Abstract
Objective
We conducted a Mendelian randomization (MR) study to disentangle the comparative effects of lipids and apolipoproteins on ischemic stroke.
Methods
Single‐nucleotide polymorphisms associated with low‐ and high‐density lipoprotein (LDL and HDL) cholesterol, triglycerides, and apolipoprotein A‐I and B (apoA‐I and apoB) at the level of genomewide significance (p < 5 × 10−8) in the UK Biobank were used as instrumental variables. Summary‐level data for ischemic stroke and its subtypes were obtained from the MEGASTROKE consortium with 514,791 individuals (60,341 ischemic stroke cases, and 454,450 non‐cases).
Results
Increased levels of apoB, LDL cholesterol, and triglycerides were associated with higher risk of any ischemic stroke, large artery stroke, and small vessel stroke in the main and sensitivity univariable MR analyses. In multivariable MR analysis including apoB, LDL cholesterol, and triglycerides in the same model, apoB retained a robust effect (p < 0.05), whereas the estimate for LDL cholesterol was reversed, and that for triglycerides largely attenuated. Decreased levels of apoA‐I and HDL cholesterol were robustly associated with increased risk of any ischemic stroke, large artery stroke, and small vessel stroke in all univariable MR analyses, but the association for apoA‐I was attenuated to the null after mutual adjustment.
Interpretation
The present MR study reveals that apoB is the predominant trait that accounts for the etiological basis of apoB, LDL cholesterol, and triglycerides in relation to ischemic stroke, in particular large artery and small vessel stroke. Whether HDL cholesterol exerts a protective effect on ischemic stroke independent of apoA‐I needs further investigation. ANN NEUROL 2020;88:1229–1236
Blood lipids are established causal factors in the development of stroke. 1 , 2 , 3 It has been shown that high concentrations of low‐density lipoprotein (LDL) cholesterol increase the risk of ischemic stroke, 2 , 3 , 4 whereas high concentrations of high‐density lipoprotein (HDL) cholesterol possibly decrease the risk of ischemic stroke, particularly small vessel stroke. 2 , 5 Furthermore, large‐scale randomized clinical trials have revealed that lowering cholesterol concentrations with statins reduces the risk of ischemic and overall stroke, 6 , 7 , 8 despite an increase in hemorrhagic stroke. 8 However, given high phenotypic and genetic correlation across different lipids and apolipoproteins, 9 it remains unclear whether one or more lipid‐related entities account for the observed associations between lipids and stroke. Disentangling the associations of atherogenic lipoprotein lipids and risk of stroke is of great public health and clinical importance. First, a better understanding of the comparative role of lipoprotein lipids in stroke not only facilitates a clearer perception of the underlying pathophysiology of stroke, but also helps to capture the most effective biomarker and corresponding agent that lipid‐modifying therapeutics should target. Second, the findings can provide an evidence basis in guiding the prevention and treatment of stroke among more than one‐quarter of the general population who has discordant apolipoprotein B (apoB) and LDL cholesterol levels, in particular those with obesity or type 2 diabetes. 10 , 11 Third, such investigation will help unify the guidelines concerning regular apoB measurement supported by European Society of Cardiology/European Atherosclerosis Society, 12 but not by the American College of Cardiology/American Heart Association. 13
The amount of cholesterol and triglycerides vary largely between lipoprotein particles, 14 , 15 , 16 which results in an imprecise quantification of the number of atherogenic lipoproteins, although the levels of LDL cholesterol and triglycerides quantifies the levels of these lipid substances carried in circulating lipoproteins. In contrast, one apoB molecule is included in each circulating atherogenic lipoprotein particle. 15 , 16 Thus, the level of apoB molecules is proportional to the number of circulating atherogenic particles in the blood. Available evidence indicates causal effects of increased LDL cholesterol, triglycerides, and apoB on increasing stroke risk 2 , 3 and a stronger effect of apoB compared to LDL cholesterol on cardiovascular disease. 17 , 18 It is plausible to assume that each lipid‐related entity played an individual causal role or that one trait, such as apoB, predominated and accounted for the associations of related lipoprotein particle entities. Confined by potential methodological limitations, such as residual confounding and reverse causality, traditional observational study designs are unable to infer causality regarding the role of lipoprotein lipids in the development of stroke. Another approach is the Mendelian randomization (MR) design, which utilizes genetic variants as instrumental variables for an exposure to determine causality of an exposure‐outcome association. Given correlations across lipid‐related traits, 9 the multivariable MR framework, as an extension to the traditional MR method, should be recommended to appraise the association of correlated multiple risk factors with the outcome of interest simultaneously. By including the genetic associations for multiple exposures in the same model, the multivariable MR can assess which traits retain causal associations with the outcome through the genetic protection against conventional biases, including unobserved confounders, reverse causality, the inherent correction for measurement error, and the avoidance of collider bias. 19 Here, we employed the traditional MR analysis to determine the associations of individual lipid‐related traits with ischemic stroke and then multivariable to MR analysis to elucidate which of the atherogenic lipid traits accounts for the etiological basis of lipoprotein lipids in relation to stroke.
