessels and nerves; ultrasound static image shows about 3 cm insertion of needle into TA at maximal insertion prior to vacuum‐assisted coring, B. 21 Each muscle core is dissected into 25 to 35 mg pieces, each of which can be used for either histologic assessments, protein analyses, RNA analyses, and we thus consider “interchangeable,” C. All small pieces are stored in liquid nitrogen (not depicted). Each piece is preserved in liquid nitrogen until sectioning. Once retrieve from liquid nitrogen, the muscle is mounted directly on the chuck. After 10 μm thick sectioning onto glass slides, we remove the excess OCT such that the remaining muscle can be replaced in the cryovial for long‐term storage in liquid nitrogen. Sections on slides are stored in −80°C, D
The skin was sterilely prepped and draped. 3‐10 mL of 1% Lidocaine was infused intradermally, subdermally, and in a wide peri‐fascial region of the muscle to be biopsied. 18 , 19 The 10‐guage core needle, in the extended position, was introduced through a 3 mm skin incision at a 30° angle and pushed through the fascia with the sharp cutting edge of the needle until the distal edge of cutting window was judged to be 5 mm past the fascia, which requires the tip of core needle to be inserted at least 25 mm into the muscle. The automated cutting and vacuum system was then engaged to obtain a core of muscle. Subsequent samples could then be obtained through reinsertion of the needle through a single skin incision. Samples were processed within minutes of biopsy (Figure 1C,D). Internal and external bleeding was controlled with local pressure for 5‐10 min after the procedure, followed by surgical strip closure of the 3 mm wound, bandage, and pressure dressing was applied for 2‐4 h. Procedural time for each biopsy was approximately 15 min.
5. CORE BIOPSY HANDLING, FREEZING, AND SECTIONING
All initial biopsy handling procedures were performed at room temperature in the procedure room immediately upon retrieval. All surfaces were cleaned with Sanizide (Safetek, USA) and RNAse Zap (Thermofisher, USA). Each core muscle biopsy was trimmed of adhered fat and connective tissue and any blood removed. Each core cylinder (20 mm length and 3 mm diameter) was cut with scalpel into three to four smaller cylinders each of about 25‐40 mg (Figure 1C). The weight of the core and each dissected piece was weighed (CS 200[TM], Ohaus). Each piece of muscle (25‐40 mg) was individually placed into a pre‐labeled 2.5 × 3 × 0.5 cm Swingsette M515 Tissue Processing and Embedding Cassette (Thermo Fisher Scientific, USA) at room temperature, closed, and then individually plunged into liquid nitrogen (LN) with a 12‐in. forceps for 30 s. Each cassette was then released to sink to the bottom of the LN tank to submerge all pieces within 10‐15 min. Biopsies were transferred into cryotubes and stored in liquid nitrogen.
For sectioning, the approximately 30 mg muscle specimens were visually oriented and adhered in Optimal Cutting Temperature compound (OCT, Sakura Finetek, USA) after equilibration within a microtome. As the initial cross‐sectional orientation of the small tissue pieces, which do not have clear anatomic references, was performed by eye, an initial 10um thick section was assessed under 20x brightfield illumination to confirm orientation. If oblique sections were obtained, which occurs about 10% of the time, the muscle is repositioned to obtain myofibers in cross‐section. After cutting, excess OCT is trimmed and samples stored in LN (Figure 1D). In this process, if samples are retrieved for additional sections, the orientation is already marked by the remaining OCT and cut surface.
6. MUSCLE BIOPSY HISTOLOGY AND IMMUNOFLUORESCENCE
Transverse 10 μm cryosections on glass slides were stained by hematoxylin and eosin (H&E). Other 10 μm thick sections were processed for immunohistochemistry (IHC) with an initial incubation in 3% bovine serum albumin (BSA) in phosphate buffered saline (PBS) for 1 h at room temperature, changed to primary antibody in PBS‐3% BSA at 4°C overnight with the following antibodies: dystrophin C‐terminal (NCL‐DYS2, 1:50, Leica Biosystems, USA) or dystrophin Rod domains (NCL‐DYS1, 1:50, Leica Biosystems, USA), Laminin (L9393, 1:25, Sigma‐Aldrich, USA), or migG1 (554 121, 1:100, BD biosciences, USA). Primary antibodies were detected by secondary antibodies: FITC donkey anti‐mouse (715‐095‐150, Jackson immunology, USA, 1:300) and Texas red anti‐rabbit (711‐076‐152, Jackson immunology, USA, 1:300). All sections were mounted in Vectashield‐DAPI (4′,6‐diamidino‐2‐phenylindole; Vector Laboratories, USA) and visualized using an Axioplan 2 microscope (Carl Zeiss Inc, USA).