Materials and Methods
Study Design
An overview of the study design and used data sources are displayed in Figure 1 and Supplementary Table S1. Genetic instruments for LDL and HDL cholesterol, triglycerides, and apoA‐I and apoB were selected based on the UK Biobank study. 9 Data for the associations of the lipid‐related traits associated single‐nucleotide polymorphisms (SNPs) with ischemic stroke and subtypes were available from the MEGASTROKE consortium. 20 The univariable MR analysis aimed to investigate the association of individual lipid‐related traits with ischemic stroke and the multivariable MR analysis aimed to compare the independent effects of correlated lipid‐related traits on ischemic stroke. We first determined which one of apoB, LDL cholesterol, and triglycerides predominantly accounted for the causal associations with ischemic stroke. We then assessed the predominant entity accounted for the inverse association of HDL‐related phenotypes with ischemic stroke (HDL cholesterol and apoA‐I). To expel the possibility of reverse causality, we performed a reverse MR analysis to examine the influence of liability to stroke on 5 lipid‐related traits. The UK Biobank study was approved by the North West Multicenter Research Ethics Committee. Original studies included in the MEGASTROKE consortium had been approved by a relevant review board. The present analyses were approved by the Swedish Ethical Review Authority.
FIGURE 1.
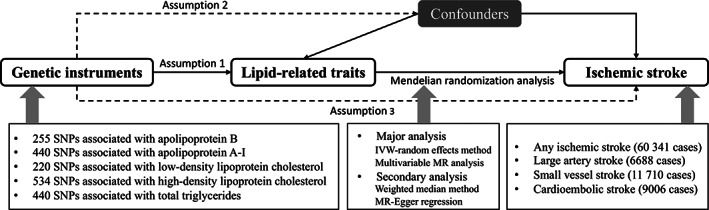
Overview of study design. There are three key assumptions for Mendelian randomization (MR). Assumption 1: the genetic variants selected as instrumental variables should be robustly associated with the lipid‐related traits. Assumption 2: the used instrumental variables should not be associated with any potential confounders. Assumption 3: the genetic variants of an exposure should affect the risk of the outcome merely through the risk factor, not via other alternative pathways. IVW = inverse variance weighted; SNP = single‐nucleotide polymorphism.
Genetic Instrument Selection
Genetic variants, in this case SNPs, associated with LDL and HDL cholesterol, triglycerides, and apoA‐I and apoB levels were extracted as instrumental variables for corresponding lipid‐related traits at the genomewide significance level (p < 5 × 10−8) from the UK Biobank study including up to 343,992 individuals of European ancestry. 9 The mean age of included participants was 56.9 years old and approximately 54% were women. The mean (standard deviation [SD]) levels were 3.57 (0.87) mmol/L for LDL cholesterol and 1.45 (0.38) mmol/L for HDL cholesterol. The median level of triglycerides was 1.50 (interquartile range = 1.11) mmol/L. The mean values for apoB and apoA‐I were 1.03 (0.24) g/L and 1.54 (0.27) g/L, respectively. Association tests were adjusted for age, sex, and a binary variable denoting the genotyping chip individuals were allocated to in UKBB. The linkage disequilibrium (LD) clumping was undertaken to select independent SNPs (r2 < 0.001) based on a reference panel of 503 Europeans from phase III (version 5) of the 1000 Genomes Project 21 and the SNP with the smallest p values for the association with the trait of interest was retained in each locus. In univariable MR analysis, we used 220 SNPs as instrument variables for LDL cholesterol, 534 SNPs for HDL cholesterol, 440 SNPs for triglycerides, 440 SNPs for apoA‐I, and 255 SNPs for apoB (Supplementary Table S2). By combing SNPs from related lipid‐traits and selecting independent SNPs (r2 < 0.01) by clump function in TwoSampleMR package, 22 we used 548 SNPs in the multivariable MR analysis of LDL cholesterol, triglycerides and apoB, and 569 SNPs in the multivariable MR analysis of HDL cholesterol and apoA‐I. We used 32 SNPs associated with stroke at the genomewide significance level as the genetic instruments for stroke in the reverse MR analysis. 20
Outcome Source
Summary‐level data for ischemic stroke and subtypes were obtained from the MEGASTROKE consortium encompassing 29 genomewide association studies (GWAS) with a final sample of 514,791 individuals (60,341 ischemic stroke cases and 454,450 non‐cases) of multi‐ancestry (European people as the majority, 86%). 20 The stroke cases were defined as rapidly developing signs of focal (or global) disturbance of cerebral function, lasting more than 24 hours or leading to death with no apparent cause other than that of vascular origin. Any ischemic stroke was defined by all stroke cases except for intracerebral hemorrhage. Any ischemic stroke included large artery ischemic stroke (LAS; 6,688 cases), cardioembolic ischemic stroke (CES; 9,006 cases), and small vessel ischemic stroke (SVS; 11,710 cases) according to the Trial of Org 10,172 in Acute Stroke Treatment criteria 23 and also included ischemic stroke of undefined subtype. Association test was performed under an additive genetic model with a minimum of sex and age as covariates. Summary‐level data for stroke based merely on European population were used in a sensitivity analysis. Summary‐level genetic data for lipids and apolipoprotein were obtained from Neale Laboratories (http://www.nealelab.is/).
Statistical Analysis
The inverse‐variance weighted method was used as major analysis. This method provides an estimate with the highest power and rely on the assumption that all SNPs are valid instrumental variables. The I2 (%) statistic was calculated to assess the heterogeneity among estimates across individual SNPs. 24 The weighted median approach and MR‐Egger regression were used as secondary analyses to examine the robustness of the results and correct for pleiotropy. The weighted median analysis can generate consistent estimates if at least 50% of the weight in the analysis comes from valid instrumental variables. 25 The MR‐Egger regression approach can detect and correct for directional pleiotropy albeit with compromised power. 25 Given genetic and phenotypic correlations across lipid‐related traits (Pearson's R ranging from −0.49 to 0.96; Supplementary Table S3), 9 we further used multivariable inverse‐variance weighted method to disentangle and compare the effects of correlated lipid‐traits on ischemic stroke and subtypes. Odd ratios (ORs) and corresponding 95% confidence intervals (CIs) for outcomes were scaled to one‐SD increase in levels of lipid‐related traits. To account for multiple testing, we considered associations with p values below 0.003 (where p = 0.05/20 [5 lipid‐related traits and 4 stroke outcomes]) to represent strong evidence of causal associations, and associations with p values below 0.05 but above 0.003 as suggestive evidence of associations in the univariable MR analysis. The multiple testing was not tailed for multivariable MR analysis due to the mutual adjustment nature of multivariable MR analysis. Statistical power was estimated using a webtool 26 and the results are shown in Supplementary Table S4. All analyses were performed using the mrrobust package 27 in Stata/SE 15.0 (Stata Statistical Software: Release 15; StataCorp LLC, College Station, TX, USA) and the TwoSampleMR 22 and Mendelian Randomization package 28 in R Software 3.6.0 (R Core Team, R Foundation for Statistical Computing, Vienna, Austria, 2019; https://www.R-project.org).
Data Availability
The datasets analyzed in this study are publicly available summary statistics. Data used can be obtained through cited papers.