7. RESULTS
7.1. Vacuum‐assisted needle biopsy system for dystrophic and healthy muscle
A total of 471 core muscle samples were obtained from 128 biopsies (Table 2). Each physician was able to obtain high‐quality tissue after their first procedure. In healthy volunteers, J.D.W. obtained a mean core weight of 153 mg vs 139 mg for S.F.N. The coefficient of variance of core weight was similar for J.D.W. (0.11) and S.F.N. (0.15). F‐test of the core weight variance between S.F.N. and J.D.W. showed no statistically significant difference (F = 0.63, 95% CI = 0.22‐1.5, P = .29). We obtained human muscle biopsy specimens across a wide age range (2‐66 y of age) with a mean age of 12.9 y. A total of 83 subjects were less than 18 y of age. Eighty‐four biopsies were performed on individuals with a diagnosed dystrophinopathy between 2 and 26 y of age.
TABLE 2.
Healthy and DMD/BMD core needle biopsy characteristics and yield
| Muscle | Weight (mg) | Cores/subjects | Male/female | Age (y) | IHC | Complication rate | |
|---|---|---|---|---|---|---|---|
| Healthy volunteers | TA | 147.67 +/− 18.61* | 45/15 | 5/10 | 21.07 +/− 2.16 | Satisfactory | 0/15 |
| VL | 146.84 +/− 23.25 | 78/29 | 13/16 | 27.00 +/− 23.25 | Satisfactory | 1/29 | |
| DMD/BMD | GN | 119.61+/− 15.47 | 132/34 | 34/0 | 6.12 +/− 3.3 | Satisfactory | 0/34 |
| TA | 116.31 +/− 23.82* | 145/38 | 38/0 | 6.52 +/− 3.3 | Satisfactory | 0/38 | |
| VL | 128.51 +/− 48.06 | 21/7 | 7/0 | 16.0 +/− 5.6 | Satisfactory | 0/7 | |
| BB | 134.01 +/− 15.90 | 3/1 | 1/0 | 6 +/− 0.0 | Satisfactory | 0/1 |
P < .001 (Studentʼs t test).
Biopsy was performed most frequently within the UCLA Clinical Translational Research Center with adjustable height bed and sufficient space for set up of muscle tissue inspection, dissection into individual pieces, labeling, and submersion into LN. However, the flexibility of the needle approach also allowed biopsy to be performed in pediatrics clinic, operating room, and inpatient hospital beds. Muscles biopsied included the medial GN, tibialis anterior (TA), vastus lateralis (VL), and BB. Some subjects as young as 6 y of age tolerated the procedure with just local anesthesia and distraction. No individuals over the age of 12 required sedation.
Complications from needle muscle biopsy were few and minor. All subjects had some degree of mild pain and discomfort with the procedure on the day of procedure and the following day regardless of biopsy location. Ambulation on the biopsied legs was possible the day of the procedure. None required more than acetaminophen to control pain. All subjects, including patients with DMD, returned to baseline function within 3 days of biopsy. There were no instances of infection. One adult (<1%) had minor local bleeding from incision after prolonged ambulation within 4 h of the procedure and required replacement of surgical strips.
Our first goal was to determine the overall mass of muscle that could be safely and reliably obtained from different muscle groups (Table 2). In general, there were no significant differences in core weights obtained from different individuals or from different muscles. We noted only a lower weight of cores obtained from the TA of individuals with DMD.
Twelve subjects had biopsies of the same muscle performed greater than 6 mo apart and demonstrated similar yields. First biopsy cores of GN had a mean weight of 124.9 mg, and the second biopsy cores had a mean weight of 118.9 mg (P = .26). First biopsy of TA had a mean weight of 106.6 mg, and second biopsy cores had a mean weight of 113.9 mg (P = .47).
Adequate amount of muscle for clinical diagnostic work‐up of a suspected inherited muscular dystrophy was obtained from all individuals biopsied. Each core was divided into three to four pieces of 25‐40 mg each, with approximate dimensions of 4 mm length by 3 mm diameter (Figure 1C). This size of muscle was sufficient for orienting on cryotome and obtaining 100 of high quality 10 μm sections from each 25‐40 mg piece. No more than four cores were needed to obtain at least 400 mg of muscle (Table 2).