Results
Univariable MR Analysis
Genetically predicated increased levels of apoB, LDL cholesterol, and triglycerides and decreased levels of apoA‐I and HDL cholesterol were significantly or suggestively associated with higher risk of acute ischemic stroke (AIS), LAS, and SVS, but not with CES. The effect sizes of the lipid‐related traits were similar for LAS and SVS than for AIS (Fig 2). Results of sensitivity analyses are displayed in Supplementary Table S5. The observed associations persisted based on data from individuals of European descent (Supplementary Table S6). We did not detect any reverse associations of genetic liability to stroke with the levels of lipids and apolipoproteins (Supplementary Table S7).
FIGURE 2.
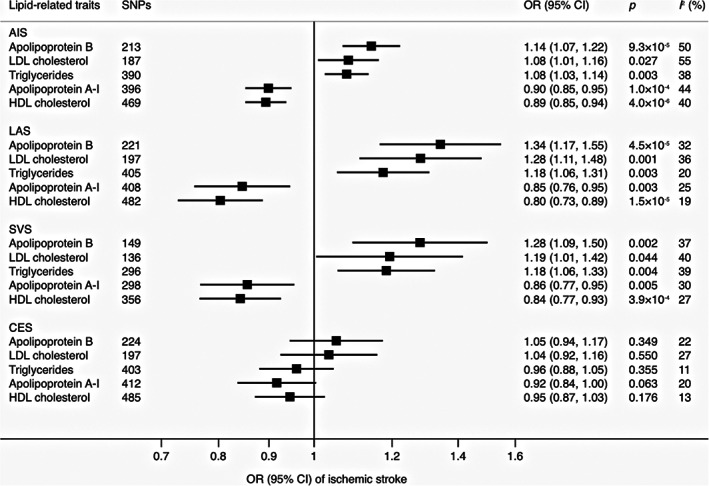
Associations of lipid‐related traits with stroke and subtypes in inverse‐variance weighted model. AIS = acute ischemic stroke; CES = cardioembolic stroke; CI = confidence interval; HDL = high‐density lipoprotein; LAS = large artery stroke; LDL = low‐density lipoprotein; OR = odds ratio; SNPs = single‐nucleotide polymorphisms; SVS = small vessel stroke.
Multivariable MR Analysis
Results of multivariable MR analysis are displayed in Figure 3 and Figure 4. In the multivariable MR analysis with mutual adjustment for apoB, LDL cholesterol, and triglycerides, apoB retained a robust causal association with AIS, LAS, and SVS, whereas the estimate for LDL cholesterol was reversed and that for triglycerides largely attenuated. The ORs of AIS, LAS, and SVS were 1.31 (95% CI = 1.01, 1.69), 1.69 (95% CI = 0.99, 2.87), and 2.18 (95% CI = 1.14, 4.18), respectively, for one‐SD increase of apoB (Fig 3). The pattern for the comparative role of apoB, LDL cholesterol, and triglycerides in ischemic stroke persisted when the analysis was confined to individuals of merely European ancestry (Supplementary Table S8). In the analysis of mutual adjustment for apoA‐I and HDL cholesterol, the magnitude of the associations for HDL cholesterol persisted or became stronger but became nonsignificant. Associations for apoA‐I became weaker or attenuated substantially to the null in the analyses of LAS and SVS. The results were consistent based on data derived from individuals of European descent and multi‐ancestries (Fig 4 and Supplementary Table S8). We did not include CES in Figures 3 and 4 because no association was detected in the univariable MR analysis of lipids and apolipoproteins with CES.
FIGURE 3.
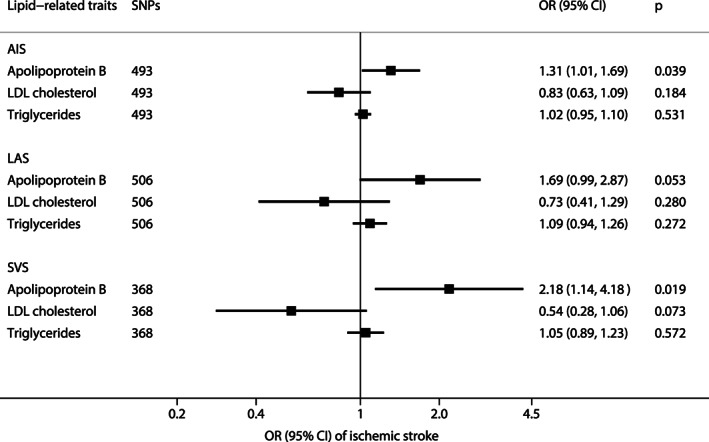
Associations of apolipoprotein B, LDL cholesterol, and triglycerides with stroke and subtypes in multivariable inverse‐variance weighted model. AIS = acute ischemic stroke; CI = confidence interval; LAS = large artery stroke; LDL = low‐density lipoprotein; OR = odds ratio; SNPs = single‐nucleotide polymorphisms; SVS = small vessel stroke.