7.2. Development of core needle muscle biopsy processing and preservation suitable for histology
We assessed different freezing techniques to improve our ability to efficiently handle and label the many separate pieces of muscle from each procedure within the procedure room to minimize risks of sample degradation. The multiple core needle biopsies from a single healthy volunteer was used to determine if each method resulted in adequate histology. For the two methods using cassettes, dissected pieces weighing approximating 30 mg were individually placed into pre‐labeled tissue cassettes and either plunged into LN directly with a 12‐in. forceps for 30 s, and then released to sink to the bottom of the LN dewar (“cassette in LN direct”) or a beaker containing an isopentane bath prechilled to −150°C within a LN bath (“Cassette in isopentane cooled in LN”) (Figure 2). A third sample was frozen without a cassette after the sample was placed on a piece of cork and plunged in a beaker containing an isopentane bath prechilled to −150°C within a LN2 bath “OCT/cork in isopentane cooled in LN.” Samples were then kept on dry ice prior to storage in cryovials in LN.
FIGURE 2.
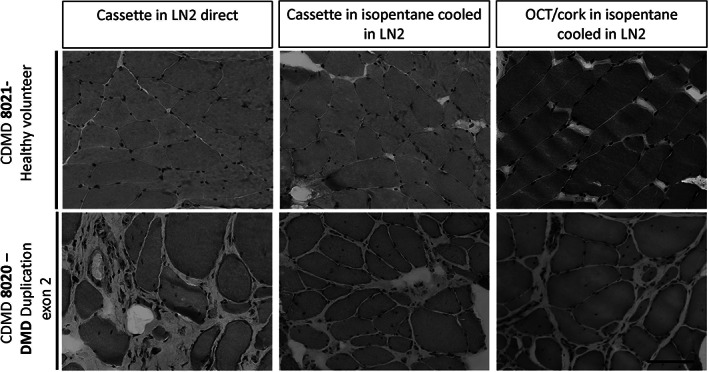
Comparison of core needle muscle biopsy histology processed using three different freezing techniques. Biopsies from the vastus lateralis of a healthy volunteer, CDMD 8021, and a patient diagnosed with dystrophinopathy, CDMD 8020 (DMD duplication of exon 2), were frozen using three different techniques: “Cassette in LN2 direct” method, where the samples were directly placed in a tissue cassette with slotted holes and plunged in LN2. “Cassette in isopentane cooled in LN2” method in which the samples were placed in the slotted tissue cassettes and plunged into isopentane pre‐chilled to −150°C using LN2; “OCT/cork in isopentane cooled in LN2” method in which the samples were mounted on cork using OCT and then plunged into isopentane pre‐chilled to −150°C in LN2, which is the most commonly used method for preserving histology of muscle. Each piece was trimmed of 100 μM depth of muscle tissue before sampling serial 10 μM sections and subsequently stained with H&E. Images were taken at 20×. Scale bar is 100 μM
Cassette‐frozen specimens were visually inspected and marked to orient appropriately in cryotome for cross‐sections, by placing a thin layer of OCT at the base of the muscle piece for adhering to the aluminum disk pre‐equilibrated to −24°C within a cryotome (Figure 1D,E). Samples were sectioned at 10 μm onto glass slides and stained with H&E or used for IHC (Figures 2, 3, 4, 5). Histologic stains were performed on muscle biopsies that were sectioned from 1‐day post freezing to 1 y post freezing.
FIGURE 3.
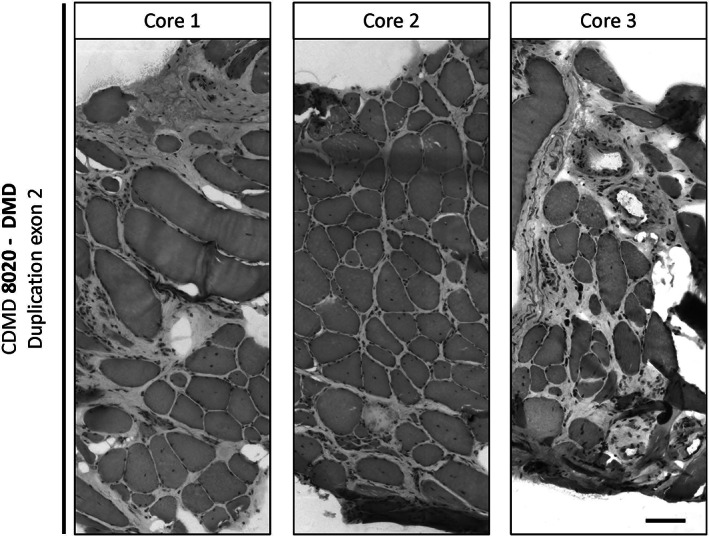
Heterogeneity of pathology across and within different muscle cores taken from the same muscle. Three cores were taken from the vastus lateralis of patient CDMD 8020 (DMD duplication exon 2) and were all frozen using the “Cassette in LN2 direct” method. Each piece was trimmed for about 100 μM depth of muscle tissue before sampling serial 10 μM sections and subsequently stained with H&E. Images were assembled as a mosaic at 20× to reveal well‐preserved structures and myofibers regardless of the degree of fibro‐fatty involvement. Scale bar is 100 μM
FIGURE 4.
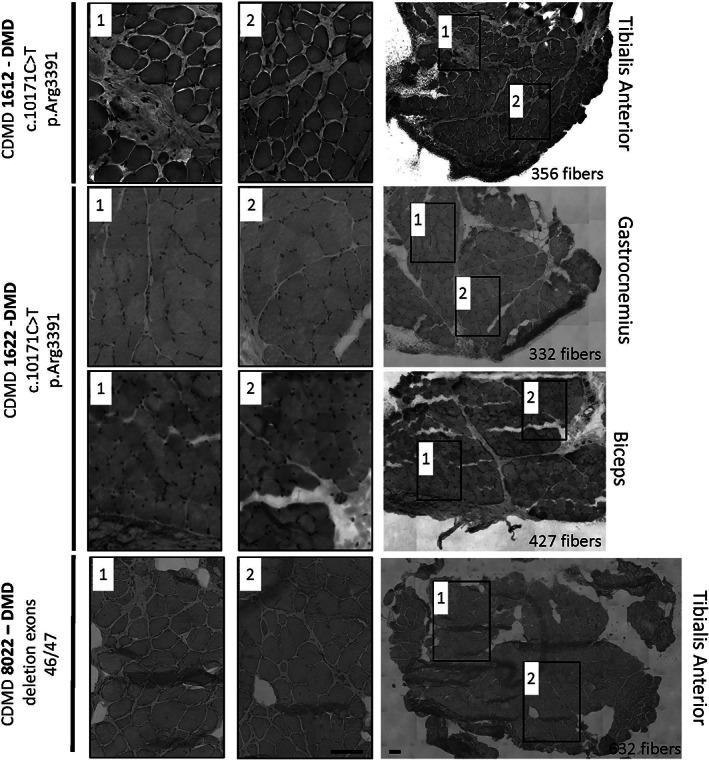
Needle muscle biopsy and “Cassette in LN2” tissue processing provides sufficient muscle for histological analysis of dystrophic and healthy muscle. Core needle biopsies processed using the “Cassette in LN2 direct” method were analyzed for histology quality and number of myofibers in different muscles: CDMD1612 (DMD c.10171C>T, p.Arg3391*) was sampled from the biceps brachii at age 6 y, CDMD1622 (DMD c.10402G>T, p.Glu3468*) was sampled from gastrocnemius at age 6 y, and CDMD8022 (DMD out of frame deletion of exons 46‐47) was sampled from tibialis anterior at age 21 y. Each piece was trimmed for about 100 μM before sampling a 10 μM section for H&E. The whole cross‐section (right panel) was counted for myofibers and the number of fibers is indicated for each cross‐section in the lower right corner of the image. Two higher magnification images (first and second images from the left) from each whole cross‐section demonstrate lack of freeze artifact or distortions in muscles with varying fibro‐fatty replacement. Images for whole cross‐sections were reconstructed as a mosaic at 20×. Scale bar is 100 μM
FIGURE 5.