FIGURE 4.
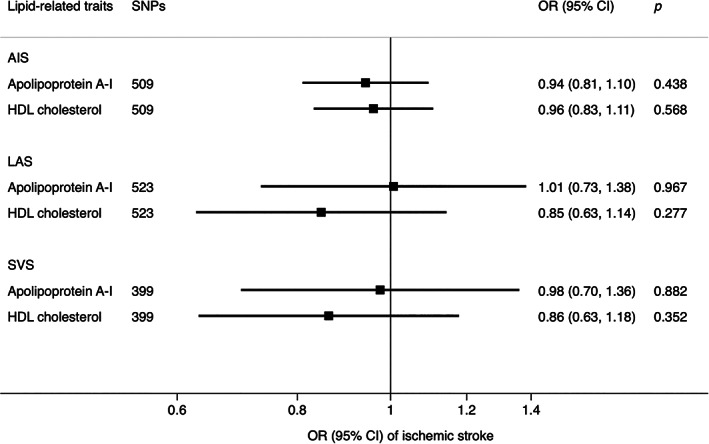
Associations of apolipoprotein A‐I and LDL cholesterol with stroke and subtypes in multivariable inverse‐variance weighted model. AIS = acute ischemic stroke; CI = confidence interval; HDL = high‐density lipoprotein; LAS = large artery stroke; OR = odds ratio; SNPs = single‐nucleotide polymorphisms; SVS = small vessel stroke.
Discussion
Principal Findings
The present study confirmed the causal effects of apoB, apoA‐I, LDL and HDL cholesterol, and triglycerides on ischemic stroke. Results of multivariable MR analyses showed that the effect of apoB on ischemic stroke remained robust, whereas the associations of LDL cholesterol and triglycerides entities with ischemic stroke attenuated markedly to the null after the adjustment. This suggests that apoB is the critical entity that underlies the positive associations of lipid‐related factors and ischemic stroke, in particular, large artery and small vessel stroke. The associations for HDL cholesterol and apoA‐I became nonsignificant after adjustment; however, the magnitude of the associations for HDL cholesterol remained or became stronger. Whether HDL cholesterol had predominant effects on ischemic stroke needs more study.
Our findings of the univariable MR investigation are overall in line with previous studies on LDL and HDL cholesterol in relation to ischemic stroke. 1 , 2 , 3 , 4 , 5 However, a previous MR study based on a smaller sample size revealed that only LDL cholesterol was statistically significantly associated with LAS and that HDL cholesterol was related to SVS. 2 That study did not observe any associations of triglycerides with ischemic stroke and subtypes, which is not consistent with the present study and a recently published MR study. 3 Discrepancy might be caused by inadequate power due to limited phenotypic variance explained by used genetic variants for the lipid trait and/or small sample size for stroke outcomes. Observational and genetic studies have reported an inverse association of apoA‐I and a positive association of apoB with ischemic stroke. 29 , 30 The present MR study confirmed those associations but extended the evidence to show that only apoB showed independent effects on stroke.