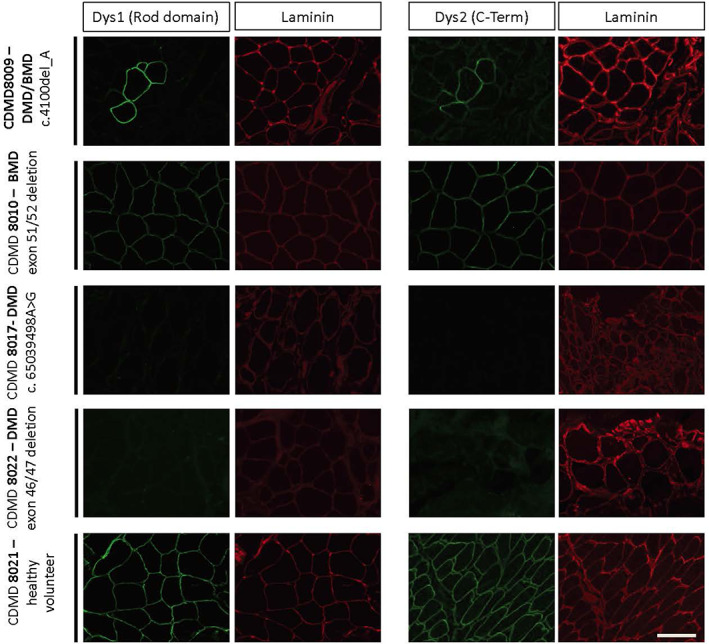
Needle biopsies processed using the “Cassette in LN2” tissue processing method is suitable for IHC staining of dystrophin. Biopsies from the vastus lateralis of a healthy volunteer (CDMD8021) and four individuals with dystrophinopathy: CDMD8022 (frameshifting DMD deletion of exons 46‐47), CDMD8017 (frameshifting DMD pseudo‐exon inclusion c.65039498A>G), CDMD8010 (DMD exon 51‐52, in frame deletion), and CDMD8009 (DMD c.4100_DelA) with slower disease progression. Frozen biopsies were sectioned, and dystrophin and laminin proteins were visualized using antibodies with specificity for the dystrophin rod domain (Dys1) or c‐terminus (Dys2) dys (green). Laminin (red) stains serve to identify the muscle sarcolemma of the in both dystrophin positive and negative samples. Images were taken at 20×. Scale bar is 100 μM [Color figure can be viewed at wileyonlinelibrary.com]
While the direct comparisons of the methods are not comprehensive, all methods resulted in good histologic appearance of the muscle. The direct comparison of these methods revealed that snap freezing using the “Cassette in LN2 direct” method is of equivalent quality for histology compared to the standard method, “OCT/cork in isopentane cooled in LN2” (Figure 2). Similarly, this method is appropriate for the assessment of DMD/BMD muscle, even with fibro‐fatty replacement (Figures 2 and 3). For practical reasons, we favor the “cassette in LN2 direct” method, which allows the labeling and handling of many specimens from each individual, and results in excellent tissue preservation. Prior reports indicate that fenestrated tubes lessen the potential for direct submersion of tissue into LN, and our data using the fenestrated tissue cassettes supports this observation. All subsequent samples presented in this manuscript were performed with the “Cassette in LN2 direct” method.
7.3. Assessment of core needle biopsies for IHC
We sought to determine if sufficient numbers of muscle fibers were observed from these relatively small pieces from DMD patients to permit immunohistochemical detection of dystrophin.
To assess the potential heterogeneity across three independent cores biopsies harvested through the same incision, we visualized muscle cores taken from the VL of a patient with significant fibro‐fatty replacement using H&E staining. There is a range of histopathologic severity observed within biopsies from the same patient with DMD (Figure 3), between different individuals, and across the four muscles surveyed (Figures 3 and 4). Adequate histopathologic examination of muscle fibers was possible even from biopsies obtained from patients presenting more with DMD and for whom no dystrophin protein production is predicted due to out of frame mutation (DMD exon 2 duplication) or nonsense mutations (DMD Arg3391* and Glu3468*) (Figures 3, 4 and 5). There is variability in the amount of fibro‐fatty replacement in dystrophic muscle, but in the samples sectioned, sufficient numbers of myofibers are observed in TA, GN, and BB and range from 332 to 632 high quality fibers per section (Figure 4) in those with a dystrophinopathy.
Similarly, dystrophin and laminin can be assessed at the sarcolemmal membrane in sections stained with fluorescently tagged antibodies specific for dystrophin or laminin, with clarity and without significant background (Figure 5). Sarcolemmal dystrophin is present at high levels in a muscle biopsy from CDMD8021, a healthy volunteer. As expected, no dystrophin was detected for CDMD8017 with an insertion a pseudo‐exon including three stop codons in DMD or for CDMD8022 (DMD exon 46‐47 deletion), whereas laminin staining is present in all (Figure 5). A higher rate of revertant fibers was observed in CDMD8009 with DMD c.4100_DelA, who lost ambulation at age 17 y, demonstrating a somewhat milder DMD progression.
CDMD8022 demonstrates the specific utility of biopsy of the relatively preserved TA in non‐ambulatory boys with DMD. This subject had lost ambulation at age 11 y and was biopsied at age 20 y. He was able to contract his TA minimally but could not substantially dorsiflex his ankle. Published MRI data demonstrates that TA has a relatively slower disease progression than most skeletal muscles. 20 Biopsy of his TA revealed substantial retention of muscle fibers (Figure 3). The amount of muscle retained would be adequate for dystrophin staining as previously performed in a trial of eteplirsen for DMD. 7
8. DISCUSSION
We add to the literature a large case series demonstrating that the 10‐gauge core needle biopsy system is a safe and effective method of obtaining skeletal muscle to aid in the diagnosis and management of muscle diseases and is particularly well suited to obtain consistent muscle biopsies in the context of some clinical trials. Our experience here indicates that the biopsy technique can be readily taught to physicians to yield consistent muscle sampling.
While open muscle biopsies are proven to be safe and have the obvious advantage of larger sample sizes, when repeated sampling of muscles is necessary, core needle biopsy provides a safe and viable option. The amount of muscle from core needle was about 400 mg in total and is sufficient for most purposes, and is remarkably consistent. In some instances, a lower mass was obtained from individuals with muscle disease relative to healthy individuals, likely due to a higher proportion of fat and fibrosis within the core volume.
While obtaining individually smaller pieces of muscle than open muscle biopsies, the core needle biopsy does offer some advantages over open muscle biopsies, including the ability to sample across slightly different areas of the same muscle from a single small surgical wound (see Table 3). Furthermore, in instances where serial muscle biopsies are required, repeat sampling of the same muscle is possible as demonstrated in a small subset of our cohort reported here.
TABLE 3.
Comparison of the advantages and disadvantages of the vacuum‐assisted biopsy system with the Bergstrom and open biopsy methods
| Advantages | Disadvantages | |
|---|---|---|
| Vacuum‐assisted core needle biopsy |
|
|
| Bergstrom |
|
|
|
Bergstrom or vacuum‐assisted core needle biopsy |
|
|
| Open biopsy |
Our system of muscle biopsy sample collection, processing and storage offers some advantages for clinical trial work. First, samples can be processed quickly in the same room as the biopsy procedure allowing immediate feedback from the processing technician to the physician performing the procedure. Second, the biopsy cores are divided into small individual pieces (approximately 25 mg/piece), typically 9‐12 per procedure, and frozen in liquid nitrogen immediately after collection. This allows easier distribution of muscle tissue to a number of assays and reduces the risks of sample loss or spoilage. Each frozen sample obtained from core needle biopsy has very similar dimensions and properties, so that technical handling of the biopsy was much more consistent than with open biopsies of variable dimensions, in our experience. Storage in LN allowed for the establishment of an efficient muscle biobank that minimized risks from subsequent sample handling and has allowed unused pieces to remain frozen and preserved indefinitely in LN for later distribution to various assays. This is important when labile biological molecules may be studied in the distant future, such as RNA. In our experience, snap freezing and storing samples using non‐volatile LN resulted in excellent samples for frozen sectioning similar to the more commonly used technique of freezing muscle in LN‐chilled isopentane.
The core needle muscle biopsy procedure was well‐tolerated and had a low complication rate, with no major complications reported thus far in the literature or in our large experience, but we and others are limited in that we only attempted to biopsy superficial muscles due to safety concerns. Issues of concern in planning the needle biopsy was size of the muscle and safety particularly relative to bleeding risk. The core needle requires that the needle be inserted about 25 mm into a muscle. Thus, some muscles, particularly in young children may be too small for this procedure. In other instances, different disease processes alter the relative benefit of biopsy of different muscles. From serial MRI of skeletal muscles of patients with DMD, the VL has a relatively rapid progression. Thus, at some stage of DMD, the VL is uninformative for biopsy as all muscle is replaced by fat and fibrosis. The GN has an intermediate progression and is a commonly biopsied muscle groups in individuals with suspected DMD. The TA has a slower progression and, thus, offers an opportunity to observe effects of treatment in DMD even in non‐ambulant children. To demonstrate this potential, we performed a core needle biopsy of the TA on a one boy who lost ambulation about 9 y prior. Despite his advanced disease, viable myofibers were observable. This may be important in tracking the longitudinal effect of dystrophin replacement strategies in DMD.
The data presented in this manuscript support the broader use of vacuum‐assisted core needle biopsy for evaluation of muscle, particularly in the context of clinical trials where consistency of sampling is critical. This study is limited as it does not include a direct comparison between vacuum‐assisted core needle biopsies and other techniques. However, Table 3 compares advantages and disadvantages of the vacuum‐assisted core needle biopsy procedure we describe here relative to suction‐modified Bergstrom needle biopsy and open biopsy, as described in the literature. An additional limitation is that the vacuum‐assisted core needle biopsy we describe can only be used to biopsy superficial muscles of sufficient size. We did not broadly compare all muscles accessible by core needle biopsy or in all dystrophies or across multiple disease. Smaller muscles are not readily biopsied by this approach and the lack of our ability to visualize the muscle may in some instances lead to inadequate sampling.