Studies on comparative effects of apoB, LDL cholesterol, and triglycerides on stroke are limited. Nevertheless, the finding of a predominant role of apoB in ischemic stroke observed in our study showed agreement with several studies on ischemic cardiovascular disease. 9 , 31 , 32 In the Copenhagen City Heart Study, even though apoB was not found to predict ischemic stroke better than LDL cholesterol in a clear pattern, women with higher levels of apoB had similar risk estimates for ischemic cerebrovascular disease and ischemic stroke compared with those with lower levels. 32 Notably, the observed dominant role of apoB does not discredit the causal roles of LDL cholesterol or triglycerides in ischemic stroke, as both LDL cholesterol and triglycerides are enveloped in atherogenic lipoproteins, each containing an apolipoprotein B molecule that cannot occur in physiological isolation. 33 Instead, our study provides genetic evidence that apoB is the necessary element in order for bad lipoprotein lipids to exert their causal effect on ischemic stroke. In other words, changes in the amount of cholesterol and triglycerides in lipoproteins that are not accompanied by commensurate changes in number of lipoprotein particles containing apoB may not affect ischemic stroke risk. Mechanically, this finding is supported by the “response to retention” hypothesis that apoB is the necessary entity for atherosclerosis to occur. 15 , 16 In detail, particles containing apoB trapped in the tunica intima of the arterial wall cause atherosclerosis. 34
Studies on comparative effects of HDL cholesterol and apoA‐I are limited. The present study found a stronger effect of HDL cholesterol than of apoA‐I on ischemic stroke. However, due to an inadequate power embedding in multivariable MR analysis, whether HDL cholesterol plays a predominant protective role in the etiology of ischemic stroke needs more investigation. High HDL cholesterol levels prevent the oxidation of LDL cholesterol and increase the reverse transport of LDL cholesterol from peripheral tissues to the liver where degradation happens. 35 These functions of HDL cholesterol lower the risk of atherogenesis and may explain why high HDL cholesterol levels reduce the risk of ischemic stroke.
Public Health and Clinical Implication
Clinical trials have demonstrated that modifying LDL cholesterol and triglycerides through angiopoietin‐like proteins 4 and proprotein convertase subtilisin/kexin type 9 inhibitor might be promising approaches to lower the risk of ischemic stroke. 36 , 37 The present study supports these current treatments. More importantly, our findings shed new light on the focus of lipid‐modifying therapies, which should be the reduction in the number of atherogenic lipoprotein particles rather than the reduction in the amount of cholesterol or triglycerides within the particles. In addition, from the preventive perspective and especially among individuals with discordant apoB and LDL cholesterol levels, we promote apoB measurement as one of routine blood lipid examination.
Strengths and Limitations
There are strengths of the present study. The major one was the multivariable MR method, which compared the roles of different correlated lipid‐related traits in ischemic stroke and exempted the findings from residual confounding and reverse causality. We used updated genetic instruments for lipid‐related traits, thereby ensuring an adequate power in analysis. The major limitation was that there were missing SNPs that might compromise the power and accuracy of analysis. However, the missing rates were not dissatisfying for AIS, LAS, and CES (all under 15%), except for SVS. Thus, the observed associations for SVS needs to be verified. In addition, a small proportion of stroke cases were from non‐European descents (around 14%), which might introduce population stratification bias. Nevertheless, the consistent findings based on data from individuals of only European ancestry indicated that there was a neglectable chance of population stratification bias twisting our findings. The CIs in the multivariable MR analysis were wide, which might reveal some degree of compromise of the precision of MR statistical model fitting strongly correlated exposures. Finally, the associations of SNPs with levels of lipid‐traits were derived from non‐fasting blood samples, which might cause inaccuracy in estimation. Nonetheless, the GWAS for lipid‐related traits found that adjustment for fasting time led to negligible alterations in the effect estimates.
Conclusions
In summary, the present MR study provides evidence supporting apoB as the predominant trait that accounts for the etiological basis of apoB, HDL cholesterol, and triglycerides in relation to ischemic stroke, in particular large artery and small vessel stroke. Whether HDL cholesterol exerts protective effects on ischemic stroke independent of apoA‐I needs further investigation.
Author Contributions
S.Y. and S.C.L. contributed to the conception and design of the study. S.Y. contributed to the acquisition and analysis of data. S.Y., B.T., J.Z., and S.C.L. contributed to drafting the text and preparing the figures.
Potential Conflicts of Interest
The authors declared no conflicts of interest.
Data Availability
The datasets analyzed in this study are publicly available summary statistics.