9. CONCLUSIONS
Muscle tissue obtained using a vacuum‐assisted core needle biopsy system in conjunction with sample processing using direct freezing in LN2, without isopentane, consistently resulted in approximately 400 mg of high‐quality muscle per individual from 3 to 4 cores per procedure, suitable for IHC and other endpoint determination. This minimally invasive biopsy technique is well‐suited to clinical research activities requiring biopsies of multiple muscles or biopsy of multiple biopsies of the same muscle at different time points.
CONFLICT OF INTEREST
The authors declare no potential conflict of interest.
ETHICAL PUBLICATION STATEMENT
We confirm that we have read the Journalʼs position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
ACKNOWLEDGMENTS
The authors acknowledge the assistance of Jacqueline Garcia Lemus and the staff of the Clinical Translational Research Center in subject scheduling and study performance. J.W. was supported by the Ruth L. Kirschstein Institutional National Research Service Award T32GM008243 at the University of California Los Angeles. Funding period: 07/2018‐06/2020. S.N.R. is supported by the supported by the NIH Training Grant in Genomic Analysis and Interpretation T32HG002536. Funding period: 7/2018‐06/2020. The work was funded from NIH P30 AR057230 to MCM and SFN, National Center for Advancing Translational Sciences, UCLA CTSI UL1TR000124, the Parent Project Muscular Dystrophy and MDA603685 to M.C.M., the Appel Pilot Grant program, and donors to the Center for Duchenne Muscular Dystrophy at UCLA.
Barthelemy F, Woods JD, Nieves‐Rodriguez S, et al. A well‐tolerated core needle muscle biopsy process suitable for children and adults. Muscle & Nerve. 2020;62:688–698. 10.1002/mus.27041
Florian Barthelemy and Jeremy D. Woods contributed equally to this work.
M. Carrie Miceli and Stanley F. Nelson contributed equally and share senior authorship.
Funding information Appel Pilot Grant program; Foundation for the National Institutes of Health, Grant/Award Number: NIH P30 AR057230; Muscular Dystrophy Association, Grant/Award Number: 603685; National Center for Advancing Translational Sciences; Parent Project Muscular Dystrophy; UCLA CTSI, Grant/Award Number: UL1TR000124; NIH Training Grant in Genomic Analysis and Interpretation, Grant/Award Number: T32HG002536; Ruth L. Kirschstein Institutional National Research Service Award, Grant/Award Number: T32GM008243
REFERENCES
- 1. Duchenne GB. The pathology of paralysis with muscular degeneration (paralysie myosclerotique), or paralysis with apparent hypertrophy. Br Med J. 1867;2(363):541‐542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kunkel LM, Beggs AH, Hoffman EP. Molecular genetics of Duchenne and Becker muscular dystrophy: emphasis on improved diagnosis. Clin Chem. 1989;35(7 Suppl):B21‐B24. [PubMed] [Google Scholar]
- 3. Alame M, Lacourt D, Zenagui R, et al. Implementation of a reliable next‐generation sequencing strategy for molecular diagnosis of dystrophinopathies. J Mol Diagn. 2016;18(5):731‐740. [DOI] [PubMed] [Google Scholar]
- 4. Aartsma‐Rus A, Ginjaar IB, Bushby K. The importance of genetic diagnosis for Duchenne muscular dystrophy. J Med Genet. 2016;53(3):145‐151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zatz M, Passos‐Bueno MR, Vainzof M. Neuromuscular disorders: genes, genetic counseling and therapeutic trials. Genet Mol Biol. 2016;39(3):339‐348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Barthélémy F, Wein N. Personalized gene and cell therapy for Duchenne muscular dystrophy. Neuromuscul Disord. 2016;28(10):803‐824. [DOI] [PubMed] [Google Scholar]
- 7. Charleston JS, Schnell FJ, Dworzak J, et al. Eteplirsen treatment for Duchenne muscular dystrophy: exon skipping and dystrophin production. Neurology. 2018;90(24):e2146‐e2154. [DOI] [PubMed] [Google Scholar]