Supporting information
Appendix S1: Supporting information
Acknowledgments
Genetic instruments for 5 lipid‐related traits were obtained from a genomewide associations study in UK Biobank (Richardson TG et al, 2020). Genetic instruments for stroke and summary‐level data for stroke and subtypes were obtained from the MEGASTROKE consortium. Summary‐level data for lipid‐related traits were obtained from the Neale Laboratory. The authors thank all investigators for sharing these data. The list of investigators of MEGASTROKE is available at http://megastroke.org/authors.html. Funding of the MEGASTROKE project is specified at megastroke.org/acknowledgements.html. The present study is funded by the Swedish Research Council for Health, Working Life, and Welfare (Forte; grant no. 2018‐00123) and the Swedish Research Council (Vetenskapsrådet; grant no. 2019‐00977). J.Z. is funded by a Vice‐Chancellor Fellowship from the University of Bristol and the UK Medical Research Council Integrative Epidemiology Unit (MC_UU_00011/1, MC_UU_00011/4).
References
- 1. Holmes MV, Millwood IY, Kartsonaki C, et al. Lipids, lipoproteins, and metabolites and risk of myocardial infarction and stroke. J Am Coll Cardiol 2018;71:620–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hindy G, Engström G, Larsson SC, et al. Role of blood lipids in the development of ischemic stroke and its subtypes: a Mendelian randomization study. Stroke 2018;49:820–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sun L, Clarke R, Bennett D, et al. Causal associations of blood lipids with risk of ischemic stroke and intracerebral hemorrhage in Chinese adults. Nat Med 2019;25:569–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kurth T, Everett BM, Buring JE, et al. Lipid levels and the risk of ischemic stroke in women. Neurology 2007;68:556–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Allara E, Morani G, Carter P, et al. Genetic determinants of lipids and cardiovascular disease outcomes: a wide‐angled Mendelian randomization investigation. Circ Genom Precis Med 2019;12:e002711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Amarenco P, Labreuche J. Lipid management in the prevention of stroke: review and updated meta‐analysis of statins for stroke prevention. Lancet Neurol 2009;8:453–463. [DOI] [PubMed] [Google Scholar]
- 7. White HD, Simes RJ, Anderson NE, et al. Pravastatin therapy and the risk of stroke. N Engl J Med 2000;343:317–326. [DOI] [PubMed] [Google Scholar]
- 8. Amarenco P, Bogousslavsky J, Callahan A, et al. Stroke prevention by aggressive reduction in cholesterol levels (SPARCL) investigators. High‐dose atorvastatin after stroke or transient ischemic attack. N Engl J Med 2006;355:549–559. [DOI] [PubMed] [Google Scholar]
- 9. Richardson TG, Sanderson E, Palmer TM, et al. Evaluating the relationship between circulating lipoprotein lipids and apolipoproteins with risk of coronary heart disease: a multivariable Mendelian randomisation analysis. PLoS Med 2020;17:e1003062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Welsh C, Celis‐Morales CA, Brown R, et al. Comparison of conventional lipoprotein tests and apolipoproteins in the prediction of cardiovascular disease. Circulation 2019;140:542–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mora S, Martin SS, Virani SS. Cholesterol insights and controversies from the UK Biobank study. Circulation 2019;140:553–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mach F, Baigent C, Catapano AL, et al. 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41:111–188. [DOI] [PubMed] [Google Scholar]
- 13. Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019;139:e1082–e1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Holmes MV, Ala‐Korpela M. What is 'LDL cholesterol. Nat Rev Cardiol 2019;16:197–198. [DOI] [PubMed] [Google Scholar]
- 15. Sniderman AD, Thanassoulis G, Glavinovic T, et al. Apolipoprotein B particles and cardiovascular disease: a narrative review. JAMA Cardiol 2019;4:1287–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sniderman AD, Pencina M, Thanassoulis G. ApoB. Circ Res 2019;124:1425–1427. [DOI] [PubMed] [Google Scholar]
- 17. Brunner FJ, Waldeyer C, Ojeda F, et al. Application of non‐HDL cholesterol for population‐based cardiovascular risk stratification: results from the multinational cardiovascular risk consortium. Lancet 2019;394:2173–2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sniderman AD, Williams K, Contois JH, et al. A meta‐analysis of low‐density lipoprotein cholesterol, non‐high‐density lipoprotein cholesterol, and apolipoprotein B as markers of cardiovascular risk. Circ Cardiovasc Qual Outcomes 2011;4:337–345. [DOI] [PubMed] [Google Scholar]
- 19. Sanderson E, Davey Smith G, Windmeijer F, Bowden J. An examination of multivariable Mendelian randomization in the single‐sample and two‐sample summary data settings. Int J Epidemiol 2019;48:713–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Malik R, Chauhan G, Traylor M, et al. Multiancestry genome‐wide association study of 520,000 subjects identifies 32 loci associated with stroke and stroke subtypes. Nat Genet 2018;50:524–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. 1000 Genomes Project Consortium , Abecasis GR, Auton A, et al. An integrated map of genetic variation from 1,092 human genomes. Nature 2012;491:56–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hemani G, Zheng J, Elsworth B, et al. The MR‐Base platform supports systematic causal inference across the human phenome. Elife 2018;7:e34408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Adams HP Jr, Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in acute stroke treatment. Stroke 1993;24:35–41. [DOI] [PubMed] [Google Scholar]
- 24. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Stat Med 2002;21:1539–1558. [DOI] [PubMed] [Google Scholar]
- 25. Burgess S, Bowden J, Fall T, et al. Sensitivity analyses for robust causal inference from Mendelian randomization analyses with multiple genetic variants. Epidemiology 2017;28:30–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Brion MJ, Shakhbazov K, Visscher PM. Calculating statistical power in Mendelian randomization studies. Int J Epidemiol 2013;42:1497–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Spiller W, Davies NM, Palmer TM. Software application profile: mrrobust—a tool for performing two‐sample summary Mendelian randomization analyses. Int J Epidemiol 2019;48:684–690. [Google Scholar]
- 28. Yavorska OO, Burgess S. MendelianRandomization: an R package for performing Mendelian randomization analyses using summarized data. Int J Epidemiol 2017;46:1734–1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dong H, Chen W, Wang X, et al. Apolipoprotein A1, B levels, and their ratio and the risk of a first stroke: a meta‐analysis and case‐control study. Metab Brain Dis 2015;30:1319–1330. [DOI] [PubMed] [Google Scholar]
- 30. Benn M, Nordestgaard BG, Jensen JS, Tybjaerg‐Hansen A. Polymorphisms in apolipoprotein B and risk of ischemic stroke. J Clin Endocrinol Metab 2007;92:3611–3617. [DOI] [PubMed] [Google Scholar]
- 31. Benn M. Apolipoprotein B levels, APOB alleles, and risk of ischemic cardiovascular disease in the general population, a review. Atherosclerosis 2009;206:17–30. [DOI] [PubMed] [Google Scholar]
- 32. Benn M, Nordestgaard BG, Jensen GB, Tybjaerg‐Hansen A. Improving prediction of ischemic cardiovascular disease in the general population using apolipoprotein B: the Copenhagen City Heart Study. Arterioscler Thromb Vasc Biol 2007;27:661–670. [DOI] [PubMed] [Google Scholar]
- 33. Ala‐Korpela M. The culprit is the carrier, not the loads: cholesterol, triglycerides and apolipoprotein B in atherosclerosis and coronary heart disease. Int J Epidemiol 2019;48:1389–1392. [DOI] [PubMed] [Google Scholar]
- 34. Borén J, Williams KJ. The central role of arterial retention of cholesterol‐rich apolipoprotein‐B‐containing lipoproteins in the pathogenesis of atherosclerosis: a triumph of simplicity. Curr Opin Lipidol 2016;27:473–483. [DOI] [PubMed] [Google Scholar]
- 35. Sacco RL, Benson RT, Kargman DE, et al. High‐density lipoprotein cholesterol and ischemic stroke in the elderly: the Northern Manhattan Stroke Study. JAMA 2001;285:2729–2735. [DOI] [PubMed] [Google Scholar]
- 36. Sabatine MS, Giugliano RP, Wiviott SD, et al. Efficacy and safety of evolocumab in reducing lipids and cardiovascular events. N Engl J Med 2015;372:1500–1509. [DOI] [PubMed] [Google Scholar]
- 37. Bouleti C, Mathivet T, Coqueran B, et al. Protective effects of angiopoietin‐like 4 on cerebrovascular and functional damages in ischaemic stroke. Eur Heart J 2013;34:3657–3668. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Supporting information
Data Availability Statement
The datasets analyzed in this study are publicly available summary statistics. Data used can be obtained through cited papers.
The datasets analyzed in this study are publicly available summary statistics.