- 8. Alfano LN, Charleston JS, Connolly AM, et al. Long‐term treatment with eteplirsen in nonambulatory patients with Duchenne muscular dystrophy. Medicine. 2019;98(26):e15858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Al‐Zaidy S, Rodino‐Klapac L, Mendell JR. Gene therapy for muscular dystrophy: moving the field forward. Pediatr Neurol. 2014;51(5):607‐618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Thavorntanaburt S, Tanboon J, Likasitwattanakul S, et al. Impact of muscle biopsy on diagnosis and management of children with neuromuscular diseases: a 10‐year retrospective critical review. J Pediatr Surg. 2018;53(3):489‐482. [DOI] [PubMed] [Google Scholar]
- 11. Tarnopolsky MA, Pearce E, Smith K, Lach B. Suction‐modified Bergström muscle biopsy technique: experience with 13,500 procedures. Muscle Nerve. 2011;43(5):716‐725. [DOI] [PubMed] [Google Scholar]
- 12. Joyce NC, Oskarsson B, Jin L‐W. Muscle biopsy evaluation in neuromuscular disorders. Phys Med Rehabil Clin N Am. 2012;23(3):609‐631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gallo A, Abraham A, Katzberg HD, Ilaalagan S, Bril V, Breiner A. Muscle biopsy technical safety and quality using a self‐contained, vacuum‐assisted biopsy technique. Neuromuscul Disord. 2018;28(5):450‐453. [DOI] [PubMed] [Google Scholar]
- 14. Billakota S, Dejesus‐Acosta C, Gable K, Massey EW, Hobson‐Webb LD. Ultrasound in EMG‐guided biopsies: a prospective, randomized pilot trial: ultrasound in EMG‐guided biopsy. Muscle Nerve. 2016;54(4):786‐788. [DOI] [PubMed] [Google Scholar]
- 15. OʼSullivan PJ, Gorman GM, Hardiman OM, Farrell MJ, Logan PM. Sonographically guided percutaneous muscle biopsy in diagnosis of neuromuscular disease: a useful alternative to open surgical biopsy. J Ultrasound Med. 2006;25(1):1‐6. [DOI] [PubMed] [Google Scholar]
- 16. Akarolo‐Anthony SN, Ogundiran TO , Nkwodimmah C, et al. Office based muscle biopsy using Vacora™ vacuum assisted biopsy system. Afr J Med Med Sci. 2012;41(3):13‐16. [PMC free article] [PubMed] [Google Scholar]
- 17. Zaum A‐K, Stüve B, Gehrig A, et al. Deep intronic variants introduce DMD pseudoexon in patient with muscular dystrophy. Neuromuscul Disord. 2017;27(7):631‐634. [DOI] [PubMed] [Google Scholar]
- 18. Hussain N, McCartney CJL, Neal JM, Chippor J, Banfield L, Abdallah FW. Local anaesthetic‐induced myotoxicity in regional anaesthesia: a systematic review and empirical analysis. Br J Anaesth. 2018;121(4):822‐841. [DOI] [PubMed] [Google Scholar]
- 19. Yagiela JA, Benoit PW, Fort NF. Mechanism of epinephrine enhancement of lidocaine‐induced skeletal muscle necrosis. J Dent Res. 1982;61(5):686‐690. [DOI] [PubMed] [Google Scholar]
- 20. Polavarapu K, Manjunath M, Preethish‐Kumar V, et al. Muscle MRI in Duchenne muscular dystrophy: evidence of a distinctive pattern. Neuromuscul Disord. 2016;26(11):768‐774. [DOI] [PubMed] [Google Scholar]
- 21. Butterfly iQ . https://www.butterflynetwork.com. Accessed September 25, 2019.
- 22. Hayot M. Skeletal muscle microbiopsy: a validation study of a minimally invasive technique. Eur Respir J. 2005;25(3):431‐440. [DOI] [PubMed] [Google Scholar]
- 23. Mikell CB, Chan AK, Stein GE, Tanji K, Winfree CJ. Muscle and nerve biopsies: techniques for the neurologist and neurosurgeon. Clin Neurol Neurosurg. 2013;115(8):1206‐1214. [DOI] [PubMed] [Google Scholar]
- 24. Yag‐Howard C. Sutures, needles, and tissue adhesives: a review for dermatologic surgery. Dermatol Surg. 2014;40:S3‐S15. [DOI] [PubMed] [Google Scholar]
- 25. Magistris MR, Kohler A, Pizzolato G, Morris MA, Barrofio A, Bernheim L. Needle muscle biopsy in the investigation of neuro muscular disorders. Muscle Nerve. 1998;21:194‐200. [DOI] [PubMed] [Google Scholar]


